Abstract
Nonalcoholic fatty liver disease (NAFLD) or metabolic dysfunction–associated steatotic liver disease (MASLD) is a significant global public health dilemma with wide-ranging social and economic implications. Diet and lifestyle modifications remain essential components of NAFLD management. The current study investigated the association between diet-related inflammation and NAFLD among 3110 Iranian adults participating in the Amol Cohort Study (AmolCS), employing the Structural Equation Modeling (SEM) approach.The inflammatory potential of the diet was quantified using an energy-adjusted dietary index (E-DII) score. Findings showed that in the total sample and separately in males, the E-DII score had a significant effect on NAFLD, with mediation through hypertension (βstandardized = 0.16, and 0.13, p < 0.001, respectively) and c-reactive protein (CRP) (βstandardized = 0.07, and 0.07, p < 0.001, respectively). In the total sample and separately in females, the E-DII score significantly affected NAFLD, with mediation through diabetes (βstandardized = 0.06, p < 0.001, and 0.07, p = 0.006, respectively). In full and both gender-specific models, dyslipidemia was a risk factor for NAFLD and partially mediated the effect of hypertension on NAFLD.
The current study concluded a mediated association between dietary inflammation and NAFLD through hypertension, CRP, diabetes, and dyslipidemia, suggesting further longitudinal studies, especially in high-risk populations. These findings underscore the complex interplay between diet, inflammation, and NAFLD in Iranian adults.
Supplementary Information
The online version contains supplementary material available at 10.1186/s41043-024-00721-1.
Keywords: Diet, Dietary inflammatory index, NAFLD, Structural equation modeling, Iranian cohort
Introduction
Nonalcoholic fatty liver disease (NAFLD) or metabolic dysfunction-associated steatotic liver disease (MASLD) presents a significant global public health dilemma, estimated to affect a quarter of the adult population worldwide and leading to a considerable burden of disease with extensive social and economic consequences [1]. In Iran, about one-third of the adult population (33%) suffers from some degree of NAFLD [2]. This prevalence is higher than the global average, estimated at around 29.8% [3]. NAFLD is linked to other highly prevalent non-communicable diseases (NCDs), including type 2 diabetes mellitus, metabolic syndrome, and cardiovascular diseases [4, 5], with a great deal of overlap in the public health and healthcare system strategies required for managing and preventing these health conditions [6]. Diet and lifestyle changes and managing underlying metabolic risk factors are the cornerstones of treatment for all patients [7, 8].
Diet plays a critical role in regulating chronic low-grade inflammation, with the Dietary Inflammatory Index (DII) as a key tool to assess the inflammatory potential of dietary patterns [9, 10]. While specific compounds’ pro- and anti-inflammatory capacities are well-documented, little is known about the impact of whole dietary patterns and foods on NAFLD [11–15]. Diets rich in inflammatory foods, including saturated fats, refined sugars, and processed ingredients, activate pro-inflammatory pathways, increasing cytokines like TNF-α and IL-6, which contribute to hepatic insulin resistance, lipid accumulation, and oxidative stress, central to NAFLD’s pathogenesis [16, 17]. Prior research has shown that diets with higher inflammatory potential significantly increase the risk of NAFLD and are linked to various metabolic disturbances such as obesity, hypertension, dyslipidemia, and diabetes [10, 18–22]. These findings underscore the critical role of dietary inflammation in the pathogenesis of NAFLD and its mediating factors.
However, most research has assumed linear relationships between DII, cardio-metabolic profiles, and NAFLD, overlooking potential non-linear connections [10, 18, 19, 23, 24]. This study uses structural equation modeling (SEM) to explore the complex pathways [25] linking DII, NAFLD, and related traits, aiming to integrate multidimensional data into a cohesive framework. Understanding these interactions could help mitigate NAFLD’s global impact. The study hypothesizes that diet modulates inflammatory mechanisms influencing NAFLD and suggests that results may guide interventional strategies to prevent and treat diet-related diseases.
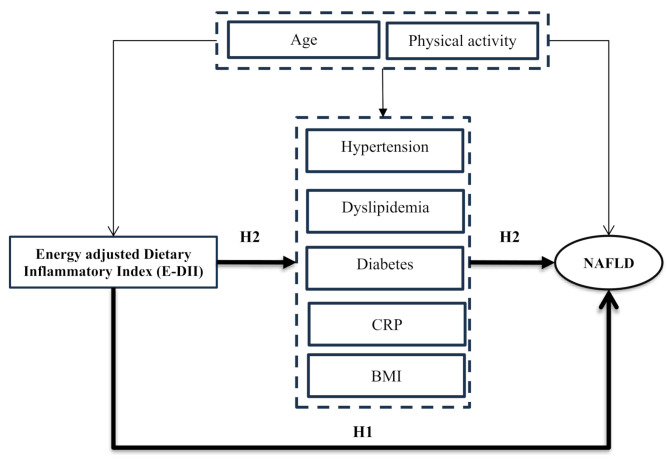
Based on the research background, a hypothetical model was proposed to estimate the association between the E-DII and NAFLD and verify the effects of mediators (Fig. 1). This study aims to test two hypotheses:1) E-DII has a significant direct association with NAFLD (H1) [16]; 2) the association between E-DII and NAFLD is mediated by BMI, HPTN, CRP, dyslipidemia, and diabetes (H2) [10, 18–22] (Fig. 1).
Fig. 1.
Proposed hypothesized model of the association between DII and NAFLD
Methods
Setting and study populations
The Amol Cohort Study (AmolCS) is a population-based cohort study in Amol to assess the prevalence and incidence rates of cardiovascular diseases (CVDs) and obesity-related metabolic disorders, including NAFLD. Amol, a medium-sized city near the Caspian Sea, lies approximately 200 km northeast of Tehran, Iran. AmolCS has been conducted in two phases (Phase 1 in 2009–2010 and Phase 2 in 2016–2017) using a multistage sampling design. Data were collected by standardized interviews, clinical examination, and tests of biological samples. The information in the second phase, which included Food Frequency Questionnaire (FFQ) data, was used in the present population-based cross-sectional study. A total of 5147 adults aged ≥ 18 were enrolled in the second phase of AmolCS (2016–2017). The ethical committee of the Iran University of Medical Sciences (IUMS) approved all procedures conducted (Approval number: IR.IUMS.REC.1401.950). Signed informed consent was acquired from all involved in the study. Further details on the project are available in the previous studies [26, 27].
We excluded participants based on the following criteria: a history of elevated alcohol intake (≥ 30 g/d for males and ≥ 20 g/d for females), viral hepatitis, hepatotoxic drugs use, significant weight changes in the three months preceding the abdominal sonography used for diagnosing NAFLD (n 486); pregnant or lactating women (n = 153); under-or over-reporting the energy intake (total energy intake of < 800 or > 4200 kcal/d for males, and < 600 or > 3500 kcal/d for females) [28] (n = 228); a CRP level of 10 mg/l or more, which may indicate infection or medication use [29] (n = 110); missing nutrition data (n = 249); missing abdominal ultrasonography data (n = 166); and missing other variables (186). As a result, we obtained a final dataset of 3,110 participants (1,398 females and 1,712 males) with complete and evaluable data. The present investigation adhered to the principles outlined in the Declaration of Helsinki, with protocols concerning human participants being approved by the ethical committee of the Iran University of Medical Sciences (IUMS) under reference number No. IR.IUMS.REC.1401.950. All participants involved in the study provided written consent before their participation.
