Abstract
Scedosporium apiospermum (S. apiospermum) can cause fungal infections in near-drowning victims, and an increasing number of cases have been reported. However, cases of bone and joint infections caused by S. apiospermum are rare. In this case, a 35-year-old otherwise healthy Chinese female presented with aspiration pneumonia and knee arthritis after accidentally falling into sewage and near-drowning and underwent macrogenomic second-generation sequencing of arthrocentesis fluid, which showed S. apiospermum. However, new lesions involving the hip joint and spine continued to develop under voriconazole monotherapy. The patient was treated with voriconazole combined with amphotericin B cholesterol sulfate lipid complex for 30 consecutive days. The patient’s symptoms improved significantly. This case highlights the robust invasiveness of S. apiospermum and the extensive spread of infection, underscoring the importance of prompt diagnosis and treatment. A combined therapeutic approach may offer a safe and efficacious option for managing S. apiospermum infection.
Keywords: near-drowning, Scedosporium apiospermum, voriconazole, amphotericin B cholesterol sulfate lipid complex, metagenomic next-generation sequencing
Introduction
Scedosporium apiospermum (S. apiospermum) is a saprophytic fungus, which is widely found in polluted environments such as sewage, decay and soil.1 As an opportunistic infectious pathogen, the severity of infection caused by S. apiospermum is closely associated with the host’s immune status. It often causes lethal infection in patients with immunodeficiency, especially those who have been using hormones for a long time, receiving solid organ or hematopoietic stem cell transplantation, or those with underlying lung disease.2 In contrast, in immunocompetent individuals, the occurrence of S. apiospermum is relatively uncommon and manifests as a localized infection, such as in the joints, skin, heart valves, and respiratory tract, often associated with local trauma, manipulation, and near-drowning.3,4
Different organ infections have different clinical manifestations, ranging from skin to various diffuse infections.2,5 Most clinical reports indicate that S. apiospermum causes lung and central nervous system infections in immunocompetent individuals.6–8 One case reported that a 13-year-old boy developed a central nervous system infection caused by S. apiospermum after near-drowning and eventually died due to delayed diagnosis.7 Shi et al9 reported a case of lumbar vertebrae infection attributed to S. apiospermum in an immunocompetent patient without a history of near-drowning. This patient achieved recovery through surgical removal of the infective focus intervention combined with voriconazole. However, reports of multi-system infections of S. apiospermum are comparatively scarce. This article reviews and analyzes a case of systemic S. apiospermum infection affecting multiple sites in an immunocompetent individual after near-drowning.
Case Presentation
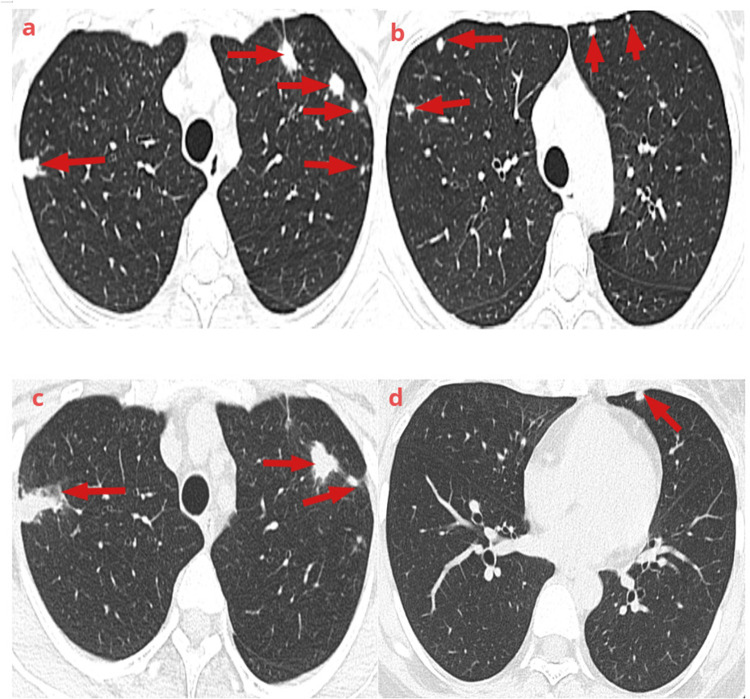
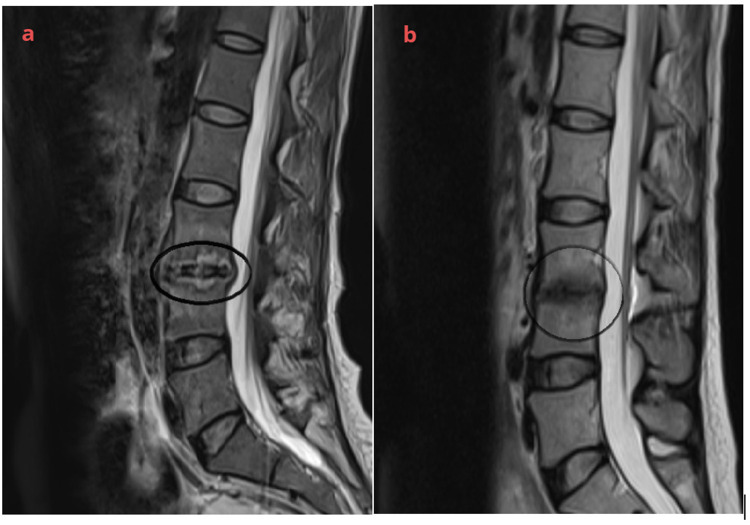
