Abstract
Background
The role of inflammation and hypoproteinemia in influencing outcomes of critically ill patients has been widely recognized. However, there is a paucity of research on the prognostic value of the platelet-to-albumin ratio (PAR) in critically ill patients. Therefore, the aim of this study is to assess the prognostic significance of PAR in this population.
Methods
Patients diagnosed with critical illnesses from January 2020 to October 2022 were retrospectively enrolled in our study. Baseline demographic and clinical data were collected for each participant. Platelet counts and albumin levels were measured at baseline, and the PAR was calculated. The area under the receiver operating characteristic curve, Kaplan–Meier survival analysis, and multivariate Cox regression analysis were used to predict 30-day mortality.
Results
Three hundred and seventy-eight patients diagnosed with critical illness were categorized into two groups: survivors (n = 299) and non-survivors (n = 79). Analysis of the 30-day outcome revealed that the area under the curve (AUC) for the PAR (AUC: 0.705; 95% CI 0.639–0.771; p < 0.001) was significantly higher than that for albumin (AUC: 0.673; 95% CI 0.609–0.736; p < 0.001), but slightly lower than that for the APACHE II score (AUC: 0.713; 95% CI 0.650–0.777; p < 0.001). In addition, the Kaplan–Meier survival analysis demonstrated a significantly higher 30-day mortality in the high-PAR group. Furthermore, Cox regression analysis identified albumin (HR: 0.936; 95% CI 0.895–0.978; p = 0.003), APACHE II score (HR: 1.225; 95% CI 1.149–1.305; p < 0.001), and high PAR (HR: 1.237; 95% CI 1.130–1.353; p < 0.001) as independent risk factors for the prognosis of critically ill patients.
Conclusions
The PAR has emerged as a significant prognostic indicator in critically ill patients, with an elevated ratio being associated with poorer clinical outcomes.
Keywords: Platelet count, Albumin, Critical illness, Mortality
Introduction
Critical illness remains a significant global challenge and a major cause of morbidity and mortality, while early diagnosis and prompt treatment can substantially enhance patient outcomes [1, 2]. Previous studies have shown that impaired physiological parameters can be reversed with timely interventions within a few days of hospital admission [3, 4]. Therefore, early identification of stratified high-risk patients can facilitate clinical interventions and improve patient prognosis.
Platelets (PLT) serve as indicators of the body's hemostatic and thrombotic function, as well as its regulation of immune inflammation [5, 6]. Albumin is synthesized by the liver. Albumin levels in some populations can be interpreted as indicators of nutritional status, while albumin as an acute response protein also reflects inflammation [7, 8]. Hypoalbuminemia, which is common in critically ill patients, is associated with worse outcomes [9, 10]. Recently, the platelet-to-albumin ratio (PAR) has emerged as a useful and potential prognostic biomarker in several diseases, including cholangiocarcinoma [11], lung cancer [12], and peritoneal dialysis [13]. Prior research has demonstrated that the prognosis of critically ill patients is influenced by an excessive host inflammatory response and hypoalbuminemia [14, 15]. Consequently, we hypothesized that PAR could be used as a prognostic indicator in critically ill patients.
To our knowledge, the prognostic significance of the association between the PAR and critically ill patients has not been previously reported. The aim of this study is to determine whether a high PAR is associated with 30-day mortality among critically ill patients, and to assess its potential as a prognostic indicator.
Methods
Patients
This retrospective cohort study was conducted in a 24-bed ICU at The Sixth Affiliated Hospital of the School of Medicine, South China University of Technology, Foshan, China. The study encompassed patients admitted to the ICU between January 2020 and October 2022. Critically ill patients were defined as individuals meeting at least two of the four systemic inflammatory response syndrome (SIRS) criteria: a heart rate exceeding 90 beats per minute, a respiratory rate surpassing 20 breaths per minute, a temperature above 38 °C or below 36 °C, and a white blood cell (WBC) count either exceeding 12,000 cells/mm3or falling below 4000 cells/mm3, or alternatively, the presence of more than 10% band cells. Furthermore, patients were also considered critically ill if they fulfilled two SIRS criteria and had a lactate level exceeding 4 mmol/L [3]. Diagnoses of critical illness were categorized according to the International Classification of Diseases, 10th Revision (ICD-10). Patients were excluded from the study if they were under 18 years of age, had cirrhosis, were pregnant, or had a hospital stay of less than 24 h. The study was approved by the Ethics Committee of the Sixth Affiliated Hospital, School of Medicine, South China University of Technology (Approval No. 2023236), and informed consent was waived due to the retrospective nature of the study.