Dietary assessment
Dietary intake was assessed using a validated 168-item semi-quantitative food-frequency questionnaire (FFQ) documented in Phase 2. Reported portion sizes and consumption frequencies were converted into daily intakes, expressed in grams, using standard household measures [30]. Nutrient and energy calculations were based on the United States Department of Agriculture (USDA) Food Composition Table (FCT), supplemented by the Iranian FCT for traditional foods not included in the USDA database [31].
Calculation of the energy-adjusted dietary inflammatory index (E-DII™)
The Dietary Inflammatory Index (DII®) was employed to assess the inflammatory potential of individuals’ dietary patterns across a spectrum from highly anti-inflammatory (described by the most negative score) to highly pro-inflammatory (defined by the most positive score). Previous literature has extensively elaborated on the DII methodology [32]. In the AmolCS, values for 32 food parameters were derived through a validated 168-item semi-quantitative FFQ [33]. Nutrient intake values were then calculated and standardized against a global reference database to create Z-scores. These standardized values were transformed into centered percentile scores multiplied by their respective inflammatory weights. These values were derived from scientific literature linking specific nutrients or food components to inflammatory biomarkers like CRP or IL-6. The sum of these weighted scores yielded the DII score for each individual [32]. In the current study, to minimize the variation induced by metabolic efficiency, physical activity levels, and body size [28], E-DII by the residual method was used. E-DII scores were determined through a density approach by adjusting DII for 1000 kcal intake [34].
Laboratory measurements
Venous blood samples were collected from participants the day before the interview, following a 12-hour fasting period, to quantify biochemical parameters. High-density lipoprotein (HDL), total cholesterol, and triglycerides were assessed by Biochemical analysis. Serum aminotransferases (AST, ALT, GGT, and ALP levels) were also quantified. The calculation of serum cholesterol levels for low-density lipoprotein (LDL) was derived from the Friedewald formula [35]. Serum concentrations of (non-hs) CRP were evaluated using the auto-analyzer and diagnostic kits. All biochemical assessments were conducted in a single laboratory facility by a pathologist blinded to the clinical findings of study participants. Standard laboratory procedures were followed, utilizing the BS-200 automatic analyzer (Mindray, China). Approximately 10% of blood samples were randomly selected and reassessed at the national reference laboratory to evaluate the accuracy of the laboratory tests. The spectrum of coefficients of variation observed across all laboratory measurements was between 1.7% and 3.8%. Pars Azmoun Pharmaceuticals, Tehran, provided the testing kits.
Definition of NAFLD diagnosed by ultrasonography
NAFLD was defined as fat accumulation in the liver, identified through imaging or liver biopsy after ruling out other evident causes of liver damage, such as excessive alcohol consumption, hepatotoxic medications, toxins, viral infections, or genetic liver disorders [36]. The current study used hepatorenal contrast (bright liver) and vascular blurring to define ultrasonographic fatty liver [37]. A solitary sonologist, unaware of the clinical condition of the individuals, performed all ultrasound scans using a 3.5 MHz convex probe (Esaote SpA, Genova, Italy). Criteria such as the obscuration of portal or hepatic veins and a notable increase in hepatic echogenicity were employed to confirm fatty liver.
Definition of diabetes
DM type 2 was defined by a history of DM or taking anti-hyperglycemic drugs (oral agents, insulin) or an FBS ≥ 125 mg/dl with no history of DM type I [38].
Measurement of other variables
A thorough anthropometric examination included height, weight, waist, and hip circumference were measured using World Health Organization guidelines, and body mass index (BMI) was calculated accordingly [39]. Smoking was defined as no smoking and past/current smoking. Physical activity was evaluated using a reliable International Physical Activity Questionnaire (IPAQ) [40]. The metabolic equivalent (MET) of physical activity energy expenditure was measured according to how long and strenuous each participant’s workout was. Following the operating procedure, blood pressure was measured utilizing a manual sphygmomanometer after 5 min of rest based on Korotkoff noise, and the average blood pressure derived from two measurement readings was used [41]. The monitors underwent calibration, and the cuffs were fitted correctly. Blood pressure was evaluated according to the recommendations in the 7th report issued by the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure [42, 43].
Statistical analysis
The data’s normality assessment was conducted using the Kolmogorov–Smirnov test. Mean ± SD represented continuous-distributed variables. We used frequency or percentage for categorical variables and the χ2 test to compare groups. A structural equation modeling (SEM) approach was employed to investigate the direct and indirect association of the dietary inflammatory index (DII) with NAFLD. The fundamental theoretical framework of SEM is depicted in Fig. 1, with NAFLD as the endogenous variable and DII as the exogenous variable. Hypertension (SBP and DBP combined), BMI, dyslipidemia (LDL, HDL, TG, and Cholesterol combined), CRP, age, and physical activity were considered key confounding and mediating variables interconnected with NAFLD. Confirmatory factor analysis (CFA) was utilized to validate the dyslipidemia measurement model by examining the relationship between the observed variable and its latent construct.
Model fitness was approached through the application of standard criteria encompassed the requirement of root-mean-square error of approximation (RMSEA) values lower than 0.08 and comparative fit index (CFI) values, goodness-of-fit index (GFI), adjusted goodness-of-fit index (AGFI), tucket lewis index (TLI) surpassing 0.90, and standardized root mean square residual (SRMSR) values lower than 0.05. Moreover, the maximum-likelihood method was employed to gauge the adequacy of the model, along with the presentation of standardized path coefficients and their respective statistical significance [44–46]. Within the realm of Structural Equation Modeling (SEM), the utilization of standardized estimates becomes imperative due to the disparate units in which each variable is gauged, thereby enhancing the interpretability and comparability of outcomes. The statistical data analysis was conducted using SPSS 24.0 and IBM SPSS Amos 24.0 (version 24.0; Amos Development Corp., Meadville, PA, USA) Statistical Software Packages. SPSS 24.0 facilitated a descriptive statistical analysis of the dataset, while SPSS Amos 24.0 was instrumental in examining structural equation modeling (SEM). All statistical tests followed a two-tailed approach, with statistical significance attributed to p-values below 0.05.
Results
Participant characteristics
A total of 3110 participants had evaluable data for these analyses, including 1398 females (44.95%). There were 1452 (46.68%) participants with NAFLD. The characteristics of these participants overall, and stratified by sex and NAFLD, are shown in Table 1. Compared with females, males were older (P < 0.001), more active (P < 0.001), smokers (P < 0.001), and were more likely to have a family history of diabetes (P = 0.01) and hypertension (P < 0.001). Furthermore, male participants presented statistically significant higher levels of WC, WHR, TG, SBP, DBP, and liver enzymes (AST, ALT, GGT) compared with females (all P < 0.001). For the other anthropometric, cardiometabolic, and liver parameters evaluated (Table 1), values were significantly greater in females than males (all P < 0.001 except P = 0.003 for SBP). The range of E-DII scores was − 4.13 to 4.45 (− 4.08 to 4.33 and − 4.13 to 4.45 in females and males, respectively), and correspondingly, their mean (SD) of dietary energy density (DED) was 1.46 (0.41) and 1.56 (0.51), respectively.
Table 1.
