Abstract
We have collected tilt series of electron micrographs from unstained clathrin cages embedded in vitreous ice. From these micrographs we have generated three-dimensional reconstructions of individual hexagonal barrels, which show details of the internal structure. Four types of preparation have been examined: (i) coated vesicles; (ii) cages reassembled from clathrin heavy and light chains; (iii) reassembled cages treated with elastase to remove the light chains; and (iv) reassembled cages treated with trypsin to remove the light chains and the terminal domains of the clathrin heavy chains. In the intact and elastase-treated cages, the clathrin extends from the vertices into the interior of the polyhedron and forms an inner shell of material. Limited digestion with trypsin removes the inner shell, which indicates that this material corresponds to the terminal domains of the clathrin heavy chains.
Full text
PDF
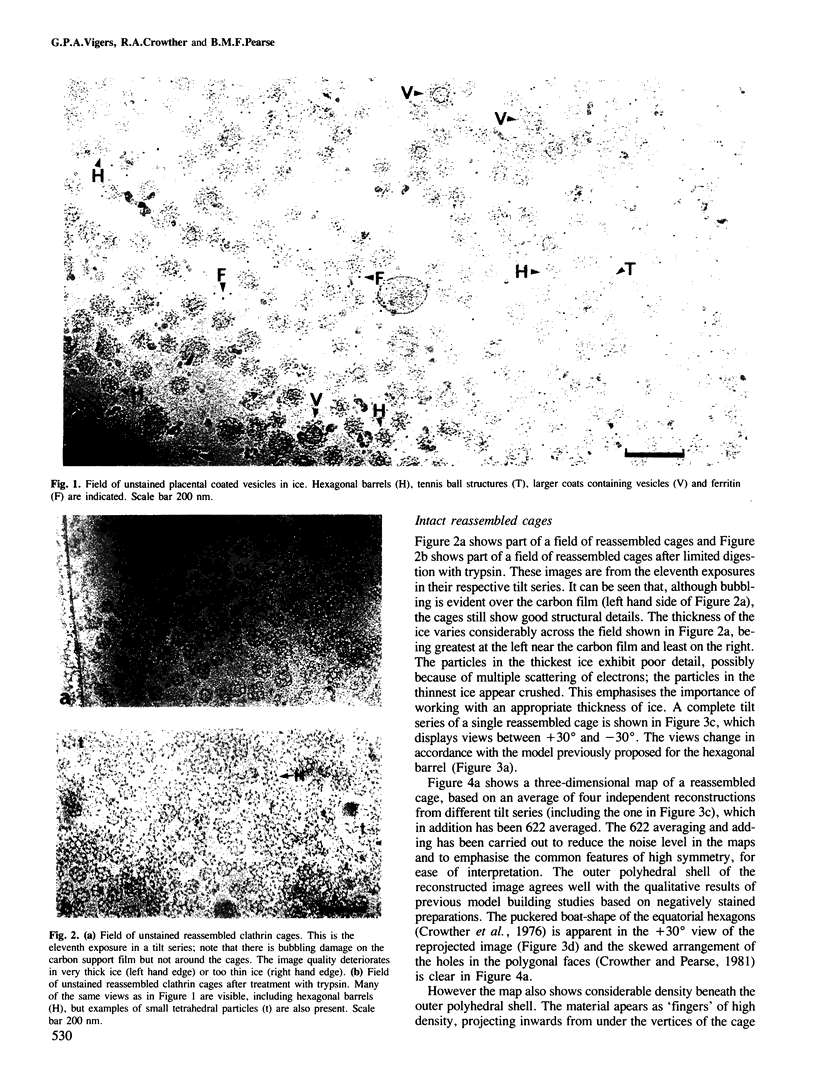
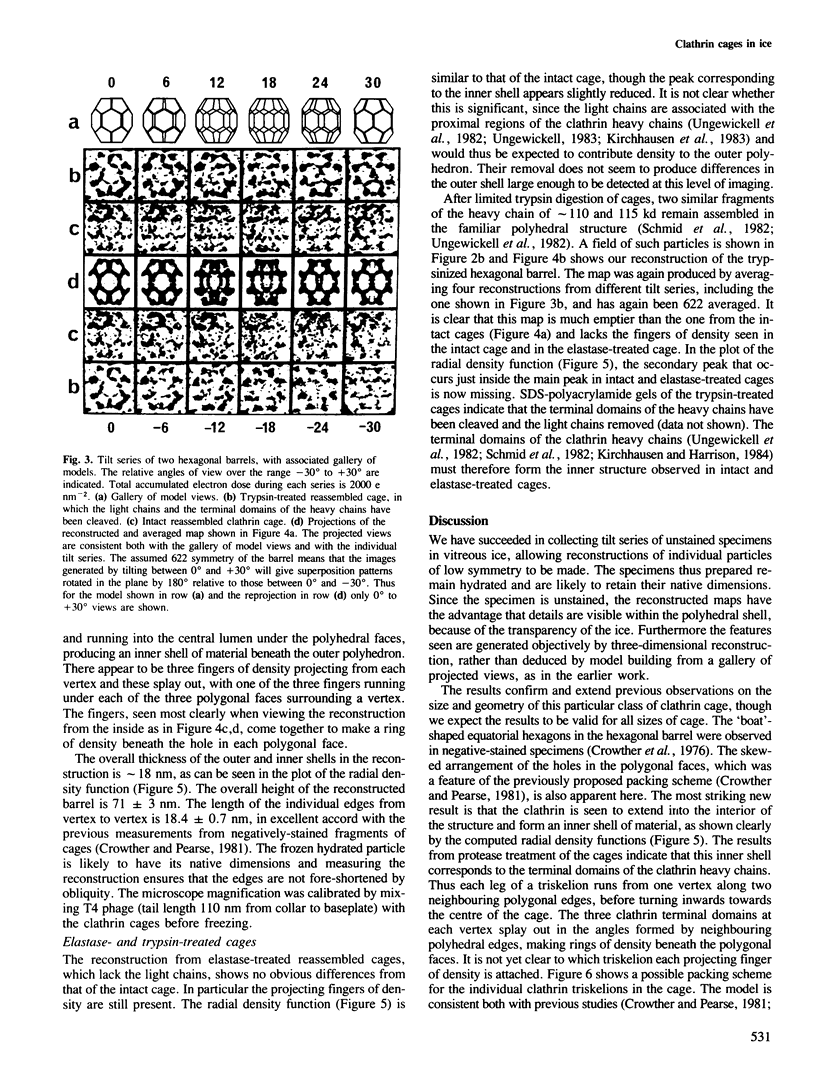
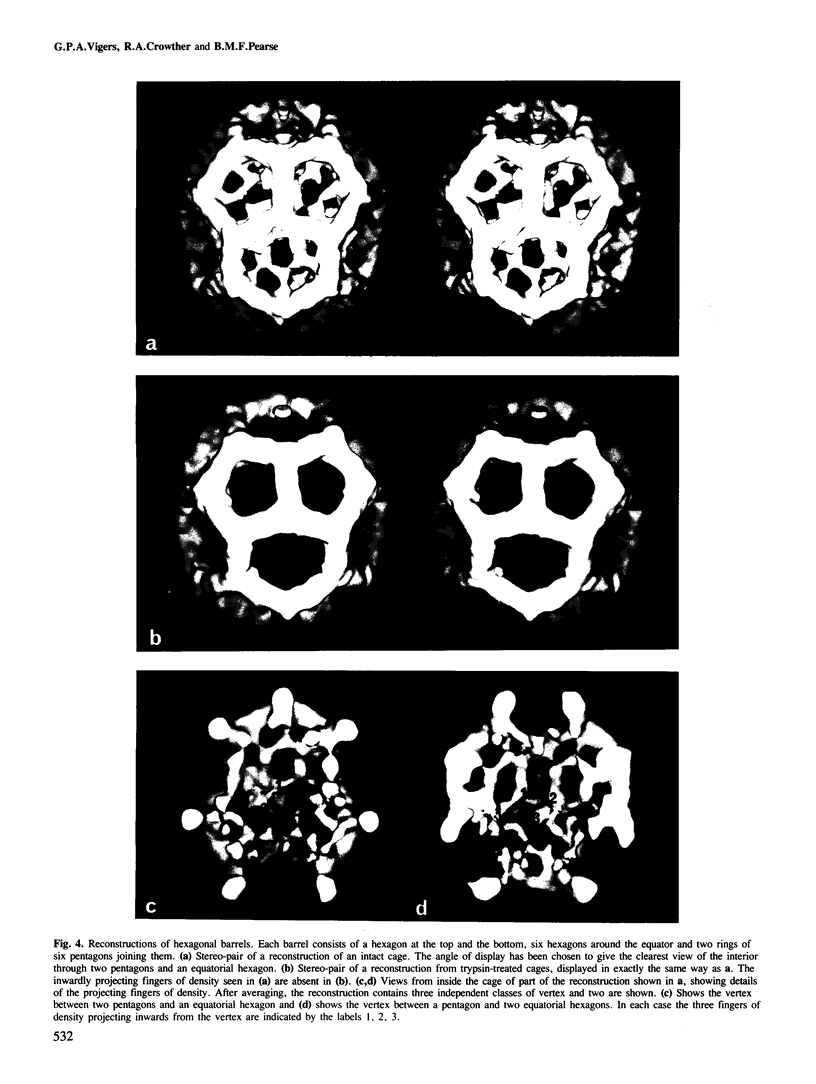
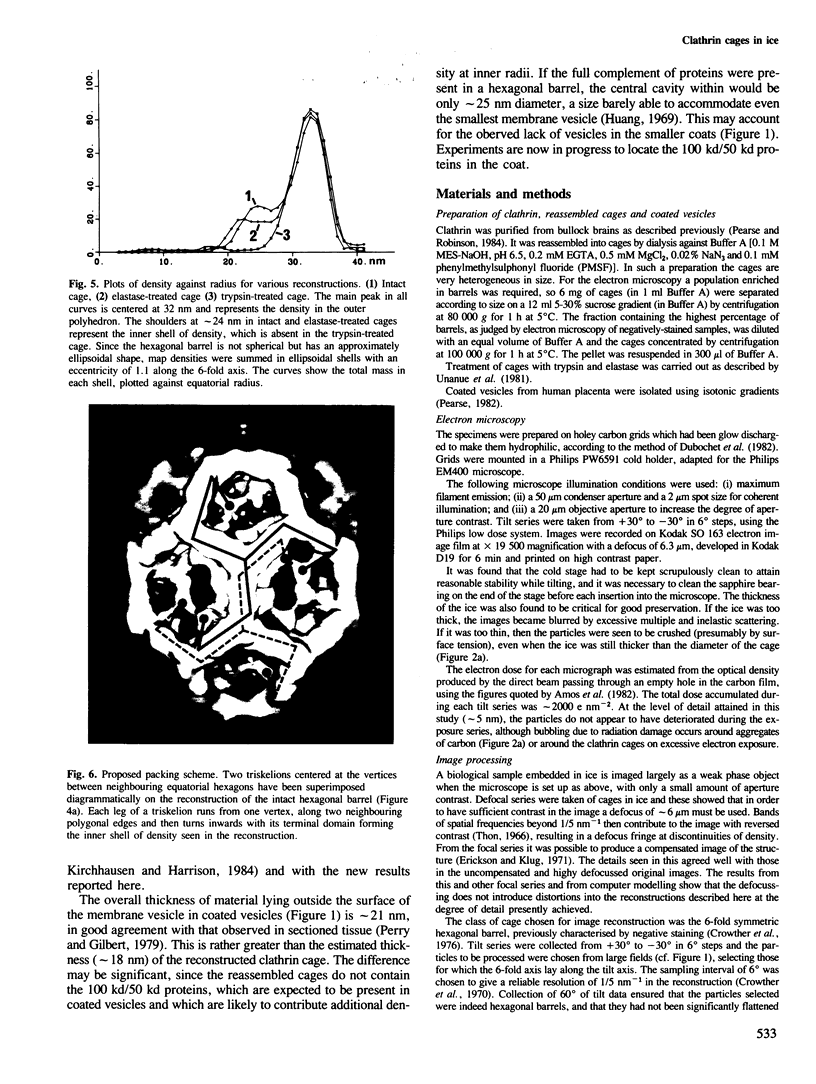

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amos L. A., Henderson R., Unwin P. N. Three-dimensional structure determination by electron microscopy of two-dimensional crystals. Prog Biophys Mol Biol. 1982;39(3):183–231. doi: 10.1016/0079-6107(83)90017-2. [DOI] [PubMed] [Google Scholar]
- Arndt U. W., Leigh J. B., Mallett J. F., Twinn K. E. A mechanical microdensitometer. J Sci Instrum. 1969 May;2(5):385–387. doi: 10.1088/0022-3735/2/5/301. [DOI] [PubMed] [Google Scholar]
