Abstract
Purpose
To evaluate the utility of apparent diffusion coefficient (ADC) values of extraocular muscles (EOMs) in differentiating activity of thyroid eye disease (TED).
Method
Forty-two TED patients who underwent diffusion tensor imaging(DTI) were retrospectively enrolled in this study, including 29 patients in analysis group and 13 patients in validation group. The mean, maximum and minimum ADC value of each EOM were regarded as ADCmean, ADCmax and ADCmin. The difference between ADCmax and ADCmin was regarded as △ADC. The correlations between ADCmean or △ADC of each EOM and clinical activity score (CAS) were assessed.
Results
In analysis group, ADCmean differed between active and inactive eyes and positively correlated with CAS in IR (P < 0.05), not in SR,LR and MR(all p > 0.05). While △ADC differed between two groups and negatively correlated with CAS in all EOMs (all P < 0.05). ADCmean predicted active disease at cut-off value of 1259.3 × 10−6mm2s−1 with sensitivity of 66.7% and specificity of 71.4% in IR[area under curve = 0.667, P < 0.05]. △ADC predicted disease activity in all EOMs [area under curve 0.658–0.746,all P < 0.05]. The cut-off values of △ADC were 382, 823,520 and 572 × 10−6mm2s−1 with sensitivity of 80.0%, 50.0%, 43.3%, 83.3% and specificity of 67.9%, 85.7%, 89.3%, 60.7% in SR, IR, MR, and LR respectively. There were no significant differences in the predictive efficacy among all cut-off values.
Conclusions
Our results showed that DTI is a valuable tool in the assessment of disease activity of TED. Both ADCmean of IR and △ADC of all four EOMs can be used in discriminating disease activity with the same predictive power.
Keywords: Thyroid eye disease, CAS, ADC, Resolve DTI, EOMs
Introduction
Thyroid eye disease(TED) is one of the most common orbital inflammatory diseases with the clinical manifestations including eyelid retraction, lid lag, proptosis, extraocular muscle restriction, exposure keratopathy, and optic neuropathy [1–3]. The whole pathological process is divided into two stages: active stage and inactive stage [4]. The active stage is characterized by infiltration and edema of inflammatory cells in the orbit. While the inactive stage is featured with fibrosis and the hyperplasia of extraocular muscles(EOMs) and fat. Patients in active stage respond well to immunosuppressive treatment. While patients in inactive stage can not benefit from that [3, 5]. Therefore, the accurate assessment of disease activity is critical to treatment selection.
Currently, the assessment of disease activity is mainly based on the Clinical activity Score (CAS), which is the sum of 7 or10 clinical items [6]. The higher the CAS score, the higher the activity. However, CAS is not without its limitations. Firstly, the score is mainly based on the clinical evaluation of the ocular surface rather than the inflammation deep in the orbit [1, 7]. Secondly CAS is a subjective parameter with poor consistency. Thus, it is necessary to find objective and reliable indicators of disease activity.
DTI is a noninvasive and advanced MRI technique, which can visualize water motion at the molecular level in three-dimensional space and provide information of directed diffusion of water [8–10]. Resolve DTI can improve quality of image and perform well in distinguishing normal anatomical structures [11]. The apparent diffusion coefficient (ADC) is a quantitative parameter of DTI, and its value increases with the increase of inflammation, edema, or tissue fluid [12–15]. In TED, previous studies have showed that ADC values of lacrimal gland was significantly higher in active patients than in inactive patients [16, 17]. This suggested that ADC may reflect inflammatory activity in TED. While, it is well known that EOMs are the main lesion sites in TED, not lacrimal gland [18, 19]. We speculated that ADC values of EOMs may be a good predictor for disease activity of TED. However, no published data was found.
Therefore, we performed orbital MRI scans with Resolve DTI sequence in TED patients to evaluate the diagnostic value of ADC values of EOMs for disease activity.
Methods
Population
From January 2020 to December 2021, TED patients who visited the First Affiliated Hospital of Chongqing Medical University were recruited. The inclusion criteria were as follows: (1) age of > 18 years and < 75 years; (2) TED patients who had undergone orbital MRI scans with Resolve DTI sequence. The exclusion criteria were as follows: (1) previous history of glucocorticoid therapy or other immunosuppressant, radiotherapy, or surgical decompression surgery;(2) other causes of orbital disease such as tumor, trauma or other immunological disorders such as Multiple sclerosis, System Lupus Erythematosus et al.;(3) image quality was inadequate for further analysis. All subjects agreed to take part in the present study and signed the consent form. The study followed the tenets of the declaration of Helsinki and was approved by the First Affiliated Hospital of Chongqing Medical University Ethical Committee. All methods and designs were performed in accordance with the consensus statement of the European Group on Graves’ Orbitopathy critera.
