Abstract
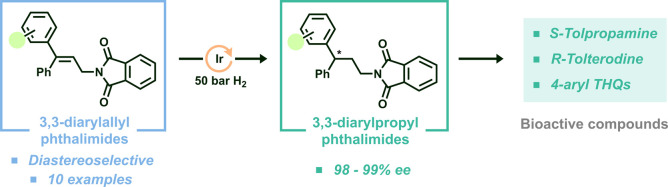
Chiral nitrogen-containing compounds are crucial for the chemical, pharmaceutical, and agrochemical industries. Nevertheless, the synthesis of certain valuable scaffolds remains underdeveloped due to the vast chemical space available. In this work, we present a diastereoselective methodology for synthesizing 3,3-diarylallyl phthalimides, which, following iridium-catalyzed asymmetric hydrogenation using Ir–UbaPHOX, yield 3,3-diarylpropyl amines with high enantioselectivity (98–99% ee). The importance of alkene purity to achieve high enantioselectivity is discussed. The synthetic utility of the chiral propylamines obtained is demonstrated through the preparation of medicinally useful bioactive compounds like the drugs tolterodine and tolpropamine and 4-aryl tetrahydroquinolines. This strategy enables the synthesis of these compounds with the highest enantioselectivity reported to date.
Chiral amines are key fragments in many biologically active compounds, including drugs, natural products, and agrochemicals.1 Furthermore, many chiral amines have been used for a wide variety of synthetic purposes like resolving agents, chiral auxiliaries, or building blocks of chiral complex molecules.2 As a result, over recent decades, synthetic chemists have been particularly focused on their asymmetric synthesis.3 Despite the widespread importance of chiral amines, traditional synthetic methods, such as resolution, are still being used. To overcome the drawbacks of these methodologies, innovative catalytic asymmetric approaches are being developed.4 Among these, the asymmetric hydrogenation (AH) of unsaturated compounds stands out as one of the most powerful tools.5 Unfortunately, due to the extension of the chemical space, the AH of certain types of amine substrates is still underdeveloped. In particular, the AH of allyl amines has received little attention because they lack a proper coordinating group.
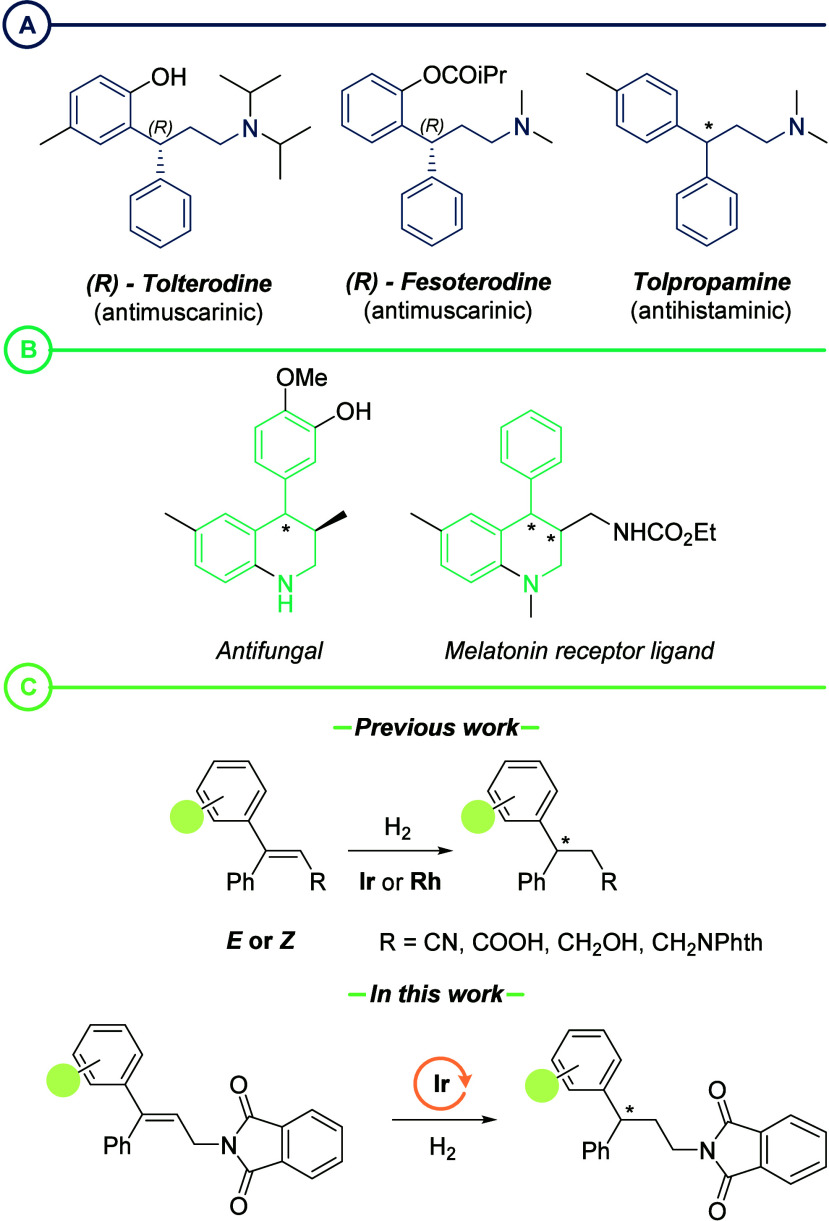
On this matter, 3,3-diarylpropyl amines rise as an interesting target. They are found in several medicinally useful bioactive compounds, including the commercially available drugs tolterodine6 and fesoterodine (Figure 1a).7 Additionally, the cyclization and functionalization of these substrates grants access to 4-aryl-substituted tetrahydroquinolines (THQs), which also hold significant relevance in the pharmaceutical industry, as reflected by their presence in numerous drugs and natural products (Figure 1b).8
Figure 1.
