Abstract
Background
Dual antiplatelet therapy is the main treatment for cardiovascular diseases (CADs). In this study, we evaluated the efficacy and safety of aspirin combined with low-dose rivaroxaban in the secondary prevention of high-risk ischemic cardiovascular diseases.
Material/Methods
In total, 168 patients who were diagnosed with acute myocardial infarction or multiple vessel disease 1 year after percutaneous coronary intervention were divided into 2 groups: the aspirin group (aspirin as acetylsalicylic acid: 100 mg once daily) and the aspirin + rivaroxaban group (aspirin: 100 mg once daily, rivaroxaban: 2.5 mg twice daily). The patients were followed up for 2 years to assess the clinical efficacy and safety of a new dual-channel antithrombotic treatment strategy.
Results
The occurrence of MACE (recurrent myocardial infarction, in-stent restenosis, coronary target vessel revascularization, stent thrombosis, heart failure, rehospitalization, and all-cause mortality) in the rivaroxaban + aspirin group was lower than that in the aspirin group (3.57% of patients received aspirin + rivaroxaban treatment vs 13.10% of patients received aspirin treatment). There were not more adverse events in the rivaroxaban + aspirin group than in the aspirin group. Compared with patients administered aspirin, the coagulation function of patients taking aspirin + rivaroxaban was significantly changed. No heart failure occurred in either group of patients with CADs.
Conclusions
Aspirin + rivaroxaban had better primary outcome and secondary outcomes in patients with a high risk of ischemia. Our results provide a basis for evaluating the efficacy and safety of drugs used in secondary prevention among patients with high risk of ischemia.
Keywords: Aspirin, Cardiovascular Diseases, Dual Anti-Platelet Therapy, Rivaroxaban, Secondary Prevention
Introduction
Coronary artery disease (CAD) is one of the most common subtypes of cardiovascular disease. It has one of the highest mortality rates in the world [1]. The progression of CAD starts with atherosclerosis and atherosclerotic plaques, which can eventually develop into acute myocardial infarction (AMI) [2,3]. Over 50% of AMI patients also have multiple vessel disease (MVD) [4]. AMI and MVD patients are at high risk of ischemia [5]. Dual antiplatelet therapy (DAPT) can improve myocardial blood supply, decrease risk of cardiomyocyte death, and maintain the patency of stents [6]. It can also prevent secondary ischemic events. Patients with AMI and MVD are generally treated with DAPT for at least 12 months, after which they may opt for single antiplatelet drug therapy [7,8]. However, medical practitioners have found that 1 year after onset some patients with a high risk of ischemia need to be repeatedly hospitalized due to frequent attacks of angina pectoris or ischemic cardiomyopathy, heart failure, and even sudden death. Therefore, a feasible treatment strategy needs to be selected.
Patients with AMI have GPIIb/IIIa (platelet marker) and fibrin, which indicates that thrombus in the coronary artery is not only platelet thrombus but also fibrin [9]. Thrombin production occurs throughout the acute and chronic stages of coronary heart disease, and it plays a prominent role [10,11]. Platelet activation commonly occurs in the acute stage; therefore, intensive antiplatelet therapy is the main treatment in the acute stage [12]. Thrombin production occurs continuously in the acute and chronic phases, providing a theoretical basis for the application of antiplatelet plus anticoagulation in the long-term follow-up phase [13,14]. Rivaroxaban is a direct inhibitor of Xa factor [15]. Rivaroxaban directly antagonizes free and bound Xa factors to reduce the activation of thrombin, thus prolonging the clotting time [16]. It blocks the formation of blood clots and also destroys formed blood clots [17]. Rivaroxaban can affect the formation of fibrin and inhibit platelet activation and aggregation [18]. Rivaroxaban is a new anticoagulant that can be administered long-term orally [19]. Rivaroxaban (2.5 mg) has been administered along with aspirin for patients with a high risk of ischemia to reduce the risk of major cardiovascular events [20]. However, studies on the clinical efficacy and safety of aspirin plus rivaroxaban in the Chinese population are limited.
In this study, we evaluated the clinical efficacy and safety of a dual-channel antithrombotic treatment strategy involving the use of low-dose rivaroxaban combined with aspirin in patients diagnosed with CAD, including 1 year after AMI and 1 year after percutaneous coronary intervention (PCI) for MVD. Our findings may provide a new option for long-term antithrombotic treatment of patients with high-risk ischemia.
Material and Methods
Study design
The Cardiovascular Outcomes for People Using dual-channel antithrombotic treatment in this study was a prospective, observational, and randomized controlled trial comparing the combination of rivaroxaban plus aspirin versus aspirin for the secondary prevention of major adverse cardiovascular events (MACE) in patients 1 year after acute myocardial infarction and those 1 year after PCI for multivessel and multisegment coronary lesions. The bleeding events and the changes of the cardiac function and coagulation function were also evaluated during the follow-up period (24 months). This clinical study was conducted at the Second Hospital of Hebei Medical University and approved by the Institutional Ethics Committee of the Second Hospital of Hebei Medical University (Shijiazhuang, China). The approval number is 2021-R423. All patients provided signed informed consent before participation.
Population
From June 2021 to March 2024, 184 patients were enrolled, including patients 1 year after acute myocardial infarction and those 1 year after PCI for multivessel and multisegment coronary lesions. Among them, 168 patients met the inclusion criteria. The patients were followed up for 2 years to assess the clinical efficacy and safety of the combination treatment involving aspirin and rivaroxaban. We aimed to provide a better treatment strategy to reduce rehospitalization and mortality rates. A flowchart illustrating the details of the study is provided in Figure 1.
Figure 1.