Baseline characteristics of the study adult participants by gender and NAFLD (n = 3110), AmolCS, 2016–2017
| Characteristic | Overall | Women (n = 1398) | Men (n = 1712) | Non NAFLD (n = 1658) | NAFLD (n = 1452) |
||
|---|---|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | P value | N (%) | N (%) | P value | |
| Family history of diseases | |||||||
| Family history of Diabetes | 1122(36.1) | 536(38.3) | 586(34.2) | 0.01 | 572(34.5) | 550(37.9) | 0.02 |
| Family history of CVDs | 217(7.0) | 104(7.4) | 113(6.6) | 0.20 | 113(6. 8) | 104(7.2) | 0.37 |
| Family history of HPTN | 1395(44.9) | 744(53.2) | 651(38.0) | < 0.001 | 660(39.8) | 735(50.6) | < 0.001 |
| Residual areas | |||||||
| Rural | 1366(43.9) | 506(36.2) | 860(50.2) | < 0.001 | 725(56.3) | 641(44.1) | 0.42 |
| Urban | 1744(56.1) | 892(63.8) | 852(49.8) | 933(56.3) | 811(55.9) | ||
| Comorbidities | |||||||
| Diabetes | 469(15.1) | 276(19.7) | 193(11.3) | < 0.001 | 168(10.1) | 301(20.7) | < 0.001 |
| Metabolic syndrome | 865(27.8) | 511(36.6) | 354(20.7) | < 0.001 | 232(14.0) | 633(43.6) | < 0.001 |
| Heart disease | 142(4.6) | 50(3.6) | 92(5.4) | 0.01 | 77(4.6) | 65(4.5) | 0.44 |
| Lifestyle factors | |||||||
| Lowering serum glucose agent’s user | 181(5.8) | 90(6.4) | 91(5.3) | 0.10 | 70(4.2) | 111(7.6) | < 0.001 |
| Lowering serum lipid agent’s user | 366(11.8) | 197(14.1) | 169(9.9) | 0.001 | 167(10.1) | 199(13.7) | 0.001 |
| Lowering hypertension agent’s user | 574(18.5) | 297(21.2) | 277(16.2) | < 0.001 | 266(16.0) | 308(21.2) | < 0.001 |
| Past/Current smoking | 443(14.2) | 8(0.6) | 435(25.4) | < 0.001 | 259(15.6) | 184(12.7) | 0.01 |
| Alcohol drinker (≥ 40 g) | 178(5.7) | 5(0.4) | 178(5.7) | < 0.001 | 85(5.1) | 93(6.4) | 0.16 |
| Physical activity (MET-h/d) | |||||||
| Low | 1069(34.4) | 525(37.6) | 544(31.8) | < 0.001 | 538(32.4) | 531(36.6) | 0.03 |
| Moderate | 1139(36.6) | 534(38.2) | 605(35.3) | 615(37.1) | 524(36.1) | ||
| high | 902(29.0) | 339(24.2) | 563(32.9) | 505(30.5) | 397(27.3) | ||
| Mean (SD) | Mean (SD) | Mean (SD) | P value | Mean (SD) | Mean (SD) | P value | |
| Age (years) | 46.97 ± 14.61 | 45.83 ± 14.03 | 47.91 ± 15.01 | < 0.001 | 45.58 ± 16.11 | 48.56 ± 12.50 | < 0.001 |
| Anthropometric parameters | |||||||
| Body mass index (BMI, kg/m2) | 28.04 ± 5.05 | 29.55 ± 5.37 | 26.81 ± 4.41 | < 0.001 | 25.89 ± 4.45 | 30.50 ± 4.55 | < 0.001 |
| Waist circumference (WC, cm) | 88.82 ± 11.34 | 87.70 ± 12.14 | 89.57 ± 10.57 | < 0.001 | 83.58 ± 10.12 | 94.79 ± 9.56 | < 0.001 |
| Waist to height (WHtR) ratio | 0.54 ± 0.07 | 0.56 ± 0.08 | 0.52 ± 0.06 | < 0.001 | 0.51 ± 0.06 | 0.58 ± 0.06 | < 0.001 |
| Waist to hip (WHR) ratio | 0.87 ± 0.08 | 0.84 ± 0.08 | 0.89 ± 0.07 | < 0.001 | 0.85 ± 0.08 | 0.90 ± 0.07 | < 0.001 |
| Cardiometabolic parameters | |||||||
| Serum triglycerides (TG, mg/dl) | 134.86 ± 91.21 | 129.07 ± 89.21 | 139.57 ± 92.57 | 0.001 | 112.52 ± 66.72 | 160.38 ± 107.33 | < 0.001 |
| Serum total cholesterol (T-C, mg/dl) | 180.70 ± 40.28 | 183.41 ± 41.86 | 178.48 ± 38.81 | 0.001 | 175.85 ± 38.01 | 186.23 ± 41.01 | < 0.001 |
| Serum high-density lipoprotein (HDL, mg/dl) | 43.67 ± 11.69 | 46.04 ± 11.85 | 41.74 ± 11.20 | < 0.001 | 44.72 ± 11.75 | 42.47 ± 11.51 | < 0.001 |
| Serum low-density lipoprotein (LDL, mg/dl) | 99.30 ± 26.29 | 99.65 ± 26.73 | 99.01 ± 25.94 | 0.49 | 97.60 ± 26.48 | 101.24 ± 25.95 | < 0.001 |
| Systolic blood pressure (SBP, mmHg) | 114.86 ± 19.27 | 113.71 ± 20.64 | 115.80 ± 18.03 | 0.003 | 111.21 ± 18.36 | 119.03 ± 19.44 | < 0.001 |
| Diastolic blood pressure (DBP, mmHg) | 71.69 ± 11.85 | 70.91 ± 12.33 | 72.32 ± 11.42 | 0.001 | 69.02 ± 11.14 | 74.72 ± 11.92 | < 0.001 |
| Fasting blood glucose (FBS, mg/dl) | 106.23 ± 35.39 | 108.98 ± 40.73 | 103.98 ± 30.18 | < 0.001 | 101.21 ± 31.33 | 111.96 ± 38.75 | < 0.001 |
| Serum C-reactive protein (CRP, mg/dl) | 3.51 ± 6.78 | 4.36 ± 7.42 | 2.83 ± 6.12 | < 0.001 | 2.94 ± 6.30 | 4.17 ± 7.24 | < 0.001 |
| Liver parameters | |||||||
| Alanine transaminase (ALT, mg/dl) | 24.01 ± 17.58 | 19.87 ± 14.04 | 27.40 ± 19.37 | < 0.001 | 19.87 ± 13.05 | 28.74 ± 20.64 | < 0.001 |
| Aspartate transaminase (AST, mg/dl) | 21.52 ± 9.19 | 19.50 ± 8.29 | 23.17 ± 9.55 | < 0.001 | 20.52 ± 8.69 | 22.67 ± 9.59 | < 0.001 |
| Gamma-glutamyl-transferase (GGT, mg/dl) | 27.19 ± 19.24 | 24.27 ± 18.74 | 29.57 ± 19.31 | < 0.001 | 23.98 ± 18.23 | 30.85 ± 19.70 | < 0.001 |
| Alkaline phosphatase (AP, mg/dl) | 197.88 ± 59.58 | 196.33 ± 67.53 | 199.15 ± 52.18 | 0.18 | 193.08 ± 61.33 | 203. 36 ± 57.04 | < 0.001 |
| Dietary parameters | |||||||
| energy-adjusted dietary index (E-DII) score | -0.79 ± 1.18 | -0.83 ± 1.17 | -0.76 ± 1.18 | 0.13 | -0.75 ± 1.18 | -0.83 ± 1.17 | 0.06 |
| Dietary energy density (DED) | 1.52 ± 0.47 | 1.46 ± 0.41 | 1.56 ± 0.51 | < 0.001 | 1.52 ± 0.49 | 1.51 ± 0.45 | 0.45 |
Data are Mean ± SD (all such values) unless indicated. Significant at P < 0.05 for independent t-test for continuous variables and chi-square test for categorical variables
Confirmatory factor analysis
The fit indices for CFA used to develop the constructs of dyslipidemia (combination of LDL, HDL, TG, and Cholesterol) had acceptable fit to the data (χ2/df = 12.144, p < 0.001, RMSEA = 0.060, SRMR = 0.038, GFI = 0.981, AGFI = 0.952, CFI = 0.960) (see supplementary Fig. 1). LDL explained a higher proportion of the variance in the dyslipidemia factor.
