Abstract
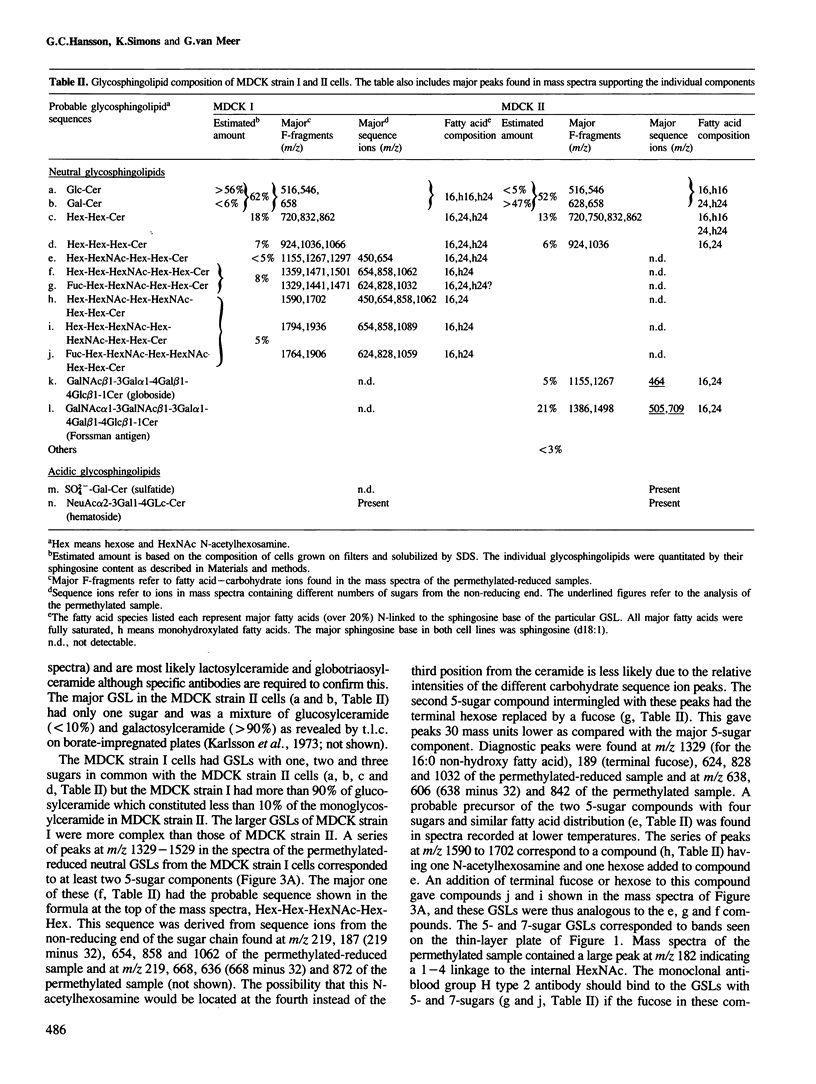
The glycosphingolipids (GSLs) of two sublines of Madin-Darby canine kidney (MDCK) cells, an epithelial cell line, were characterized by t.l.c., antibody overlay and mass spectrometry. The major characteristic which distinguishes the two MDCK cell strains is their trans-epithelial electrical resistance which is typically of the order of 3000 ohm.cm2 for strain I and 100 ohm.cm2 for strain II cells. Strain I and II cells were equally rich in glycolipids, the cellular GSL/phospholipid ratio being 0.04. However, while the phospholipid patterns were identical, the GSLs showed striking differences, and each cell strain expressed appreciable amounts of GSLs that were not found in the other strain. Both cell types possessed neutral GSLs with one, two or three carbohydrate moieties. The monoglycosylceramide accounted for 50% of the total GSLs in each strain. However, while in strain I cells over 90% of this monoglycosylceramide was monoglucosylceramide, in strain II cells over 90% consisted of monogalactosylceramide. In addition, MDCK strain II cells selectively expressed GSLs belonging to the globo series (26% of its neutral GSLs), including globoside and Forssman antigen, a globoside derivative. MDCK strain I cells, on the other hand, expressed another series of GSLs with 4-7 carbohydrate moieties characterized by the common sequence Hex-HexNAc-Hex-Hex-Cer. The presence of two fucosylated GSLs in these series was established. Both MDCK strain I and II cells contained negatively charged GSLs, the major component of which was the ganglioside GM3. MDCK strain II cells in addition expressed sulfatide, the sulfated derivative of galactosylceramide.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angström J., Breimer M. E., Falk K. E., Griph I., Hansson G. C., Karlsson K. A., Leffler H. Separation and characterization of hematosides with different sialic acids and ceramides from rat small intestine. Different composition of epithelial cells versus non-epithelial tissue and of duodenum versus jejunum-ileum. J Biochem. 1981 Oct;90(4):909–921. doi: 10.1093/oxfordjournals.jbchem.a133579. [DOI] [PubMed] [Google Scholar]
- Balcarova-Ständer J., Pfeiffer S. E., Fuller S. D., Simons K. Development of cell surface polarity in the epithelial Madin-Darby canine kidney (MDCK) cell line. EMBO J. 1984 Nov;3(11):2687–2694. doi: 10.1002/j.1460-2075.1984.tb02194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker G., Simmons N. L. Identification of two strains of cultured canine renal epithelial cells (MDCK cells) which display entirely different physiological properties. Q J Exp Physiol. 1981 Jan;66(1):61–72. doi: 10.1113/expphysiol.1981.sp002529. [DOI] [PubMed] [Google Scholar]
- Bouhours J. F., Glickman R. M. Rat intestinal glycolipids. II. Distribution and biosynthesis of glycolipids and ceramide in villus and crypt cells. Biochim Biophys Acta. 1976 Jul 20;441(1):123–133. doi: 10.1016/0005-2760(76)90287-3. [DOI] [PubMed] [Google Scholar]