Clinical and biochemical measurements
External and funduscopic exam, visual acuity, intraocular pressure (IOP) (noncontact tonometer CT-1, Topcon, Japan) and exophthalmometry (66vision Technology Co, Ltd, Suzhou, China) were evaluated by an ophthalmologist in all patients. The diagnosis and evaluation of TED followed the consensus statement of the European Group on Graves’ eye (EUGOGO) criteria [6]. The CAS consists of seven findings: spontaneous retrobulbar pain; pain on attempted up or down gaze; redness of the conjunctiva; redness of the eyelids; inflammation of the caruncle and/or plica; swelling of the eyelids; and conjunctival edema. Each finding was scored 1 point. A summed CAS score of ≥ 3 was considered as active TED.
Image acquisition
MRI scan was performed in all patients using a 3.0-T MRI system (Skyra, Siemens, Germany) with a 20-channel head coil. Patients were asked to rest in supine position and look at a fixed site with eyes closed in order to reduce motion-related artifacts. Orbital coronal DTI images and conventional images were collected respectively. The ADC values in the superior rectus (SR), inferior rectus(IR), medial rectus(MR) and lateral rectus(LR) were measured on the DTI images which scanned by the routine scan protocol (repetition time (TR) = 4170 ms, echo time (TE) = 70 ms, FOV = 180 mm × 180 mm, slice thickness = 2.5 mm, interval between layers = 0.25 mm, scanning time = 283 s).
Magnetic resonance image (MRI) analysis
Siemens post-processing workstation was used to analyze DTI for measurement. With an MRI scan of the eye, there were 3–5 consecutive sections of each EOM. ROIs were drawn manually on each section of each EOM in areas with obvious inflammation visible to observers. Since it’s difficult to distinguish the superior rectus muscle and the levator palpebrae superioris from each other, they were measured together. After ROIs were placed, ADC values of each section were obtained in each eye (Fig. 1). The mean, maximum and minimum ADC values of all sections in each EOM were regarded as ADCmean, ADCmax and ADCmin. The difference between ADCmax and ADCmin was regarded as △ADC. The measurements were performed by two independent observers. The results of the two observers had excellent inter-reader agreement with an intra-class correlation coefficient (ICC) of 0.98. Then the results of the observer 1 were used for further statistical analysis.
Fig. 1.
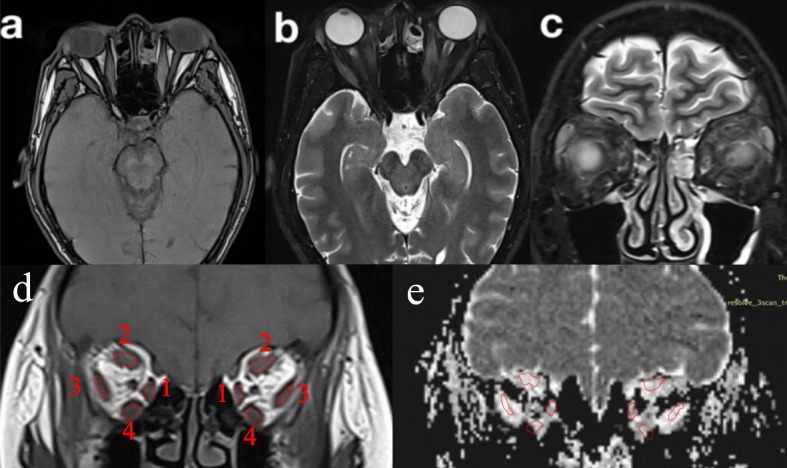
Orbital MRI of a 58-year-old male with TAO. a-b Axial T1- and T2-weighted imaging shows the orbit. c,d Coronal T2- and T1-weighted imaging shows the quantitative measurements of the EOMs. e ADC map shows the quantitative measurements of the EOMs.The number from 1 to 4 represent medial rectus, superior rectus, lateral rectus and inferior rectus respectively.MRI, magnetic resonance imaging;TAO, thyroid associated ophthalmopathy; EOMs, extraoclar muscls; ADC, apparent diffusion coefficient
Statistical analysis
SPSS (version 26.0) was used for statistical analysis. All results were reported as the mean ± standard deviation. The Shapiro–Wilk test was conducted to test the normality of all continuous variables. Means of continuous variable were compared using the Unpaired t-test, or Mann–Whitney Test (when the data were not normally distributed). Pearson correlation test was used to evaluate the correlations between CAS and ADC. The receiver operating characteristic (ROC) curve was done to determine the cut-off point of the ADC value of EOMs used to differentiate TED from active and inactive eyes with calculation of area under the curve (AUC). The sensitivity and specificity were also obtained with further analysis. Statistical analysis regarding the ROC were conducted using the MedCalc software. Differences were statistically significant at P < 0.05.