Association between E-DII and NAFLD
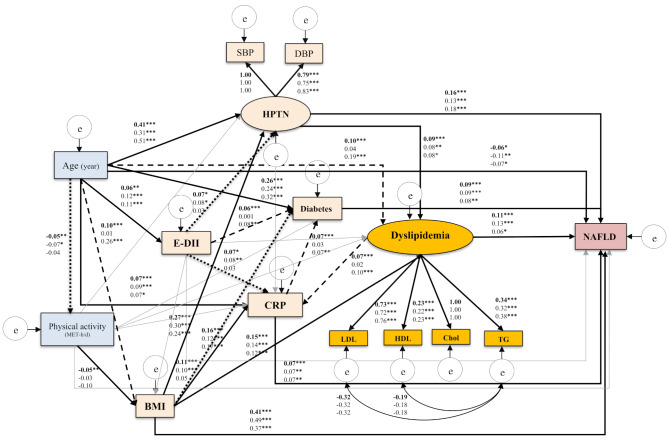
The SEM output of the Model in the three sub-samples (overall population, males, and females) is presented in Fig. 2, which depicts the relationships between the E-DII and NAFLD while considering the main confounders and mediators.
Fig. 2.
Final structural model of the relationship between energy adjusted dietary inflammatory index (E-DII) and NAFLD. The full-model fit indices: χ2/df = 12.144, p < 0.001, GFI = 0.981, AGFI = 0.952, CFI = 0.960, TLI = 0.920, RMSEA = 0.060, SRMR = 0.038. Note: The values on the paths represent standardized regression coefficients. The upper-faced numbers refer to the full sample, whereas the numerical values below refer to male and female sub-samples. Arrows in bold represent statistically significant associations (*P < 0.05; ** P < 0.01; *** P < 0.001). Square dot arrows refer to statistically significant associations in both total sample and males sub-sample, whereas, dash arrows refer to statistically significant associations in both the total sample and the female sub-sample
E-DII had no significant direct effect on NAFLD in all three models (overall model: βstandardized = 0.024, P = 0.234, 95% CI [0.034, 0.15]; males model: βstandardized = 0.049, P = 0.069, 95% CI [0.020, 0.069]; females model: βstandardized = -0.017, P = 0.582, 95% CI [-0.039, 0.023]). Therefore, hypothesis 1 of the present study was not supported.
However, hypothesis 2 was supported in the current study. E-DII score had a significant effect on NAFLD, which was mediated through hypertension (in both sexes: of E-DII to hypertension: ( βstandardized = 0.07, P = 0.04; and of hypertension to NAFLD: βstandardized = 0.16, p < 0.001; in males: of E-DII to hypertension: βstandardized = 0.08, P = 0.004; of hypertension to NAFLD: βstandardized = 0.13, p < 0.001), CRP (in both sexes: of E-DII to CRP: βstandardized = 0.07, p < 0.001; of CRP to NAFLD: βstandardized = 0.07, p < 0.001; in males: of E-DII to CRP: βstandardized = 0.08, P = 0.008; and of CRP to NAFLD: βstandardized = 0.07, p = 0.003), and diabetes (in both sexes: of E-DII to diabetes: βstandardized = 0.06, p < 0.001; of diabetes to NAFLD: βstandardized = 0.09, p < 0.001; in females: of E-DII to diabetes: βstandardized = 0.07, P = 0.006; and of diabetes to NAFLD: βstandardized = 0.08, p = 0.002). For females, results for both the hypertension and CRP were null. The effect of E-DII on diabetes was also ineffective in men.
In full and both gender-specific models, dyslipidemia was a risk factor for NAFLD (in both sexes combined: βstandardized = 0.11, p < 0.001; in males: βstandardized = 0.13, p < 0.001, and in females: βstandardized = 0.06, p = 0.034), and partially mediated the effect of hypertension on NAFLD (in both sexes combined: βstandardized = 0.09, p < 0.001; in males: βstandardized = 0.09, p < 0.001, and in females: βstandardized = 0.08, p = 0.014).
The goodness-of-fit metrics presented evidence that the models (full sample, males and females) perfectly fit the data: full model, χ2/df = 12.144, p < 0.001, RMSEA = 0.060, SRMR = 0.038, GFI = 0.981, AGFI = 0.952, CFI = 0.960, and TLI = 0.920. Males model, χ2/df = 7.053, p < 0.001, RMSEA = 0.057, SRMR = 0.043, GFI = 0.976, AGFI = 0.942, CFI = 0.958, TLI = 0.915. Females model, χ2/df = 7.905, p < 0.001, RMSEA = 0.066, SRMR = 0.043, GFI = 0.975, AGFI = 0.940, CFI = 0.951, TLI = 0.910.
Discussion
In this study, no direct association was observed between E-DII and NAFLD. To further understand the association between them, we proposed a hypothesis to evaluate whether some cardio-metabolic and inflammatory factors play a role as mediators. The results of our study supported the mediation hypothesis that E-DII through hypertension, CRP, dyslipidemia, and diabetes is associated with NAFLD evaluated by ultrasound.
The link between DII and NAFLD has been confirmed by growing evidence from both observational and interventional studies suggesting that anti-inflammatory diets might have hepatoprotective effects that improve NAFLD severity [47–49]. Zhang et al., in the analysis based on nationwide survey data including 10,052 US adults, found a significant association between inflammatory potential diet and Fatty Liver Index (FLI) by showing that individuals in the highest DII quartile had a 52% increased likelihood of fatty liver than those in the lowest DII quartile (OR 1.52, 95% CI 1.27–1.83, P trend <0.001) [12].
In our study, adherence to a pro-inflammatory diet was associated with a greater intake of energy, carbohydrates, saturated fat, MUFA, cholesterol, and total fat, as well as a low intake of fiber, vitamins (except for Vitamin B-12), and minerals. Furthermore, a pro-inflammatory diet was also associated with low intakes of caffeine, garlic, pepper, onion, and green/black tea (see supplementary Table 1).