- Brasitus T. A., Schachter D. Lipid dynamics and lipid-protein interactions in rat enterocyte basolateral and microvillus membranes. Biochemistry. 1980 Jun 10;19(12):2763–2769. doi: 10.1021/bi00553a035. [DOI] [PubMed] [Google Scholar]
- Breimer M. E., Hansson G. C., Karlsson K. A., Leffler H. Blood group type glycosphingolipids from the small intestine of different animals analysed by mass spectrometry and thin-layer chromatography. A note on species diversity. J Biochem. 1981 Sep;90(3):589–609. doi: 10.1093/oxfordjournals.jbchem.a133513. [DOI] [PubMed] [Google Scholar]
- Breimer M. E., Hansson G. C., Karlsson K. A., Leffler H. Glycosphingolipids and the differentiation of intestinal epithelium. Exp Cell Res. 1981 Sep;135(1):1–13. doi: 10.1016/0014-4827(81)90293-7. [DOI] [PubMed] [Google Scholar]
- Breimer M. E., Hansson G. C., Karlsson K. A., Leffler H. Glycosphingolipids of rat tissues. Different composition of epithelial and nonepithelial cells of small intestine. J Biol Chem. 1982 Jan 10;257(1):557–568. [PubMed] [Google Scholar]
- Breimer M. E., Hansson G. C., Karlsson K. A., Leffler H., Pimlott W., Samuelsson B. E. Selected ion monitoring of glycospingolipid mixtures. Identification of several blood group type glycolipids in the small intestine of an individual rabbit. Biomed Mass Spectrom. 1979 Jun;6(6):231–241. doi: 10.1002/bms.1200060603. [DOI] [PubMed] [Google Scholar]
- Brockhaus M., Magnani J. L., Blaszczyk M., Steplewski Z., Koprowski H., Karlsson K. A., Larson G., Ginsburg V. Monoclonal antibodies directed against the human Leb blood group antigen. J Biol Chem. 1981 Dec 25;256(24):13223–13225. [PubMed] [Google Scholar]
- Chapelle S., Gilles-Baillien M. Phospholipids and cholesterol in brush border and basolateral membranes from rat intestinal mucosa. Biochim Biophys Acta. 1983 Sep 20;753(2):269–271. doi: 10.1016/0005-2760(83)90017-6. [DOI] [PubMed] [Google Scholar]
- Feizi T. Demonstration by monoclonal antibodies that carbohydrate structures of glycoproteins and glycolipids are onco-developmental antigens. Nature. 1985 Mar 7;314(6006):53–57. doi: 10.1038/314053a0. [DOI] [PubMed] [Google Scholar]
- Forstner G. G., Wherrett J. R. Plasma membrane and mucosal glycosphingolipids in the rat intestine. Biochim Biophys Acta. 1973 Jun 21;306(3):446–459. doi: 10.1016/0005-2760(73)90183-5. [DOI] [PubMed] [Google Scholar]
- Fuller S., von Bonsdorff C. H., Simons K. Vesicular stomatitis virus infects and matures only through the basolateral surface of the polarized epithelial cell line, MDCK. Cell. 1984 Aug;38(1):65–77. doi: 10.1016/0092-8674(84)90527-0. [DOI] [PubMed] [Google Scholar]
- Gaush C. R., Hard W. L., Smith T. F. Characterization of an established line of canine kidney cells (MDCK). Proc Soc Exp Biol Med. 1966 Jul;122(3):931–935. doi: 10.3181/00379727-122-31293. [DOI] [PubMed] [Google Scholar]
- HAKOMORI S. A RAPID PERMETHYLATION OF GLYCOLIPID, AND POLYSACCHARIDE CATALYZED BY METHYLSULFINYL CARBANION IN DIMETHYL SULFOXIDE. J Biochem. 1964 Feb;55:205–208. [PubMed] [Google Scholar]
- Hakomori S. Glycosphingolipids in cellular interaction, differentiation, and oncogenesis. Annu Rev Biochem. 1981;50:733–764. doi: 10.1146/annurev.bi.50.070181.003505. [DOI] [PubMed] [Google Scholar]
