Abstract
Background
Antipsychotic-induced weight gain (AIWG) is a common side effect of antipsychotic drugs and may lead to cardiometabolic comorbidities. There is an urgent public health need to identify patients at high risk of AIWG and determine potential biomarkers for AIWG.
Methods
In the Sequential Multiple-Assignment Randomized Trials to Compare Antipsychotic Treatments (SMART-CAT) trail, first-episode schizophrenia patients were randomly assigned to olanzapine, risperidone, perphenazine, amisulpride or aripiprazole for 8 weeks. We applied absolute quantitative lipidomics at baseline and after 8 weeks of antipsychotic treatment in 80 patients. To evaluate the effects of AIWG on lipid profile, 25 patients with ≥ 7% weight changes (weight gain, WG) and 28 patients with <|3|% weight changes (weight stable, WS) were investigated, separately.
Results
We found that baseline CerP(d40:3) and PC(20:1_22:6) were positively associated with weight changes at follow-up (r > 0.4, pFDR < 0.05). Additionally, baseline CerP(d40:3) and PC(20:1_22:6) independently predicted rapid weight gain, with receiver operating curve (ROC) of 0.76 (95% CI: 0.63–0.90), and 0.75 (95% CI: 0.62–0.88), respectively. Compared with baseline, levels of 45 differential lipid metabolites (fold change > 1.2, VIP > 1 and pFDR < 0.05) were significantly higher in the WG group. Interestingly, no differential lipid metabolites were identified in the WS group. The LASSO regression model identified 18 AIWG lipid signatures, including 2 cholesterol esters (ChEs), 1 diglyceride (DG), 12 phosphatidylcholines (PCs), 1 phosphatidylglycerol (PG), 1 phosphatidylinositol (PI), and 1 sphingomyelin (SM), with the ChE(16:1) contributing the most. Furthermore, the level changes of ChE(16:1) were positively associated with weight gain(r = 0.67, pFDR < 0.05).
Conclusion
Our findings indicate that lipid profile may serve as predictors of rapid weight gain in schizophrenia and provide useful markers for AIWG intervention.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12888-024-06413-8.
Keywords: Schizophrenia, Antipsychotic, Weight gain, Lipid
Introduction
Antipsychotic-Induced Weight Gain (AIWG) is a major side effect of antipsychotic treatment, and negatively impacts on quality of life, treatment adherence and may result in metabolic syndrome and excess mortality in severe mental illness [1–3]. Recent studies have focused on the reversibility of AIWG, nevertheless, AIWG can only be reversed to a limited extent by reducing, switching, or discontinuing antipsychotics [4, 5]. Effective management of AIWG during initial treatment is critical for prompt correction of AIWG progression and its associated metabolic complications. Thus, there is an urgent need for better understand the molecular mechanisms behind AIWG in order to develop more precise prevention strategies.
A two-stage genome-wide association study in Han Chinese patients with schizophrenia highlighted that lipid pathway dysfunction plays a role in the mechanism of AIWG [6]. Lipidomics, a rapid development of high-throughput metabolomics techniques, has paved the way for directly measureing disease- or intervention-derived shifts in metabolism. Emerging data demonstrates that exposure to antipsychotic drugs could alter lipid metabolism. In female patients with first-episode schizophrenia, 4-week antipsychotic treatment was characterized by elevated levels of lysophosphatidylcholine (LysoPC), lysophosphatidylethanolamine (LysoPE), as well as reduced carnitine levels [7]. Notably, changes in LysoPC(14:0) level independently contributed to olanzapine-induced weight gain [7]. After 7-month antipsychotic treatment, the levels of 11 glycerophospolipids (GPLs) [2 LysoPCs, 9 phosphatidylcholines (PCs)] and 2 sphingomyelins (SMs)] changed in individuals with first-episode psychosis [8], however the relationship between these altered lipid profiles and weight gain remains unknown. In particular, a molecular signature consisting of 2 triacylglycerols (TGs) was found to predict one year weight gain in individuals with psychotic disorders [9]. Recent research has confirmed that olanzapine exposure leads to weight gain and liver lipid accumulation through up-regulation of genes related to lipid synthesis and down-regulation of genes involved in lipolysis [10]. On the other hand, lipid alterations have been documented in plasma and adipocytes from obese individuals. Compared to lean controls, obese females and males had a 31% and 34% increase in total ceramide content in their adipocytes, respectively [11]. Plasma lipidomics reported higher levels of dhCer, Cer(d18:0_22:0), 12 TG species, and DG(18:0_20:4) among overweight/obese patients [12].
The aforementioned studies hold promise for the identification of potential lipid contributors to AIWG. The aim of this study is to identify schizophrenia patients who have a high risk of rapid AIWG, and to characterize the early-stage lipid profile and biomarkers of AIWG. Here we present a comprehensive serum lipid profile analysis in first-episode drug-naive patients with schizophrenia (FES) before and after 8 weeks of antipsychotic monotherapy based on the Sequential Multiple-Assignment Randomized Trials to Compare Antipsychotic Treatments (SMART-CAT) trial [13]. Taking into account the above evidence, we hypothesized that lipid profiles may serve as predictors and useful markers for AIWG in individuals with schizophrenia.
