Abstract
Background
Amphetamine-type stimulant (ATS) abuse is strongly associated with an elevated risk of HIV infection and transmission. Antiretroviral therapy (ART) serves as the primary approach for managing HIV infection and AIDS progression. However, ATS abuse diminishes the efficacy of ART in HIV/AIDS patients, amplifying the vulnerability to immunological non-response (INR) and ultimately increasing the incidence rate and mortality of opportunistic infections. Currently, no effective interventions targeting INR exist. Acupuncture has demonstrated promise in bidirectionally modulating the body’s immune response and may be beneficial for INR in HIV/AIDS combined with ATS abuse. Nevertheless, further research and comprehensive evaluation are imperative to substantiate these findings.
Methods
This study is a two-center, randomized, non-acupoint controlled, single-blind clinical trial. It will be conducted in two large drug rehabilitation centers in western China, involving 114 INR patients receiving ART. The participants will be randomly assigned to either the Transcutaneous Electrical Acupoint Stimulation (TEAS) + ART group or the sham-TEAS + ART group, in a 1:1 ratio. Both groups will receive a 48-week treatment. The primary outcome measure assessed after treatment is the CD4 + T cell count. Secondary outcome measures include the immune reconstitution efficiency of HIV patients, CD4/CD8 ratio, CD4 + CD45RA + and CD4 + CD45RO + counts, CD4 + CD28 + counts, CD4 + CD38 + and CD8 + CD38 + counts, CD4 + ki67 + and CD8 + ki67 + counts, JC mitochondrial membrane potential testing, the incidence of opportunistic infections, and the HIV/AIDS PRO scale. Adverse events occurring during the study observation period will be documented.
Discussion
This study will investigate the effect of TEAS on immune reconstitution in patients with amphetamine abuse and HIV infection.
Trial registration
Chinese Clinical Trial Registry, ChiCTR 2300076363. Registered on October 7, 2023, https://www.chictr.org.cn/.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12906-024-04724-7.
Keywords: Complementary medicine, Amphetamine abuse, HIV/AIDS, immunological non-responder, protocol
Background
Acquired Immune Deficiency Syndrome (AIDS) is a serious immune system disease caused by human immunodeficiency virus (HIV) infection. It is distinguished by a progressive decline in CD4 + T cell count and functional impairment. Consequently, the immune system undergoes severe damage, leading to various opportunistic infections and tumors [1]. In China, the outbreak of HIV/AIDS originated from intravenous drugs [2, 3]. However, the rising prevalence of amphetamine-type stimulant (ATS) abuse has contributed to significant changes in the behavior of drug users. ATS consumption leads to heightened dopamine secretion, alters mental states, reduces self-control, and increases risky sex [4, 5]. As a consequence, the proportion of HIV transmission through sexual routes has been steadily increasing over the years [6, 7]. According to China’s latest diagnostic report, more than 95% of AIDS-infected people are sexually infected [8]. This demonstrates a strong correlation between ATS abuse and HIV transmission [9], with ATS abuse serving as an independent risk factor for HIV exposure [10, 11].
Currently, antiretroviral therapy (ART) serves as the primary approach to managing HIV infection and AIDS, transforming AIDS into a chronic and controllable disease [12, 13]. However, a significant proportion (approximately 10-40%) of HIV/AIDS patients fail to achieve adequate recovery of their peripheral blood CD4 + T cell count even with sufficient ART, leading to immunological non-response (INR) [14–17]. INR appears to be more prevalent among individuals who abuse ATS and are infected with HIV. On one hand, HIV-infected ATS abusers tend to exhibit higher viral loads compared to non-users. Therefore, initiation of ART in ATS abusers is associated with delayed viral suppression, and an increased likelihood of drug resistance mutations, resulting in CD4 + T cell depletion, immune activation, and accelerated disease progression [18, 19]. On the other hand, ATS abuse can also impede patients’ access to medical services and hinder adherence to treatment regimens, thus diminishing the overall effectiveness of ART interventions [18].
The primary clinical manifestation of INR in HIV/AIDS is long-term immunodeficiency, which significantly increases the risk of opportunistic infections and mortality [16, 20]. In recent years, there has been growing interest in implementing effective interventions to address INR in HIV patients. While certain studies suggest that drug therapies can enhance the immune response by suppressing abnormal immune activation [21], numerous studies have demonstrated the limited therapeutic efficacy of immunomodulators such as statins [22], mesalazine [23], rifaximin [24], and rapamycin [25] in improving INR. Currently, there is a scarcity of effective intervention measures for INR.