- Hakomori S., Kannagi R. Glycosphingolipids as tumor-associated and differentiation markers. J Natl Cancer Inst. 1983 Aug;71(2):231–251. [PubMed] [Google Scholar]
- Hansson C. G., Karlsson K. A., Samuelsson B. E. The identification of sulphatides in human erythrocyte membrane and their relation to sodium-potassium dependent adenosine triphosphatase. J Biochem. 1978 Mar;83(3):813–819. doi: 10.1093/oxfordjournals.jbchem.a131977. [DOI] [PubMed] [Google Scholar]
- Hansson G. C., Karlsson K. A., Larson G., McKibbin J. M., Blaszczyk M., Herlyn M., Steplewski Z., Koprowski H. Mouse monoclonal antibodies against human cancer cell lines with specificities for blood group and related antigens. Characterization by antibody binding to glycosphingolipids in a chromatogram binding assay. J Biol Chem. 1983 Apr 10;258(7):4091–4097. [PubMed] [Google Scholar]
- Hansson G. C. The subcellular localization of the glycosphingolipids in the epithelial cells of rat small intestine. Biochim Biophys Acta. 1983 Sep 7;733(2):295–299. doi: 10.1016/0005-2736(83)90536-9. [DOI] [PubMed] [Google Scholar]
- Hise M. K., Mantulin W. W., Weinman E. J. Fluidity and composition of brush border and basolateral membranes from rat kidney. Am J Physiol. 1984 Sep;247(3 Pt 2):F434–F439. doi: 10.1152/ajprenal.1984.247.3.F434. [DOI] [PubMed] [Google Scholar]
- Ishizuka I., Tadano K., Nagata N., Niimura Y., Nagai Y. Hormone-specific responses and biosynthesis of sulfolipids in cell lines derived from mammalian kidney. Biochim Biophys Acta. 1978 Jul 17;541(4):467–482. doi: 10.1016/0304-4165(78)90156-3. [DOI] [PubMed] [Google Scholar]
- Kannagi R., Cochran N. A., Ishigami F., Hakomori S., Andrews P. W., Knowles B. B., Solter D. Stage-specific embryonic antigens (SSEA-3 and -4) are epitopes of a unique globo-series ganglioside isolated from human teratocarcinoma cells. EMBO J. 1983;2(12):2355–2361. doi: 10.1002/j.1460-2075.1983.tb01746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson K. A. Carbohydrate composition and sequence analysis of a derivative of brain disialoganglioside by mass spectrometry, with molecular weight ions at m-e 2245. Potential use in the specific microanalysis of cell surface components. Biochemistry. 1974 Aug 27;13(18):3643–3647. doi: 10.1021/bi00715a003. [DOI] [PubMed] [Google Scholar]
- Karlsson K. A., Leffler H., Samuelsson B. E. Characterization of the forssman glycolipid hapten of horse kidney by mass spectrometry. J Biol Chem. 1974 Aug 10;249(15):4819–4823. [PubMed] [Google Scholar]
- Karlsson K. A., Samuelsson B. E., Steen G. O. The lipid composition and Na+-K+-dependent adenosine-triphosphatase activity of the salt (nasal) gland of eider duck and herring gull. A role for sulphatides in sodium-ion transport. Eur J Biochem. 1974 Jul 15;46(2):243–258. doi: 10.1111/j.1432-1033.1974.tb03617.x. [DOI] [PubMed] [Google Scholar]
- Karlsson K. A., Samuelsson B. E., Steen G. O. The sphingolipid composition of bovine kidney cortex, medulla and papilla. Biochim Biophys Acta. 1973 Sep 25;316(3):317–335. doi: 10.1016/0005-2760(73)90072-6. [DOI] [PubMed] [Google Scholar]