(a) Examples of commercially available drugs containing a 3,3-diarylpropyl amine core. (b) Examples of biologically active compounds with a 4-aryl-substituted THQ core. (c) Previous AH approaches to access 3,3-diarylpropyl amines and strategy envisaged in this study.
So far, catalytic asymmetric methods to synthesize 3,3-diarylpropyl compounds rely mainly on two strategies. The most widely used is the enantioselective rhodium-catalyzed 1,4-conjugate addition of arylboronic acids to β-aryl-α,β-unsaturated esters.9 This strategy provides good results in terms of enantioselectivity when meta- or para-substituted boronic acids are employed. However, when it comes to ortho-substituted compounds, the selectivity decreases. Other organometallic nucleophiles and α,β-unsaturated groups have been used without success.10 The alternative strategy involves metal-catalyzed AH (Figure 1c), the reaction employed herein. Currently, this approach is dominated by rhodium catalysts.11 Recently, the Rh-catalyzed hydrogenation of a single diarylallyl phthalimide was reported to provide 86% ee.11e The unsatisfactory results obtained when synthesizing ortho- or para-substituted compounds make the use of this metal far from ideal. Iridium is another metal frequently used in AH. However, to our knowledge, only two studies on iridium-catalyzed AH of this class of compounds have been reported, both with suboptimal enantioselectivites.12 Therefore, a general and highly enantioselective methodology for the synthesis of 3,3-diarylpropyl amines is desirable.
Here we describe a novel approach based on the iridium-catalyzed AH of 3,3-diarylallyl phthalimides.13 Our strategy grants access to the desired motifs with optimal enantioselectivities regardless of the aryl substitution. We also show that the resulting substrates can be easily derivatized to obtain medicinally useful bioactive compounds like the drugs tolterodine and tolpropamine14 and 4-aryl THQs.
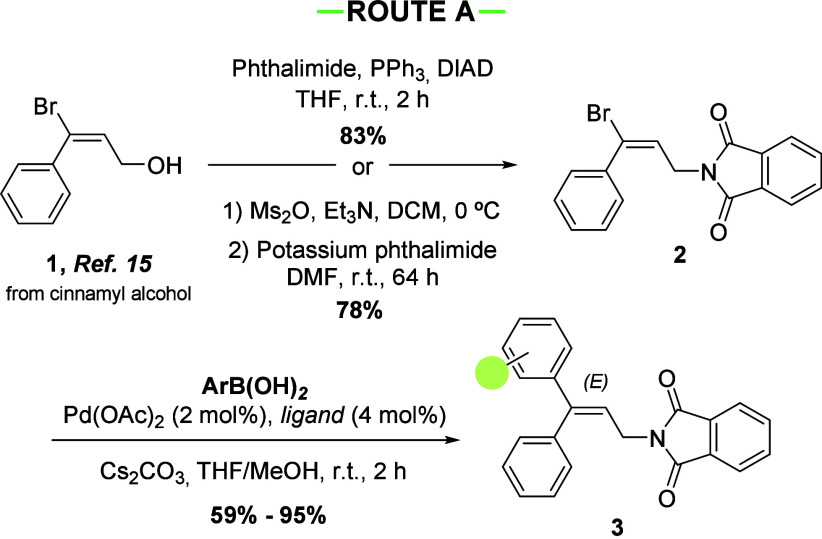
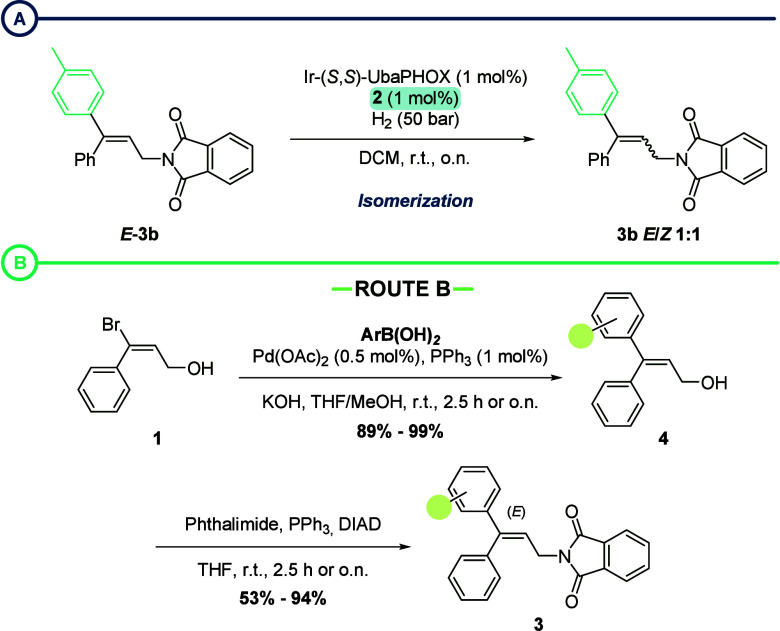
The synthesis of 3,3-diarylallyl phthalimides was envisaged from allylic alcohol 1, which is easily accessible in a stereoselective manner from E-cinnamyl alcohol by Monteiro’s procedure.15 From compound 1, the phthalimide and aryl fragments can be introduced in any order. Initially, we introduced the phthalimide first. This can be done either by a Mitsunobu reaction or by substitution on the corresponding mesylate. Both procedures afforded vinyl bromide 2 in excellent yield (Scheme 1). Next, the Suzuki coupling with different boronic acids provided a diverse array of 3,3-diarylallyl phthalimides 3 in good yields.
Scheme 1. Synthesis of 3,3-Diarylallyl Phthalimides 3 by Route A.