Methods
Participants
One hundred and five patients who met Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V) criteria for schizophrenia, schizophreniform or schizoaffective disorder were recruited from Shanghai Mental Health Center (SMHC). The inclusion criteria were as follows: (1) aged between 16 and 45 years; (2) hospitalized patients; (3) first episode and illness duration < 3 years (4) drug-naïve, or continuous use of the same antipsychotic drug < 14 days, and the cumulative antipsychotic treatment < 42 days; (4) PANSS total score > 70 and at least one of the following items (delusions, conceptual disorganization, hallucinatory behavior, grandiosity, or suspiciousness/persecution) score ≥ 4. Participants with diagnoses of schizophreniform disorder at study enrollment were subsequently confirmed as schizophrenia 6 months later. Exclusion criteria included unstable clinical condition, significant medical conditions or any other psychiatric or neurological disorders, current substance abuse, modified electric convulsive therapy, pregnancy, or breastfeeding. The study was approved by the Institutional Review Board of SMHC (Reference no.:2018-37R), and written informed consent was obtained from all patients.
Study design
This randomized, controlled study was based on the SMART-CAT trial [13] and conducted between January 1, 2021 and December 31, 2021. After enrollment, eligible patients were randomly assigned to receive oral olanzapine, risperidone, amisulpride, aripiprazole or perphenazine for 8 weeks. The detailed dosing for the pharmacological treatment is listed in the supplementary material. Clinical symptoms were assessed using the Positive and Negative Syndrome Scale (PANSS) [14] and the Clinical Global Impression (CGI)-severity scale [15]. After 8 weeks treatment, non-responders (defined as PANSS reduction rate < 40%) or patients with intolerable side effects were excluded in the analysis. Body weight and laboratory tests [fasting blood glucose(GLU), triglycerides, high density lipoprotein cholesterol (HDL-C), and low density lipoprotein cholesterol (LDL-C)] were collected at baseline and 8 weeks follow-up. Out of the 105 patients enrolled, 2 patients withdrew their consent, and 23 patients switched treatments due to poor efficacy. Finally, 80 patients completed 8 weeks of treatment and entered the final analysis, comprising 71 drug-naïve patients and 9 patients who had received antipsychotics for a duration of 1–3 days.
Serum absolute quantitative lipidomics and data processing
Fasting serum samples of FES patients were collected for lipidomics measurement before and after 8 weeks antipsychotic treatment. Blood samples were obtained using standard venipuncture technique between 06:00–06:30 a.m. A volume of 5 ml blood was sampled in anticoagulant-free tubes and kept for 1 h at 4 °C prior to serum isolation (centrifugation at 3000 rpm for 15 min at 4 °C). Serum samples were frozen and stored under identical conditions at − 80 °C until processing.
The experiments and data processing were supported by Shanghai Applied Protein Technology Co., and the details has been published elsewhere [16], including sample preparation and lipid extraction (Tert-butyl Methyl Ether method), lipid analysis (LC-MS/MS method) and identification of lipids (LipidSearch software).
Statistical analysis
All lipidomics data were processed with normalization and integration using the Perato scaling method for further analysis. In order to investigate the association between pre-therapeutic serum lipids and rapid weight gain in FES patients, we first performed pearson correlation and then independently tested each potential predictor of future weight gain by the receiver operating curve (ROC) analysis. Multiple comparisons were corrected using the false discovery rate (FDR). To evaluate the effects of AIWG on lipid profile, the paired student’s t-test was used to select the differential features of AIWG in the WG and WS group, separately. A multivariate analysis was performed with orthogonal partial least squares discriminant analysis (OPLS-DA) by SIMPCA-P 16.1 (Umetrics, Umea, Sweden). Lipids with significant differences were identified based on a combination of statistically significant thresholds of variable importance in projection (VIP) calculated by OPLS-DA > 1, fold change (FC) > 1.2 or FC < 0.8 and pFDR < 0.05 (paired student’s t-test). Subsequently, the least absolute shrinkage and selection operator (LASSO) regression was developed to select lipid profile and potential lipid biomarkers associated with AIWG.