- Kawai K., Fujita M., Nakao M. Lipid components of two different regions of an intestinal epithelial cell membrane of mouse. Biochim Biophys Acta. 1974 Nov 18;369(2):222–233. [PubMed] [Google Scholar]
- LEDUC E. H., TANAKA N. A study of the cellular distribution of Forssman antigen in various species. J Immunol. 1956 Sep;77(3):198–212. [PubMed] [Google Scholar]
- Levine Y. K., Wilkins M. H. Structure of oriented lipid bilayers. Nat New Biol. 1971 Mar 17;230(11):69–72. doi: 10.1038/newbio230069a0. [DOI] [PubMed] [Google Scholar]
- Louvard D. Apical membrane aminopeptidase appears at site of cell-cell contact in cultured kidney epithelial cells. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4132–4136. doi: 10.1073/pnas.77.7.4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio B., Cumar F. A., Caputto R. Surface behaviour of gangliosides and related glycosphingolipids. Biochem J. 1978 Jun 1;171(3):559–565. doi: 10.1042/bj1710559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnani J. L., Brockhaus M., Smith D. F., Ginsburg V. Detection of glycolipid ligands by direct binding of carbohydrate-binding proteins to thin-layer chromatograms. Methods Enzymol. 1982;83:235–241. doi: 10.1016/0076-6879(82)83016-4. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Fredman P., Svennerholm L. Receptor ganglioside content of three hosts for Sendai virus. MDBK, HeLa, and MDCK cells. Biochim Biophys Acta. 1984 Aug 8;775(1):7–16. doi: 10.1016/0005-2736(84)90228-1. [DOI] [PubMed] [Google Scholar]
- Matlin K. S., Simons K. Sorting of an apical plasma membrane glycoprotein occurs before it reaches the cell surface in cultured epithelial cells. J Cell Biol. 1984 Dec;99(6):2131–2139. doi: 10.1083/jcb.99.6.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKibbin J. M., Spencer W. A., Smith E. L., Mansson J. E., Karlsson K. A., Samuelsson B. E., Li Y. T., Li S. C. Lewis blood group fucolipids and their isomers from human and canine intestine. J Biol Chem. 1982 Jan 25;257(2):755–760. [PubMed] [Google Scholar]
- Meier P. J., Sztul E. S., Reuben A., Boyer J. L. Structural and functional polarity of canalicular and basolateral plasma membrane vesicles isolated in high yield from rat liver. J Cell Biol. 1984 Mar;98(3):991–1000. doi: 10.1083/jcb.98.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molitoris B. A., Simon F. R. Renal cortical brush-border and basolateral membranes: cholesterol and phospholipid composition and relative turnover. J Membr Biol. 1985;83(3):207–215. doi: 10.1007/BF01868695. [DOI] [PubMed] [Google Scholar]
- Naoi M., Lee Y. C., Roseman S. Rapid and sensitive determination of sphingosine bases and sphingolipids with fluorescamine. Anal Biochem. 1974 Apr;58(2):571–577. doi: 10.1016/0003-2697(74)90226-7. [DOI] [PubMed] [Google Scholar]
- Ojakian G. K., Herzlinger D. A. Analysis of epithelial cell surface polarity with monoclonal antibodies. Fed Proc. 1984 May 15;43(8):2208–2216. [PubMed] [Google Scholar]