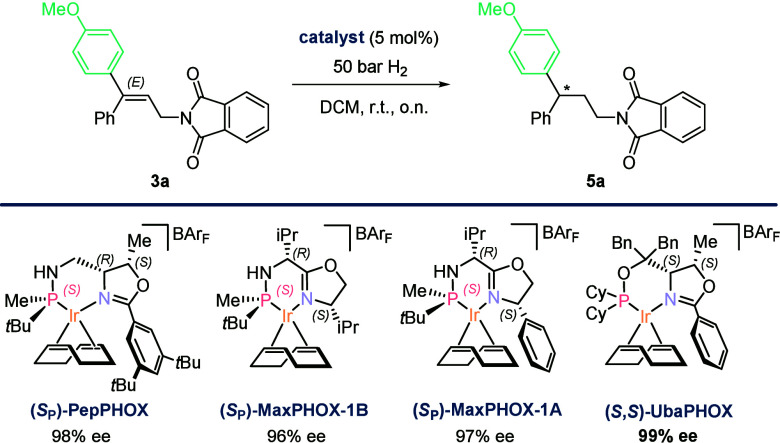
The initial catalyst screening for AH was carried out using different catalysts developed by our group, like the Ir–MaxPHOX16 and Ir–PepPHOX17 families, on substrate 3a (Scheme 2). Although these catalysts afforded excellent results, the best enantioselectivity (99% ee) was obtained using commercially available Ir–(S,S)-UbaPHOX.18 For full details, see the Supporting Information (SI).
Scheme 2. Summarized Screening of Catalysts for the AH of 3a.
The catalysts shown afforded full conversion. See the SI for the complete catalyst screening results.
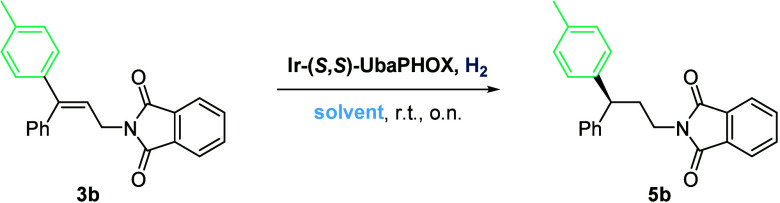
Next, we studied the optimization of the hydrogenation conditions with p-methyl-substituted substrate 3b and Ir-UbaPHOX catalyst (Table 1). Dichloromethane (DCM), trifluorotoluene (TFT), and dichloroethane (DCE) all provided 96–97% ee (Table 1, entries 1–3). Toluene also provided comparable selectivity but with a significant loss of activity (Table 1, entry 4). The use of a weakly coordinating solvent such as ethyl acetate (EtOAc) was detrimental in terms of conversion (Table 1, entry 5). The use of greener solvents such as dimethyl carbonate (DMC) and propylene carbonate (PC) was also attempted without success due to the poor solubility of the allyl phthalimide (Table 1, entries 6 and 7). Regarding the hydrogen pressure, the best results were obtained at 50 bar (Table 1, entry 1). Decreasing the pressure to 10 bar resulted in a slight decrease in selectivity (Table 1, entry 8), and when the pressure was set at 3 bar, the reaction did not reach full conversion (Table 1, entry 9). Finally, the catalyst loading was decreased to 1 mol % with no loss of selectivity (Table 1, entry 10).
Table 1. Optimization of Pressure, Solvent, and Catalyst Loading Parametersa.
While screening the reaction conditions with 3b, we encountered a few reproducibility issues. In some instances, the hydrogenation was not complete, and the isomerized starting material E/Z-3b was recovered. After closer inspection, we realized that this occurred with non-recrystallized samples of 3b. HPLC-MS analysis of such batches revealed that they contained small amounts of the previous bromoalkene intermediate 2 (see the SI for more details). To confirm that the presence of the bromoalkene was responsible for the isomerization, a 1 mol % loading of 2 was added to a recrystallized batch of E-3b (Scheme 3a). Hydrogenation at 1 mol % catalyst in this case was completely suppressed, and the isomerized alkene was recovered. This observation confirmed that any bromoalkene impurity was extremely detrimental for the conversion and selectivity of the AH process. To avoid the presence of 2, we tackled the synthesis of alkene substrates 3 via an alternative route. Reversing the order of the reactions, the arylboronic acids were introduced first via a Suzuki coupling, and the phthalimide group was incorporated later using a Mitsunobu reaction (Scheme 3b). After column chromatography and/or recrystallization, the desired E-3,3-diarylallyl phthalimides 3 were obtained as single diastereomers. Notably, the AH of 3a synthesized via Route B resulted in an increase in selectivity from 96% to 98% ee (Table 1, entry 11).
Scheme 3. (a) Isomerization Induced by the Presence of 2; (b) Synthesis of 3,3-Diarylallyl Phthalimides 3 by Route B.