Results
Baseline demographic and clinical characteristics
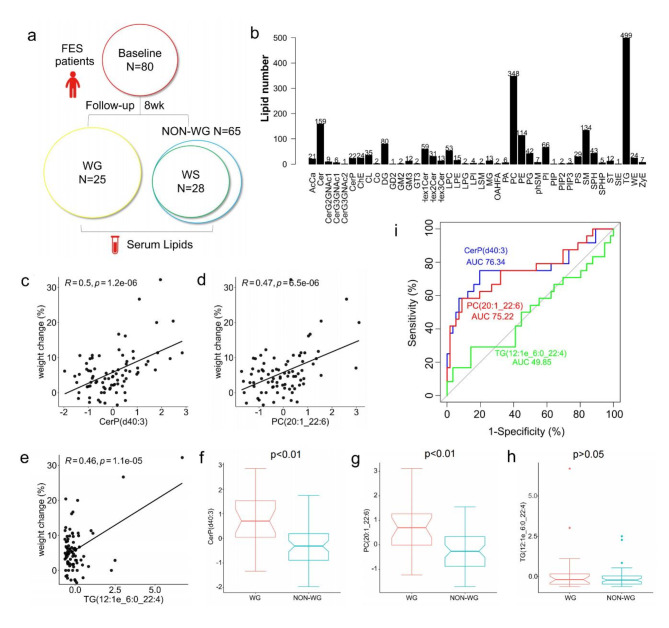
After 8 weeks of antipsychotic therapy, 25 (31.25%) patients had clinically significant weight gain (≥ 7% increases from baseline) and 28 patients had stable weight (< |3|% weight change), Fig. 1a. In the WG group, 8 patients were treated with olanzapine, 5 with risperidone, 7 with amisulpride, 3 with perphenazine, and 2 with aripiprazole. In the WS group, 7 with olanzapine, 5 with risperidone, 5 with amisulpride, 6 with perphenazine, 5 with aripiprazole. There was no statistical differences in drug types between the WG and WS groups (p = 0.640). The demographic and clinical characteristics of patients at baseline are summarized in Table 1. There were no statistically significant differences between WG and WS in terms of age, education, PANSS total and subscales, HDL-C, LDL-C and GLU (all p > 0.05). We found significant group differences in sex, BMI, body weight, WC and TG (all p < 0.05).
Fig. 1.
(a) Study setting. (b) Lipid class. (c) (d) (e) Correlation of pre-therapeutic lipid metabolites with weight change (%) (8-week follow-up vs. baseline) in the FES. (f) (g) (h) Difference in pre-therapeutic lipid levels between WG and NON-WG. (i) Pre-therapeutic lipid metabolites serve as predictors for rapid weight gain in the FES. WG, weight gain; NON-WG, non-weight gain; WS, weight stable
Table 1.
Demographic and clinical characteristics
| ALL N = 80 |
WG N = 25 |
WS N = 28 |
t/χ²(p) | |
|---|---|---|---|---|
| Age (years) | 27.20 ± 7.19 | 26.25 ± 7.48 | 29.19 ± 7.95 | -1.346(0.158) |
| Sex (M/F) | 27/53 | 4/21 | 13/15 | 5.613(0.018) |
| Education (years) | 14.58 ± 2.61 | 14.86 ± 2.49 | 13.71 ± 2.84 | 1.462(0.151) |
| PANSS-total | 85.26 ± 14.52 | 86.56 ± 13.37 | 83.07 ± 13.52 | 0.943(0.350) |
| PANSS-P | 24.4 ± 4.65 | 23.48 ± 4.63 | 24.50 ± 4.57 | -0.806(0.424) |
| PANSS-N | 17.76 ± 8.08 | 19.40 ± 7.89 | 16.82 ± 6.85 | 1.273(0.209) |
| PANSS-G | 43.10 ± 7.79 | 43.68 ± 7.12 | 41.75 ± 8.06 | 0.919(0.362) |
| CGI-S | 5.25 ± 0.70 | 5.12 ± 0.78 | 5.36 ± 0.62 | -1.229(0.225) |
| BMI (kg/m2) | 21.91 ± 3.83 | 20.18 ± 2.88 | 23.92 ± 3.80 | -4.006(< 0.001) |
| Body weight (kg) | 61.24 ± 12.94 | 54.12 ± 9.67 | 68.03 ± 13.20 | -4.331(< 0.001) |
| WC (cm) | 80.77 ± 10.66 | 75.70 ± 8.85 | 85.60 ± 9.59 | -3.793(< 0.001) |
| HDL-C (mmol/L) | 1.60 ± 0.38 | 1.70 ± 0.36 | 1.54 ± 0.44 | 1.439(0.156) |
| LDL-C(mmol/L) | 2.30 ± 0.81 | 2.26 ± 0.76 | 2.39 ± 0.74 | -0.634(0.529) |
| TG (mmol/L) | 0.92 ± 0.40 | 0.78 ± 0.28 | 1.02 ± 0.49 | -2.150(0.036) |
| GLU (mmol/L) | 4.90 ± 0.63 | 4.79 ± 0.64 | 5.06 ± 0.65 | -1.510(0.137) |
Note: WG, weight gain; WS, weight stable; PANSS, Positive and Negative Syndrome Scale, which includes positive symptoms, negative symptoms and general psychopathology subscales; CGI-S, Clinical Global Impression-Severity; BMI, body mass index; WC, Waist Circumference; HDL-c, High Density Lipoprotein Cholesterol; LDL-C: Low Density Lipoprotein Cholesterol; TG: triglyceride; GLU, Fasting Blood Glucose
Pre-therapeutic lipid metabolites predict the antipsychotic induced weight gain
A total of 1913 lipid metabolites were detected using untargeted lipidomics absolute-quantitative analysis (Fig. 1b). Among these, three pre-therapeutic lipid metabolites correlated positively with future weight change (r > 0.4, pFDR < 0.05, Fig. 1c-e). Compared with controls, patients with weight gain (WG) demonstrated elevated levels of CerP(d40:3) and PC(20:1_22:6) (p < 0.01) at baseline(Fig. 1f and g), while the level of TG(12:1e_6:0_22:4) remained similar between the two groups (Fig. 1h). Correlation between pre-therapeutic lipid CerP(d40:3) and PC(20:1_22:6) and laboratory tests in FES patients are provided in supplementary Tables 3–4.