A 35-year-old healthy Chinese woman accidentally fell into sewage in October 2023 and choked on it. She was admitted to the hospital for relevant examinations and diagnosed with aspiration pneumonia (Streptococcus pneumoniae infection). After 5 days of anti-infective treatment, symptoms such as chest tightness and cough improved, but she gradually felt pain and discomfort in right knee joint. The examination revealed that the skin temperature of the right knee was normal, there was no local redness or swelling, the joint movement was limited, and the patellar slip test was positive. A right knee magnetic resonance imaging (MRI) examination showed joint effusion and drainage of pale yellow fluid. The joint fluid was examined using metagenomic next-generation sequencing technology (mNGS), which revealed the presence of S. apiospermum (number of sequences: 3). The patient was discharged from the hospital seven days after antifungal treatment with voriconazole. However, the patient presented with lower back and right hip pain with restricted movement of the right lower limb one week after discharge, which led to her admission to our hospital. The breath sounds were clear on the auscultation of both lungs, no pathological murmurs were heard on cardiac auscultation, and no abnormalities were found during abdominal physical examination. The physiological curvature of the spine was normal, lower back tenderness was positive, straight leg raise test of both lower limbs was negative, muscle strength of the right lower limb was weak (grade 2), and dorsal extensor and plantar flexor muscles of the foot were normal. Her white blood cell count was 7.7×109/L, C-reactive protein (CRP) level was 45.26 mg/L, erythrocyte sedimentation rate (ESR) level was 70 mm/h, and procalcitonin level was 0.03 ng/L. The patient’s blood and sputum cultures, (1,3) -β-D dextran test (G test), and galactomannan test (GM test) were all negative for fungi. Rheumatoid factor, thyroid function, tuberculosis-infected T cells, and liver and kidney functions were within normal reference ranges. Computed tomography (CT) scanning of her head showed no obvious abnormalities, and the chest CT showed nodules scattered in both lungs, suggesting inflammatory lesions (Figure 1a and b). Lumbar spine and hip enhanced MRI showed pathological changes in the L3 and L4 vertebrae, and L3 /L4 intervertebral disks, right hip, and surrounding soft tissue, and a small amount of fluid in the right hip joint cavity, indicating vertebral osteomyelitis, intervertebral disk inflammation, and right hip arthritis (Figure 2a). The patient refused to undergo a bronchoalveolar lavage fluid examination, and a small amount of fluid in the hip cavity could not undergo arthrocentesis. Based on the history of sewage near-drowning and S. apiospermum infection of the knee joint, infections of the lungs, lumbar spine, and right hip were caused by the spread of this fungus. The patient was diagnosed with multiple S. apiospermum infections. During hospitalization, voriconazole (0.2 g, iv, Q12h) combined with amphotericin B cholesterol sulfate complex (initial dose 150 mg, iv, QD, gradually increased to 300 mg, iv, QD) antifungal treatment was given for 30 consecutive days. The pain in the patient’s waist, right hip, and knee joints was gradually relieved. The patient continued maintenance therapy with voriconazole (0.2 g, oral, BID.) after discharge. Two months later, lumbar spine and hip MRI indicated that pathological changes and joint cavity effusion were significantly reduced (Figure 1c and d). The chest CT suggested that the nodular lesions were partially absorbed and reduced in size (Figure 2b). Currently, the patient is still being treated with voriconazole.