Data collection
Data on patient characteristics such as age, sex, underlying diseases, severity of illness as measured by the Acute Physiology and Chronic Health Evaluation II (APACHE II), mechanical ventilation, primary diagnosis, 30-day mortality, and laboratory test results were extracted from the hospital's electronic medical record system.
Laboratory parameters
Blood samples were taken within 24 h of ICU admission. Complete blood count was performed using a Sysmex XN-9000 hematology analyzer (Sysmex, Kobe, Japan). Serum albumin levels were measured using a Beckman Coulter AU5800 chemistry analyzer (Beckman Coulter Inc., Brea, CA). Laboratory parameters assessed included white blood cells, neutrophils, lymphocytes, eosinophils, hemoglobin, platelet count and albumin. The platelet-to-albumin ratio (PAR) was calculated by dividing the absolute platelet count by the serum albumin level.
Sample size calculation
The sample size was determined based on the expected 30-day mortality rates of 30% in the high PAR group and 15% in the low PAR group. With a significance level of 5% and power of 80%, it was estimated that 236 critically ill patients were needed for the study.
Statistical analysis
Normally distributed data were expressed as mean and standard deviation (mean ± SD), whereas non-normally distributed data were presented as median and interquartile range [M (Q1, Q3)]. The Mann–Whitney U test was used to compare continuous variables between the two groups. Categorical data were also described as numbers and percentages [n (%)], and the chi-squared test was used to compare proportions across groups. Receiver operating characteristic (ROC) curve analysis was performed to assess the predictive accuracy of the biomarkers for mortality. Comparisons of ROC AUC values were made using De-Long's method [16]. Survival curves for mortality were estimated and plotted using the Kaplan–Meier method, and differences were assessed using the log-rank test. Cox proportional hazards regression was used to identify factors associated with mortality. Data processing and analysis were performed using SPSS software, version 26.0 (IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
A total of 378 patients were enrolled, including survivor group (n = 299) and non-survivor group (n = 79). Baseline data are presented in Table 1. When comparing the survivor group to the non-survivor group, we observed that the survivor group had a higher incidence of chronic obstructive pulmonary disease (7.69% vs. 1.27%; p = 0.037), as well as higher leucocyte counts (12.74 × 109/L [interquartile range (IQR) 8.64–17.75 × 109/L] vs. 10.37 × 109/L [IQR 6.45–16.19 × 109/L]; p = 0.026) and neutrophil counts (10.48 × 109/L [interquartile range (IQR) 6.65–15.49 × 109/L] vs. 8.74 × 109/L [IQR 5.04–14.22 × 109/L]; p = 0.040). In addition, the median albumin level was significantly higher in the survivor group (33.42 vs. 27.10 g/L; p < 0.001). In contrast, APACHE II score (19.26 ± 4.74 vs. 23.55 ± 5.30; p < 0.001), and the median PAR (5.43 vs. 6.79; p < 0.001) in survivor group were substantially decreased than in non-survivor group. We thus aimed to investigate the prognostic value of PAR.
Table 1.