The independent signatures CerP(d40:3) and PC(20:1_22:6) displayed a good ability to predict rapid weight gain in individuals with schizophrenia, with an area under the receiver operating characteristic curve (AUROC) of 0.76 (95% CI: 0.63–0.90), and 0.75 (95% CI: 0.62–0.88), respectively (Fig. 1i, Supplementary Table 4.). However, baseline TG(12:1e_6:0_22:4) cannot predict rapid weight gain with AUC = 0.50 (95%CI: 0.35–0.65, p = 0.98). In addition, we used the Delong test to evaluate the best predictor for weight gain, the prediction value was similar between baseline CerP(d40:3) and baseline PC(20:1_22:6) (Z = 0.159, p = 0.873).
Lipid metabolites in response to antipsychotic induced weight gain
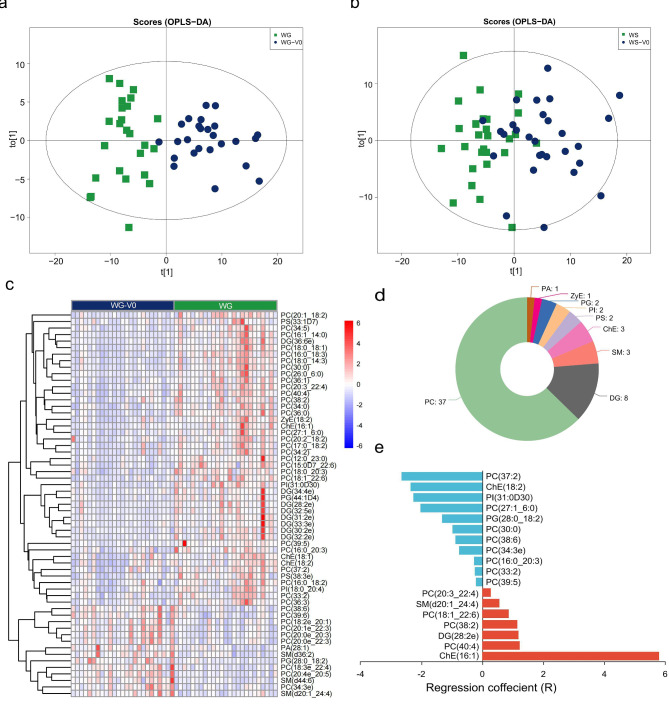
To investigate the dynamics of lipid metabolism during antipsychotic treatment, serum samples for each patient were collected at the two time points: before treatment and at the 8wk of treatment. The OPLS-DA model of the two groups are displayed in Fig. 2a and b. Following screening with VIP value > 1, pFDR < 0.05, and FC > 1.2 or FC < 0.8 criteria, a total of 59 differential lipid metabolites were identified in the WG group after treatment (Fig. 2c, Supplementary Table 5). Among these, levels of 45 differential lipid metabolites were significantly higher in the WG group compared to baseline. The most abundant subclass was phosphatidylcholine (PC) (37 lipid molecules), followed by diglyceride (DG) and sphingomyelin (SM) with 8 and 3 lipid molecules, respectively (Fig. 2d). After screening with VIP value > 1, pFDR < 0.05, and FC > 1.2 or FC < 0.8 criteria, no differential lipid metabolites were observed in the WS group after treatment. The LASSO regression model identified 18 lipid signatures related to AIWG, including 2 cholesterol ester (ChEs), 1DG, 12PCs, 1 phosphatidylglycerol (PG), 1 phosphatidylinositol (PI), and 1 SM, with the ChE(16:1) contributing the most (Fig. 2e, Supplementary Table 6). Moreover, partial correlation analysis revealed a positive association between changes in ChE(16:1) level and rapid weight gain(r = 0.67, pFDR < 0.05). Additionally, changes in ChE(16:1) level could distinguish different degrees of weight gain in the WG group, as patients with weight gains over 15% showed larger level changes than those with gains under 15% (2.03 ± 0.46 vs. 3.94 ± 0.85, p = 0.002).
Fig. 2.