Results
A total of 42 TED patients were enrolled in this study, including 29 patients in analysis group and 13 patients in validation group. In analysis group,the mean age was 47.78 ± 5.15 years. The duration of TED was 19.04 ± 15.49 months. Nine of them were male (31.03%). Five of them were smoker (17.24%).
The clinical characteristics of active and inactive eyes
Fifty-eight eyes of 29 TED patients in analysis group were divided into active (n = 28) and inactive eyes (n = 30) based on CAS. As shown in Table 1, compared with inactive eyes, active eyes had much worse eyesight, higher intraocular pressure and degree of exophthalmos.
Table 1.
The clinical characteristics of active and inactive eyes
| Active eyes(n = 28) | Inactive eyes(n = 30) | P-value | |
|---|---|---|---|
| Eyesight | 0.58 ± 0.14 | 0.83 ± 0.10 | 0.010# |
| Intraocular pressure(mmHg) | 20.75 ± 1.89 | 17.43 ± 0.77 | 0.002* |
| Exophthalmos(mm) | 20.63 ± 1.07 | 18.00 ± 0.99 | 0.001# |
| CAS | 3.82 ± 0.33 | 1.17 ± 0.14 | 0.000# |
Data represented as mean ± SD; n number
*: the Unpaired t-test
#: Mann–Whitney Test
P- value < 0.05 was considered to be significant
ADC values of each EOM in active and inactive eyes
As shown in Table 2, ADCmean differed between the two groups in IR (1289.45 ± 90.44 × 10−6mm2s−1 vs 1161.18 ± 88.87 × 10−6mm2s−1; P = 0.029), not in SR, LR and MR (all p > 0.05). △ADC differed between the two groups in all four EOMs (
Table 2.
The ADCmean and △ADC value of each EOM in active and inactive eyes
| Active eyes(n = 28) | Inactive eyes(n = 30) | P-Value | |
|---|---|---|---|
| ADCmean of SR | 1412.21 ± 89.56 | 1508.57 ± 108.62 | 0.170* |
| ADCmean of IR | 1289.45 ± 90.44 | 1161.18 ± 88.87 | 0.029# |
| ADCmean of MR | 1526.72 ± 80.57 | 1396.41 ± 124.84 | 0.079* |
| ADCmean of LR | 1297.67 ± 106.56 | 1256.01 ± 108.15 | 0.577* |
| △ADC of SR | 392.18 ± 101.04 | 555.7 ± 80.23 | 0.001# |
| △ADC of IR | 581.46 ± 162.67 | 880.77 ± 193.43 | 0.039# |
| △ADC of MR | 344.32 ± 78.81 | 476.13 ± 93.04 | 0.032* |
| △ADC of LR | 677.39 ± 187.22 | 1154.97 ± 295.04 | 0.011# |
ADCmean: the average ADC value of five planes, SR Superior rectus, IR Inferior rectus, MR Medial rectus, LR Lateral rectus, n number
Data represented as mean ± SD
*: The Unpaired t-test
#: Mann–Whitney Test
Q- value < 0.05 was considered to be significant
SR: 392.18 ± 101.04 × 10−6mm2s−1 vs. 555.7 ± 80.23 × 10−6mm2s−1, P = 0.001;
IR: 581.46 ± 162.67 × 10−6mm2s−1 vs. 880.77 ± 193.43 × 10−6mm2s−1, P = 0.039;
MR: 344.32 ± 78.81 × 10−6mm2s−1 vs. 476.13 ± 93.04 × 10−6mm2s−1, P = 0.032;
LR: 677.39 ± 187.22 × 10−6mm2s−1 vs. 1154.97 ± 295.04 × 10−6mm2s−1,P = 0.011).