- Crowther R. A., Finch J. T., Pearse B. M. On the structure of coated vesicles. J Mol Biol. 1976 Jun 5;103(4):785–798. doi: 10.1016/0022-2836(76)90209-6. [DOI] [PubMed] [Google Scholar]
- Crowther R. A., Pearse B. M. Assembly and packing of clathrin into coats. J Cell Biol. 1981 Dec;91(3 Pt 1):790–797. doi: 10.1083/jcb.91.3.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert P. F. The reconstruction of a three-dimensional structure from projections and its application to electron microscopy. II. Direct methods. Proc R Soc Lond B Biol Sci. 1972 Jul 25;182(1066):89–102. doi: 10.1098/rspb.1972.0068. [DOI] [PubMed] [Google Scholar]
- Heuser J. Three-dimensional visualization of coated vesicle formation in fibroblasts. J Cell Biol. 1980 Mar;84(3):560–583. doi: 10.1083/jcb.84.3.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. Studies on phosphatidylcholine vesicles. Formation and physical characteristics. Biochemistry. 1969 Jan;8(1):344–352. doi: 10.1021/bi00829a048. [DOI] [PubMed] [Google Scholar]
- Kirchhausen T., Harrison S. C., Parham P., Brodsky F. M. Location and distribution of the light chains in clathrin trimers. Proc Natl Acad Sci U S A. 1983 May;80(9):2481–2485. doi: 10.1073/pnas.80.9.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhausen T., Harrison S. C. Structural domains of clathrin heavy chains. J Cell Biol. 1984 Nov;99(5):1725–1734. doi: 10.1083/jcb.99.