As a component of traditional Chinese medicine, acupuncture has the potential to modulate immune cells and immune molecules bidirectionally, thereby enhancing overall immunity and disease resistance [26, 27]. Transcutaneous electrical acupoint stimulation (TEAS) integrates traditional Chinese medicine principles with modern electrical stimulation techniques [28]. Applying electrical stimulation to specific acupoints can potentially regulate the patient’s immune microenvironment through multiple pathways and targets, ultimately improving immune function and facilitating recovery [29]. Previous studies have demonstrated that TEAS can regulate the quantities of CD4 + and CD8 + cells, boost immunity, and reduce the risk of infection [30]. Additionally, TEAS has shown efficacy in enhancing immune function in perioperative patients [31], mitigating bone marrow suppression following chemotherapy [32], and improving postoperative immune function in lung cancer patients [33]. Considering the disease characteristics of INR patients, TEAS may serve as a promising novel intervention strategy to promote immune reconstitution. Investigating the impact of TEAS on INR in HIV/AIDS and ATS abuse patients can provide valuable insights for its clinical application.
Methods
Study design
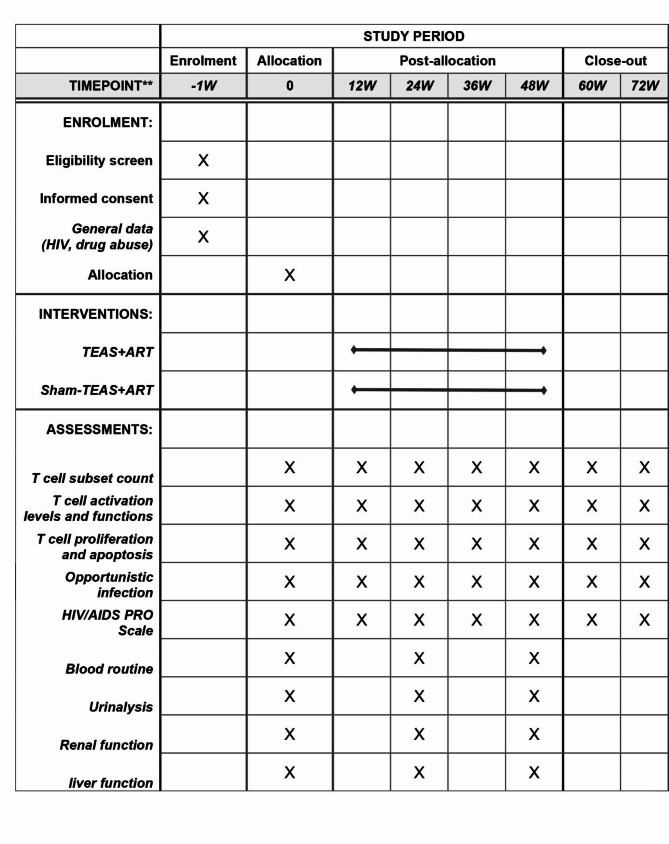
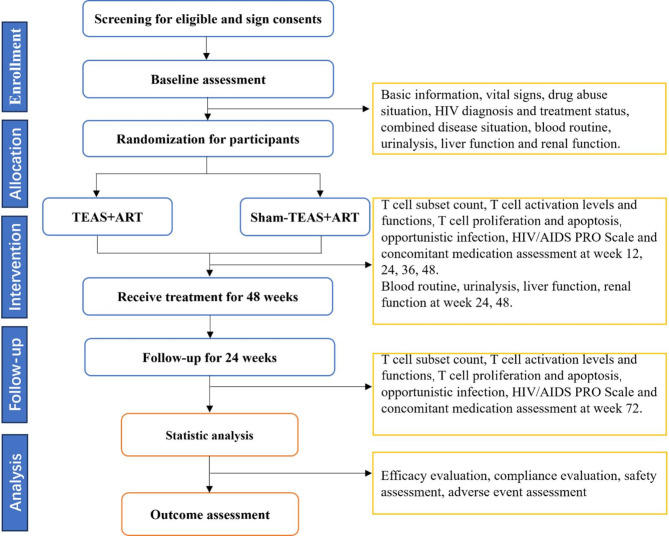
This is a two-center, randomized, non-acupoint controlled, single-blind clinical trial designed to demonstrate the efficacy of TEAS in improving INR among HIV/AIDS combined with ATS abusers. The study will be conducted at the Deyang Women’s Drug Rehabilitation Center and Ziyang Men’s Drug Rehabilitation Center in Sichuan, China, with drug users from all over the country. Patients who simultaneously satisfy ATS abuse and HIV immunological non-response will be included in our study and randomly divided into TEAS group and sham-TEAS group in a 1:1 ratio. The total observation period for this study will span 73 weeks, consisting of a 1-week baseline period, a 48-week treatment period, and a 12-week follow-up period. The study protocol follows the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) [34]. The SPIRIT schedule is shown in Fig. 1, and the flowchart is shown in Fig. 2.
Fig. 1.
The SPIRIT schedule
Fig. 2.