Correlations between ADC values and CAS
As shown in Table 3, ADCmean of IR showed positive correlation with CAS (P = 0.043, R = 0.27). △ADC showed negative correlation with CAS in SR (P = 0.011, R = −0.330), IR(P = 0.019, R = −0.306), MR(P = 0.032, R = −0.282) and LR(P = 0.008, R = −0.345).
Table 3.
The association between ADC values of each EOM and CAS
| r | P-Value | |
|---|---|---|
| ADCmean of IR | 0.27 | 0.043 |
| △ADC of SR | −0.33 | 0.011 |
| △ADC of IR | −0.31 | 0.019 |
| △ADC of MR | −0.28 | 0.032 |
| △ADC of LR | −0.35 | 0.008 |
EOM extraocular muscle, CAS clinical activity score, SR Superior rectus, IR Inferior rectus, MR Medial rectus, LR Lateral rectus, △ADC the difference between the maximum and minimum ADC value
Pearson correlation test was used to evaluate the correlations
P-value < 0.05 was considered to be significant
Receiver operating characteristic (ROC) cure analysis
To determine the predictive efficiency and diagnostic cut-off value, we performed ROC curve analysis in Table 4.The cut-off value of ADCmean used to predict active disease was > 1259.3 × 10−6mm2s−1 with a sensitivity of 66.7% and a specificity of 71.4% in IR (AUC:0.667, P = 0.029). △ADC performed well in discriminating activity in all four EOMs (SR:AUC = 0.746, P = 0.001; IR:AUC = 0.675, P = 0.022; MR:AUC = 0.658, P = 0.039 and LR:AUC = 0.695, P = 0.011). The cut-off value of △ADC were > 382 × 10−6mm2s−1 in SR, > 823 × 10−6mm2s−1 in IR, > 520 × 10−6mm2s−1 in MR and > 572 × 10−6mm2s−1 in LR. And the sensitivity were 80.0%, 50.0%,43.3%,83.3% and the specificity were 67.9%,85.7%,89.3%,60.7% in SR, IR, MR, and LR respectively.
Table 4.
Predictive performance of ADC values of each EOM between the active and inactive eyes
| AUC | Cut-off | Sensitivity % | Sensitivity % | P-value | Validation of Cut-offs | ||
|---|---|---|---|---|---|---|---|
| Sensitivity% | Specificity% | ||||||
| ADCmean of IR | 0.667 | 1259.3 | 66.70 | 71.4 | 0.029 | 68.42 | 57.14 |
| △ADC of SR | 0.746 | 382.00 | 80.00 | 67.90 | 0.001 | 68.42 | 71.43 |
| △ADC of IR | 0.675 | 823.00 | 50.00 | 85.70 | 0.022 | 63.16 | 57.14 |
| △ADC of MR | 0.658 | 520.00 | 43.30 | 89.30 | 0.039 | 68.42 | 57.14 |
| △ADC of LR | 0.695 | 572.00 | 83.30 | 60.70 | 0.011 | 68.42 | 85.71 |
AUC Area under curve, EOM extraocular muscle, △ADC the difference between the maximum and minimum ADC value, SR Superior rectus, IR Inferior rectus, MR Medial rectus, LR Lateral rectus
P value < 0.05 was considered to be significant
The diagnostic accuracy of the identified cut-off values was further validated in validation group in Table 4 (n = 26). Twenty-six eyes were classified into active and inactive group according to the identified cut-offs and matched with their actual CAS. The diagnostic sensitivity and specificity for the cut-off of ADCmean of IR was 68.42% and 57.14%. The diagnostic sensitivity and specificity for the cut-off of △ADC were 68.42%, 63.16%, 68.42%, 68.42% and 71.43%, 57.14%, 57.14%, 85.71% in SR, IR, MR, and LR respectively.
The predictive efficacy between different △ADC values
To find the best predictor, the predictive efficacy of different ADC were compared in Table 5. There were no significant differences in the predictive efficacy of △ADC cut-off values among SR, IR, MR, LR (all P > 0.05). Also there were no significant differences in the predictive efficacy between ADCmean of IR and △ADC cut-off values (all P > 0.05).
Table 5.