Figure 1.
Lung computed tomography findings: (a and b) Multiple nodules and patchy shadows were seen in both lungs (red arrow) (November 20, 2023). (c and d) The number of lesions was decreased in both lungs, changes in lungs during the recovery period (red arrow) (February 23, 2024).
Figure 2.
Lumbar spine magnetic resonance imaging: (a) pathological changes in L3 and L4 vertebral, and L3/L4 intervertebral disks, showing vertebral osteomyelitis and diskitis (black circle) (November 20, 2023). (b) Changes in lumbar vertebrae during the recovery period (black circle) (February 23, 2024).
Discussion
S. apiospermum belongs to the asexual form of Pseudallescheria boydii, which is widely distributed in various environments. It has been reported that the risk factors for S. apiospermum infection in immunocompetent patients are surgery or trauma.10 In this case, the patient had no chronic underlying diseases, and was an immunocompetent host. There was a history of sewage near-drowning and a possible history of exposure to S. apiospermum. This fungus can rapidly enter the lungs through the respiratory tract, where it grows and multiplies and then travels through the bloodstream to other organs and tissues, causing invasive pneumonia, vertebral osteomyelitis, diskitis, knee joint infections and hip joint infections. The mNGS of the joint cavity effusion was suggestive of S. apiospermum infection; therefore, the diagnosis was clear.
S. apiospermum can invade almost every tissue and organ of the human body, and its clinical characteristics vary. The most common site of infection is the lungs, and clinical symptoms usually include fever, cough, expectoration, hemoptysis, chest pain, and dyspnea.11 Imaging changes were similar to pulmonary aspergillosis, such as nodular lesions with or without cavities, focal infiltration, or bilateral diffuse infiltration.12 It is relatively rare to cause bone and joint infections, but once it is invaded, it can cause arthritis, discitis and osteomyelitis.13 However, the clinical symptoms and imaging findings are nonspecific, making early diagnosis difficult. Pathogen identification is a key step in diagnosis and treatment. The diagnosis of S. apiospermum infection mainly relies on histology, cytology, and fungal culture from infected site specimens. Conventional culture methods are time-consuming, extremely low sensitivity, and fungi are relatively rare. Moreover, the morphology of S. apiospermum is variable,14 and laboratory personnel lack awareness of it, which can easily lead to missed diagnoses, making diagnosis very difficult. The G and GM tests have low sensitivity and specificity for diagnosing S. apiospermum infections and are not recommended as diagnostic criteria.4,15 It has been reported that the median time to diagnosis of S. apiospermum infection was 28 days,16 but this seriously delays the optimal time for antifungal treatment. In the present case, the patient developed aspiration pneumonia. Routine sputum culture revealed only a Streptococcus pneumoniae. The inflammatory marker ESR and CRP levels were only slightly elevated. The G and GM test results were negative. S. apiospermum was detected in the joint effusion using mNGS. This does not exclude lung lesions combined with S. apiospermum infection, which was treated in a timely manner and resulted in a better prognosis, suggesting that mNGS can be used as one of the methods for early diagnosis of S. apiospermum infection, and there have been several case reports of S. apiospermum infections diagnosed with the help of mNGS.17–19 As a new method for detecting pathogenic microorganisms, mNGS can quickly identify pathogens and provide valuable diagnosis, thereby avoiding delays in treatment.