(a) (b) OPLS-DA scores plots distinguishing patients before (Blue dots) and after (Green square) treatments of the WG group and the WS group. (c) (d) Heatmap and lipid class of the 59 differential metabolites of the WG group. (e) Regression coefficient of the LASSO model. WG, weight gain; WS, weight stable
Discussion
Our first major finding indicates that serum lipids, specifically CerP and PC may be valuable molecular predictors for identifying individuals who are prone to rapid weight gain. Glutamate and dopamine are crucial neurotransmitters associated with the etiology of schizophrenia [17]. The dysregulation of dopamine and glutamate affects the brain’s reward circuitry, leading to excessive food intake and weight gain [18]. Various antipsychotics can differ considerably in their propensity of weight gain [19, 20]. Furthermore, there is also substantial variability in weight gain and lipid changes among the FES individuals with respect to antipsychotic drugs [20]. As a novel finding, we have demonstrated the lipid signature associated with an increased susceptibility to AIWG in FES patients, independently of diverse antipsychotic medication. This suggests that specific lipid disturbances in early psychosis may also contribute to the development of metabolic comorbidities.
In recent years, ceramides and their metabolizing enzymes have emerged as key signaling molecules implicated in obesity [21]. The increased expression of ceramide kinase (CerK) and ceramide 1-phosphate C1P formation during adipogenesis are consistent with the substantial reduction of ceramide levels during differentiation of 3T3-L1 preadipocytes, as CerK produces C1P from ceramides [22, 23]. Recent evidence has revealed that CerK expression was increased during adipogenic differentiation, while CerK knockdown resulted in impaired adipogenesis as determined by reduced lipid droplet formation and the TG content of cells [24]. In addition, administering exogenous C1P to cells undergoing adipocyte differentiation caused a significant decrease in TG levels, along with decreased lipid droplet formation and leptin release, suggesting a possible anti-adipogenic role of exogenous C1P [25]. Phosphatidylcholine (PC) is the most abundant phospholipids in all mammalian cell membranes [26]. Previous studies have demonstrated that PC contributes to fatty acid oxidation and metabolism, while reducing cholesterol or fatty acids absorption in the gastrointestinal tract [27]. Treatment with soybean PC alleviated obesity induced by high fat diet and its its associated hyperlipidemic changes [28]. Children with obesity exhibited elevated concentrations of branch-chained amino acids and various lipid metabolites, such as PC, ChE, TG [29]. A prospective, multicenter cohort study found that 1 PC, 9 phosphatidylethanolamines (PEs) and 1 phosphatidylserine (PS) were associated with increased risk of incident metabolic syndrome [30]. The above results suggest a potential association between CerP and PC with an increased susceptibility to AIWG. This distinctive lipid signature may be used to identify individuals who are at increased risk of developing metabolic comorbidities in FES. Our findings might provide novels insights into the development of pharmaceuticals targeting AIWG.
In our study, weight gain with ≥ 7% leads to disturbances in lipid metabolism, characterized by elevated levels of 48 differential lipid metabolites, mostly PC. Furthermore, among the 18 lipid signatures, elevated levels of ChE contribute most to the pathology of AIWG. Meanwhile, changes in ChE level can differentiate the severity of AIWG. Cholesterol homeostasis is crucial for proper cellular and systemic functions [31]. Cholesterol esterification serves as a mechanism to avoid cellular toxicity from the overabundance of unesterified cholesterol. Acyl-coenzyme A: cholesterol acyltransferases (ACATs) catalyze the formation of ChE from free cholesterol to regulate intracellular cholesterol homeostasis. The ACAT inhibitor, avasimibe, can potentially ameliorate body weight with increased energy expenditure in high-fat diet-induced obese mice [32]. Elevated plasma ChE levels are common manifestations of obesity, as well as a risk factor of insulin resistance [12, 29]. In a large population-based cohort, there is an association between ChE levels and BMI [33]. Between classes, ChE displayed a strong correlation with PC in our study. Therefore, ChE elevation in AIWG observed in our work may be associated with enhanced cholesterol homeostasis and increased PC levels. It is worth noting that the brain is the most cholesterol-rich organ in the body, and brain cholesterol metabolism may play an important role in food intake [34]. The Melanocortin-4 receptor (MC4R) is a G-protein-coupled receptor expressed in the hypothalamus where it controls feeding behavior. Membrane cholesterol is reduced in the hypothalamus of insulin-deficient diabetic mice, leading to increased food intake and weight gain [34]. The reduced membrane cholesterol specifically inhibits MC4R constitutive endocytosis and impairs responsiveness to α-MSH, indicating changes in membrane lipid composition can affect appetite control [35]. Treatment with risperidone induced hyperphagia and weight gain, reduced MC4R expression; and administration of MC4R-specific agonist mitigated hyperphagia and obesity in both risperidone- and olanzapine-fed mice [36]. Hence, these findings provide hope for cholesterol metabolism as the potential mechanism-based therapy for individuals affected by AIWG. Furthermore, it is crucial to closely monitor ChE concentrations in FES as they may serve as an important target for managing AIWG and preventing obesity.