Flowchart of the study
Diagnostic criteria
ATS abuse
According to the diagnostic criteria for ATS abuse withdrawal in the Diagnostic Manual of the American Psychiatric Association (DSM-5). [35]
Criterion A: The cessation of or reduction in prolonged use of amphetamine-type substances.
Criterion B: Dysphoric mood and at least two of the following physiological changes, developing within a few hours to several days after Criterion A: Fatigue; Vivid, unpleasant dreams; Insomnia or hypersomnia; Increased appetite; Psychomotor agitation or retardation.
Criterion C: The symptoms in Criterion B cause clinically significant distress or impairment in social, occupational, or other important areas of functioning.
Criterion D: The symptoms are not attributable to another medical condition or better explained by another mental disorder.
HIV immunological non-response
Referring to the diagnostic criteria for INR in previous relevant studies [16, 36].
CD4 + T lymphocyte count < 200 cells/µL in patients who have been receiving antiretroviral therapy (ART) for more than 1 year but less than 2 years.
CD4 + T lymphocyte count < 350 cells/µL in patients who have been receiving ART for more than 2 years.
After a prolonged period of treatment (e.g., 4–7 years), if the CD4 + T lymphocyte count does not exceed a specific threshold (e.g., > 350 cells/µL or 500 cells/µL) and HIV-RNA remains below the detection limit.
Inclusion criteria
The study includes individuals between the ages of 18 and 65, irrespective of gender.
Participants should have a history of previous ATS abuse and meet the diagnostic criteria for ATS withdrawal.
HIV patients should have received standard ART for a minimum duration of 1 year.
Participants should meet the diagnostic criteria for INR.
Following treatment, the viral load should be consistently controlled at a level below 50 copies/mL for at least 1 year.
All participants are required to provide voluntary informed consent by signing a consent form.
Exclusion criteria
Presence of uncontrolled serious opportunistic infections.
Coexistence of other serious organic diseases, such as tumors, cardiovascular diseases, cirrhosis, or mental illnesses, such as schizophrenia.
Participation in other clinical trials within the past 3 months.
Receipt of treatment other than the ART regimen within the past 1 month.
Coexistence of liver and kidney dysfunction.
Participants who meet the inclusion criteria but satisfy any of the above conditions will be excluded from the study.
Sample size
According to relevant literature, the standard deviation of CD4 + T cell count in INR patients receiving ART is reported to be 137.77 [37]. This study is designed as a superiority trial, according to the sample size formula [38], α = 0.05, β = 0.2, unilateral test. The minimum effect size is set at 50 [39]. The sample size of n = 47 is initially determined. However, considering a 20% potential dropout rate, we have decided to collect 57 patients per group.
Recruitment
Recruitment of participants is scheduled to commence in October 2023 and is expected to be completed by September 2024. Due to the poor compliance of HIV patients with combined ATS abuse receiving ART therapy, we have decided to select the drug rehabilitation center as the research site. All HIV-infected patients in the center will receive or are currently receiving ART treatment. We will publicly recruit potential participants through posters, public lectures, and survey questionnaires.
Randomization and allocation concealment
Stratified block randomization will be implemented in this study to ensure a balanced allocation of subjects across study centers. The randomization process involved dividing eligible subjects into two groups, namely the TEAS group and the sham-TEAS group, in a 1:1 ratio. We will use a computer system to generate a random sequence before starting to recruit patients. The randomization plan will be managed and maintained by a third-party randomization team, independent of the study investigators. The randomization scheme will be securely enclosed in a sealed opaque envelope with a unique code. The envelope will be handed over to the recruiting researcher. At the time of subject enrollment, the envelope corresponding to the subject’s assigned number will be opened to reveal the group allocation information.
Blinding
In this experiment, a blinding method will be employed for the participants to ensure they are unaware of the type of TEAS. However, due to the nature of the TEAS treatment, it is not possible to blind the acupuncture doctor. Nevertheless, the blinding assessment will be followed, and efficacy evaluation, as well as statistical analysis, will be conducted by third-party individuals who are not involved in the trial, achieving separation of research, evaluation, and statistics. To facilitate the blind evaluation process, the acupoints or non-acupoints will be labeled as A1, A2, B1, and B2 in the Case Report Form. The specific acupoints or non-acupoints will not be mentioned in the tables to further ensure blinding during the evaluation process.
Interventions
ART
Both groups of patients refer to the ART treatment scheme recommended in the 2021 Chinese HIV/AIDS Diagnosis and Treatment Guide [40].