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepault J., Leonard K. Three-dimensional structure of unstained, frozen-hydrated extended tails of bacteriophage T4. J Mol Biol. 1985 Apr 5;182(3):431–441. doi: 10.1016/0022-2836(85)90202-5. [DOI] [PubMed] [Google Scholar]
- Milligan R. A., Brisson A., Unwin P. N. Molecular structure determination of crystalline specimens in frozen aqueous solutions. Ultramicroscopy. 1984;13(1-2):1–9. doi: 10.1016/0304-3991(84)90051-2. [DOI] [PubMed] [Google Scholar]
- Pearse B. M., Bretscher M. S. Membrane recycling by coated vesicles. Annu Rev Biochem. 1981;50:85–101. doi: 10.1146/annurev.bi.50.070181.000505. [DOI] [PubMed] [Google Scholar]
- Pearse B. M. Coated vesicles from human placenta carry ferritin, transferrin, and immunoglobulin G. Proc Natl Acad Sci U S A. 1982 Jan;79(2):451–455. doi: 10.1073/pnas.79.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse B. M., Robinson M. S. Purification and properties of 100-kd proteins from coated vesicles and their reconstitution with clathrin. EMBO J. 1984 Sep;3(9):1951–1957. doi: 10.1002/j.1460-2075.1984.tb02075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry M. M., Gilbert A. B. Yolk transport in the ovarian follicle of the hen (Gallus domesticus): lipoprotein-like particles at the periphery of the oocyte in the rapid growth phase. J Cell Sci. 1979 Oct;39:257–272. doi: 10.1242/jcs.39.1.257. [DOI] [PubMed] [Google Scholar]