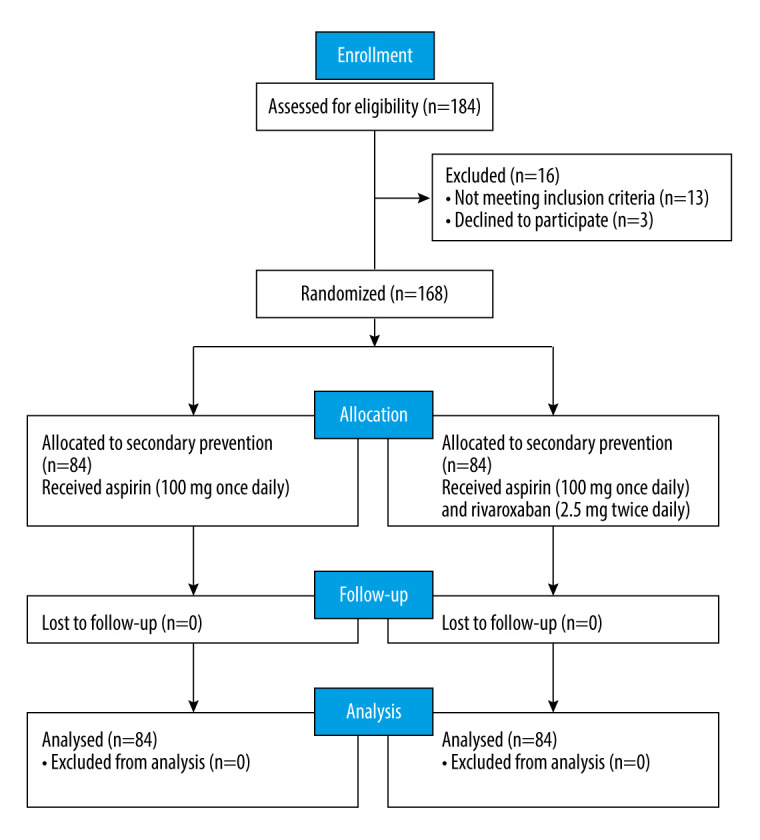
CONSORT diagram of participants screening in this study. (The figure was created by Microsoft Office PowerPoint, version 2021, Microsoft).
Eligibility
Eligible patients who were determined to be qualified according to standard and biochemical indicators were enrolled and evaluated in the trial. The inclusion criteria were: 1) patients who were 40–75 years old; 2) those clinically diagnosed with AMI and with MVD 1 year after PCI; 3) those who provided signed informed consent to participate in this study. The exclusion criteria were: 1) AMI patients with a history of less than 1 year; 2) NYHA class III or IV, or left ventricular ejection fraction <30%; 3) history of hemorrhagic diseases, such as gastrointestinal bleeding, or cerebral hemorrhage; 4) severe stomach diseases, such as peptic ulcer; 5) uncontrollable or recurrent arrhythmias; 6) uncontrollable hypertension, defined as systolic blood pressure >180 mmHg or diastolic blood pressure >110 mmHg; 7) severe renal insufficiency, defined as eGFR <30 mL/min/1.73 m2; 8) active liver disease or liver dysfunction, defined as AST or ALT> 3 times the upper limit of the normal value; 9) allergic to the drugs used in this study; 10) pregnant or lactating women; 11) other serious complications, such as the presence of malignant tumors. All patients provided written informed consent before the trial. During the study, patients could withdraw from the study if any of the following occurred: 1) serious adverse drug reactions in the course of medication; 2) he patient died in the course of the study; 3) other serious diseases during the study, such as serious impairments in liver and kidney function; 4) requested to withdraw from the study due to other reasons; 5) were lost to follow-up.
Randomization and Interventions
Using a randomizer tool (https://www.randomizer.org/), 168 patients were randomly allocated to either the aspirin + rivaroxaban group or the aspirin group in a 1: 1 ratio. An assistant who was not involved in screening the participants managed the randomization procedure. The patients in the aspirin group received aspirin (100 mg once daily). The patients in the aspirin + rivaroxaban group received aspirin (100 mg once daily) and rivaroxaban (2.5 mg twice daily).
Outcomes
Primary outcome was the incidence of MACE within 2 years of follow-up, including recurrent myocardial infarction, in-stent restenosis, coronary target vessel revascularization, stent thrombosis, heart failure, rehospitalization, and all-cause mortality.
Secondary outcomes were 1) the effect of the new dual-channel antithrombotic treatment strategy on the cardiac function of the patients; 2) the effect of rivaroxaban on the coagulation function of the patients.
We used the clinical safety index to assess serious life-threatening bleeding events, such as severe gastrointestinal bleeding, intracranial hemorrhage, and blood in the urine, as per the BARC bleeding classification standard of grades 3 and 5, as well as bleeding events which were not serious bleeding, but also had adverse effects on the patients, and met the BARC bleeding classification standard of 1 stroke grade 2. Measurement and evaluation methods used were telephone follow-up and outpatient follow-up registration.
Follow-Up Data Collection
Information on major adverse cardiovascular events and bleeding events was obtained every month via telephone calls. Every 3 months, the patients visited the outpatient clinic for re-examination, and various blood test indices (eg, blood routine, coagulation routine, myocardial enzyme, troponin, platelet aggregation rate, BNP) were recorded; additionally, cardiac ultrasound examination (each cardiac chamber size) was performed. Based on these tests and indices, the changes in cardiac function were monitored.