Polyphenol-rich foods such as coffee, tea, fruits, and vegetables have been shown to have anti-inflammatory effects [34]. A long-term diet that prioritizes plant-based foods, low-fat dairy, and fish while avoiding processed meats and sugar-sweetened beverages can prevent intestinal inflammatory processes via the gut microbiome [50]. Furthermore, minerals such as magnesium can regulate enzymes involved in liver lipid metabolism and may improve chronic metabolic disorders by reducing low-grade inflammation [51, 52]. An elevated risk of NAFLD has already been linked to a lack of adherence to these recommendations [11–13, 49].
The present study is the first to explore the association between E-DII and NAFLD using the SEM approach. Structural equation modeling has evolved to allow for non-linear relations and multiple pathways between DII and NAFLD [25]. The study found that the E-DII affects NAFLD through hypertension, CRP, and dyslipidemia in the total sample and males. Additionally, diabetes mediated the relationship between E-DII and NAFLD in the total sample and among females. Evidence from the studies has suggested the correlation between hypertension and hepatic steatosis [53, 54] and confirmed that the increase in hypertension could predict the occurrence and development of NAFLD [55, 56]. Another study conducted by Zhang et al. revealed that the level of DII was significantly positively correlated with SBP and DBP in US adults [57], which is consistent with our findings. Studies indicated that hypertension contributes to oxidative stress, systemic inflammation, and endothelial dysfunction, which promote hepatic insulin resistance and fat accumulation [58]. Hypertension can also exacerbate liver fibrosis by increasing renin-angiotensin system activity, leading to profibrotic signaling [59, 60]. This pathway highlights the role of hypertension as both a contributor to and a potential mediator in the progression of NAFLD.
The underlying mechanisms of the association between E-DII and NAFLD through hypertension, diabetes, and dyslipidemia might be explained by the fact that gut microbial metabolites like trimethylamine N-oxide and short-chain fatty acids act on downstream cellular targets contribute to hypertension [61–64]. Dietary contents affect the composition and function of the gut microbiota, and previous research has also confirmed the cardio-metabolic disease risk-reducing effects of anti-inflammatory dietary patterns by shaping the gut microbiome [63, 65, 66]. Disturbances in lipid metabolism, insulin resistance, and gut-derived endotoxins contribute to producing and releasing pro-inflammatory cytokines, inhibiting insulin receptor signaling and leading to steatosis and fibrosis [67, 68]. Evidence supports an inverse association between DII and lipid profiles [69, 70]. Pro-inflammatory diets may activate the NF-KB pathways, simulate TG production in the liver, and alter the balance of the lipid profile [71].
CRP’s role as a mediator is consistent with its ability to act as a regulator of nitric oxide production in the endothelium and coordinate the production and secretion of various cytokines, increasing the pro-inflammatory activity of different adipokines. MetS was associated with oxidative stress and chronic low-grade inflammation [72], and NAFLD is one criterion of MetS [73].
The association of E-DII with NAFLD through hypertension, CRP, and dyslipidemia was not observed in females, which means other determinants might be involved. Previous studies have emphasized that women are less affected by environmental stress (such as oxidative stress) than men [74]. Estrogen has antioxidant and anti-inflammatory properties in females, which may significantly contribute to this difference [75]. Therefore, eating habits with inflammatory potential may have less impact on the development of NAFLD in females. Our results emphasize the importance of gender-specific interventions to control NAFLD and also the pivotal role of anti-inflammatory diets in preventing hypertension, low-grade systemic inflammation, and dyslipidemia among high-risk individuals.
As far as we know, the present study is the first to investigate the relationship between DII and NAFLD through the SEM technique. One of its significant strengths is the temporal alignment of dietary data collection, blood sampling, and sonographic findings, which greatly enhances causal inference [76]. Despite its strengths and novelty, the present findings should be interpreted in the context of several limitations. First, the potential issue of reverse causation could arise due to the cross-sectional design employed in the study. The second limitation of the study is the single assessment of inflammatory markers (CRP) as risk factors. However, CRP is the most commonly measured biomarker of systemic inflammation in research examining the relationship between the dietary inflammatory index and various diseases [77]. This could be attributed to the extended half-life of CRP in the serum compared to other low-grade inflammatory biomarkers [78]. In addition, our study was limited to the population of the north of Iran. Finally, this study used liver ultrasonography, associated with lower accuracy than liver biopsy, to identify NAFLD, which may cause the misclassification. However, ultrasonography is considered a safe, accurate, and convenient tool for evaluating NAFLD in epidemiological surveys [79].
Conclusions
Our findings suggest that hypertension, CRP, and dyslipidemia mediate the association between dietary inflammatory potential and NAFLD. Therefore, a pro-inflammatory diet may exacerbate the effects of hypertension, CRP, and dyslipidemia and, as a result, may increase the risk of NAFLD. Altogether, these results demonstrate the importance of constantly assessing the dietary intake in patients with hypertension and dyslipidemia to improve diet quality and reduce its complication rate in the population. However, substantiating the efficacy of the DII requires further longitudinal studies, particularly among populations at high risk of chronic diseases.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1: Fig. S1 Confirmatory factor analysis (CFA) of the measurement model of dyslipidemia components. The full-model fit indices had acceptable fit to the data: χ2/df = 41.815, p < 0.001, GFI = 0.993, AGFI = 0.934, CFI = 0.988, TLI = 0.931, RMSEA = 0.080, SRMR = 0.026
Supplementary Material 2: table 1 dietary intakes of study participants across tertiles of DII score (N = 3110), AmolCS, 2016–2017a Data are presented as mean ± standard deviation.
Acknowledgements
We wish to convey our gratitude to the participants, the healthcare leaders, and the GILDRC team (//www.gildrc.ac.ir), for their valuable contribution to this study. Additionally, we express our gratitude to the Iran University of Medical Sciences (IUMS) for providing funding for this research (grant NO: 1401-3-75-22501).
Author contributions
The research idea and structure were created by FZ, HA, and AD. NM and AD had full access to the data and ensured analysis accuracy. MM, EG, and BA contributed to gathering the data. The data was analyzed and interpreted by AD, SE, NM, JRH, and SP. The initial manuscript draft was written by AD, BA, and SE. All authors reviewed the manuscript for important content and approved the final version. HA is responsible for overseeing the entire paper as the guarantor.
Funding
The study was financed by the Iran University of Medical Sciences (IUMS) with grant number 1401-3-75-22501. Dr. Hébert received backing from grant U01 CA272977-01 from the United States National Cancer Institute. The funders were not involved in designing the study, collecting and analyzing data, interpreting results, or writing the report.
Data availability
The data gathered and analyzed in this study can be obtained from the corresponding author upon reasonable request. Interested researchers may reach out to the corresponding author, Dr. Hossein Ajdarkosh, email address: ajdarkosh.h@iums.ac.ir.
Declarations
Ethics approval and consent to participate
The investigation followed the guidelines specified in the Declaration of Helsinki and received approval from the ethical committee of the Iran University of Medical Sciences (IUMS) with the reference code IR.IUMS.REC.1401.950. Each individual involved in the study provided written consent after receiving comprehensive information regarding the study’s aims and procedures.
Competing interests
The authors declare no competing interests.
Consent for publication
Not applicable.