- Op den Kamp J. A. Lipid asymmetry in membranes. Annu Rev Biochem. 1979;48:47–71. doi: 10.1146/annurev.bi.48.070179.000403. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Mirsky R., Fields K. L., Lisak R. P., Dorfman S. H., Silberberg D. H., Gregson N. A., Leibowitz S., Kennedy M. C. Galactocerebroside is a specific cell-surface antigenic marker for oligodendrocytes in culture. Nature. 1978 Aug 24;274(5673):813–816. [PubMed] [Google Scholar]
- Richardson J. C., Scalera V., Simmons N. L. Identification of two strains of MDCK cells which resemble separate nephron tubule segments. Biochim Biophys Acta. 1981 Feb 18;673(1):26–36. [PubMed] [Google Scholar]
- SVENNERHOLM L. CHROMATOGRAPHIC SEPARATION OF HUMAN BRAIN GANGLIOSIDES. J Neurochem. 1963 Sep;10:613–623. doi: 10.1111/j.1471-4159.1963.tb08933.x. [DOI] [PubMed] [Google Scholar]
- Siddiqui B., Hakomori S. A revised structure for the Forssman glycolipid hapten. J Biol Chem. 1971 Sep 25;246(18):5766–5769. [PubMed] [Google Scholar]
- Skipski V. P. Thin-layer chromatography of neutral glycosphingolipids. Methods Enzymol. 1975;35:396–425. doi: 10.1016/0076-6879(75)35178-1. [DOI] [PubMed] [Google Scholar]
- Sung S. S., Esselman W. J., Sweeley C. C. Structure of a pentahexosylceramide (Forssman hapten) from canine intestine and kidney. J Biol Chem. 1973 Sep 25;248(18):6528–6533. [PubMed] [Google Scholar]
- Thompson T. E., Tillack T. W. Organization of glycosphingolipids in bilayers and plasma membranes of mammalian cells. Annu Rev Biophys Biophys Chem. 1985;14:361–386. doi: 10.1146/annurev.bb.14.060185.002045. [DOI] [PubMed] [Google Scholar]
- Tokuyasu K. T. A technique for ultracryotomy of cell suspensions and tissues. J Cell Biol. 1973 May;57(2):551–565. doi: 10.1083/jcb.57.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentich J. D. Morphological similarities between the dog kidney cell line MDCK and the mammalian cortical collecting tubule. Ann N Y Acad Sci. 1981;372:384–405. doi: 10.1111/j.1749-6632.1981.tb15490.x. [DOI] [PubMed] [Google Scholar]
- Willison K. R., Stern P. L. Expression of a Forssman antigenic specificity in the preimplantation mouse embryo. Cell. 1978 Aug;14(4):785–793. doi: 10.1016/0092-8674(78)90334-3. [DOI] [PubMed] [Google Scholar]
- Yang H. J., Hakomori S. I. A sphingolipid having a novel type of ceramide and lacto-N-fucopentaose 3. J Biol Chem. 1971 Mar 10;246(5):1192–1200. [PubMed] [Google Scholar]
- Zalc B., Helwig J. J., Ghandour M. S., Sarlieve L. Sulfatide in the kidney: how is this lipid involved in sodium chloride transport? FEBS Lett. 1978 Aug 1;92(1):92–96. doi: 10.1016/0014-5793(78)80729-7. [DOI] [PubMed] [Google Scholar]
- van Meer G., Simons K. Viruses budding from either the apical or the basolateral plasma membrane domain of MDCK cells have unique phospholipid compositions. EMBO J. 1982;1(7):847–852. doi: 10.1002/j.1460-2075.1982.tb01258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bonsdorff C. H., Fuller S. D., Simons K. Apical and basolateral endocytosis in Madin-Darby canine kidney (MDCK) cells grown on nitrocellulose filters. EMBO J. 1985 Nov;4(11):2781–2792. doi: 10.1002/j.1460-2075.1985.tb04004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]