TEAS and sham-TEAS
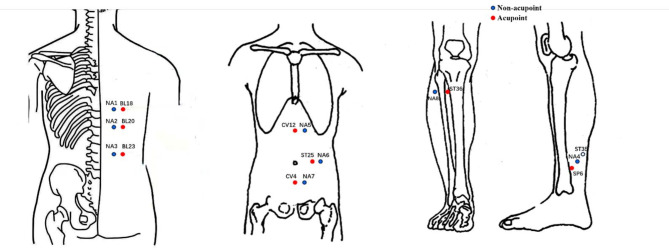
TEAS operation: Before treatment, the patients will be instructed to empty their bladder and lie flat on the bed. The TEAS electrode patch will be applied to the corresponding acupoint. The selection of acupoints is based on previous research [36, 41–43]. Detailed information about acupoint location is shown in Table 1; Fig. 3. The current intensity will begin at 0, and each button press will increase the intensity by 1 mA. The intensity of stimulation will be adjusted to a range of 15–25 mA, based on the patient’s maximum tolerance. The stimulation frequency will be set at 2/100Hz, with a density wave alternating every 3 s. The operation will be performed by two qualified Traditional Chinese Medicine practitioners trained in acupuncture techniques. TEAS recommends replacing a set of acupoint stimuli every three months. Based on the duration of this research experiment, it is suggested to alternate between two sets of acupoints. The left and right acupoints will be used alternatively, with only one side acupoint used during each treatment session.
Table 1.
Non-acupoints and acupoints were used in the trial
| Treatment group | Acupoint group | Non-acupoint/Acupoint | Location |
|---|---|---|---|
| Sham-TEAS | A1 | Non-acupoint 1 | Under the spinous process of the 9th thoracic vertebra, 2.25 cun horizontally apart from GV8 (Jinsuo) |
| Non-acupoint 2 | Under the spinous process of the 11th thoracic vertebra, 2.25 cun horizontally apart from GV 6 (Jizhong) | ||
| Non-acupoint 3 | Under the spinous process of the 2nd lumbar vertebra, 2.25 cun horizontally apart from GV4 (Mingmen) | ||
| Non-acupoint 4 | The midpoint of the connection between SP 6 (Sanyinjiao) and KI 9 (Zhubin) | ||
| A2 | Non-acupoint 5 | 1.25 cun horizontally apart from the CV12 (Zhongwan) | |
| Non-acupoint 6 | 1 cun horizontally apart from the ST25 (Tianshu) | ||
| Non-acupoint 7 | 1.25 cun horizontally apart from the CV4 (Guanyuan) | ||
| Non-acupoint 8 | On the lateral side of the lower leg, 1 cun lateral to ST36 (Zusanli), between the Stomach Meridian of Foot-Yangming and Gallbladder Meridian of Foot-Shaoyang (needled bilaterally) | ||
| TEAS | B1 | BL18 (Ganshu) | Under the spinous process of the 9th thoracic vertebra, 3 cun horizontally apart from GV8 (Jinsuo) |
| BL20 (Pishu) | Under the spinous process of the 11th thoracic vertebra, 3 cun horizontally apart from GV 6 (Jizhong) | ||
| BL23 (Shenshu) | Under the spinous process of the 2nd lumbar vertebra, 3 cun horizontally apart from GV4 (Mingmen) | ||
| SP6 (Sanyinjiao) | On the medial side of the shank, 3 cun above the medial malleolus, by the posterior border of the tibia (needled bilaterally) | ||
| B2 | CV12 (Zhongwan) | On the anterior median line of the upper abdomen, 4 cun above the navel. | |
| ST25 (Tianshu) | 3 cun horizontally next to the navel | ||
| CV4 (Guanyuan) | On the anterior median line of the lower abdomen, 3 cun below the navel. | ||
| ST36 (Zusanli) | On the anterior lateral side of the shank, 3 cun below ST35 (Dubi), one horizontally placed finger distance lateral to the anterior border of the tibia (the middle finger) (needled bilaterally) |
Fig. 3.
Location of acupoints and non-acupoints
Sham-TEAS operation: Before treatment, the patient will be informed to empty the bladder and lie flat on the bed. The electrode patch will be applied to the corresponding non-acupoint skin, and detailed information about acupoint positions is shown in Tables 1and Fig. 3. The current intensity does not exceed 5 mA and only produces a slight sensation under non-acupoint conditions to ensure psychological comfort and simulate the treatment experience. Two sets of acupoints are used alternately, with only one acupoint used during each treatment period. The operation will be carried out by two qualified doctors who have received acupuncture technical training.
Treatment time and observation period
ART takes medication according to the prescribed treatment plan. Each acupoint receives 30 min of TEAS treatment, three times a week. The total observation period for this study will span 73 weeks, consisting of a 1-week baseline period, a 48-week treatment period, and a 24-week follow-up period.
Outcome measures
Primary outcome measurement
CD4 + T cell count: It is a crucial indicator for assessing the immune function recovery in individuals with HIV [44]. It holds significant importance in understanding the immune status and disease progression, determining disease staging, evaluating treatment effectiveness, and identifying potential complications in HIV-infected patients [40].