Statistical Analysis
All statistical analyses were performed using SPSS (version 22.0; SPSS Inc., Chicago, USA). All figures were created by GraphPad Prism, version 8.0 (GraphPad Software, Inc.). The Shapiro-Wilk test was performed to determine whether the data followed a normal distribution. The differences in count data between groups were determined by the chi-square test or Fisher’s exact test. For data that followed a normal distribution, the differences in various parameters between the groups were analyzed by the t test. For data that did not follow a normal distribution, the differences in various parameters between the 2 groups were determined by the Wilcoxon test or Mann-Whitney U test. Covariance analysis was performed to compare the data of the 2 groups after intervention. The median time of primary efficacy outcome was analyzed by the Kaplan-Meier method, and the difference was evaluated by performing log rank tests. All differences between groups were considered to be statistically significant at P<0.05.
Results
Participants
The baseline characteristics of patients are provided in Table 1. The mean age of patients was 61.14 ±7.65 years, and over 60% were males. The BMI of patients was in the normal range in both groups. More than half of the patients engaged in smoking and drinking. No significant differences were observed between the aspirin and aspirin + rivaroxaban groups for comorbidities, including hypertension, diabetes, and chronic obstructive pulmonary disease. Furthermore, no difference in the CK level was found between the 2 groups. Similar results were recorded for the levels of cTnT, NT-proBNP, AST, and ALT in the 2 groups. In total, 65.76% of patients had a history of acute myocardial infarction (73.81% in the aspirin group and 70.24% in the aspirin + rivaroxaban group). Over 60% of patients had a history of multivessel and muti-segment coronary artery disease in both groups. In the aspirin and aspirin + rivaroxaban groups, 66.67% and 69.05%, respectively, of patients had never used an ACE inhibitor or ARB. A few patients had previously used a calcium channel blocker and diuretic in both groups. Beta-blocker was used by 65.48% of patients in the aspirin group and 70.24% of patients in the aspirin + rivaroxaban group. In total, 35.71% and 32.14% of patients in the aspirin and aspirin + rivaroxaban groups, respectively, had previously used non-trial PPI. These results indicated there was no significant difference in baseline characteristics between the 2 groups.
Table 1.
Clinical characteristics of participants at baseline.
| Characteristic | Aspirin group (n=84) | Aspirin + rivaroxaban group (n=84) | P value |
|---|---|---|---|
| Age (year) | 61.96±7.52 | 60.31±7.74 | 0.217 |
| Gender | 0.527 | ||
| Male | 53 (63.1%) | 49 (58.33%) | |
| Female | 31 (36.9%) | 35 (41.67%) | |
| BMI (kg/m2) | 24.46±3.28 | 23.66±3.66 | |
| Smoking | 45 (53.57%) | 47 (55.95%) | 0.822 |
| Drink | 48 (57.14%) | 43 (51.19%) | 0.439 |
| Comorbidities | |||
| Hypertension | 51 (60.71%) | 56 (66.67%) | 0.422 |
| Diabetes | 27 (32.14%) | 32 (38.1%) | 0.419 |
| Chronic obstructive pulmonary disease | 11 (13.1%) | 9 (10.71%) | 0.634 |
| CK (u/l) | 272.28±34.05 | 263.88±35.09 | 0.117 |
| cTnT (ug/ml) | 68.16±7.55 | 67.41±11.48 | 0.618 |
| NT-proBNP (pg/ml) | 351.21±49.98 | 347.44±51.92 | 0.632 |
| History of acute myocardial infarction | 62 (73.81%) | 59 (70.24%) | 0.606 |
| History of multiple branches and segments of coronary artery lesions | 55 (65.48%) | 54 (64.29%) | 0.872 |
| Number of supports | 0.976 | ||
| ALT (U/L) | 54.17±8.6 | 55.38±9.64 | 0.392 |
| AST (U/L) | 25.55±5.3 | 25.24±6.08 | 0.533 |
| Medication | |||
| ACE inhibitor or ARB | 56 (66.67%) | 58 (69.05%) | 0.741 |
| Calcium-channel blocker | 21 (25%) | 19 (22.62%) | 0.717 |
| Diuretic | 23 (27.38%) | 24 (28.57%) | 0.864 |
| Beta-blocker | 55 (65.48%) | 59 (70.24%) | 0.509 |
| Lipid-lowering agent | 84 (100%) | 84 (100%) | |
| NSAID | 84 (100%) | 84 (100%) | |
| Non-trial PPI | 30 (35.71%) | 27 (32.14%) | 0.625 |
BMI – body mass index; CK – creatine kinase; cTnT – cardiac troponin; NT-proBNP – N-terminal fragment B-type natriuretic peptide; ALT – alanine aminotransferase; AST – aspartate aminotransferase; ACE – angiotensin converting enzyme; ARB – angiotensin receptor blocker; NSAID – nonsteroidal anti-inflammatory drugs; PPI – proton pump inhibitors.
Primary Efficacy Outcome
As shown in Table 2, MACE (recurrent myocardial infarction, in-stent restenosis, coronary target vessel revascularization, stent thrombosis, heart failure, rehospitalization, and all-cause mortality) occurred in 3 patients (3.57%) in the aspirin + rivaroxaban group and 11 patients (13.10%) in the aspirin group (P<0.05). These results suggest that aspirin + rivaroxaban treatment reduced the occurrence of MACE among CAD patients to a greater extent than aspirin treatment. Additionally, we evaluated the efficacy of the aspirin + rivaroxaban in males and females. During the follow-up, MACE occurred in 7 male patients (8.33%) in the aspirin group and in 2 male patients (2.38%) in the aspirin + rivaroxaban group, and the incidence rates of MACE were similar by randomized treatment arm (P>0.05, Table 3). Similarly, aspirin + rivaroxaban had no observed effect on MACE in females compared with the aspirin treatment (P>0.05, Table 4). These findings demonstrate that compared with aspirin alone, the combination of rivaroxaban and aspirin appears to be consistently more effective in both women and men.