Interestingly, no differential lipid metabolites were identified in the weight stable patients. Sevel studies have reported an association between weight gain or increased serum lipids and improved antipsychotic response [37–39]. Marked differences exist between antipsychotics regarding metabolic side-effects, with olanzapine and clozapine exhibiting the worst profiles and aripiprazole the most benign profiles [20]. In a population of drug-naïve schizophrenia patients, changes in the levels of Cer, PC, phosphatidic acid (PA), PE, PI, PG and PS were observed after 6 weeks antipsychotic treatment, and PS levels may be a potential biomarker for poor response [40]. Notably, marked differences exist among antipsychotics in terms of the affected numbers of lipids, with risperidone treatment affected more classes of lipids compared to olanzapine and quetiapine [40]. In our study, we have controlled the confounding influence of treatment efficacy and suggest that changes in lipids seem to be the result of metabolic disturbances by antipsychotics.
Our study has some limitations that should be considered. The small sample size precluded subgroup analysis, including those regarding specific antipsychotics and gender. Furthermore, external independent validation will be necessary to confirm our findings. Despite the limited number of samples, samples used in this study are all drug-naïve hospitalized patients, which may help mitigate potential confounding effects from medications at baseline, diet, physical exercise, and sleep schedule during treatment. Thus, future studies with a larger sample size considering possible confounding factors for the metabolites identified in the present work may possibly provide novel mechanisms underlying the pathophysiology of AIWG.
Conclusion
The results of the present study provide evidence for the utility lipids metabolomics strategies in exploring the pathophysiology of AIWG, as well as in the identification of individuals at high risk of rapid weight gain and valuable markers for AIWG intervention.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank all the subjects who participated in this study, and the staff of the SMHC for their assistance with recruitment.
Author contributions
SZZ and ZLZ: Conceptualization, Methodology, Formal analysis, Investigation, Writing- Original Draft. THG:Investigation, Resources, Data curation. XPK and KMZ: Resources, Data curation. XL, DYK, and LC: Investigation, Methodology. QX: Conceptualization, Resources, Supervision, Methodology. DTL: Conceptualization, Resources, Funding acquisition, Data curation, Investigation, Methodology, Project administration. All authors contributed to and have approved the final manuscript.
Funding
This work was supported by Key Program of SMHC Clinical Research Center (CRC2017ZD03), National Natural Science Foundation of China [82171496, 82371504], Medical innovation research project of science and technology innovation action of Shanghai Science and Technology Commission [22Y11903400]. It was also supported by Multidisciplinary Cross Research Foundation of Shanghai Jiao Tong University [YG2024QNA55] and Shanghai three-year action plan to strengthen the construction of public health system-Outstanding young talents project [GWVI-11.2-YQ39].
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Shanghai Mental Health Center. All participants provided written informed consents. All procedures carried out in the study comply with the Declaration of Helsinki for experiments involving humans.
Consent for publication
No individual data is presented, and consent to publication is therefore not applicable.
Competing interests
The authors declare no competing interests.
Conflict of interest
All authors have no competing interest to disclose, financial or otherwise.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Suzhen Zhang and Zhaolin Zhai contributed equally to this work.
Contributor Information
Qiong Xiang, Email: xiangqiongxl@126.com.
Dengtang Liu, Email: liudengtang@smhc.org.cn.
References