Secondary outcome measurement
Efficiency of immune reconstitution
The evaluation criteria for assessing the efficiency of immune reconstitution are outlined in the Chinese Medicine Diagnosis and Treatment Plan for AIDS (Adults) (2016 Edition), developed by experts organized by the China Administration of Traditional Chinese Medicine and the China Health and Family Planning Commission.
Effective: An increase in CD4 + T cell count of ≥ 50/µl or ≥ 30% compared to the baseline.
Stable: CD4 + T cell count shows an increase or decrease of less than 50/µl or less than 30% compared to the baseline.
Invalid: A decrease in CD4 + T cell count of ≥ 50/µl or ≥ 30% compared to the baseline.
T cell subset
CD4+/CD8+: The CD4+/CD8 + ratio is a crucial indicator of immune status that reflects the activation level of T cells. It holds significant importance in predicting the risk of CD4 cell depletion in HIV patients undergoing ART [45].
CD4 + CD45RA + and CD4 + CD45RO+: Following ART treatment, the early-stage immune reconstitution primarily involves the expansion of peripheral memory CD4 + T cells, while the later stage relies on the replenishment of naïve CD4 + T cells. Therefore, the quantification of CD4 + CD45RA + and CD4 + CD45RO + subsets is highly relevant for assessing the reconstruction of immune function [46].
T cell activation levels and functions
CD4 + CD28+: CD28 is a critical receptor on T lymphocytes that plays a vital role in their activation. Research has indicated a significant reduction in both the number and percentage of CD4 + CD28 + cells in individuals infected with HIV-1 compared to the general population [47].
CD4 + CD38 + and CD8 + CD38+: The expression levels of CD4 + CD38 + and CD8 + CD38 + molecules serve as crucial indicators reflecting the level of immune activation in the body [48, 49].
T cell proliferation and apoptosis
CD4 + ki67+, CD8 + ki67+: Ki67 is a non-histone protein found in the nucleus that serves as an important marker of cell proliferation. The expression of Ki67 indicates the presence of cells in the proliferative stage [50].
JC mitochondrial membrane potential detection/CD4+, JC mitochondrial membrane potential detection/CD8+: reflect the apoptosis rate of CD4 + T cells and CD8 + T cells.
Opportunistic infection
According to the Chinese AIDS Diagnosis and Treatment Guidelines (2021 Edition), the diagnosis of all opportunistic infections will be confirmed based on clinical manifestations and relevant auxiliary examinations. This diagnostic process involves the collaboration of two or more attending physicians.
HIV/AIDS PRO scale
The HIV/AIDS PRO Scale will be used as a tool to evaluate the quality of life and health status of HIV/AIDS patients.
Data management
Researchers will be required to complete CRF in a timely, accurate, complete, standardized, and truthful manner. To establish a database, EpiData2.1 software will be utilized. Two well-trained data entry personnel perform parallel double entry of the data from the CRF table. After the parallel double entry is completed, the EpiData function is employed to compare and verify the consistency of the double entry. In cases where inconsistencies are identified during the comparison, manual verification will be conducted to compare and correct the data concerning the CRF data on an individual basis. Once the data verification process is completed, it will be submitted to the project team for review. The data administrator then will announce the locking of the data and securely store the password. The finalized data will be subsequently transferred to the statistical department for further statistical analysis.
Statistical analysis
Statistical analysis will utilize SPSS 25.0 software. A significance level of P < 0.05 will be considered statistically significant for assessing differences. Continuous variables will be described using mean ± standard deviation, median, P25, P75, maximum, and minimum values. Categorical variables will be presented as percentages. Compare two groups of demographic data and other pre-treatment efficacy evaluation indicators between groups to measure comparability. Group t-tests are used for metric data that conform to a normal distribution, while nonparametric tests (Wilcoxon Test) are used if not. If there are significant differences in age, gender, disease type, or other relevant factors between the two groups before the experiment, or if there are significant factors that influence efficacy, these variables should be considered as covariates. In such cases, covariance analysis or logistic regression analysis will be performed. Primary per-protocol (PP) and intention-to-treat (ITT) analyses will be utilized for the main and overall indicators. We will use multiple imputations to process missing data.
Interim analyses
There will be no interim analysis.
Quality control
To ensure the progression of the research, a clinical training meeting will be conducted prior to the clinical trial. This meeting will focus on training the project implementation plan and various standard operating procedures. All methods of TEAS will be recorded on video to maintain consistency in operation. Furthermore, all acupuncturists involved in the study will possess valid licenses and a minimum of 2 years of clinical practice experience in acupuncture. To ensure data and material security, all research documents (such as screening tables, CRF, and treatment records) and treatment materials will be stored in locked storage units at restricted study locations. Weekly checks of the CRF and records of acupuncture treatments will be conducted at each research site. Additionally, a quality control review will be performed every 3 months at each site, generating a comprehensive report on the research process’s overall quality. Regular meetings among the primary researchers will be held to discuss and address any issues that arise during the observation period.