The predictive efficacy between different ADC values
| △ADC | ADCmean of IR vs △ADC of each EOM | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SR vs IR | SR vs MR | SR vs LR | IR vs MR | IR vs LR | MR vs LR | IR vs SR | IR vs IR | IR vs MR | IR vs LR | |
| P-value | 0.416 | 0.367 | 0.514 | 0.840 | 0.835 | 0.350 | 0.374 | 0.927 | 0.929 | 0.794 |
△ADC the difference between the maximum and minimum ADC value, SR Superior rectus, IR Inferior rectus, MR Medial rectus, LR Lateral rectus
Delong’s test was used to evaluate the predictive efficacy between different △ADC
P-value < 0.05 was considered to be significant
Discussion
Our study demonstrated that △ADC of all four EOMs and ADCmean of IR could predict disease activity in TED. This was the first study to show that DTI parameters of a single EOM can be used to assess disease activity of TED.
Previous studies have shown that inflammation can increase ADC values of tissue [12–14, 20, 22]. The active stage of TED is characterized by lymphocytic cell infiltration, edema and inflammation. The inactive stage is characterized by adipogenesis and myofibrillogenesis. During the active stage, the inflammation of EOMs is more severe and even. In active stage, the inflammation is reduced, uneven distribution.
ADCmean is the average ADC value of 3–5 layers of the whole muscle. We found that ADCmean was positively correlated with CAS in IR which was in line with previous studies.
But this relationship was not found in SR, LR and MR. The reason for the difference results was not clear. There may be several explanations for that. Firstly, IR was reported to be the most susceptible muscle among all four EOMs [18, 19, 21]. Secondly, we thought that the inflammations of EOMs might be not uniform in each part, therefore, using ADCmean to represent the entire EOMs may result in some loss of information.
We thought that △ADC could reflect the heterogeneity of water diffusion in different parts of EOMs and might better reflect the inflammation of EOMs, which is the difference between ADC maximum and ADC minimum. Therefore, we calculated △ADC of each EOM and assessed the correlations between △ADC and CAS. As expected, △ADC differed between active and inactive eyes and negatively correlated with CAS in all four EOMs in our study.
To prove our results, we calculated the cut-off value of △ADC and ADCmean and validate them in another 26 TED eyes. Our data showed that both △ADC of all four EOMs and ADCmean of IR could predict disease activity with good sensitivity and specificity.
To find the best predictor, the predictive effects of △ADC and ADCmean were compared.We found the same predictive value for disease activity among △ADC values of four EOMs, as well as between △ADC and ADCmean. Therefore for clinical applications, both △ADC of any EOM or ADCmean of IR can be selected.
The present study had several limitations. First, this was a retrospective study and sample size was relatively small. Second, due to the difficulties in achieving the histological analysis of EOMs, the disease activity was based on the CAS. Therefore, although the identified cut-offs were validated in a new group of patients, the accuracy of the predicting values need to be validated in future large population.
Conclusion
Our results showed that DTI is a valuable tool in the assessment of disease activity of TED. Both ADCmean of IR and △ADC of all four EOMs can be used in discriminating disease activity with the same predictive power.
Acknowledgements
We appreciate very much for all the healthcare staff and our participants.
Authors' contributions
Liang liang contributed to the diagnosis and assessment of TED. Cheng yang Tang and Qian Huang performed parameter measurements. Ming qiao zhang and Cheng yang Tang contributed to data analysis. Cheng yang Tang and Long jian contributed to manuscript preparation. Xiaoya zheng, and Longjian contributed to the conception of the study. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Funding
This study is supported by Chongqing Health Commission NO:2020MSXM119).