Invasive fungal infections caused by S. apiospermum are characterized by strong invasiveness, wide infection sites, resistance to multiple antifungal drugs, and a poor prognosis. Early treatment improves prognosis. Treatment of S. apiospermum infection is characterized by a long course of treatment, pan-drug resistance, and easy relapse.14 Voriconazole is recommended as a first-line treatment drug.20 Yan et al21 reported a case involving an elderly male patient with normal immune function who developed multiple infections in the lungs, brain, and thoracic spine due to S. apiospermum after near-drowning, and the symptoms were alleviated after voriconazole treatment. S. apiospermum is also sensitive to posaconazole, itraconazole, and amphotericin B.22,23 However, despite sufficient treatment with voriconazole, new lesions or abscesses may appear, such as brain abscesses, ocular and corneal infections, vertebral inflammation and arthritis.24 Thus, two or even three antifungal drugs can be used in combination when necessary depending on the location and severity of the infection. Previous case reports have shown good therapeutic effects using the combined treatment of voriconazole and terbinafine, and long-term consolidation therapy with voriconazole was used during the recovery period.25,26 In vitro studies showed that micafungin combined with voriconazole had a significant therapeutic effect on S. apiospermum.11,27 It has also been reported that voriconazole combined with liposomal amphotericin B has achieved good results.13 Nakamura et al28 also reported that amphotericin B had a relatively low inhibitory concentration against S. apiospermum, and when combined with voriconazole successfully treated lung and brain abscesses. In vitro confirmed that amphotericin B plus azoles enhanced antifungal drug activity.29 In this case, new lesions continued to develop despite antifungal therapy with voriconazole monotherapy; therefore, amphotericin B cholesterol sulfate was combined and achieved good results. However, studies have also shown that S. apiospermum isolates have variable susceptibility to amphotericin B,16,25,30 so the efficacy of amphotericin B needs further study. In addition to combined antifungal treatment, surgical intervention also has significant effects. There are case reports of surgical debridement of the central nervous system, respiratory system, bone marrow and other tissues combined with voriconazole to achieve good results.17,25 Han et al17 reported a case of pulmonary S. apiospermum infection, which was improved by voriconazole combined with a thoracoscopic right upper lobectomy. In addition, studies have shown that antifungal drugs combined with immune adjuvants (such as recombinant gamma interferon and granulocyte colony-stimulating factor) have satisfactory efficacy in the treatment of disseminated infections caused by S. apiospermum.31,32 Currently, the majority of research concerning S. apiospermum infections following near-drowning is comprised of case reports. There still needed a large number of prospective studies into various aspects such as the treatment duration, side effects of antifungal medications, drug resistance, and optimal timing for drug withdrawal. The current treatment options are mostly based on case reports and lack evidence-based medical evidence. There is currently no standardized treatment regimen for combination treatment, and whether combination therapy can be used as the first-choice treatment for disseminated infections remains to be verified. In this case, the patient was still receiving voriconazole with no significant side effects and underwent regular follow-up examinations. In the later stages, the right time to discontinue medication was chosen according to the degree of recovery from the disease.
Conclusion
In conclusion, severe invasive fungal infections can occur in near-drowning victims. The invasive force of S. apiospermum is strong, and the infection site is wide; therefore, it is necessary to diagnose and treat it as soon as possible. Combination treatment with voriconazole and amphotericin B cholesterol sulfate lipid complex may be a safe and effective treatment for systemic S. apiospermum infections. Although the diagnosis of scedosporiosis may be difficult, a fast and correct etiological diagnosis could improve the patient’s chance of recovery in any case.
Acknowledgments
The authors thank the patient for her cooperation in the diagnostic process.
Funding Statement
There is no funding to report.
Ethics Approval and Informed Consent
This study was approved by the Ethics Committee of Yijishan Hospital of Wannan Medical College. Written informed consent for the publication of images and other clinical information were obtained from the patient. We confirm that institutional approval was not required to publish the case details, as all patient information has been anonymized and there are no identifying details included in the manuscript. The authors confirm that the study complied with the Declaration of Helsinki.
Disclosure
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflict of interest.