Patient and public involvement
The development, outcome measures, study design, recruitment, and implementation of this study did not incorporate patient priorities, experiences, or preferences. The results will not be shared with the study participants. Moreover, the burden of intervention will not be assessed by trial participants. The findings of this study will be disseminated to the study participants exclusively through our hospital’s website. We will keep the personal information of any participants confidential.
Trial status
This study is currently in the recruitment stage. Since December 2023, the participants have exhibited remarkable motivation, and the recruitment process has proceeded seamlessly.
Discussion
This article presents a comprehensive introduction to a parallel randomized controlled trial aimed at evaluating the efficacy of TEAS on INR in patients of ATS abuse combined with HIV/AIDS. Currently, there is a lack of effective interventions to achieve satisfactory therapeutic outcomes in improving immune reconstitution. Acupuncture has been demonstrated to bidirectionally regulate the effect on the body’s immune system. TEAS combines traditional Chinese medicine theory with modern electrical stimulation technology to regulate immune function, improve disease symptoms, and promote rehabilitation by applying electrical stimulation to specific acupoints. Previous studies have reported the beneficial effects of TEAS on various immune system disorders [30–33]. Therefore, it is believed that TEAS can effectively enhance reconstitution in ATS abuse combined with HIV/AIDS.
The significance of this study lies in the absence of reports on the effectiveness of TEAS in immune reconstitution among ATS abuse combined with HIV/AIDS. With TEAS being highly accessible and readily available, it is expected to improve immune function. However, this study does have certain limitations. Firstly, due to the nature of acupuncture, blinding is not feasible when conducting TEAS or sham TEAS. Therefore, blind method evaluation and statistical analysis are adopted, with efficacy evaluation and statistical analysis being performed by a third party not involved in the experiment. Secondly, the longer observation period of this study may result in subjects being unable to complete follow-up or provide complete data due to various reasons. This could lead to missing data, potentially impacting the integrity and reliability of the results. Lastly, the study is primarily conducted in two cities in Sichuan, China, and the participant recruitment may not fully represent other cities in China or Western countries, limiting the generalizability of the research findings to the wider population.
To ensure comprehensive reporting, this study will adhere to the Guidelines for Comprehensive Reporting Standard Test Report and the recommended standards for reporting intervention measures in acupuncture clinical trials [51, 52]. The proposed revisions to the protocol will be subject to discussion among all members of the research team in a meeting, aiming to reach a consensus. The revised protocol will be submitted to the Ethics Committee of Chengdu University of Traditional Chinese Medicine Affiliated Hospital, and if deemed necessary, the participants will be informed accordingly. The aim is to provide high-quality evidence for the use of TEAS in the treatment of immune unresponsive individuals with ATS combined with HIV infection. The results of this study will be presented at selected conferences and scientific meetings and will be published in peer-reviewed journals.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors express sincere gratitude to all the doctors, nurses, and staff members who actively participated in this study and made valuable contributions. Additionally, thanks for the financial support provided by the Sichuan Provincial Science and Technology Plan project.
Abbreviations
- ATS
Amphetamine-type stimulant
- ART
Antiretroviral therapy
- INR
Immunological non-response
- TEAS
Transcutaneous Electrical Acupoint Stimulation
- AIDS
Acquired Immune Deficiency Syndrome
- HIV
Human immunodeficiency virus
Author contributions
Conceptualization: YLR; Data Curation: XZ; Funding Acquisition: YLR and JL; Investigation: TL and SZ; Methodology: YLR and JL; Project Administration: ZLL and SJW; Resources: JL and ZLL; Software: XZ; Supervision: YLR and JL; Visualization: TL; Writing – Original Draft Preparation: JL and TL; Writing – Review & Editing: TL and YLR.