Baseline characteristics by 30-day survival status
| Characteristic | Survivor group (n = 299) |
Non-survivor group (n = 79) |
p value |
|---|---|---|---|
| Age, years | 68 (54,77) | 68 (48,76) | 0.464 |
| Male (n, %) | 203 (67.89) | 58 (73.42) | 0.345 |
| BMI (kg/m2) | 22.51 ± 0.38 | 23.11 ± 0.43 | 0.521 |
| APACHE II | 19.26 ± 4.74 | 23.55 ± 5.30 | < 0.001 |
| Underlying diseases | |||
| Diabetes mellitus (n, %) | 87 (29.10) | 30 (37.97) | 0.129 |
| Hypertension (n, %) | 135 (45.15) | 28 (35.44) | 0.121 |
| Chronic obstructive pulmonary disease (n, %) | 23 (7.69) | 1 (1.27) | 0.037 |
| Chronic renal insufficiency (n, %) | 35 (11.71) | 13 (16.46) | 0.259 |
| Chronic cholecystitis (n, %) | 14 (4.68) | 3 (3.80) | 0.974 |
| Admission diagnosis | |||
| Pneumonia (n, %) | 63 (21.07) | 18 (22.80) | 0.731 |
| Severe burn or trauma (n, %) | 43 (14.38) | 8 (10.12) | 0.330 |
| Acute myocardial infarction (n, %) | 67 (22.41) | 22 (27.85) | 0.304 |
| Sepsis (n, %) | 65 (21.74) | 20 (25.32) | 0.498 |
| Mechanical ventilation (n, %) | 176 (58.86) | 52 (65.82) | 0.261 |
| Vasoactive drug (n, %) | 110 (36.79) | 38 (48.10) | 0.067 |
| Leucocytes count (109/L) | 12.74 (8.64, 17.75) | 10.37 (6.45, 16.19) | 0.026 |
| Neutrophil count (109/L) | 10.48 (6.65, 15.49) | 8.74 (5.04, 14.22) | 0.040 |
| Lymphocyte count (109/L) | 0.92 (0.50, 1.53) | 0.78 (0.43, 1.38) | 0.335 |
| Eosinophil count (109/L) | 0.01 (0.00, 0.05) | 0.01 (0.00, 0.04) | 0.392 |
| Hemoglobin (g/L) | 104.83 ± 29.69 | 98.63 ± 33.14 | 0.109 |
| Platelet count (109/L) | 194 (114, 244) | 191 (126, 258) | 0.711 |
| Albumin (g/L) | 33.42 ± 6.57 | 27.10 ± 6.80 | < 0.001 |
| PAR | 5.43 (3.62, 7.30) | 6.79 (4.71, 10.94) | < 0.001 |
Data are expressed as mean ± SD, n (%) or median (IQR), as appropriate; PAR, platelet-to-albumin ratio
Predicting outcomes by biomarkers
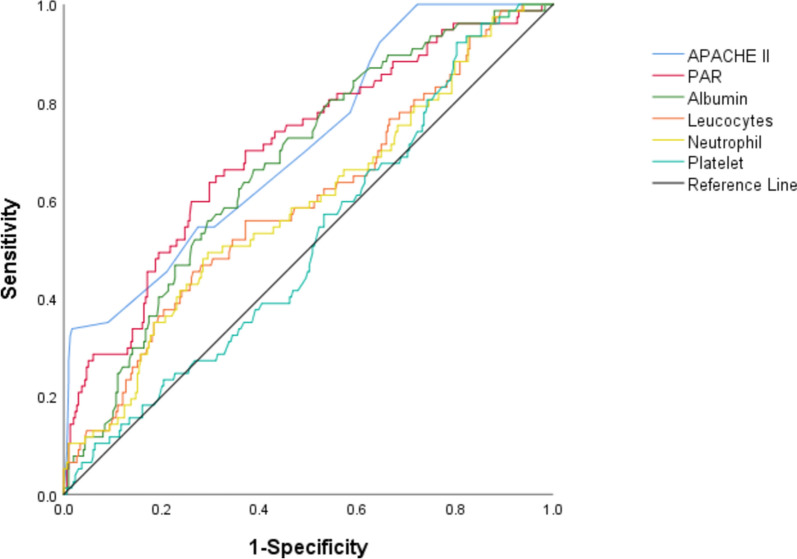
According to the ROC analysis results for mortality prediction, using De-Long's method to compare areas under the curve (AUC), PAR exhibited an AUC of 0.705, which was significantly higher than the AUC for Leucocytes (0.581), neutrophils (0.575), and albumin (0.673) alone (p < 0.05). Nonetheless, the AUC for PAR was marginally lower than that of the APACHE II score, which was 0.713. The ROC curves for all biomarkers to predict outcomes are given in Fig. 1 and Table 2.
Fig. 1.