Disclosure
Dr. James R. Hébert is the primary shareholder of Connecting Health Innovations LLC (CHI), which has secured authorization for his innovation, the dietary inflammatory index (DII®), from the University of South Carolina. CHI plans to use this invention to create computer and smartphone applications for patient counseling and dietary intervention in clinical settings. CHI also holds the exclusive rights to the E-DII™. The content of this paper is not related to CHI’s work, and CHI has not influenced this project in any way.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stefan N, Häring H-U, Cusi K. Non-alcoholic fatty liver disease: causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol. 2019;7:313–24. [DOI] [PubMed] [Google Scholar]
- 2.Tabaeian SP, Rezapour A, Azari S, Martini M, Saran M, Behzadifar M, Shahabi S, Sayyad A, Tahernejad A, Bragazzi N. Prevalence of non-alcoholic fatty liver disease in Iran: a systematic review and meta-analysis. J Clin Experimental Hepatol. 2024;14:101209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Le MH, Yeo YH, Li X, Li J, Zou B, Wu Y, Ye Q, Huang DQ, Zhao C, Zhang J. 2019 global NAFLD prevalence: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2022;20:2809–17. e2828. [DOI] [PubMed] [Google Scholar]
- 4.Li H, Shi Z, Chen X, Wang J, Ding J, Geng S, Sheng X, Shi S. Relationship between six insulin resistance surrogates and nonalcoholic fatty liver Disease among older adults: a cross-sectional study. Diabetes Metabolic Syndrome Obes 2023:1685–96. [DOI] [PMC free article] [PubMed]
- 5.Hardy T, Oakley F, Anstee QM, Day CP. Nonalcoholic fatty liver disease: pathogenesis and disease spectrum. Annu Rev Pathol. 2016;11:451–96. [DOI] [PubMed] [Google Scholar]
- 6.Lazarus JV, Mark HE, Anstee QM, Arab JP, Batterham RL, Castera L, Cortez-Pinto H, Crespo J, Cusi K, Dirac MA. Advancing the global public health agenda for NAFLD: a consensus statement. Nat Reviews Gastroenterol Hepatol. 2022;19:60–78. [DOI] [PubMed] [Google Scholar]
- 7.Berná G, Romero-Gomez M. The role of nutrition in non‐alcoholic fatty liver disease: pathophysiology and management. Liver Int. 2020;40:102–8. [DOI] [PubMed] [Google Scholar]
- 8.Fernández T, Viñuela M, Vidal C, Barrera F. Lifestyle changes in patients with non-alcoholic fatty liver disease: a systematic review and meta-analysis. PLoS ONE. 2022;17:e0263931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shivappa N, Steck SE, Hurley TG, Hussey JR, Hébert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17:1689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doustmohammadian A, Amirkalali B, Esfandyari S, Motamed N, Maadi M, Shivappa N, Gholizadeh E, Hébert JR, Zamani F. The association between dietary inflammatory index (DII) scores and c-reactive protein (CRP) and nonalcoholic fatty liver disease (NAFLD) in a general population cohort. Clin Nutr ESPEN. 2024;60:156–64. [DOI] [PubMed] [Google Scholar]
- 11.Ramírez-Vélez R, García‐Hermoso A, Izquierdo M, Correa‐Rodríguez M. The Dietary Inflammatory Index and hepatic health in the US adult population. J Hum Nutr Dietetics. 2022;35:968–79. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Z, Wang L, Lin Z, Yan W, Chen J, Zhang X, Ye W, Li J, Li Z. Dietary inflammatory index and risk of non-alcoholic fatty liver disease and advanced hepatic fibrosis in US adults. Front Nutr. 2023;10:1102660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farhadnejad H, Tehrani AN, Jahromi MK, Teymoori F, Mokhtari E, Salehi-Sahlabadi A, Mirmiran P. The association between dietary inflammation scores and non-alcoholic fatty liver diseases in Iranian adults. BMC Gastroenterol. 2022;22:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doustmohammadian A, Pishgar E, Clark CC, Sobhrakhshankhah E, Nikkhah M, Faraji AH, Motamed N, Mansourian MR, Amirkalali B, Maadi M. Empirically-derived dietary patterns in relation to non-alcoholic fatty liver diseases among adult participants in Amol, Northern Iran: a structural equation modeling Approach. Front Nutr 2022, 9. [DOI] [PMC free article] [PubMed]
- 15.Doustmohammadian A, Clark CC, Maadi M, Motamed N, Sobhrakhshankhah E, Ajdarkosh H, Mansourian MR, Esfandyari S, Hanjani NA, Nikkhoo M. Favorable association between Mediterranean diet (MeD) and DASH with NAFLD among Iranian adults of the Amol Cohort Study (AmolCS). Sci Rep. 2022;12:2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X, Ruan J, He Y, Xu A, Fang Y, Zhang Q, Gu L, Liu X. Dietary inflammatory index and the risks of non-alcoholic fatty liver disease: a systematic review and meta-analysis. Front Nutr. 2024;11:1388557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polak-Szczybyło E, Tabarkiewicz J. Influence of dietary and lifestyle factors on levels of inflammatory markers (IL-6, IFN-γ and TNF-α) in obese subjects. Cent Eur J Immunol 2024, 49. [DOI] [PMC free article] [PubMed]
- 18.Kumar R, Porwal Y, Dev N, Kumar P, Chakravarthy S, Kumawat A. Association of high-sensitivity C-reactive protein (hs-CRP) with non-alcoholic fatty liver disease (NAFLD) in Asian indians: a cross-sectional study. J Family Med Prim Care. 2020;9:390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soltanieh S, Salavatizadeh M, Poustchi H, Yari Z, Mansour A, Khamseh ME, Malek M, Alaei-Shahmiri F, Hekmatdoost A. The association of dietary inflammatory index (DII) and central obesity with non-alcoholic fatty liver disease (NAFLD) in people with diabetes (T2DM). Heliyon 2023, 9. [DOI] [PMC free article] [PubMed]
- 20.Doustmohammadian A, Nouri Saeidlou S, Esfandyari S, Gholizadeh E, Maadi M, Motamed N, Ajdarkosh H, Khoonsari M, Clark CC, Zamani F. Dietary acid load (DAL), glycated hemoglobin A1c (HbA1c), and metabolic syndrome (MeS) mediate the association of the adherence to the dietary approaches to stopping hypertension (DASH) and mediterranean diet (MeD) with nonalcoholic fatty liver disease. Front Nutr. 2022;9:921415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aslani Z, Sadeghi O, Heidari-Beni M, Zahedi H, Baygi F, Shivappa N, Hébert JR, Moradi S, Sotoudeh G, Asayesh H. Association of dietary inflammatory potential with cardiometabolic risk factors and diseases: a systematic review and dose–response meta-analysis of observational studies. Diabetol Metab Syndr. 2020;12:1–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang X, Tao S, Peng J, Zhao J, Li S, Wu N, Wen Y, Xue Q, Yang CX, Pan XF. High-sensitivity C‐reactive protein and risk of type 2 diabetes: a nationwide cohort study and updated meta‐analysis. Diab/Metab Res Rev. 2021;37:e3446. [DOI] [PubMed] [Google Scholar]