Table 2.
Primary outcome of both groups.
| Outcomes | Aspirin group (n=84) | Aspirin + rivaroxaban group (n=84) | P value |
|---|---|---|---|
| Recurrent myocardial infarction | 2 (2.38%) | 1 (1.19%) | 1.000 |
| Stent restenosis | 2 (2.38%) | 0 (0.0%) | 0.497 |
| Coronary target vessel revascularization | 1 (1.19%) | 0 (0.0%) | 1.000 |
| Intrastent thrombosis | 1 (1.19%) | 0 (0.0%) | 1.000 |
| Heart failure | 2 (2.38%) | 1 (1.19%) | 1.000 |
| Readmission | 5 (5.95%) | 3 (3.57%) | 0.720 |
| All-cause death | 3 (3.57%) | 1 (1.19%) | 0.620 |
| Total MACE | 11 (13.10) | 3 (3.57%) | 0.026 |
MACE – major adverse cardiovascular events.
Table 3.
Primary outcome of male patients.
| Outcomes | Aspirin group (n=84) | Aspirin + rivaroxaban group (n=84) | P value |
|---|---|---|---|
| Recurrent myocardial infarction | 2 (2.38%) | 1 (1.19%) | 1.000 |
| Stent restenosis | 2 (2.38%) | 0 (0.0%) | 0.497 |
| Coronary target vessel revascularization | 1 (1.19%) | 0 (0.0%) | 1.000 |
| Intrastent thrombosis | 0 (0.0%) | 0 (0.0%) | 1.000 |
| Heart failure | 0 (0.0%) | 0 (0.0%) | |
| Readmission | 3 (3.57%) | 2 (2.38%) | 0.650 |
| All-cause death | 2 (2.38%) | 0 (0.0%) | 0.497 |
| Total MACE | 7 (8.33%) | 2 (2.38%) | 0.087 |
MACE – major adverse cardiovascular events.
Table 4.
Primary outcome of female patients.
| Outcomes | Aspirin group (n=84) | Aspirin + rivaroxaban group (n=84) | P value |
|---|---|---|---|
| Recurrent myocardial infarction | 0 (0.0%) | 0 (0.0%) | |
| Stent restenosis | 0 (0.0%) | 0 (0.0%) | |
| Coronary target vessel revascularization | 0 (0.0%) | 0 (0.0%) | |
| Intrastent thrombosis | 1 (1.19%) | 0 (0.0%) | 1.000 |
| Heart failure | 2 (2.38%) | 1 (1.19%) | 1.000 |
| Readmission | 2 (2.38%) | 1 (1.19%) | 1.000 |
| All-cause death | 1 (1.19%) | 1 (1.19%) | 1.000 |
| Total MACE | 4 (4.76%) | 1 (1.19%) | 0.173 |
MACE – major adverse cardiovascular events.
The cumulative incidence curves for the time to MACE are shown in Figure 2. The incidence of MACE was significantly lower in patients treated with aspirin and rivaroxaban compared to those treated with aspirin alone after 24 months (P<0.05). At data cutoff, 14 patients had progressive disease, including 3 (3.57%) in the aspirin + rivaroxaban group and 11 (13.10%) in the aspirin group (log rank P=0.024).
Figure 2.
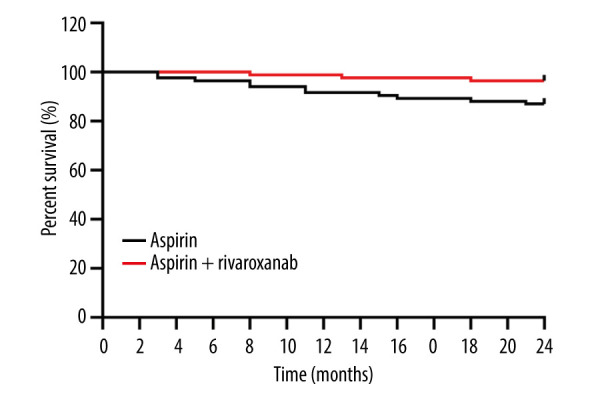
Cumulative survival curve of primary efficacy outcome. (The figure was created by GraphPad Prism, version 8.0, GraphPad Software, Inc.).
Drug Safety
We also analyzed the drug safety of aspirin plus rivaroxaban (Table 5) and found that aspirin and rivaroxaban did not impair liver and renal functions; therefore, these organs were not assessed to determine the safety of the drug. No patients were allergic to aspirin or rivaroxaban. Additionally, only 1 patient developed life-threatening severe intracranial hemorrhage in the aspirin group, while 2 patients in the aspirin + rivaroxaban group showed complications (1 had gastrointestinal bleeding, and the other had intracranial hemorrhage). The difference in the total incidence rate of adverse events between the aspirin and aspirin + rivaroxaban groups was not significant (P>0.05).
Table 5.
Adverse events of both groups.
| Outcomes | Aspirin group (n=84) | Aspirin + rivaroxaban group (n=84) | P value |
|---|---|---|---|
| Allergy | 0 (0.0%) | 0 (0.0%) | 1.000 |
| Gastrointestinal bleeding | 0 (0.0%) | 1 (1.19%) | 1.000 |
| Intracranial hemorrhage | 1 (1.19%) | 1 (1.19%) | 1.000 |
| Urinary bleeding | 0 (0.0%)0 | 0 (0.0%) | 1.000 |
| Meets the 3–5 levels of the BARC bleeding grading criteria | 1 (1.19%) | 1 (1.19%) | 1.000 |
| Meets the 1–2 levels of the BARC bleeding grading criteria | 1 (1.19%) | 2 (2.38%) | 1.000 |
| Total | 2 (2.38%) | 3 (3.57%) | 0.650 |
BARC – bleeding academic research consortium.