Receiver operating characteristic curve analysis for mortality in patients. PAR, platelet-to-albumin ratio
Table 2.
AUC using ROC curve analyses to predict mortality in patients
| Parameter | AUC (95% CI) | SE | p value |
|---|---|---|---|
| Leucocytes | 0.581 (0.508–0.654) | 0.037 | 0.026 |
| Neutrophil | 0.575 (0.502–0.649) | 0.038 | 0.040 |
| Platelet | 0.516 (0.447–0.586) | 0.035 | 0.658 |
| Albumin | 0.673 (0.609–0.736) | 0.032 | < 0.001 |
| PAR | 0.705 (0.639–0.771) | 0.034 | < 0.001 |
| APACHE II | 0.713 (0.650–0.777) | 0.032 | < 0.001 |
AUC area under the curve, CI confidence interval, PAR platelet-to-albumin ratio, APACHE II Acute Physiology and Chronic Health Evaluation II, ROC receiver operating characteristic
Survival of patients by PAR level and multivariate Cox regression analysis of factors associated with 30-day mortality
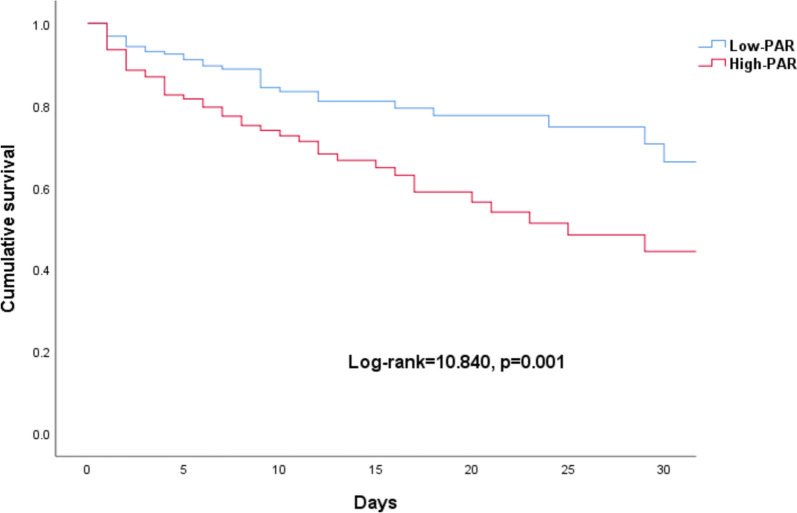
To explore whether the level of PAR affected patient outcome, we used the X-tile to calculate the optimal threshold of PAR. Then patients were divided into the low PAR group (PAR < 6.27) and the high PAR group (PAR ≥ 6.27). Our findings showed a high PAR group demonstrated a significantly higher 30-day mortality in the Kaplan–Meier survival analyses (Fig. 2). Furthermore, cox regression analysis revealed that albumin (HR: 0.936; 95% CI 0.895–0.978; p = 0.003), APACHE II score (HR: 1.225; 95% CI 1.149–1.305, p < 0.001) and high PAR (HR: 1.237; 95% CI 1.130–1.353, p < 0.001) were identified as independent risk factors for the prognosis of critically ill patients (Table 3).
Fig. 2.
Survival to day 30 by baseline ratio of PAR. PAR, platelet-to-albumin ratio
Table 3.
Multivariable Cox regression analysis for 30-day mortality
| Variables | HR (95% CI) | p value |
|---|---|---|
| Leucocytes count (109/L) | 0.910 (0.704–1.177) | 0.473 |
| Neutrophil count (109/L) | 1.049 (0.795–1.384) | 0.735 |
| Albumin (g/L) | 0.936 (0.895–0.978) | 0.003 |
| PAR ≥ 6.27 | 1.237 (1.130–1.353) | < 0.001 |
| Chronic obstructive pulmonary disease | 0.185 (0.023–1.481) | 0.112 |
| APACHE II | 1.225 (1.149–1.305) | < 0.001 |
PAR platelet-to-albumin ratio, HR Hazard ratio, CI confidence interval, APACHE II Acute Physiology and Chronic Health Evaluation II
Discussion
The prognosis of critically ill patients was significantly influenced by the presence of inflammation and hypoproteinemia. In this study, our findings demonstrated that a novel inflammation-based prognostic indicator, the PAR, was effective in predicting outcomes. Specifically, the high PAR group exhibited significantly higher 30-day mortality and served as an independent risk factor for the prognosis of critically ill patients.