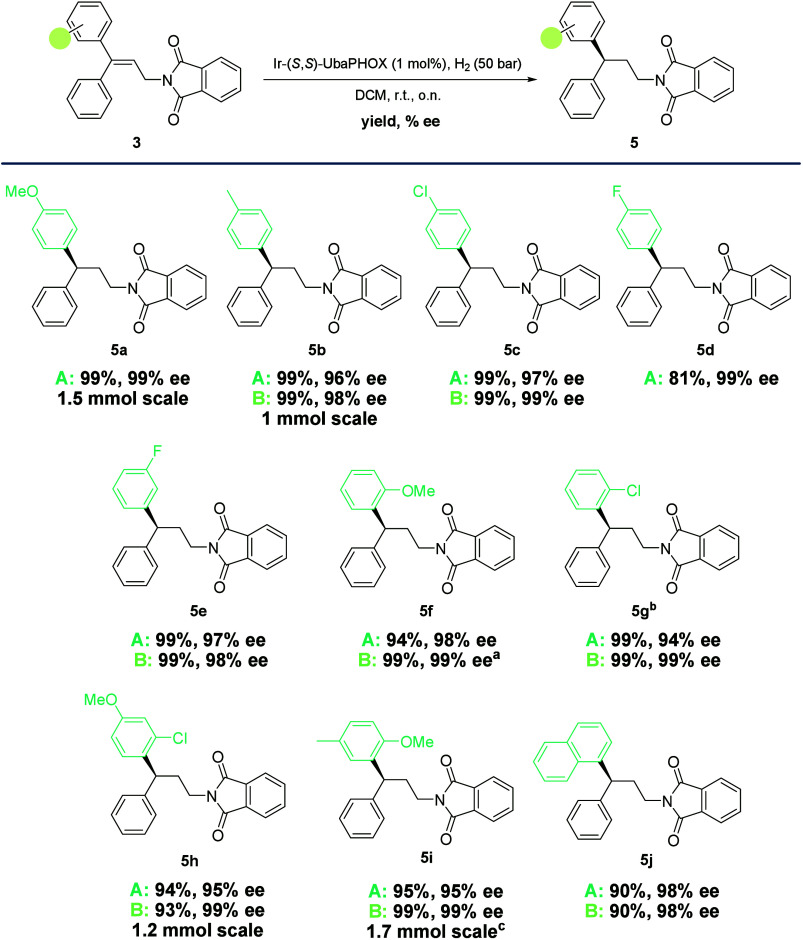
Using both routes, a set of 3,3-diarylallyl phthalimides with different substituents on one of the aryl groups (3a–3j) were prepared. These substrates were subjected to AH at 1 mol % under the optimized conditions (Scheme 4). All olefins bearing para substituents (3a–3d) on the aryl ring gave enantioselectivities ranging from 98% to 99% ee. When this substitution was placed at the meta position (3e), 98% ee was achieved. Example 3f with ortho substitution yielded 99% ee. Similarly, 3g also provided 99% ee but required a longer reaction time and an increase in the catalyst loading. The transformation also proved to be effective with disubstituted compounds. In this regard, 3h and 3i were successfully hydrogenated, achieving 99% ee. Finally, the naphthyl substituted substrate 3j was also attempted, yielding 98% ee. It was observed that compounds synthesized through Route B consistently provided higher selectivity. This observation confirmed that trace amounts of 2 that remained on substrate 3 were responsible for partial isomerization of the substrate, thus resulting in a decrease in selectivity.19 Hydrogenation of substrates containing acetyl, furan, and thiophene moieties provided low conversion and selectivity (see the SI). This is most likely due to coordination of these moieties to the iridium center, resulting in catalyst deactivation.
Scheme 4. Scope of the Catalytic Hydrogenation of 3,3-Diarylallyl Phthalimides 3.
98% ee was obtained when the catalyst loading was decreased to 0.5 mol %.
2 mol % catalyst loading and 64 h reaction time were used.
Large-scale hydrogenation was performed with Ir–(R,R)-UbaPHOX to yield (R)-5i.
See the SI for the molarity values used in each reaction. All substrates provided complete conversion, except for 5h (Route B; 97%). The ee values were determined by HPLC analysis on a chiral stationary phase.
Examples 3a, 3b, 3g, 3h, and 3i were also hydrogenated on larger scales ranging from 0.5 to 1.7 mmol (150–650 mg) of starting material without loss of selectivity. Example 3f was also hydrogenated at a 0.5 mol % catalyst loading with a minimal decrease in enantioselectivity (98% ee). The stereochemistry of all the products was predicted to be S using Andersson’s quadrant model (see the SI).20 This was later confirmed by comparison of the sign of the optical rotation of 10g (vide infra) with the literature.21 The stereochemical outcome was assumed to be the same for all substrates.
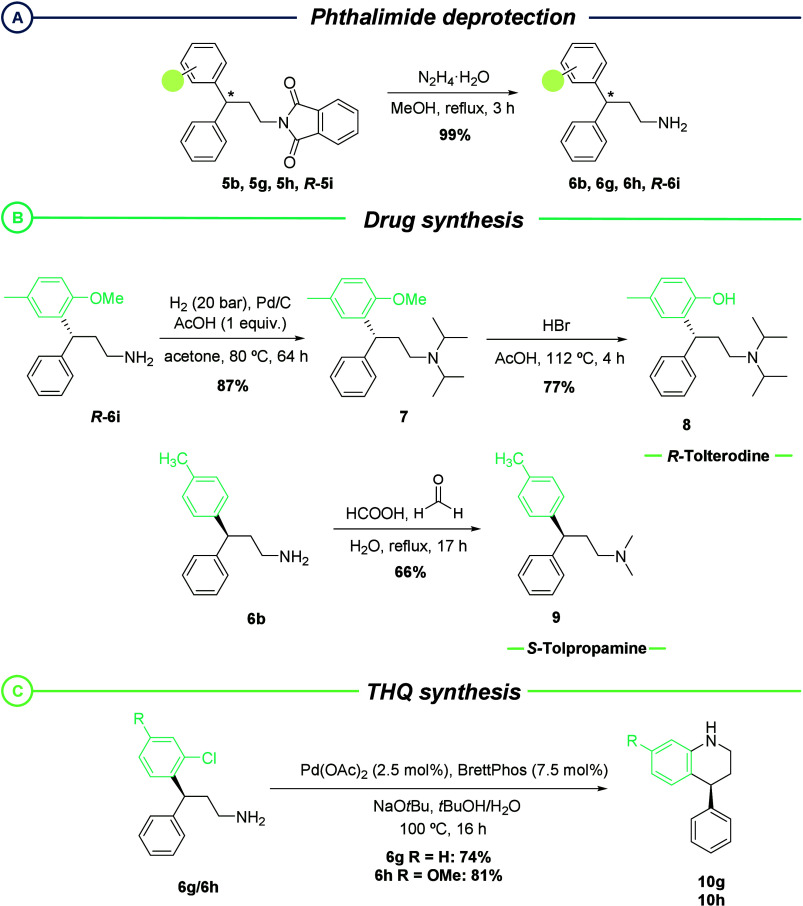
We next proceeded to demonstrate the usefulness of the present methodology by applying it to the synthesis of biologically active compounds of pharmacological interest (Scheme 5). First, the deprotected primary amine derivatives of 5 were readily obtained in quantitative yield by phthalimide deprotection using hydrazine (Scheme 5a). (R)-Tolterodine is a commercially available drug that has been synthesized on numerous occasions using racemic resolution,22 chiral auxiliaries,23 rhodium-catalyzed 1,4-conjugate addition,9 or AH on coumarins.24 Nonetheless, none of these approaches contemplate iridium-catalyzed AH as the key step. Starting from (R)-6i (Scheme 5b), obtained using Ir–(R,R)-UbaPHOX, the free amine was alkylated with two isopropyl groups using acetone and Pd/C under H2 pressure to yield 7. A final deprotection of the phenol group provided (R)-tolterodine (8) in optically pure form. Tolpropamine, an antihistaminic drug, has only been described as a racemate, and no asymmetric synthesis has been previously reported. Here, starting from 6b (Scheme 5b), the free amine was dimethylated via an Eschweiler–Clarke reaction to yield (S)-tolpropamine (9).