Funding
This study is supported by the major R&D project of the Sichuan Provincial Department of Science and Technology of China (approval No. 2018SZ0071).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The research protocol was approved by the Medical Ethics Committee of Chengdu University of Traditional Chinese Medicine Affiliated Hospital on August 30, 2023, with approval number 2023 kl-124-1. Participants provide written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Protocol version
20230818, v4.0.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fenwick C, et al. T-cell exhaustion in HIV infection. Immunol Rev. 2019;292(1):149–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dou Z, et al. Trends in Mortality and Prevalence of Reported HIV/AIDS Cases - China, 2002–2021. China CDC Wkly. 2023;5(42):943–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luo W, et al. Needle and syringe exchange programmes and prevalence of HIV infection among intravenous drug users in China. Addiction. 2015;110(Suppl 1):61–7. [DOI] [PubMed] [Google Scholar]
- 4.Colfax G, et al. Amphetamine-group substances and HIV. Lancet. 2010;376(9739):458–74. [DOI] [PubMed] [Google Scholar]
- 5.Kulsudjarit K. Drug problem in southeast and southwest Asia. Ann N Y Acad Sci. 2004;1025:446–57. [DOI] [PubMed] [Google Scholar]
- 6.Marshall BD, et al. Pathways to HIV risk and vulnerability among lesbian, gay, bisexual, and transgendered methamphetamine users: a multi-cohort gender-based analysis. BMC Public Health. 2011;11:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pitpitan EV, et al. Mood, Meth, Condom Use, and Gender: Latent Growth Curve Modeling Results from a Randomized Trial. AIDS Behav. 2018;22(9):2815–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prevention CCFD. Core information on HIV prevention in 2020. Health Guide. 2021;27(01):54. [Google Scholar]
- 9.Colfax G, Shoptaw S. The methamphetamine epidemic: implications for HIV prevention and treatment. Curr HIV/AIDS Rep. 2005;2(4):194–9. [DOI] [PubMed] [Google Scholar]
- 10.Ober A, et al. Factors associated with event-level stimulant use during sex in a sample of older, low-income men who have sex with men in Los Angeles. Drug Alcohol Depend. 2009;102(1–3):123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng WS, et al. Binge use and sex and drug use behaviors among HIV(-), heterosexual methamphetamine users in San Diego. Subst Use Misuse. 2010;45(1–2):116–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodger AJ, et al. Sexual Activity Without Condoms and Risk of HIV Transmission in Serodifferent Couples When the HIV-Positive Partner Is Using Suppressive Antiretroviral Therapy. JAMA. 2016;316(2):171–81. [DOI] [PubMed] [Google Scholar]
- 13.Deeks SG, et al. International AIDS Society global scientific strategy: towards an HIV cure 2016. Nat Med. 2016;22(8):839–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erikstrup C, et al. T-cell dysfunction in HIV-1-infected patients with impaired recovery of CD4 cells despite suppression of viral replication. J Acquir Immune Defic Syndr. 2010;53(3):303–10. [DOI] [PubMed] [Google Scholar]
- 15.Lok JJ, et al. Long-term increase in CD4 + T-cell counts during combination antiretroviral therapy for HIV-1 infection. AIDS. 2010;24(12):1867–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang X, et al. Incomplete immune reconstitution in HIV/AIDS patients on antiretroviral therapy: Challenges of immunological non-responders. J Leukoc Biol. 2020;107(4):597–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelley CF, et al. Incomplete peripheral CD4 + cell count restoration in HIV-infected patients receiving long-term antiretroviral treatment. Clin Infect Dis. 2009;48(6):787–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ellis RJ, et al. Increased human immunodeficiency virus loads in active methamphetamine users are explained by reduced effectiveness of antiretroviral therapy. J Infect Dis. 2003;188(12):1820–6. [DOI] [PubMed] [Google Scholar]
- 19.Passaro RC, et al. The Complex Interaction Between Methamphetamine Abuse and HIV-1 Pathogenesis. J Neuroimmune Pharmacol. 2015;10(3):477–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li T, et al. Reduced thymic output is a major mechanism of immune reconstitution failure in HIV-infected patients after long-term antiretroviral therapy. Clin Infect Dis. 2011;53(9):944–51. [DOI] [PubMed] [Google Scholar]
- 21.Piconi S, et al. Hydroxychloroquine drastically reduces immune activation in HIV-infected, antiretroviral therapy-treated immunologic nonresponders. Blood. 2011;118(12):3263–72. [DOI] [PubMed] [Google Scholar]
- 22.Mokgalaboni K et al. A Systematic Review and Meta-Analysis on the Impact of Statin Treatment in HIV Patients on Antiretroviral Therapy. Int J Environ Res Public Health, 2023. 20(9). [DOI] [PMC free article] [PubMed]
- 23.Somsouk M, et al. The immunologic effects of mesalamine in treated HIV-infected individuals with incomplete CD4 + T cell recovery: a randomized crossover trial. PLoS ONE. 2014;9(12):e116306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tenorio AR, et al. Rifaximin has a marginal impact on microbial translocation, T-cell activation and inflammation in HIV-positive immune non-responders to antiretroviral therapy - ACTG A5286. J Infect Dis. 2015;211(5):780–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heredia A, et al. Reduction of CCR5 with low-dose rapamycin enhances the antiviral activity of vicriviroc against both sensitive and drug-resistant HIV-1. Proc Natl Acad Sci U S A. 2008;105(51):20476–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu F et al. Acupuncture & its ability to restore and maintain immune homeostasis. QJM, 2023. [DOI] [PubMed]
- 27.Wang M, et al. The immunomodulatory mechanisms for acupuncture practice. Front Immunol. 2023;14:1147718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chi YL, et al. Transcutaneous Electrical Acupoint Stimulation for Improving Postoperative Recovery, Reducing Stress and Inflammatory Responses in Elderly Patient Undergoing Knee Surgery. Am J Chin Med. 2019;47(7):1445–58. [DOI] [PubMed] [Google Scholar]
- 29.J, C. and C. W, Mechanisms of acupuncture in regulating immune function. Liaoning J Traditional Chin Med, 2006(02): pp. 210–1.