Albumin, a negative acute-phase immune protein synthesized by the liver, has multiple physiological effects, involving regulation of colloid osmotic pressure, binding and transportation of various substances (for example, drugs, hormones) within the blood, antioxidant properties, nitric oxide modulation and buffer capabilities [17, 18]. Previous research has shown a correlation between hypoalbuminemia and unfavorable prognosis in critically ill patients, including those with acute myocardial infarction, sepsis, and burns [19–21]. In the present study, a significant reduction in serum albumin levels was observed in the non-survivor group in comparison with the survivor group. Although albumin levels are associated with 30-day mortality in critically ill patients, the area under the receiver operating characteristic curve for albumin is merely 0.673, suggesting a relatively limited predictive value. This finding may be attributed to factors such as inflammation, hypoalbuminemia, and the specific disease type that impact albumin levels [22, 23].
Platelets are a crucial component of blood cells, playing a vital role in the coagulation process. Several factors, such as infection, surgery, trauma, and early tissue cell damage, can trigger the direct or indirect activation of platelets [24]. Numerous studies have demonstrated that platelet count can serve as a sensitive prognostic indicator in critically ill patients [25–27]. However, our study found no significant difference in platelet count between the survivor group and non-survivor group. It is hypothesized that fluctuations in platelet count may be contributing to this condition. To minimize the impact of potential confounding factors, our study evaluated the PAR as a novel indicator derived from routine laboratory tests, which may offer valuable insights into the prognosis of critically ill patients.
In this study, our findings indicated that the area under the curve (AUC) for the PAR was significantly higher than that for albumin alone in predicting 30-day mortality. However, it was slightly inferior to the AUC of the APACHE II score. Moreover, an increased PAR was associated with a poorer prognosis in critically ill patients. Shirai et al. were the first to report on the prognostic significance of PAR in a study involving 107 patients with post-operative pancreatic ductal carcinoma, demonstrating that a PAR value greater than 46.4 was an independent risk factor for patient prognosis [28]. Similarly, Yang et al. conducted a study with 405 peritoneal dialysis patients, followed up for 24 months, and discovered that a baseline high PAR ratio was associated with tube placement failure and patient death [13]. In addition, a recent study on critically ill patients with colorectal cancer showed a higher mortality rate at 28 days with a PAR value of 8.6 or higher [23]. These findings suggest that PAR can serve as an effective prognostic indicator for various diseases. However, despite its potential, there is a lack of research on PAR in critically ill patients. In the current study, we observed a significantly higher PAR value in the non-survivor group and found that baseline PAR was associated with an increased likelihood of mortality within 30 days among critically ill patients. Furthermore, our research encompassed a wide variety of critical illnesses, making the results potentially more applicable to the intensive care unit setting. Taken together, our research results confirm that baseline high PAR is an independent prognostic factor for critically ill patients.
Our study had several limitations. First, as a single-center retrospective study with a relatively small sample size of critically ill patients, it may suffer from selection bias, thereby limiting the generalizability of our findings to other disease populations. Second, despite our efforts to account for a wide range of confounding variables, interventions such as continuous renal replacement therapy and antibiotic therapy, which are known to affect patient mortality, may still influence the prognostic significance of PAR. Nevertheless, it is important to note that PAR remains a clinically relevant and readily available metric for application in real-world clinical practice [29–31].
Conclusions
The study demonstrated that the PAR greater than 6.27 correlates significantly with adverse outcomes among critically ill patients. These findings provide a biological rationale for interventions aimed at improving the prognosis of patients with critical illnesses.
Acknowledgements
The authors are grateful to Prof. De-Bing Huang for his statistical assistance and supervision.
Author contributions
Zhi-Ying Deng collected and analyzed the data and prepared the manuscript. Ping Chen collected and analyzed the data and prepared the manuscript. Qing-Nian Wu collected and analyzed the data and revised the manuscript. Chun-Lin Liu designed the study, prepared and revised the manuscript. Shi-Qiang Guo analyzed the data, and revised the manuscript. All authors read and approved the final manuscript.