Scheme 5. (a) Deprotection of Phthalimides 5; (b) Asymmetric Synthesis of (R)-Tolterodine and (S)-Tolpropamine; (c) Cyclization of Hydrogenated Amines 6g/6h to Provide THQs 10g/10h.
Ultimately, regarding the promising activity of 4-aryl THQs against biological targets, we envisioned their asymmetric synthesis from the cyclization of o-chloro-substituted 3,3-diarylpropyl phthalimides 5g/5h (Scheme 5c). After deprotection of the phthalimides, a Buchwald–Hartwig cyclization yielded the desired 4-aryl THQs. Comparison of the optical rotation of 10g with literature data confirmed not only the absolute configuration of the hydrogenation products but also that no racemization occurred during the deprotection and cyclization reactions.20 To the best of our knowledge, the approach described herein provides the best enantioselectivity in the synthesis of such compounds reported to date. All of these applications demonstrate the versatility of the chiral diarylpropyl amine intermediates obtained using our methodology.
In summary, here we describe a novel methodology to prepare 3,3-diarylallyl phthalimides 3 as single diastereomers. Iridium-catalyzed asymmetric hydrogenation of these compounds provides the corresponding 3,3-diarylpropyl amines with high enantioselectivity. During optimization of the reaction, it was found that bromoalkene impurities induced the isomerization of the alkene starting material, thus lowering the selectivity of the overall process. Using a synthetic route that minimizes the bromoalkene impurities in the starting material, the final chiral propylamines were obtained with selectivity ranging from 98 to 99% ee. The scope of the reaction has been shown to tolerate distinct functional groups and substitutions patterns. The synthetic utility of 3,3-diarylpropyl phthalimides 5 has been proven by preparing tolpropamine, tolterodine, and 4-aryl THQs, achieving the highest enantioselectivities reported to date.
Acknowledgments
This work was supported by a grant awarded to A.R. and X.V. from the Ministerio de Ciencia, Innovación y Universidades (PID2023-147298NB-I00 funded by MCIU/AEI/10.13039/501100011033/FEDER, UE). IRB Barcelona is the recipient of institutional funding from MICINN through the Centers of Excellence Severo Ochoa Award and from the CERCA Program of the Catalan Government. We thank the Generalitat de Catalunya for Grant 2021 SGR 00866. M.S. thanks the Fundación Ramón Areces for a predoctoral fellowship, and Y.W. thanks MICINN for a predoctoral fellowship.
Data Availability Statement
The data underlying this study are available in the published article and its online Supporting Information.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.4c04076.