Blood Coagulation Function
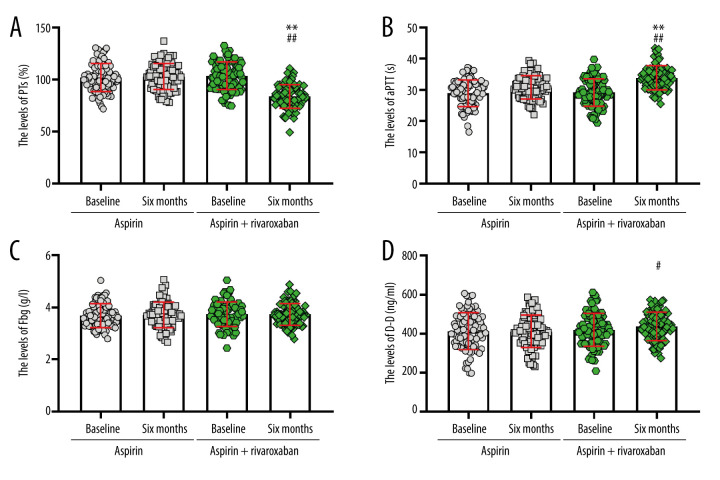
To determine the anticoagulant effect of aspirin and rivaroxaban, we analyzed the blood coagulation function of patients with different treatment conditions. At baseline, no significant difference was found in prothrombin time (PT) levels between the 2 groups. Within-group analyses showed a significant decrease in PT levels at 6 months of intervention compared to baseline values in the aspirin + rivaroxaban group (P<0.05) but not in the aspirin group (P>0.05). After adjusting the baseline data, the PT levels in the aspirin + rivaroxaban group were significantly lower than in the aspirin group (F=105.634, P<0.01) (Figure 3A). Before administering aspirin or aspirin + rivaroxaban, no significant difference was found in the partial thromboplastin time (aPTT) and D dimer (D-D) (P>0.05, Figure 3B, 3C). From baseline to month 6, the aPTT levels increased significantly in the aspirin + rivaroxaban group, but the levels did not change significantly in the aspirin group. Moreover, aspirin + rivaroxaban was more effective than aspirin in improving aPTT levels at 6 months after the first visit (F=27.461, P<0.01, Figure 3B). However, D-D showed the opposite trend to aPTT (F=2.355, P>0.05, Figure 3C). No significant differences were observed in fibrinogen (Fbg) and platelets between the 2 groups at baseline (all P>0.05). After administering aspirin or aspirin + rivaroxaban for 6 months, no significant change was recorded in Fbg levels (F=0.142, P>0.05, Figure 3D). Moreover, patients administered aspirin or aspirin + rivaroxaban did not show significant differences in platelet levels (F=0.780, P>0.05, Figure 4).
Figure 3.
Changes in coagulation factor levels before and after aspirin or aspirin + rivaroxaban intervention, including (A) prothrombin time (PTs), (B) partial thromboplastin time (aPTT), (C) fibrinogen (Fbg), and (D) D dimer (D-D) (The figure was created by GraphPad Prism, version 8.0, GraphPad Software, Inc.).
Figure 4.
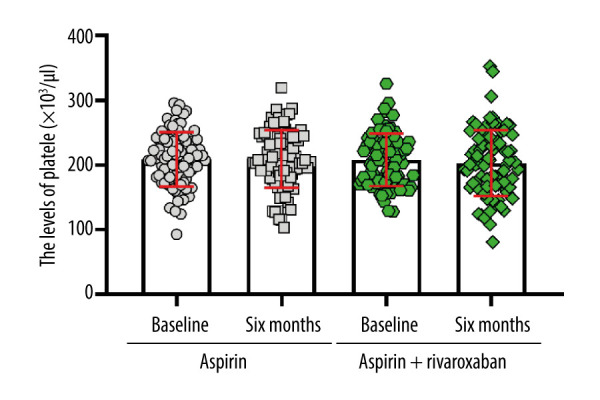
Changes in platelets levels before and after aspirin or aspirin + rivaroxaban intervention. (The figure was created by GraphPad Prism, version 8.0, GraphPad Software, Inc.).
Cardiac Function Analysis
We evaluated the changes in cardiac function by performing cardiac ultrasonography (Figure 5). The left ventricular ejection fraction (LVEF) increased significantly in the aspirin + rivaroxaban group after treatment compared to the corresponding value at baseline (P<0.01). However, no significant difference in the LVEF was recorded for the aspirin group between pretreatment and post-treatment conditions. Additionally, the LVEF levels of the aspirin + rivaroxaban group were 2.34% higher than those recorded in the aspirin group after treatment (F=1.941, P>0.05). These findings indicated that aspirin and rivaroxaban were helpful to improve cardiac function, but did not reach statistical significance.
Figure 5.
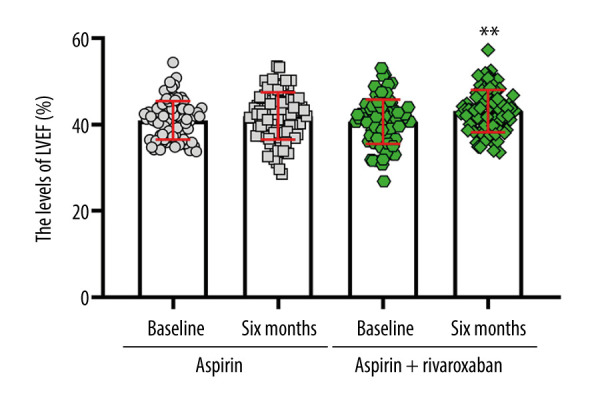
The effect of aspirin or aspirin + rivaroxaban on left ventricular ejection fraction (LVEF). (The figure was created by GraphPad Prism, version 8.0, GraphPad Software, Inc.).