- 23.DeFilippis AP, Blaha MJ, Martin SS, Reed RM, Jones SR, Nasir K, Blumenthal RS, Budoff MJ. Nonalcoholic fatty liver disease and serum lipoproteins: the multi-ethnic study of atherosclerosis. Atherosclerosis. 2013;227:429–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flisiak-Jackiewicz M, Bobrus-Chociej A, Wasilewska N, Lebensztejn DM. From nonalcoholic fatty liver Disease (NAFLD) to Metabolic Dysfunction-Associated fatty liver disease (MAFLD)—New Terminology in Pediatric patients as a step in Good Scientific Direction? J Clin Med. 2021;10:924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Little TD, Card NA, Bovaird JA, Preacher KJ, Crandall CS. Structural equation modeling of mediation and moderation with contextual factors. Model Contextual Eff Longitud Stud. 2007;1:207–30. [Google Scholar]
- 26.Zamani F, Sohrabi M, Alipour A, Motamed N, Saeedian FS, Pirzad R, Abedi K, Maadi M, Ajdarkosh H, Hemmasi G. Prevalence and risk factors of cholelithiasis in Amol city, northern Iran: a population based study. Arch Iran Med. 2014;17:0–0. [PubMed] [Google Scholar]
- 27.Doustmohammadian A, Clark CC, Maadi M, Motamed N, Sobhrakhshankhah E, Ajdarkosh H, Mansourian MR, Esfandyari S, Hanjani NA, Nikkhoo M. Favorable association between Mediterranean diet (MeD) and DASH with NAFLD among Iranian adults of the Amol Cohort Study (AmolCS). Sci Rep. 2022;12:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65:S1220–8. [DOI] [PubMed] [Google Scholar]
- 29.Pearson T, Centers for Disease Control and Prevention; American Heart Association. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. [DOI] [PubMed] [Google Scholar]
- 30.Ghaffarpour M, Houshiar-Rad A, Kianfar H. The manual for household measures, cooking yields factors and edible portion of foods. Tehran: Nashre Olume Keshavarzy. 1999;7:42–58. [Google Scholar]
- 31.Kiefte-de Jong JC, Li Y, Chen M, Curhan GC, Mattei J, Malik VS, Forman JP, Franco OH, Hu FB. Diet-dependent acid load and type 2 diabetes: pooled results from three prospective cohort studies. Diabetologia. 2017;60:270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shivappa N, Steck SE, Hurley TG, Hussey JR, Hebert JR. Designing and developing a literature-derived population-based dietary inflammatory index. Public Health Nutr. 2014;17:1689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mirmiran P, Esfahani FH, Mehrabi Y, Hedayati M, Azizi F. Reliability and relative validity of an FFQ for nutrients in the Tehran lipid and glucose study. Public Health Nutr. 2010;13:654–62. [DOI] [PubMed] [Google Scholar]
- 34.Hébert JR, Shivappa N, Wirth MD, Hussey JR, Hurley TG. Perspective: the Dietary Inflammatory Index (DII)-Lessons learned, improvements made, and future directions. Adv Nutr. 2019;10:185–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin SS, Blaha MJ, Elshazly MB, Toth PP, Kwiterovich PO, Blumenthal RS, Jones SR. Comparison of a novel method vs the Friedewald equation for estimating low-density lipoprotein cholesterol levels from the standard lipid profile. JAMA. 2013;310:2061–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–31. [DOI] [PubMed] [Google Scholar]
- 37.Osawa H, Mori Y. Sonographic diagnosis of fatty liver using a histogram technique that compares liver and renal cortical echo amplitudes. J Clin Ultrasound. 1996;24:25–9. [DOI] [PubMed] [Google Scholar]
- 38.Petersmann A, Müller-Wieland D, Müller UA, Landgraf R, Nauck M, Freckmann G, Heinemann L, Schleicher E. Definition, classification and diagnosis of diabetes mellitus. Exp Clin Endocrinol Diabetes. 2019;127:S1–7. [DOI] [PubMed] [Google Scholar]
- 39.World Health Organization. Obesity: Preventing and managing the global epidemic 2000;10. [PubMed]
- 40.Hagströmer M, Oja P, Sjöström M. The International Physical Activity Questionnaire (IPAQ): a study of concurrent and construct validity. Public Health Nutr. 2006;9:755–62. [DOI] [PubMed] [Google Scholar]
- 41.Zelber-Sagi S, Salomone F, Mlynarsky L. The Mediterranean dietary pattern as the diet of choice for non-alcoholic fatty liver disease: evidence and plausible mechanisms. Liver Int. 2017;37:936–49. [DOI] [PubMed] [Google Scholar]
- 42.Velasco N, Contreras A, Grassi B. The Mediterranean diet, hepatic steatosis and nonalcoholic fatty liver disease. Curr Opin Clin Nutr Metab Care. 2014;17:453–7. [DOI] [PubMed] [Google Scholar]
- 43.Romero-Gómez M, Zelber-Sagi S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol. 2017;67:829–46. [DOI] [PubMed] [Google Scholar]
- 44.Ryu E. Model fit evaluation in multilevel structural equation models. Front Psychol. 2014;5:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bae B-R. Structural equation modeling with Amos 19: principles and practice. Seoul: Chungram Books 2011:1–668.
- 46.Sahoo M. Structural equation modeling: threshold criteria for assessing model fit. Methodological issues in management research: advances, challenges, and the way ahead. Emerald Publishing Limited; 2019. pp. 269–76.
- 47.Li R, Chen Z. Validation and comparison of two Dietary indexes for Predicting nonalcoholic fatty liver disease in US adults. J Nutr. 2022;152:2865–76. [DOI] [PubMed] [Google Scholar]
- 48.Darbandi M, Hamzeh B, Ayenepour A, Rezaeian S, Najafi F, Shakiba E, Pasdar Y. Anti-inflammatory diet consumption reduced fatty liver indices. Sci Rep. 2021;11:22601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abdallah J, Assaf S, Das A, Hirani V. Effects of anti-inflammatory dietary patterns on non-alcoholic fatty liver disease: a systematic literature review. Eur J Nutr 2023:1–16. [DOI] [PMC free article] [PubMed]
- 50.van Woudenbergh GJ, Theofylaktopoulou D, Kuijsten A, Ferreira I, van Greevenbroek MM, van der Kallen CJ, Schalkwijk CG, Stehouwer CD, Ocke MC, Nijpels G. Adapted dietary inflammatory index and its association with a summary score for low-grade inflammation and markers of glucose metabolism: the Cohort study on diabetes and atherosclerosis maastricht (CODAM) and the Hoorn study. Am J Clin Nutr. 2013;98:1533–42. [DOI] [PubMed] [Google Scholar]
- 51.Song Y, Ridker PM, Manson JE, Cook NR, Buring JE, Liu S. Magnesium intake, C-reactive protein, and the prevalence of metabolic syndrome in middle-aged and older US women. Diabetes Care. 2005;28:1438–44. [DOI] [PubMed] [Google Scholar]
- 52.Lupo MG, Biancorosso N, Brilli E, Tarantino G, Adorni MP, Vivian G, Salvalaio M, Dall’Acqua S, Sut S, Neutel C. Cholesterol-lowering action of a novel nutraceutical combination in uremic rats: insights into the molecular mechanism in a hepatoma cell line. Nutrients. 2020;12:436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lonardo A, Nascimbeni F, Mantovani A, Targher G. Hypertension, diabetes, atherosclerosis and NASH: cause or consequence? J Hepatol. 2018;68:335–52. [DOI] [PubMed] [Google Scholar]
- 54.Ma J, Hwang S-J, Pedley A, Massaro JM, Hoffmann U, Chung RT, Benjamin EJ, Levy D, Fox CS, Long MT. Bi-directional analysis between fatty liver and cardiovascular disease risk factors. J Hepatol. 2017;66:390–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsuneto A, Hida A, Sera N, Imaizumi M, Ichimaru S, Nakashima E, Seto S, Maemura K, Akahoshi M. Fatty liver incidence and predictive variables. Hypertens Res. 2010;33:638–43. [DOI] [PubMed] [Google Scholar]