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
We confirmed that informed consent was obtained from all subjects, all subjects recruited agreed to take part in the present study. The study was approved by the First Affiliated Hospital of Chongqing Medical University Ethical Committee. All methods and designs were performed in accordance with the consensus statement of the European Group on Graves’ Orbitopathy critera.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lingam RK, Mundada P, Lee V. Novel use of non-echo-planar diffusion weighted MRI in monitoring disease activity and treatment response in active Grave’s eye: an initial observational cohort study. Orbit. 2018;37:325–30. [DOI] [PubMed] [Google Scholar]
- 2.Hodgson NM, Rajaii F. Current understanding of the progression and management of associated eye: a systematic review. Ophthalmol Ther. 2020;9:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Şahlı E, Gündüz K. Thyroid-associated Ophthalmopathy. Türk Oftalmoloji Dergisi. 2017;47:94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartley GB. Rundle and his curve. Arch Ophthalmol. 2011;129:356–8. [DOI] [PubMed] [Google Scholar]
- 5.Bartalena L, Piantanida E, Gallo D, Lai A, Tanda ML. Epidemiology, natural history, risk factors, and prevention of Graves’ eye. Front Endocrinol. 2020;11:615993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luigi B, Kahaly George J, Lelio B, Dayan Colin M, Anja E, Claudio M, Wiersinga WM, The 2021 European Group On Graves’ eye (EUGOGO) clinical practice guidelines for the medical management of Graves’ eye. Eur J Endocrinol. 2021;2021(185):G43–67. [DOI] [PubMed] [Google Scholar]
- 7.Dolman PJ. Grading severity and activity in thyroid eye disease. Ophthalmic Plast Reconstr Surg. 2018;34:S34–40. [DOI] [PubMed] [Google Scholar]
- 8.Min J, Park M, Choi JW, Jahng GH, Moon WJ. Inter-Vendor and Inter-Session reliability of diffusion tensor imaging: implications for multicenter clinical imaging studies. Korean J Radiol. 2018;19:777–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vo NQ, Hoang NT, Nguyen DD, Nguyen TH, Le TB, Le NT, Nguyen TT. Quantitative parameters of diffusion tensor imaging in the evaluation of carpal tunnel syndrome. Quant Imaging Med Surg. 2022;12:3379–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thiel TA, Schweitzer J, Xia T, Bechler E, Valentin B, Steuwe A, Boege F, Westenfeld R, Wittsack HJ, Ljimani A. Evaluation of radiographic contrast-induced nephropathy by functional diffusion weighted imaging. J Clin Med. 2021;10:4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu XQ, Liu J, Hu H, et al. Improve the image quality of orbital 3 T diffusion-weighted magnetic resonance imaging with readoutsegmented echo-planar imaging. Clin Imaging. 2016;40:793–6. [DOI] [PubMed] [Google Scholar]
- 12.Kamper L, Dreger NM, Brandt AS, Haage P. Diffusion-weighted MRI and PET-CT in the follow up of chronic periaortitis. Int J Cardiovasc Imaging. 2018;34:1779–85. [DOI] [PubMed] [Google Scholar]
- 13.Zappa M, Doblas S, Cazals-Hatem D, Van Beers BE. Quantitative MRI in murine radiation-induced rectocolitis: comparison with histopathological inflammation score. NMR Biomed. 2018;31:e3897. [DOI] [PubMed] [Google Scholar]
- 14.Bray TJP, Sakai N, Dudek A, Hall-Craggs MA. Histographic analysis of oedema and fat in inflamed bone marrow based on quantitative MRI. Eur Radiol. 2020;30:5099–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aggarwal A, Das CJ, Khanna N, Sharma R, Srivastava DN, Goyal V, Netaji A. Role of diffusion tensor imaging in the evaluation of ulnar nerve involvement in leprosy. Br J Radiol. 2022;95:20210290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao Y, Wu H, Wu Q, Chen L, Chen W, Xu X, Wu F. Quantitative measurement of diffusion tensor imaging based on readout-segmented echo planar imaging of lacrimal glands in evaluating disease activity of thyroid-associated ophthalmopathy. Chin J Med Imaging. 2022;30:447–50. [Google Scholar]
- 17.Chen L, Hu H, Chen W, Wu Q, Zhou J, Chen HH, Xu XQ, Shi HB, Wu FY. Usefulness of readout-segmented EPI-based diffusion tensor imaging of lacrimal gland for detection and disease staging in thyroid-associated ophthalmopathy. BMC Ophthalmol. 2021;21:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mei Y, Bai-xue DU, Yu-jiao WA, Wei-min HE. Clinical analysis of 2 170 cases of thyroid-associated ophthalmopathy involving extraocular muscles. Sichuan Da Xue Xue BaoYi Xue Ban. 2021;52:510–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Du B, Wang Y, Yang M, He W. Clinical features and clinical course of thyroid-associated ophthalmopathy: a case series of 3620 Chinese cases. Eye (Lond). 2021;35:2294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feeney C, Lingam RK, Lee V, Rahman F, Nagendran S. Non-EPI-DWI for detection, disease monitoring, and clinical decision-making in thyroid eye disease. Am J Neuroradiol. 2020;41:1466–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Savino G, Mattei R, Salerni A, Fossataro C, Pafundi PC. Long-term follow-up of surgical treatment of thyroid eye disesaserestrictive strabismus. Front Endocrinol. 2022;13:1030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen HH, Hu H, Chen W, Yang T. Thyroid-Associated eye: evaluating microstructural changes of extraocular muscles and optic nerves using readout-segmented echo-planar imaging-based diffusion tensor imaging. Korean J Radiol. 2020;21:332–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.