References
- 1.Masukane S, Kitahara Y, Okumoto J, et al. The effective treatment of lung infection due to Scedosporium prolificans with voriconazole and surgery. Intern Med. 2017;56(8):973–977. doi: 10.2169/internalmedicine.56.7447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodriguez-Tudela JL, Berenguer J, Guarro J, et al. Epidemiology and outcome of Scedosporium prolificans infection, a review of 162 cases. Med Mycol. 2009;47(4):359–370. doi: 10.1080/13693780802524506 [DOI] [PubMed] [Google Scholar]
- 3.Ramirez-Garcia A, Pellon A, Rementeria A, et al. Scedosporium and Lomentospora: an updated overview of underrated opportunists. Med Mycol. 2018;56(suppl_1):102–125. doi: 10.1093/mmy/myx113 [DOI] [PubMed] [Google Scholar]
- 4.Rougeron A, Giraud S, Alastruey-Izquierdo A, et al. Ecology of Scedosporium species: present knowledge and future research. Mycopathologia. 2018;183(1):185–200. doi: 10.1007/s11046-017-0200-2 [DOI] [PubMed] [Google Scholar]
- 5.Yao Y, Xu Q, Liang W, et al. Multi-organ involvement caused by Scedosporium apiospermum infection after near drowning: a case report and literature review. BMC Neurol. 2024;24(1):124. doi: 10.1186/s12883-024-03637-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tortorano AM, Richardson M, Roilides E, et al. ESCMID and ECMM joint guidelines on diagnosis and management of hyalohyphomycosis: Fusarium spp. Scedosporium spp. and others. Clin Microbiol Infect. 2014;20(Suppl 3):27–46. doi: 10.1111/1469-0691.12465 [DOI] [PubMed] [Google Scholar]
- 7.Lee MG, Choi JG, Son BC. Scedosporium apiospermum: an emerging fatal cause of fungal abscess and ventriculitis after near-drowning. Asian J Neurosurg. 2018;13(3):792–796. doi: 10.4103/ajns.AJNS_236_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakadate T, Nakamura Y, Yamauchii K, et al. Two cases of severe pneumonia after the 2011 Great East Japan Earthquake. West Pac Surveill Response J. 2012;3(4):67–70. doi: 10.5365/WPSAR.2012.3.2.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi XW, Li ST, Lou JP, et al. Scedosporium apiospermum infection of the lumbar vertebrae: a case report. World J Clin Cases. 2022;10(10):3251–3260. doi: 10.12998/wjcc.v10.i10.3251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castiglioni B, Sutton DA, Rinaldi MG, et al. Pseudallescheria boydii (anamorph Scedosporium apiospermum). Infection in solid organ transplant recipients in a tertiary medical center and review of the literature. Medicine. 2002;81(5):333–348. doi: 10.1097/00005792-200209000-00001 [DOI] [PubMed] [Google Scholar]
- 11.Lackner M, de Hoog GS, Verweij PE, et al. Species-specific antifungal susceptibility patterns of Scedosporium and Pseudallescheria species. Antimicrob Agents Chemother. 2012;56(5):2635–2642. doi: 10.1128/AAC.05910-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cortez KJ, Roilides E, Quiroz-Telles F, et al. Infections caused by Scedosporium spp. Clin Microbiol Rev. 2008;21(1):157–197. doi: 10.1128/CMR.00039-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He XH, Wu JY, Wu CJ, et al. Scedosporium apiospermum infection after near-drowning. Chin Med J. 2015;128(15):2119–2123. doi: 10.4103/0366-6999.161401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ference EH, Kubak BM, Zhang P, et al. Successful treatment of Scedosporium Sinusitis in two lung transplant recipients: review of the literature and recommendations for management. Allergy Rhinol. 2019;10:2152656719827253. doi: 10.1177/2152656719827253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donnelly JP, Chen SC, Kauffman CA, et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis. 2020;71(6):1367–1376. doi: 10.1093/cid/ciz1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katragkou A, Dotis J, Kotsiou M, et al. Scedosporium apiospermum infection after near-drowning. Mycoses. 2007;50(5):412–421. doi: 10.1111/j.1439-0507.2007.01388.x [DOI] [PubMed] [Google Scholar]