Discussion
Patients with AMI and MVD after PCI need antiplatelet drugs with various clinical characteristics, especially to balance the risk of bleeding and ischemia [21]. Aspirin and rivaroxaban are commonly used to treat cardiovascular diseases [22]. Aspirin can irreversibly inhibit platelet cyclooxygenase (COX-1), resulting in a decrease in the production of TXA2, thus inhibiting platelet aggregation [23]. Rivaroxaban can selectively and competitively inhibit free and bound Xa factors and prothrombin activity and it can prolong activated partial thromboplastin time and prothrombin time in a dose-dependent manner [3,24,25]. The main difference between rivaroxaban and sulfonated heparin sodium/heparin is that it does not need the participation of antithrombin III and can directly antagonize free and bound Xa factors to reduce the activation of thrombin, thus prolonging the clotting time. It can also block the formation of blood clots and destroy formed blood clots. The efficacy and safety of rivaroxaban + aspirin or aspirin alone in reducing the risk of myocardial infarction, stroke, and cardiovascular death in patients with coronary artery disease (CAD) or PAD was evaluated in another study [26]. In another study, DCAT (rivaroxaban + aspirin) significantly reduced the risk of MACE by 24% [25]. By comparing the all-cause mortality between the COMPASS study and the traditional intensive antiplatelet therapy study, only the DCAT regimen was found to improve the overall survival rate of patients with CAD or PAD. Therefore, the 2019 ESC-CCS guidelines were based on the COMPASS study and recommended the administration of rivaroxaban (2.5 mg) combined with aspirin for secondary prevention of CCS (CAD patients with a course of more than 1 year or more vessel lesions after myocardial infarction) [27]. Concerning antithrombotic therapy, the latest NSTE-ACS management guide of ESC in 2020 recommended administering DCAT (rivaroxaban 2.5 mg + aspirin 75–100 mg) as one of the alternative antithrombotic options for long-term secondary prevention of NSTE-ACS in patients with high ischemic risk IIaA, moderate ischemic risk (IIbA), no high risk of bleeding, or life-threatening bleeding [8]. However, the secondary prevention effect of aspirin and rivaroxaban is rarely included in the Chinese population, only in studies on the Asian population. For example, Liang’s COMPASS study showed that only 15.58% of the patients enrolled were Asian and only 14.42% of patients were from the Asia-Pacific region [28]. Therefore, whether the above results are suitable for Chinese patient needs to be confirmed by collecting more data from Chinese patients.
First, we evaluated the major adverse cardiovascular events (MACE) in both groups. Treatment with aspirin and rivaroxaban decreased the risk of MACE in AMI and MVD patients significantly; this treatment technique performed better for patients with MACE than for patients with CAD and lower-extremity peripheral artery disease (LE-PAD) [29]. Our results showed that combined therapy of aspirin and rivaroxaban improved overall survival in patients with AMI and MVD. These findings provide further credible evidence for determining the efficacy of aspirin + rivaroxaban in cardiovascular disease. Women may exhibit different symptoms of cardiovascular disease than men, which could be attributed to various biological and social factors [30]. Notably, compared with aspirin treatment, our study showed that aspirin + rivaroxaban therapy reduced the incidence of MACE in both male and female populations, but the difference was not statistically significant. This is consistent with the research results of Liang et al [28]. Studies have found that bleeding events can seriously affect the prognosis of patients. The BARC, TIMI, and GUSTO bleeding scores are the 3 most commonly used in the clinical evaluation of bleeding risk among patients [31]. The BARC bleeding score is more detailed and effective for evaluating patients than the other 2 scoring systems. Therefore, we used the BARC bleeding score to evaluate the bleeding condition of the patients in this study. Bleeding occurred in a few patients in the aspirin + rivaroxaban group, and no significant difference was recorded in the evaluation of BARC bleeding between the 2 groups. Our results were different from those reported in another study [25]. Aspirin can directly destroy the digestive tract mucosa and cause gastrointestinal bleeding [32]. Although no significant difference in bleeding events between the groups was recorded in our study, the bleeding of patients administered DCAT needs to be closely monitored. However, we also found that the aPTT, PTs, and D-D were significantly different between the aspirin plus rivaroxaban group and the aspirin group, as reported in other studies [29,33]. Our results indicated that the intervention of aspirin plus rivaroxaban increased the risk of bleeding, based on the assessments of the effects of antiplatelet and anticoagulant factors in AMI and MVD patients. This suggests that high-risk ischemic patients may respond uniquely to antiplatelet drugs; however, the specific cause could not be determined. Hence, further studies are needed to evaluate its significance. These findings indicate that when patients are administered aspirin + rivaroxaban, their bleeding status must be closely monitored to prevent excessive bleeding from causing secondary injury. Our results showed that aspirin plus rivaroxaban did not affect cardiac function. Our findings were similar to those of a study by Branch on rivaroxaban with or without aspirin in patients with heart failure and chronic coronary or peripheral artery disease [34]. Our study is the first to evaluate the efficacy and safety of aspirin and rivaroxaban in secondary prophylaxis of coronary heart disease patients at high risk of ischemia (AMI and MVD). In addition, our study provides a reference for the implementation of DAPT based on antithrombotic and anticoagulant therapy in patients with cardiovascular disease, and also provides a new option for long-term antithrombotic strategies in such patients at high risk of ischemia.