Screening of catalysts for the AH; HPLC-MS chromatogram of non-recrystallized 3b; experimental procedures and spectroscopic data; 1H, 13C, and 19F NMR spectra; and HPLC chromatograms of racemic and enantioenriched compounds (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Höhne M.; Bornscheuer U. T. Biocatalytic Routes to Optically Active Amines. ChemCatChem 2009, 1, 42–51. 10.1002/cctc.200900110. [DOI] [Google Scholar]; b Chiral Drugs: Chemistry and Biological Action; Lin G.-Q., You Q.-D., Cheng J.-F., Eds.; Wiley, 2011. [Google Scholar]
- a Hodgson D. M.; Gibbs A. R.; Lee G. P. Enantioselective Desymmetrisation of Achiral Epoxides. Tetrahedron 1996, 52, 14361–14384. 10.1016/0040-4020(96)00888-5. [DOI] [Google Scholar]; b Nugent T. C.; El-Shazly M. Chiral Amine Synthesis - Recent Developments and Trends for Enamide Reduction, Reductive Amination, and Imine Reduction. Adv. Synth. Catal. 2010, 352, 753–819. 10.1002/adsc.200900719. [DOI] [Google Scholar]
- Stereoselective Formation of Amines; Wei L., Zhang X., Eds.; Topics in Current Chemistry, Vol. 343; Springer, 2014. [DOI] [PubMed] [Google Scholar]
- a Comprehensive Asymmetric Catalysis; Jacobsen E. N., Pfaltz A., Yamamoto H., Eds.; Springer, 2004. [Google Scholar]; b Caprio V.; Williams J. M. J.. Catalysis in Asymmetric Synthesis, 2nd ed.; Wiley, 2009. [Google Scholar]; c Catalytic Asymmetric Synthesis; Takahiko A., Ojima I., Eds.; Wiley, 2022. [Google Scholar]
- a Kim A. N.; Stoltz M. Recent Advances in Homogeneous Catalysts for the Asymmetric Hydrogenation of Heteroarenes. ACS Catal. 2020, 10, 13834–13851. 10.1021/acscatal.0c03958. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Cabré A.; Verdaguer X.; Riera A. Recent Advances in the Enantioselective Synthesis of Chiral Amines via Transition Metal-Catalyzed Asymmetric Hydrogenation. Chem. Rev. 2022, 122, 269–339. 10.1021/acs.chemrev.1c00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staskin D.; Herschorn S.; Fialkov J.; Tu L. M.; Walsh T.; Schermer C. R. A Prospective, Double-Blind, Randomized, Two-Period Crossover, Multicenter Study to Evaluate Tolerability and Patient Preference between Mirabegron and Tolterodine in Patients with Overactive Bladder (PREFER Study). Int. Urogynecol. J. 2018, 29, 273–283. 10.1007/s00192-017-3377-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi M.; Takano Y.; Torita Y.; Malhotra B.; Chiba K. Physiological Based Pharmacokinetic Modeling to Estimate in Vivo Ki of Ketoconazole on Renal P-Gp Using Human Drug-Drug Interaction Study Result of Fesoterodine and Ketoconazole. Drug. Metab. Pharmacokinet. 2018, 33, 90–95. 10.1016/j.dmpk.2017.11.005. [DOI] [PubMed] [Google Scholar]
- a Romero-Bohórquez A. R.; Kouznetsov V. V.; Zacchino S. A. Synthesis and in Vitro Evaluation of Antifungal Properties of Some 4-Aryl-3-Methyl-1,2,3,4-Tetrahydroquinolines Derivatives. Univ. Sci. 2014, 20, 177–189. 10.11144/Javeriana.SC20-2.siea. [DOI] [Google Scholar]; b Harland A. A.; Bender A. M.; Griggs N. W.; Gao C.; Anand J. P.; Pogozheva I. D.; Traynor J. R.; Jutkiewicz E. M.; Mosberg H. I. Effects of N -Substitutions on the Tetrahydroquinoline (THQ) Core of Mixed-Efficacy μ-Opioid Receptor (MOR)/δ-Opioid Receptor (DOR) Ligands. J. Med. Chem. 2016, 59, 4985–4998. 10.1021/acs.jmedchem.6b00308. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Rivara S.; Scalvini L.; Lodola A.; Mor M.; Caignard D.-H.; Delagrange P.; Collina S.; Lucini V.; Scaglione F.; Furiassi L.; Mari M.; Lucarini S.; Bedini A.; Spadoni G. Tetrahydroquinoline Ring as a Versatile Bioisostere of Tetralin for Melatonin Receptor Ligands. J. Med. Chem. 2018, 61, 3726–3737. 10.1021/acs.jmedchem.8b00359. [DOI] [PubMed] [Google Scholar]
- a Paquin J.-F.; Stephenson C. R. J.; Defieber C.; Carreira E. M. Catalytic Asymmetric Synthesis with Rh–Diene Complexes: 1,4-Addition of Arylboronic Acids to Unsaturated Esters. Org. Lett. 2005, 7, 3821–3824. 10.1021/ol051533l. [DOI] [PubMed] [Google Scholar]; b Itoh T.; Mase T.; Nishikata T.; Iyama T.; Tachikawa H.; Kobayashi Y.; Yamamoto Y.; Miyaura N. 1,4-Addition of Arylboronic Acids to β-Aryl-α,β-Unsaturated Ketones and Esters Catalyzed by a Rhodium(I)–Chiraphos Complex for Catalytic and Enantioselective Synthesis of Selective Endothelin A Receptor Antagonists. Tetrahedron 2006, 62, 9610–9621. 10.1016/j.tet.2006.07.075. [DOI] [Google Scholar]; c Sörgel S.; Tokunaga N.; Sasaki K.; Okamoto K.; Hayashi T. Rhodium/Chiral Diene-Catalyzed Asymmetric 1,4-Addition of Arylboronic Acids to Arylmethylene Cyanoacetates. Org. Lett. 2008, 10, 589–592. 10.1021/ol702879u. [DOI] [PubMed] [Google Scholar]; d Yasukawa T.; Suzuki A.; Miyamura H.; Nishino K.; Kobayashi S. Chiral Metal Nanoparticle Systems as Heterogeneous Catalysts beyond Homogeneous Metal Complex Catalysts for Asymmetric Addition of Arylboronic Acids to α,β-Unsaturated Carbonyl Compounds. J. Am. Chem. Soc. 2015, 137, 6616–6623. 10.1021/jacs.5b02213. [DOI] [PubMed] [Google Scholar]; e Zullo V.; Iuliano A. Rh-Catalyzed Asymmetric Conjugate Addition of Arylboronic Acids to 3-Arylpropenoates: Enantioselective Synthesis of (R)-Tolterodine. Eur. J. Org. Chem. 2019, 2019, 1377–1384. 10.1002/ejoc.201801690. [DOI] [Google Scholar]