Availability of data and materials
Data are available on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the Sixth Affiliated Hospital, School of Medicine, South China University of Technology (Approval No.2023236).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bowker SL, Williams K, Volk A, Auger L, Lafontaine A, Dumont P, Wingert A, Davis A, Bialy L, Wright E, et al. Incidence and outcomes of critical illness in indigenous peoples: a systematic review and meta-analysis. Crit Care. 2023;27(1):285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falvey JR, Cohen AB, O’Leary JR, Leo-Summers L, Murphy TE, Ferrante LE. Association of social isolation with disability burden and 1-year mortality among older adults with critical illness. JAMA Intern Med. 2021;181(11):1433–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akilli NB, Yortanlı M, Mutlu H, Günaydın YK, Koylu R, Akca HS, Akinci E, Dundar ZD, Cander B. Prognostic importance of neutrophil-lymphocyte ratio in critically ill patients: Short- and long-term outcomes. Am J Emerg Med. 2014;32(12):1476–80. [DOI] [PubMed] [Google Scholar]
- 4.Beni CE, Arbabi S, Robinson BRH, O’Keefe GE. Early fluid is less fluid: Comparing early versus late ICU resuscitation in severely injured trauma patients. Crit Care Explor. 2024;6(7): e1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Athale J, Danner R. In critically ill patients with COVID-19, antiplatelet therapy did not increase organ support-free days at 21 d. Ann Internal Med. 2022;175(7):JC80. [DOI] [PubMed] [Google Scholar]
- 6.Thomas MR, Storey RF. The role of platelets in inflammation. Thromb Haemost. 2015;114(3):449–58. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Z, Pereira SL, Luo M, Matheson EM. Evaluation of blood biomarkers associated with risk of malnutrition in older adults: a systematic review and meta-analysis. Nutrients. 2017;9(8):829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He H, Liu D, Ince C. Colloids and the microcirculation. Anesth Analg. 2018;126(5):1747–54. [DOI] [PubMed] [Google Scholar]
- 9.Thongprayoon C, Cheungpasitporn W, Radhakrishnan Y, Petnak T, Qureshi F, Mao MA, Kashani KB. Impact of hypoalbuminemia on mortality in critically ill patients requiring continuous renal replacement therapy. J Crit Care. 2022;68:72–5. [DOI] [PubMed] [Google Scholar]
- 10.Kovacevic T, Miljkovic B, Mikov M, Stojisavljevic Satara S, Dragic S, Momcicevic D, Kovacevic P. The effect of hypoalbuminemia on the therapeutic concentration and dosage of vancomycin in critically ill septic patients in low-resource countries. Dose Response. 2019;17(2):1559325819850419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saito N, Shirai Y, Horiuchi T, Sugano H, Shiba H, Sakamoto T, Uwagawa T, Yanaga K. Preoperative platelet to albumin ratio predicts outcome of patients with cholangiocarcinoma. Anticancer Res. 2018;38(2):987–92. [DOI] [PubMed] [Google Scholar]
- 12.Guo M, Sun T, Zhao Z, Ming L. Preoperative platelet to albumin ratio predicts outcome of patients with non-small-cell lung cancer. Ann Thorac Cardiovasc Surg. 2021;27(2):84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Y, Yuan J, Liu L, Qie S, Yang L, Yan Z. Platelet-to-albumin ratio: a risk factor associated with technique failure and mortality in peritoneal dialysis patients. Ren Fail. 2021;43(1):1359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ingels C, Langouche L, Dubois J, Derese I, Vander Perre S, Wouters PJ, Gunst J, Casaer M, Güiza F, Vanhorebeek I, et al. C-reactive protein rise in response to macronutrient deficit early in critical illness: sign of inflammation or mediator of infection prevention and recovery. Intensive Care Med. 2022;48(1):25–35. [DOI] [PubMed] [Google Scholar]
- 15.Sharma K, Mogensen KM, Robinson MK. Pathophysiology of critical illness and role of nutrition. Nutr Clin Pract. 2019;34(1):12–22. [DOI] [PubMed] [Google Scholar]