This study had some limitations. First, although aspirin + rivaroxaban treatment was found to be more effective in treating AMI, a comprehensive analysis of the AMI subgroups was lacking. Second, the mechanism underlying the effectiveness of the combined therapy (aspirin + rivaroxaban) in treating AMI and MVD needs to be determined. Furthermore, since only some patients have received platelet aggregation test or light transmission aggregation assay (LTA), more extensive tests are still needed in the follow-up. Finally, multicenter clinical studies with a large sample size need to be conducted to validate our findings.
Conclusions
To summarize, we compared the effectiveness of 2 antithrombotic treatment methods (aspirin combined with rivaroxaban vs aspirin alone) in patients diagnosed with AMI (1 year) and MVD after PCI. Patients administered aspirin + rivaroxaban showed a significant decrease in the occurrence of adverse cardiovascular events but not a significant increase in the bleeding events. These results suggest that administering a combination of aspirin and rivaroxaban is an effective and safe strategy for treating patients with high-risk ischemia.
Footnotes
Conflict of interest: None declared
Publisher’s note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher
Ethics Approval and Consent to Participate: This study was approved by the Institutional Ethics Committee of the Second Hospital of Hebei Medical University (Shijiazhuang, China). The approval number is 2021-R423. Written informed consent was obtained from every participant in the present study.
Declaration of Figures’ Authenticity: All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
Financial support: This study was funded by the Health Commission of Hebei Province (No. 20221015)
References
- 1.Malakar AK, Choudhury D, Halder B, et al. A review on coronary artery disease, its risk factors, and therapeutics. J Cell Physiol. 2019;234(10):16812–23. doi: 10.1002/jcp.28350. [DOI] [PubMed] [Google Scholar]
- 2.Dong C, Yang Y, Wang Y, et al. Gut microbiota combined with metabolites reveals unique features of acute myocardial infarction patients different from stable coronary artery disease. J Adv Res. 2023;46:101–12. doi: 10.1016/j.jare.2022.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo Y, Jiang H, Wang J, et al. 5mC modification patterns provide novel direction for early acute myocardial infarction detection and personalized therapy. Front Cardiovasc Med. 2022;9:1053697. doi: 10.3389/fcvm.2022.1053697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stähli BE, Varbella F, Schwarz B, et al. Rationale and design of the MULTISTARS AMI Trial: A randomized comparison of immediate versus staged complete revascularization in patients with ST-segment elevation myocardial infarction and multivessel disease. Am Heart J. 2020;228:98–108. doi: 10.1016/j.ahj.2020.07.016. [DOI] [PubMed] [Google Scholar]
- 5.Khaled S, Jaha N, Shalaby G. Clinical characteristics and short-term outcomes of patients presenting with acute myocardial infarction having multi-vessel disease – a single Middle-Eastern tertiary-care center experience. Indian Heart J. 2022;74(1):28–33. doi: 10.1016/j.ihj.2021.11.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma R, Kumar P, Prashanth SP, et al. Dual antiplatelet therapy in coronary artery disease. Cardiol Ther. 2020;9(2):349–61. doi: 10.1007/s40119-020-00197-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ibánez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Rev Esp Cardiol (Engl Ed) 2017;70(12):1082. doi: 10.1016/j.rec.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Collet JP, Thiele H, Barbato E, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42(14):1289–367. doi: 10.1093/eurheartj/ehaa575. [DOI] [PubMed] [Google Scholar]
- 9.Yamashita A, Sumi T, Goto S, et al. Detection of von Willebrand factor and tissue factor in platelets-fibrin rich coronary thrombi in acute myocardial infarction. Am J Cardiol. 2006;97(1):26–28. doi: 10.1016/j.amjcard.2005.07.105. [DOI] [PubMed] [Google Scholar]
- 10.Gerotziafas GT, Zografos T, Pantos I, et al. Prospective assessment of biomarkers of hypercoagulability for the identification of patients with severe coronary artery disease. The ROADMAP-CAD Study. Clin Appl Thromb Hemost. 2020;26:1076029620964590. doi: 10.1177/1076029620964590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franchi F, Angiolillo DJ. Novel antiplatelet agents in acute coronary syndrome. Nat Rev Cardiol. 2015;12(1):30–47. doi: 10.1038/nrcardio.2014.156. [DOI] [PubMed] [Google Scholar]
- 12.Cohen M, Iyer D. The “dual-pathway” strategy after acute coronary syndrome: Rivaroxaban and antiplatelet agents in the ATLAS ACS 2-TIMI 51 trial. Cardiovasc Ther. 2014;32(5):224–32. doi: 10.1111/1755-5922.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Angiolillo DJ, Capodanno D, Goto S. Platelet thrombin receptor antagonism and atherothrombosis. Eur Heart J. 2010;31(1):17–28. doi: 10.1093/eurheartj/ehp504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petzold T, Thienel M, Dannenberg L, et al. Rivaroxaban reduces arterial thrombosis by inhibition of FXa-driven platelet activation via protease activated receptor-1. Circ Res. 2020;126(4):486–500. doi: 10.1161/CIRCRESAHA.119.315099. [DOI] [PubMed] [Google Scholar]