- Schmid S. L., Matsumoto A. K., Rothman J. E. A domain of clathrin that forms coats. Proc Natl Acad Sci U S A. 1982 Jan;79(1):91–95. doi: 10.1073/pnas.79.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor K. A., Glaeser R. M. Electron diffraction of frozen, hydrated protein crystals. Science. 1974 Dec 13;186(4168):1036–1037. doi: 10.1126/science.186.4168.1036. [DOI] [PubMed] [Google Scholar]
- Unanue E. R., Ungewickell E., Branton D. The binding of clathrin triskelions to membranes from coated vesicles. Cell. 1981 Nov;26(3 Pt 1):439–446. doi: 10.1016/0092-8674(81)90213-0. [DOI] [PubMed] [Google Scholar]
- Ungewickell E. Biochemical and immunological studies on clathrin light chains and their binding sites on clathrin triskelions. EMBO J. 1983;2(8):1401–1408. doi: 10.1002/j.1460-2075.1983.tb01598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungewickell E., Unanue E. R., Branton D. Functional and structural studies on clathrin triskelions and baskets. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 2):723–731. doi: 10.1101/sqb.1982.046.01.069. [DOI] [PubMed] [Google Scholar]
- Unwin P. N., Ennis P. D. Two configurations of a channel-forming membrane protein. Nature. 1984 Feb 16;307(5952):609–613. doi: 10.1038/307609a0. [DOI] [PubMed] [Google Scholar]
- Zaremba S., Keen J. H. Assembly polypeptides from coated vesicles mediate reassembly of unique clathrin coats. J Cell Biol. 1983 Nov;97(5 Pt 1):1339–1347. doi: 10.1083/jcb.97.5.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]