- 56.Sorrentino P, Terracciano L, D’angelo S, Ferbo U, Bracigliano A, Vecchione R. Predicting fibrosis worsening in obese patients with NASH through parenchymal fibronectin, HOMA-IR, and hypertension. Official J Am Coll Gastroenterology| ACG. 2010;105:336–44. [DOI] [PubMed] [Google Scholar]
- 57.Zhang X, Guo Y, Yao N, Wang L, Sun M, Xu X, Yang H, Sun Y, Li B. Association between dietary inflammatory index and metabolic syndrome: analysis of the NHANES 2005–2016. Front Nutr. 2022;9:991907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eryd SA, Gudbjörnsdottir S, Manhem K, Rosengren A, Svensson A-M, Miftaraj M, Franzén S, Björck S. Blood pressure and complications in individuals with type 2 diabetes and no previous cardiovascular disease: national population based cohort study. BMJ 2016, 354. [DOI] [PMC free article] [PubMed]
- 59.Maeda T. The causal relationship between non-alcoholic fatty liver disease, hypertension, and cardiovascular diseases: implications for future research. Hypertens Res. 2024;47:2580–2. [DOI] [PubMed] [Google Scholar]
- 60.Aneni EC, Oni ET, Martin SS, Blaha MJ, Agatston AS, Feldman T, Veledar E, Conçeicao RD, Carvalho JA, Santos RD. Blood pressure is associated with the presence and severity of nonalcoholic fatty liver disease across the spectrum of cardiometabolic risk. J Hypertens. 2015;33:1207–14. [DOI] [PubMed] [Google Scholar]
- 61.Bartolomaeus H, Balogh A, Yakoub M, Homann S, Markó L, Höges S, Tsvetkov D, Krannich A, Wundersitz S, Avery EG. Short-chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation. 2019;139:1407–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ge X, Zheng L, Zhuang R, Yu P, Xu Z, Liu G, Xi X, Zhou X, Fan H. The gut microbial metabolite trimethylamine N-oxide and hypertension risk: a systematic review and dose–response meta-analysis. Adv Nutr. 2020;11:66–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zmora N, Suez J, Elinav E. You are what you eat: diet, health and the gut microbiota. Nat Reviews Gastroenterol Hepatol. 2019;16:35–56. [DOI] [PubMed] [Google Scholar]
- 64.Poll BG, Cheema MU, Pluznick JL. Gut microbial metabolites and blood pressure regulation: focus on SCFAs and TMAO. Physiology. 2020;35:275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang DD, Nguyen LH, Li Y, Yan Y, Ma W, Rinott E, Ivey KL, Shai I, Willett WC, Hu FB. The gut microbiome modulates the protective association between a Mediterranean diet and cardiometabolic disease risk. Nat Med. 2021;27:333–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rinott E, Meir AY, Tsaban G, Zelicha H, Kaplan A, Knights D, Tuohy K, Scholz MU, Koren O, Stampfer MJ. The effects of the Green-Mediterranean diet on cardiometabolic health are linked to gut microbiome modifications: a randomized controlled trial. Genome Med. 2022;14:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ziolkowska S, Binienda A, Jabłkowski M, Szemraj J, Czarny P. The interplay between insulin resistance, inflammation, oxidative stress, base excision repair and metabolic syndrome in nonalcoholic fatty liver disease. Int J Mol Sci. 2021;22:11128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Targher G, Corey KE, Byrne CD, Roden M. The complex link between NAFLD and type 2 diabetes mellitus—mechanisms and treatments. Nat Reviews Gastroenterol Hepatol. 2021;18:599–612. [DOI] [PubMed] [Google Scholar]
- 69.Pasdar Y, Moradi F, Cheshmeh S, Sedighi M, Saber A, Moradi S, Bonyani M, Najafi F. Major dietary patterns and dietary inflammatory index in relation to dyslipidemia using cross-sectional results from the RaNCD cohort study. Sci Rep. 2023;13:19075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vajdi M, Farhangi MA, Mahmoudi-Nezhad M. Dietary inflammatory index significantly affects lipids profile among adults: an updated systematic review and meta-analysis. Int J Vitam Nutr Res 2020. [DOI] [PubMed]
- 71.Martin A, Lang S, Goeser T, Demir M, Steffen H-M, Kasper P. Management of dyslipidemia in patients with non-alcoholic fatty liver disease. Curr Atheroscler Rep. 2022;24:533–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.O’Donoghue G, Kennedy A, Puggina A, Aleksovska K, Buck C, Burns C, Cardon G, Carlin A, Ciarapica D, Colotto M. Socio-economic determinants of physical activity across the life course: a DEterminants of DIet and physical ACtivity(DEDIPAC) umbrella literature review. PLoS ONE. 2018;13:e0190737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sy RG, Llanes EJB, Reganit PFM, Castillo-Carandang N, Punzalan FER, Sison OT, Khaing NEE, Poulton R, Woodward M, Tai ES. Socio-demographic factors and the prevalence of metabolic syndrome among filipinos from the LIFECARE cohort. J Atheroscler Thromb. 2014;21:S9–17. [DOI] [PubMed] [Google Scholar]
- 74.Kander MC, Cui Y, Liu Z. Gender difference in oxidative stress: a new look at the mechanisms for cardiovascular diseases. J Cell Mol Med. 2017;21:1024–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Knowlton A, Lee A. Estrogen and the cardiovascular system. Pharmacol Ther. 2012;135:54–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hébert JR, Hurley TG, Steck SE, Miller DR, Tabung FK, Peterson KE, Kushi LH, Frongillo EA. Considering the value of dietary assessment data in informing nutrition-related health policy. Adv Nutr. 2014;5:447–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hart MJ, Torres SJ, McNaughton SA, Milte CM. Dietary patterns and associations with biomarkers of inflammation in adults: a systematic review of observational studies. Nutr J. 2021;20:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wirtz DC, Heller KD, Miltner O, Zilkens KW, Wolff JM. Interleukin-6: a potential inflammatory marker after total joint replacement. Int Orthop. 2000;24:194–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen BR, Pan CQ. Non-invasive assessment of fibrosis and steatosis in pediatric non-alcoholic fatty liver disease. Clin Res Hepatol Gastroenterol. 2022;46:101755. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Fig. S1 Confirmatory factor analysis (CFA) of the measurement model of dyslipidemia components. The full-model fit indices had acceptable fit to the data: χ2/df = 41.815, p < 0.001, GFI = 0.993, AGFI = 0.934, CFI = 0.988, TLI = 0.931, RMSEA = 0.080, SRMR = 0.026
Supplementary Material 2: table 1 dietary intakes of study participants across tertiles of DII score (N = 3110), AmolCS, 2016–2017a Data are presented as mean ± standard deviation.
Data Availability Statement
The data gathered and analyzed in this study can be obtained from the corresponding author upon reasonable request. Interested researchers may reach out to the corresponding author, Dr. Hossein Ajdarkosh, email address: ajdarkosh.h@iums.ac.ir.