- 15.Costa OS, Connolly SJ, Sharma M, et al. Andexanet alfa versus four-factor prothrombin complex concentrate for the reversal of apixaban- or rivaroxaban-associated intracranial hemorrhage: A propensity score-overlap weighted analysis. Crit Care. 2022;26(1):180. doi: 10.1186/s13054-022-04043-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Songqun H, Chunling W, Zhifu G, et al. Effects of rivaroxaban on activated clotting time in catheter ablation for atrial fibrillation in Chinese patients. J Interv Card Electrophysiol. 2020;59(3):509–16. doi: 10.1007/s10840-019-00650-8. [DOI] [PubMed] [Google Scholar]
- 17.Ageno W, Bertù L, Bucherini E, et al. Rivaroxaban treatment for six weeks versus three months in patients with symptomatic isolated distal deep vein thrombosis: Randomised controlled trial. BMJ. 2022;379:e072623. doi: 10.1136/bmj-2022-072623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazzone PM, Capodanno D. Low dose rivaroxaban for the management of atherosclerotic cardiovascular disease. J Thromb Thrombolysis. 2023;56(1):91–102. doi: 10.1007/s11239-023-02821-x. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y, Qin L, Yang J, et al. Changes in thromboelastography to predict ecchymosis after knee arthroplasty: A promising guide for the use of anticoagulants. Front Surg. 2022;9:871776. doi: 10.3389/fsurg.2022.871776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.AlKhalfan F, Kerneis M, Nafee T, et al. D-dimer levels and effect of rivaroxaban on those levels and outcomes in patients with acute coronary syndrome (an ATLAS ACS-TIMI 46 trial substudy) Am J Cardiol. 2018;122(9):1459–64. doi: 10.1016/j.amjcard.2018.07.032. [DOI] [PubMed] [Google Scholar]
- 21.Bonaca MP, Goto S, Bhatt DL, et al. Prevention of stroke with ticagrelor in patients with prior myocardial infarction: Insights from PEGASUS-TIMI 54 (Prevention of Cardiovascular Events in Patients with Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin-Thrombolysis in Myocardial Infarction 54) Circulation. 2016;134(12):861–71. doi: 10.1161/CIRCULATIONAHA.116.024637. [DOI] [PubMed] [Google Scholar]
- 22.Hiatt WR, Bonaca MP, Patel MR, et al. Rivaroxaban and aspirin in peripheral artery disease lower extremity revascularization: Impact of concomitant clopidogrel on efficacy and safety. Circulation. 2020;142(23):2219–30. doi: 10.1161/CIRCULATIONAHA.120.050465. [DOI] [PubMed] [Google Scholar]
- 23.Mirabito Colafella KM, Neuman RI, Visser W, et al. Aspirin for the prevention and treatment of pre-eclampsia: A matter of COX-1 and/or COX-2 inhibition? Basic Clin Pharmacol Toxicol. 2020;127(2):132–41. doi: 10.1111/bcpt.13308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Millesimo M, Elia E, Marengo G, et al. Antithrombotic strategy in secondary prevention for high-risk patients with previous acute coronary syndrome: Overlap between the PEGASUS eligibility and the COMPASS eligibility in a large multicenter registry. Am J Cardiovasc Drugs. 2023;23(1):77–87. doi: 10.1007/s40256-022-00554-5. [DOI] [PubMed] [Google Scholar]
- 25.Eikelboom JW, Connolly SJ, Bosch J, et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377(14):1319–30. doi: 10.1056/NEJMoa1709118. [DOI] [PubMed] [Google Scholar]
- 26.Eikelboom JW, Bosch J, Connolly SJ, et al. Long-term treatment with the combination of rivaroxaban and aspirin in patients with chronic coronary or peripheral artery disease: Outcomes during the open label extension of the COMPASS trial. Eur Heart J Cardiovasc Pharmacother. 2022;8(8):786–95. doi: 10.1093/ehjcvp/pvac023. [DOI] [PubMed] [Google Scholar]
- 27.Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407–77. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 28.Liang Y, Zhu J, Liu L, et al. Efficacy and safety of rivaroxaban plus aspirin in women and men with chronic coronary or peripheral artery disease. Cardiovasc Res. 2021;117(3):942–49. doi: 10.1093/cvr/cvaa100. [DOI] [PubMed] [Google Scholar]
- 29.Anand SS, Bosch J, Eikelboom JW, et al. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: An international, randomised, double-blind, placebo-controlled trial. Lancet. 2018;391(10117):219–29. doi: 10.1016/S0140-6736(17)32409-1. [DOI] [PubMed] [Google Scholar]
- 30.Kouvari M, Yannakoulia M, Souliotis K, et al. Challenges in sex- and gender-centered prevention and management of cardiovascular disease: Implications of genetic, metabolic, and environmental paths. Angiology. 2018;69(10):843–53. doi: 10.1177/0003319718756732. [DOI] [PubMed] [Google Scholar]
- 31.Lettino M, Leonardi S, De Maria E, et al. Antiplatelet and antithrombotic treatment for secondary prevention in ischaemic heart disease. Eur J Prev Cardiol. 2017;24(3 Suppl):61–70. doi: 10.1177/2047487317707854. [DOI] [PubMed] [Google Scholar]
- 32.Zhao R, Coker OO, Wu J, et al. Aspirin reduces colorectal tumor development in mice and gut microbes reduce its bioavailability and chemopreventive effects. Gastroenterology. 2020;159(3):969–83e4. doi: 10.1053/j.gastro.2020.05.004. [DOI] [PubMed] [Google Scholar]
- 33.Pistrosch F, Matschke JB, Schipp D, et al. Rivaroxaban compared with low-dose aspirin in individuals with type 2 diabetes and high cardiovascular risk: A randomised trial to assess effects on endothelial function, platelet activation and vascular biomarkers. Diabetologia. 2021;64(12):2701–12. doi: 10.1007/s00125-021-05562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Branch KR, Probstfield JL, Eikelboom JW, et al. Rivaroxaban with or without aspirin in patients with heart failure and chronic coronary or peripheral artery disease: The COMPASS trial. Circulation. 2019;140(7):529–37. doi: 10.1161/CIRCULATIONAHA.119.039609. [DOI] [PMC free article] [PubMed] [Google Scholar]