- 1.CU C, S M, V N. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: a large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry. 2018;16(2):163–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dayabandara M, Hanwella R, Ratnatunga S, Seneviratne S, Suraweera C, de Silva VA. Antipsychotic-associated weight gain: management strategies and impact on treatment adherence. Neuropsychiatr Dis Treat. 2017;13:2231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Y, Wang Y, Fang X, Zhang Y, Song L, Zhang C. Association of the HTR2C-759 C/T polymorphism and antipsychotic-induced weight gain: a meta-analysis. Gen Psychiatr. 2020;33(3):e100192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vazquez-Bourgon J, Mayoral-van Son J, Gomez-Revuelta M, Juncal-Ruiz M, de la Ortiz-Garcia V, Tordesillas-Gutierrez D, Ayesa-Arriola R, Bioque M, Crespo-Facorro B. Treatment discontinuation impact on long-term (10-Year) weight gain and lipid metabolism in first-episode psychosis: results from the PAFIP-10 cohort. Int J Neuropsychopharmacol. 2021;24(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Speyer H, Westergaard C, Albert N, Karlsen M, Sturup AE, Nordentoft M, Krogh J. Reversibility of antipsychotic-Induced Weight Gain: a systematic review and Meta-analysis. Front Endocrinol (Lausanne). 2021;12:577919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liao Y, Yu H, Zhang Y, Lu Z, Sun Y, Guo L, Guo J, Kang Z, Feng X, Sun Y et al. Genome-wide association study implicates lipid pathway dysfunction in antipsychotic-induced weight gain: multi-ancestry validation. Mol Psychiatry 2024. [DOI] [PubMed]
- 7.Liu JH, Chen N, Guo YH, Guan XN, Wang J, Wang D, Xiu MH. Metabolomics-based understanding of the olanzapine-induced weight gain in female first-episode drug-naive patients with schizophrenia. J Psychiatr Res. 2021;140:409–15. [DOI] [PubMed] [Google Scholar]
- 8.Leppik L, Parksepp M, Janno S, Koido K, Haring L, Vasar E, Zilmer M. Profiling of lipidomics before and after antipsychotic treatment in first-episode psychosis. Eur Arch Psychiatry Clin Neurosci. 2020;270(1):59–70. [DOI] [PubMed] [Google Scholar]
- 9.Lamichhane S, Dickens AM, Sen P, Laurikainen H, Borgan F, Suvisaari J, Hyotylainen T, Howes O, Hietala J, Oresic M. Association between circulating lipids and Future Weight Gain in individuals with an At-Risk Mental State and in First-Episode Psychosis. Schizophr Bull. 2021;47(1):160–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang X, Shen Y, Yun L, Wang X, Feng J, Yang G, Meng X, Zhang J, Su X. The antipsychotic drug olanzapine altered lipid metabolism in the common carp (Cyprinus carpio L.): insight from the gut microbiota-SCFAs-liver axis. Sci Total Environ. 2023;856(Pt 1):159054. [DOI] [PubMed] [Google Scholar]
- 11.Blachnio-Zabielska AU, Koutsari C, Tchkonia T, Jensen MD. Sphingolipid content of human adipose tissue: relationship to adiponectin and insulin resistance. Obes (Silver Spring). 2012;20(12):2341–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tonks KT, Coster AC, Christopher MJ, Chaudhuri R, Xu A, Gagnon-Bartsch J, Chisholm DJ, James DE, Meikle PJ, Greenfield JR, et al. Skeletal muscle and plasma lipidomic signatures of insulin resistance and overweight/obesity in humans. Obes (Silver Spring). 2016;24(4):908–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li X, Guo X, Fan X, Feng T, Wang C, Yao Z, Xu X, Chen Z, Wang H, Xie S, et al. Sequential multiple-assignment randomized trials to compare antipsychotic treatments (SMART-CAT) in first-episode schizophrenia patients: Rationale and trial design. Schizophr Res. 2021;230:87–94. [DOI] [PubMed] [Google Scholar]
- 14.Kay SR, Fiszbein A, Opler LA. THE POSITIVE AND NEGATIVE SYNDROME SCALE (PANSS) FOR SCHIZOPHRENIA. Schizophrenia Bull 1987, 13(2):261–76. [DOI] [PubMed]
- 15.Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont). 2007;4(7):28–37. [PMC free article] [PubMed] [Google Scholar]
- 16.Fu Y, Chen N, Wang Z, Luo S, Ding Y, Lu B. Degradation of lipid droplets by chimeric autophagy-tethering compounds. Cell Res. 2021;31(9):965–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howes O, McCutcheon R, Stone J. Glutamate and dopamine in schizophrenia: an update for the 21st century. J Psychopharmacol. 2015;29(2):97–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Panizzutti B, Bortolasci CC, Spolding B, Kidnapillai S, Connor T, Richardson MF, Truong TTT, Liu ZSJ, Gray L, Kim JH et al. Biological mechanism(s) underpinning the association between antipsychotic drugs and Weight Gain. J Clin Med 2021, 10(18). [DOI] [PMC free article] [PubMed]
- 19.Huhn M, Nikolakopoulou A, Schneider-Thoma J, Krause M, Samara M, Peter N, Arndt T, Bäckers L, Rothe P, Cipriani A, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394(10202):939–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pillinger T, McCutcheon RA, Vano L, Mizuno Y, Arumuham A, Hindley G, Beck K, Natesan S, Efthimiou O, Cipriani A, et al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta-analysis. Lancet Psychiat. 2020;7(1):64–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Kruining D, Luo Q, van Echten-Deckert G, Mielke MM, Bowman A, Ellis S, Oliveira TG, Martinez-Martinez P. Sphingolipids as prognostic biomarkers of neurodegeneration, neuroinflammation, and psychiatric diseases and their emerging role in lipidomic investigation methods. Adv Drug Deliv Rev. 2020;159:232–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Millner A, Running L, Colon-Rosa N, Aga DS, Frasor J, Atilla-Gokcumen GE. Ceramide-1-Phosphate Is involved in Therapy-Induced Senescence. ACS Chem Biol. 2022;17(4):822–8. [DOI] [PubMed] [Google Scholar]