- 30.X. S, et al. Effects of transcutaneous acupoint electrical stimulation on immune function in traumatized rats. Mod Traditional Chin Med. 2016;36(06):110–2. [Google Scholar]
- 31.Ao L, et al. Effects of transcutaneous electrical acupoint stimulation on perioperative immune function and postoperative analgesia in patients undergoing radical mastectomy: A randomized controlled trial. Experimental And Therapeutic Medicine; 2021;21:3. [DOI] [PMC free article] [PubMed]
- 32.Hou LL, Gu F, Zhou C. Transcutaneous electrical acupoint stimulation ameliorates chemotherapy-induced bone marrow suppression in lung cancer patients. J Thorac Oncol. 2016;11(4):S77–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu HX, et al. Effects of transcutaneous acupoint electrical stimulation on the imbalance of Th1, Th2, Th17 and Treg cells following thoracotomy of patients with lung cancer. Experimental Therapeutic Med. 2016;11(2):495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan AW, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346:e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Battle DE. Diagnostic and Statistical Manual of Mental Disorders (DSM). Codas. 2013;25(2):191–2. [DOI] [PubMed] [Google Scholar]
- 36.Anti-AIDS BOCA. Expert Consensus on the Integrated Traditional Chinese and Western Medicine Treatment for Immunological Dysfunction in HIV/AIDS. Chin J Traditional Chin Med. 2020;35(02):281–4. [Google Scholar]
- 37.Li T, et al. Tripterygium wilfordii Hook F extract in cART-treated HIV patients with poor immune response: a pilot study to assess its immunomodulatory effects and safety. HIV Clin Trials. 2015;16(2):49–56. [DOI] [PubMed] [Google Scholar]
- 38.Christensen E. Methodology of superiority vs. equivalence trials and non-inferiority trials. J Hepatol. 2007;46(5):947–54. [DOI] [PubMed] [Google Scholar]
- 39.Zhang W, Ruan L. Recent advances in poor HIV immune reconstitution: what will the future look like? Front Microbiol. 2023;14:1236460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diseases CSOI, Prevention CCFD. Chinese Guideline for the Diagnosis and Treatment of HIV/AIDS (2021 edition). Journal of Peking University Medicine, 2022;13(02):203–226.
- 41.Zheng H, et al. Acupuncture as a treatment for functional dyspepsia: design and methods of a randomized controlled trial. Trials. 2009;10:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.X Y, et al. A review of non-traditional acupoint selection methods in acupuncture research at home and abroad. Chin J Traditional Chin Med. 2009;50(08):748–50. [Google Scholar]
- 43.Gao J. Effects of moxibustion on immune function and gut microbiota diversity in 200 patients with immunological dysfunction in HIV/AIDS. 2022.China Academy of Chinese Medical Sciences. p. 115.
- 44.Li CX, et al. The predictive role of CD4(+) cell count and CD4/CD8 ratio in immune reconstitution outcome among HIV/AIDS patients receiving antiretroviral therapy: an eight-year observation in China. BMC Immunol. 2019;20(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Serrano-Villar S, Deeks SG. CD4/CD8 ratio: an emerging biomarker for HIV. Lancet HIV. 2015;2(3):e76–7. [DOI] [PubMed] [Google Scholar]
- 46.Hellerstein MK, et al. Subpopulations of long-lived and short-lived T cells in advanced HIV-1 infection. J Clin Invest. 2003;112(6):956–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ostrowski SR, et al. A low level of CD4 + CD28 + T cells is an independent predictor of high mortality in human immunodeficiency virus type 1-infected patients. J Infect Dis. 2003;187(11):1726–34. [DOI] [PubMed] [Google Scholar]
- 48.Song CB, et al. CD4(+)CD38(+) central memory T cells contribute to HIV persistence in HIV-infected individuals on long-term ART. J Transl Med. 2020;18(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tuaillon E, et al. Close association of CD8+/CD38 bright with HIV-1 replication and complex relationship with CD4 + T-cell count. Cytometry B Clin Cytom. 2009;76(4):249–60. [DOI] [PubMed] [Google Scholar]
- 50.Pardons M, et al. Single-cell characterization and quantification of translation-competent viral reservoirs in treated and untreated HIV infection. PLoS Pathog. 2019;15(2):e1007619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moher D, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.MacPherson H, et al. Revised STandards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA): extending the CONSORT statement. Acupunct Med. 2010;28(2):83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.