- 17.Han J, Liang L, Li Q, et al. Diagnosis of pulmonary Scedosporium apiospermum infection from bronchoalveolar lavage fluid by metagenomic next-generation sequencing in an immunocompetent female patient with normal lung structure: a case report and literature review. BMC Infect Dis. 2024;24(1):308. doi: 10.1186/s12879-024-09140-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang Y, Xiong X, Zhang C, et al. Scedosporium apiospermum invasive rhinosinusitis in an elderly patient: diagnosis and treatment. Heliyon. 2022;8(12):e12476. doi: 10.1016/j.heliyon.2022.e12476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao W, Han P, Xu Z, et al. Pulmonary scedosporiosis in a patient with acute hematopoietic failure: diagnosis aided by next-generation sequencing. Int J Infect Dis. 2019;85:114–116. doi: 10.1016/j.ijid.2019.05.033 [DOI] [PubMed] [Google Scholar]
- 20.Chen X, Lei W, Cai J, et al. Genotypic diversity and antifungal susceptibility of Scedosporium species from clinical settings in China. Mycoses. 2022;65(12):1159–1169. doi: 10.1111/myc.13507 [DOI] [PubMed] [Google Scholar]
- 21.Yan P, Chen J, Wang H, et al. A systemic infection involved in lung, brain and spine caused by Scedosporium apiospermum species complex after near-drowning: a case report and literature review. BMC Infect Dis. 2024;24(1):342. doi: 10.1186/s12879-023-08279-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oliveira Fde M, Unis G, Hochhegger B, et al. Scedosporium apiospermum eumycetoma successfully treated with oral voriconazole: report of a case and review of the Brazilian reports on scedosporiosis. Rev Inst Med Trop Sao Paulo. 2013;55:121–123. doi: 10.1590/s0036-46652013000200010 [DOI] [PubMed] [Google Scholar]
- 23.Wang H, Wan Z, Li R, et al. Molecular identification and susceptibility of clinically relevant Scedosporium spp. in China. Biomed Res Int. 2015;2015:109656. doi: 10.1155/2015/109656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lackner M, Hagen F, Meis JF, et al. Susceptibility and diversity in the therapy-refractory genus scedosporium. Antimicrob Agents Chemother. 2014;58(10):5877–5885. doi: 10.1128/AAC.03211-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Troke P, Aguirrebengoa K, Arteaga C, et al. Treatment of scedosporiosis with voriconazole: clinical experience with 107 patients. Antimicrob Agents Chemother. 2008;52(5):1743–1750. doi: 10.1128/AAC.01388-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henao-Martínez AF, Castillo-Mancilla JR, Barron MA, et al. Combination antifungal therapy in the treatment of Scedosporium apiospermum central nervous system infections. Case Rep Infect Dis. 2013;2013:589490. doi: 10.1155/2013/589490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heyn K, Tredup A, Salvenmoser S, et al. Effect of voriconazole combined with micafungin against Candida, Aspergillus, and Scedosporium spp. and Fusarium solani. Antimicrob Agents Chemother. 2005;49(12):5157–5159. doi: 10.1128/AAC.49.12.5157-5159.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamura Y, Utsumi Y, Suzuki N, et al. Multiple Scedosporium apiospermum abscesses in a woman survivor of a tsunami in northeastern Japan: a case report. J Med Case Rep. 2011;5:526. doi: 10.1186/1752-1947-5-526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodríguez MM, Calvo E, Serena C, et al. Effects of double and triple combinations of antifungal drugs in a murine model of disseminated infection by Scedosporium prolificans. Antimicrob Agents Chemother. 2009;53(5):2153–2155. doi: 10.1128/AAC.01477-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cuenca-Estrella M, Gomez-Lopez A, Mellado E, et al. Head-to-head comparison of the activities of currently available antifungal agents against 3,378 Spanish clinical isolates of yeasts and filamentous fungi. Antimicrob Agents Chemother. 2006;50:917–921. doi: 10.1128/AAC.50.3.917-921.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luna-Rodríguez CE, González GM, Flores-Maldonado OE, et al. Early production of proinflammatory cytokines in response to Scedosporium apiospermum during murine pulmonary infection. Microb Pathog. 2022;170:105718. doi: 10.1016/j.micpath.2022.105718 [DOI] [PubMed] [Google Scholar]
- 32.Muñoz P, Marín M, Tornero P, et al. Successful outcome of Scedosporium apiospermum disseminated infection treated with voriconazole in a patient receiving corticosteroid therapy. Clin Infect Dis. 2000;31(6):1499–1501. doi: 10.1086/317496 [DOI] [PubMed] [Google Scholar]