- a Tokunaga N.; Hayashi T. Highly Enantioselective 1,4-Addition of Arylzinc Reagents to 3-Arylpropenals Catalyzed by a Rhodium–Binap Complex in the Presence of Chlorotrimethylsilane. Tetrahedron: Asymmetry 2006, 17, 607–613. 10.1016/j.tetasy.2006.01.036. [DOI] [Google Scholar]; b Monti C.; Gennari C.; Piarulli U. Rh-Catalyzed Enantioselective Conjugate Addition of Arylboronic Acids with a Dynamic Library of Chiral Tropos Phosphorus Ligands. Chem.—Eur. J. 2007, 13, 1547–1558. 10.1002/chem.200600960. [DOI] [PubMed] [Google Scholar]; c Hu S.-B.; Chen Z.-P.; Zhou J.; Zhou Y.-G. Electronically Deficient (Rax,S, S)-F12-C3-TunePhos and Its Applications in Asymmetric 1,4-Addition Reactions. Tetrahedron Lett. 2016, 57, 1925–1929. 10.1016/j.tetlet.2016.03.072. [DOI] [Google Scholar]; d Yao J.; Liu N.; Yin L.; Xing J.; Lu T.; Dou X. Catalytic Asymmetric Synthesis of Chiral Phenols in Ethanol with Recyclable Rhodium Catalyst. Green Chem. 2019, 21, 4946–4950. 10.1039/C9GC02420D. [DOI] [Google Scholar]; e Casotti G.; Rositano V.; Iuliano A. Enantioselective Conjugate Addition of Stabilized Arylzinc Iodide to Enones: An Improved Protocol of the Hayashi Reaction. Adv. Synth Catal. 2021, 363, 1126–1131. 10.1002/adsc.202001141. [DOI] [Google Scholar]
- a Wang X.; Guram A.; Caille S.; Hu J.; Preston J. P.; Ronk M.; Walker S. Highly Enantioselective Hydrogenation of Styrenes Directed by 2′-Hydroxyl Groups. Org. Lett. 2011, 13, 1881–1883. 10.1021/ol200422p. [DOI] [PubMed] [Google Scholar]; b Li Y.; Dong K.; Wang Z.; Ding K. Rhodium(I)-Catalyzed Enantioselective Hydrogenation of Substituted Acrylic Acids with Sterically Similar β, β -Diaryls. Angew. Chem., Int. Ed. 2013, 52, 6748–6752. 10.1002/anie.201302349. [DOI] [PubMed] [Google Scholar]; c Yan Q.; Kong D.; Li M.; Hou G.; Zi G. Highly Efficient Rh-Catalyzed Asymmetric Hydrogenation of α,β-Unsaturated Nitriles. J. Am. Chem. Soc. 2015, 137, 10177–10181. 10.1021/jacs.5b06418. [DOI] [PubMed] [Google Scholar]; d Wu Z.; Laffoon S. D.; Hull K. L. Asymmetric Synthesis of γ-Branched Amines via Rhodium-Catalyzed Reductive Amination. Nat. Commun. 2018, 9, 1185. 10.1038/s41467-018-03535-y. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Zhang J.; Chen T.; Wang Y.; Zhou F.; Zhang Z.; Gridnev I. D.; Zhang W. Asymmetric Hydrogenation of γ -branched Allylamines for the Efficient Synthesis of γ-chirogenic Amines. Nat. Sci. 2021, 1, e10021 10.1002/ntls.10021. [DOI] [Google Scholar]
- a Tolstoy P.; Engman M.; Paptchikhine A.; Bergquist J.; Church T. L.; Leung A. W.-M.; Andersson P. G. Iridium-Catalyzed Asymmetric Hydrogenation Yielding Chiral Diarylmethines with Weakly Coordinating or Noncoordinating Substituents. J. Am. Chem. Soc. 2009, 131, 8855–8860. 10.1021/ja9013375. [DOI] [PubMed] [Google Scholar]; b Li J.-Q.; Liu J.; Krajangsri S.; Chumnanvej N.; Singh T.; Andersson P. G. Asymmetric hydrogenation of allylic alcohols using Ir–N,P-complexes. ACS Catal. 2016, 6, 8342–8349. 10.1021/acscatal.6b02456. [DOI] [Google Scholar]
- Previous work by our group on allyl phthalimides:; Cabré A.; Romagnoli E.; Martínez-Balart P.; Verdaguer X.; Riera A. Highly Enan-tioselective Iridium-Catalyzed Hydrogena-tion of 2-Aryl Allyl Phthalimides. Org. Lett. 2019, 21, 9709–9713. 10.1021/acs.orglett.9b03865. [DOI] [PubMed] [Google Scholar]
- Meindl W. Antimykobakterielle Antihistaminika. Arch. Pharm. 1989, 322, 493–497. 10.1002/ardp.19893220808. [DOI] [PubMed] [Google Scholar]
- Limberger J.; Claudino T. S.; Monteiro A. L. Stereoselective Synthesis of (E)-3,3-Diaryl and (E)-3-Aryl-3-Aryloxy Allylamines and Allylalcohols from Trans-Cinnamyl Chloride and Alcohol. RSC Adv. 2014, 4, 45558–45565. 10.1039/C4RA08036J. [DOI] [Google Scholar]