- 23.Choi KM, Lee Ys Fau - Choi M-H, Choi Mh Fau - Sin D-M, Sin Dm Fau - Lee S, Lee S, Fau - Ji S-Y. Ji Sy Fau - Lee MK, Lee Mk Fau - Lee Y-M, Lee Ym Fau - Yun Y-P, Yun Yp Fau - Hong J-T, Hong Jt Fau - Yoo H-S et al: inverse relationship between adipocyte differentiation and ceramide level in 3T3-L1 cells. Biol Pharm Bull. 2011;34:1347–5215. (Electronic)). [DOI] [PubMed] [Google Scholar]
- 24.Ordonez M, Presa N, Trueba M, Gomez-Munoz A. Implication of Ceramide kinase in Adipogenesis. Mediators Inflamm. 2017;2017:9374563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ordonez M, Presa N, Dominguez-Herrera A, Trueba M, Gomez-Munoz A. Regulation of adipogenesis by ceramide 1-phosphate. Exp Cell Res. 2018;372(2):150–7. [DOI] [PubMed] [Google Scholar]
- 26.van der Veen JN, Kennelly JP, Wan S, Vance JE, Vance DE, Jacobs RL. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim Biophys Acta Biomembr. 2017;1859(9 Pt B):1558–72. [DOI] [PubMed] [Google Scholar]
- 27.Jiang Y, Noh SK, Koo SI. Egg phosphatidylcholine decreases the lymphatic absorption of cholesterol in rats. J Nutr. 2001;131(9):2358–63. [DOI] [PubMed] [Google Scholar]
- 28.Lee HS, Nam Y, Chung YH, Kim HR, Park ES, Chung SJ, Kim JH, Sohn UD, Kim HC, Oh KW, et al. Beneficial effects of phosphatidylcholine on high-fat diet-induced obesity, hyperlipidemia and fatty liver in mice. Life Sci. 2014;118(1):7–14. [DOI] [PubMed] [Google Scholar]
- 29.Szczerbinski L, Wojciechowska G, Olichwier A, Taylor MA, Puchta U, Konopka P, Paszko A, Citko A, Goscik J, Fiehn O et al. Untargeted metabolomics analysis of the Serum Metabolic Signature of Childhood Obesity. Nutrients 2022, 14(1). [DOI] [PMC free article] [PubMed]
- 30.Chen S, Wu Q, Zhu L, Zong G, Li H, Zheng H, Zeng R, Lin X, Sun L. Plasma glycerophospholipid profile, erythrocyte n-3 PUFAs, and metabolic syndrome incidence: a prospective study in Chinese men and women. Am J Clin Nutr. 2021;114(1):143–53. [DOI] [PubMed] [Google Scholar]
- 31.Luo J, Yang H, Song BL. Mechanisms and regulation of cholesterol homeostasis. Nat Rev Mol Cell Biol. 2020;21(4):225–45. [DOI] [PubMed] [Google Scholar]
- 32.Zhu Y, Kim SQ, Zhang Y, Liu Q, Kim KH. Pharmacological inhibition of acyl-coenzyme A:cholesterol acyltransferase alleviates obesity and insulin resistance in diet-induced obese mice by regulating food intake. Metabolism. 2021;123:154861. [DOI] [PubMed] [Google Scholar]
- 33.Weir JM, Wong G, Barlow CK, Greeve MA, Kowalczyk A, Almasy L, Comuzzie AG, Mahaney MC, Jowett JB, Shaw J, et al. Plasma lipid profiling in a large population-based cohort. J Lipid Res. 2013;54(10):2898–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suzuki R, Lee K, Jing E, Biddinger SB, McDonald JG, Montine TJ, Craft S, Kahn CR. Diabetes and insulin in regulation of brain cholesterol metabolism. Cell Metab. 2010;12(6):567–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McDaniel FK, Molden BM, Mohammad S, Baldini G, McPike L, Narducci P, Granell S, Baldini G. Constitutive cholesterol-dependent endocytosis of melanocortin-4 receptor (MC4R) is essential to maintain receptor responsiveness to alpha-melanocyte-stimulating hormone (alpha-MSH). J Biol Chem. 2012;287(26):21873–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li L, Yoo ES, Li X, Wyler SC, Chen X, Wan R, Arnold AG, Birnbaum SG, Jia L, Sohn JW et al. The atypical antipsychotic risperidone targets hypothalamic melanocortin 4 receptors to cause weight gain. J Exp Med 2021, 218(7). [DOI] [PMC free article] [PubMed]
- 37.Garlow SJ, Purselle DC, Heninger M. Cocaine and alcohol use preceding suicide in African American and white adolescents. J Psychiatr Res. 2007;41(6):530–6. [DOI] [PubMed] [Google Scholar]
- 38.Kim DD, Barr AM, Fredrikson DH, Honer WG, Procyshyn RM. Association between Serum Lipids and antipsychotic response in Schizophrenia. Curr Neuropharmacol. 2019;17(9):852–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang TL, Chen JF. Serum lipid profiles and schizophrenia: effects of conventional or atypical antipsychotic drugs in Taiwan. Schizophr Res. 2005;80(1):55–9. [DOI] [PubMed] [Google Scholar]
- 40.de Almeida V, Alexandrino GL, Aquino A, Gomes AF, Murgu M, Dobrowolny H, Guest PC, Steiner J, Martins-de-Souza D. Changes in the blood plasma lipidome associated with effective or poor response to atypical antipsychotic treatments in schizophrenia patients. Prog Neuropsychopharmacol Biol Psychiatry. 2020;101:109945. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.