- 16.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–45. [PubMed] [Google Scholar]
- 17.Arroyo V, García-Martinez R, Salvatella X. Human serum albumin, systemic inflammation, and cirrhosis. J Hepatol. 2014;61(2):396–407. [DOI] [PubMed] [Google Scholar]
- 18.Pesonen E, Vlasov H, Suojaranta R, Hiippala S, Schramko A, Wilkman E, Eränen T, Arvonen K, Mazanikov M, Salminen US, et al. Effect of 4% albumin solution vs ringer acetate on major adverse events in patients undergoing cardiac surgery with cardiopulmonary bypass: a randomized clinical trial. JAMA. 2022;328(3):251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furukawa M, Kinoshita K, Yamaguchi J, Hori S, Sakurai A. Sepsis patients with complication of hypoglycemia and hypoalbuminemia are an early and easy identification of high mortality risk. Intern Emerg Med. 2019;14(4):539–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang W, Li C, Wang Z, Wang H, Zhou N, Jiang J, Ni L, Zhang X, Wang D. Decreased serum albumin level indicates poor prognosis of COVID-19 patients: hepatic injury analysis from 2,623 hospitalized cases. Sci China Life Sci. 2020;63(11):1678–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melinyshyn A, Callum J, Jeschke MC, Cartotto R. Albumin supplementation for hypoalbuminemia following burns: unnecessary and costly! J Burn Care Res. 2013;34(1):8–17. [DOI] [PubMed] [Google Scholar]
- 22.Gatta A, Verardo A, Bolognesi M. Hypoalbuminemia. Intern Emerg Med. 2012;7(Suppl 3):S193–9. [DOI] [PubMed] [Google Scholar]
- 23.Li A, Wang Z, Lv Q, Ling Y. Prognostic utility of platelet-to-albumin ratio among critically ill patients with colorectal cancer: a propensity score matching study. J Oncol. 2022;2022:6107997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Griffin B, Wu C, O’Horo J, Faubel S, Jalal D, Kashani K. The association of platelet decrease following continuous renal replacement therapy initiation and increased rates of secondary infections. Crit Care Med. 2021;49(2):e130–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He Z, Wang H, Wang S, Li L. Predictive value of platelet-to-albumin ratio (PAR) for the cardiac-associated acute kidney injury and prognosis of patients in the intensive care unit. Int J Gen Med. 2022;15:8315–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haksoyler V, Topkan E. High pretreatment platelet-to-albumin ratio predicts poor survival results in locally advanced nasopharyngeal cancers treated with chemoradiotherapy. Ther Clin Risk Manag. 2021;17:691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gui Y, Xu Y, Yang P. Predictive value of the platelet-to-albumin ratio (PAR) on the risk of death at admission in patients suffering from severe fever with thrombocytopenia syndrome. J Inflamm Res. 2021;14:5647–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shirai Y, Shiba H, Haruki K, Horiuchi T, Saito N, Fujiwara Y, Sakamoto T, Uwagawa T, Yanaga K. Preoperative platelet-to-albumin ratio predicts prognosis of patients with pancreatic ductal adenocarcinoma after pancreatic resection. Anticancer Res. 2017;37(2):787–93. [DOI] [PubMed] [Google Scholar]
- 29.Zhao J, Feng J, Ma Q, Li C, Qiu F. Prognostic value of inflammation biomarkers for 30-day mortality in critically ill patients with stroke. Front Neurol. 2023;14:1110347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang Z, Zheng Q, Yu Y, Zheng H, Wu Y, Wang Z, Liu L, Zhang M, Liu T, Li H, et al. Prognostic significance of platelet-to-albumin ratio in patients with esophageal squamous cell carcinoma receiving definitive radiotherapy. Sci Rep. 2022;12(1):3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hao P, Feng S, Suo M, Wang S, Wu X. Platelet to albumin ratio: A risk factor related to prognosis in patients with non-ST-segment elevation acute coronary syndrome undergoing percutaneous coronary intervention. Int J Cardiol. 2023;395: 131588. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on reasonable request.