- a Orgué S.; Flores-Gaspar A.; Biosca M.; Pàmies O.; Diéguez M.; Riera A.; Verdaguer X. Chem. Commun. 2015, 51, 17548–17551. 10.1039/C5CC07504A. [DOI] [PubMed] [Google Scholar]; b Salomó E.; Orgué S.; Riera A.; Verdaguer X. Angew. Chem., Int. Ed. 2016, 55, 7988–7992. 10.1002/anie.201602219. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Salomó E.; Rojo P.; Hernández-Lladó P.; Riera A.; Verdaguer X. P-Stereogenic and Non-P-Stereogenic Ir–MaxPHOX in the Asymmetric Hydrogenation of N -Aryl Imines. Isolation and X-Ray Analysis of Imine Iridacycles. J. Org. Chem. 2018, 83, 4618–4627. 10.1021/acs.joc.8b00361. [DOI] [PubMed] [Google Scholar]; d Cabré A.; Riera A.; Verdaguer X. P-Stereogenic Amino-Phosphines as Chiral Ligands: From Privileged Intermediates to Asymmetric Catalysis. Acc. Chem. Res. 2020, 53, 676–689. 10.1021/acs.accounts.9b00633. [DOI] [PubMed] [Google Scholar]; e Biosca M.; de la Cruz-Sánchez P.; Faiges J.; Margalef J.; Salomó E.; Riera A.; Verdaguer X.; Ferré J.; Maseras F.; Besora M.; Pàmies O.; Diéguez M. P-Stereogenic Ir-MaxPHOX: A Step toward Privileged Catalysts for Asymmetric Hydrogenation of Nonchelating Olefins. ACS Catal. 2023, 13, 3020–3035. 10.1021/acscatal.2c05579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo P.; Molinari M.; Cabré A.; García-Mateos C.; Riera A.; Verdaguer X. Iridium-Catalyzed Asymmetric Hydrogenation of 2,3-Diarylallyl Amines with a Threonine-Derived P-Stereogenic Ligand for the Synthesis of Tetrahydroquinolines and Tetrahydroisoquinolines. Angew. Chem., Int. Ed. 2022, 61, e202204300 10.1002/anie.202204300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaltz A.; Blankenstein J.; Hilgraf R.; Hörmann E.; McIntyre S.; Menges F.; Schönleber M.; Smidt S.; Wüstenberg B.; Zimmermann N. Iridium-Catalyzed Enantioselective Hydrogenation of Olefins. Adv. Synth. Catal. 2003, 345, 33–43. 10.1002/adsc.200390027. [DOI] [Google Scholar]
- We believe that the presence of 2 in the reaction mixture results in an oxidative addition reaction on iridium. This should lead to a new metal complex that efficiently catalyzes the isomerization but not hydrogenation
- a Källström K.; Hedberg C.; Brandt P.; Bayer A.; Andersson P. G. Rationally Designed Ligands for Asymmetric Iridium-Catalyzed Hydrogenation of Olefins. J. Am. Chem. Soc. 2004, 126, 14308–14309. 10.1021/ja0464241. [DOI] [PubMed] [Google Scholar]; b Church T. L.; Rasmussen T.; Andersson P. G. Enantioselectivity in the Iridium-Catalyzed Hydrogenation of Unfunctionalized Olefins. Organometallics 2010, 29, 6769–6781. 10.1021/om100899u. [DOI] [Google Scholar]
- Rueping M.; Theissmann T.; Stoeckel M.; Antonchick A. P. Direct Enantioselective Access to 4-Substituted Tetrahydroquinolines by Catalytic Asymmetric Transfer Hydrogenation of Quinolines. Org. Biomol. Chem. 2011, 9, 6844–6850. 10.1039/c1ob05870c. [DOI] [PubMed] [Google Scholar]
- a De Castro K. A.; Ko J.; Park D.; Park S.; Rhee H. Reduction of Ethyl Benzoylacetate and Selective Protection of 2-(3-Hydroxy-1-Phenylpropyl)-4-Methylphenol: A New and Facile Synthesis of Tolterodine. Org. Process Res. Dev. 2007, 11, 918–921. 10.1021/op7001134. [DOI] [Google Scholar]; b Dirat O.; Bibb A. J.; Burns C. M.; Checksfield G. D.; Dillon B. R.; Field S. E.; Fussell S. J.; Green S. P.; Mason C.; Mathew J.; Mathew S.; Moses I. B.; Nikiforov P. I.; Pettman A. J.; Susanne F. The Lactol Route to Fesoterodine: An Amine-Promoted Friedel–Crafts Alkylation on Commercial Scale. Org. Process Res. Dev. 2011, 15, 1010–1017. 10.1021/op200107g. [DOI] [Google Scholar]
- a Andersson P. G.; Schink H. E.; Österlund K. Asymmetric Total Synthesis of (+)-Tolterodine, a New Muscarinic Receptor Antagonist, via Copper-Assisted Asymmetric Conjugate Addition of Aryl Grignard Reagents to 3-Phenyl-Prop-2-Enoyl-Oxazolidinones. J. Org. Chem. 1998, 63, 8067–8070. 10.1021/jo981259r. [DOI] [Google Scholar]; b Zhi W.; Li J.; Zou D.; Wu Y.; Wu Y. Palladium-Catalyzed Diastereoselective Synthesis of β,β-Diarylpropionic Acid Derivatives and Its Application to the Total Synthesis of (R)-Tolterodine and the Enantiomer of a Key Intermediate for MK-8718. Tetrahedron Lett. 2018, 59, 537–540. 10.1016/j.tetlet.2017.12.082. [DOI] [Google Scholar]
- a Hedberg C.; Andersson P. Catalytic Asymmetric Total Synthesis of the Muscarinic Receptor Antagonist (R)-Tolterodine. Adv. Synth. Catal. 2005, 347, 662–666. 10.1002/adsc.200404234. [DOI] [Google Scholar]; b Chen G.; Tokunaga N.; Hayashi T. Rhodium-Catalyzed Asymmetric 1,4-Addition of Arylboronic Acids to Coumarins: Asymmetric Synthesis of (R)-Tolterodine. Org. Lett. 2005, 7, 2285–2288. 10.1021/ol0507367. [DOI] [PubMed] [Google Scholar]; c Jagdale A. R.; Sudalai A. Co-Catalyzed Mild and Chemoselective Reduction of Phenyl Esters with NaBH4: A Practical Synthesis of (R)-Tolterodine. Tetrahedron Lett. 2008, 49, 3790–3793. 10.1016/j.tetlet.2008.04.007. [DOI] [Google Scholar]; d Gallagher B. D.; Taft B. R.; Lipshutz B. H. Asymmetric Conjugate Reductions of Coumarins. A New Route to Tolterodine and Related Coumarin Derivatives. Org. Lett. 2009, 11, 5374–5377. 10.1021/ol9020404. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its online Supporting Information.