Abstract
Introduction
Growth hormone (GH) is crucial for immune system development and regulation, potentially benefiting COVID-19 outcomes. However, there are limited studies on the role of GH treatment in COVID-19 in children with short stature.
Methods
We conducted a survey study to evaluate the association between GH treatment and COVID-19 risk in short stature children aged 7 to 18 years. Two groups were defined: GH Treated and GH Untreated. The primary endpoint was the proportion of children with COVID-19 histories. Secondary endpoints included the presence, severity, and duration of COVID-19 symptoms. Exploratory endpoints included the frequency of common colds after GH treatment. We further performed two-sample Mendelian randomization (MR) analyses to explore the causal relationship between GH levels and COVID-19 susceptibility, hospitalization, and severity using genome-wide association study summary-level data.
Results
Of the 201 children, 113 (56.2%) reported COVID-19 history, and 149 (74.1%) used GH. The mean age was 11.02 ± 2.10 years. GH treatment was associated with a somewhat lower proportion of COVID-19 history (−9.77%, 95% confidence interval [CI] −26.41% to 6.87%; P = 0.289), and the odds ratio (OR) is 0.58 (95% CI 0.29 to 1.14, P = 0.120) after adjusting for confounders. Among the 113 children with COVID-19 histories, the highest body temperature was significantly lower in the GH Treated group (P = 0.040). In the MR analyses, for one unit increase in GH level, the OR was 0.95 (95% CI 0.92 to 0.99, P = 0.022) for COVID-19 susceptibility, 0.86 (95% CI 0.77 to 0.96, P = 0.007) for COVID-19 hospitalization, and 0.95 (95% CI 0.84 to 1.07, P = 0.392) for COVID-19 severity.
Conclusion
GH treatment was associated with somewhat decreased COVID-19 susceptibility but was not statistically significant. Higher GH levels were causally associated with a significantly lower rate of COVID-19 susceptibility and hospitalization.
Keywords: Mendelian randomization analysis, growth hormone treatment, COVID-19, children with short stature
Background
The coronavirus disease 2019 (COVID-19) pandemic is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), with more than 765 million confirmed cases, including more than 6.9 million deaths by May 2023.1 Both COVID-19 susceptibility and severity have been reported to be age-dependent, with the 5 to 17 age group having fewer cases, hospitalizations, and deaths compared to adults and the elderly.2 Younger children, schoolchildren, and adolescents usually have fewer and milder symptoms and are less likely to experience severe COVID-19 than adults.3
Children generally have higher levels of growth hormone (GH) than adults. Normally, daily GH secretion peaks at puberty and gradually declines, with a rate of approximately 15% each decade.4 The variation in COVID-19 susceptibility and severity across age groups led us to hypothesize that this may be related to GH levels; specifically, adolescent populations with higher levels of GH may be associated with lower susceptibility to and severity of COVID-19. Previous studies have shown that there were interactions between GH levels and the immune system functions, which are complex and bidirectional.5 GH has been demonstrated to exert potential protective immunomodulatory effects in in vitro and animal studies; however, it is important to note that in humans, even moderate to severe growth hormone deficiency (GHD) has not typically been associated with significant immune dysfunction.6 Cytokine release has been shown to be affected by GH treatment in both short prepubertal non-GHD children7 and GHD children,8 suggesting a direct effect of GH on the immune system. GH levels have been linked to COVID-19 in a few studies. GH has been reported to alleviate inflammation in a BEAS-2B COVID-19-like proinflammatory cell model.9 The impairment of the growth hormone–insulin-like growth factor 1 (IGF-1) axis may contribute to the pathogenesis and prognosis of COVID-19, particularly in elderly male patients with obesity, who may benefit from recombinant GH treatment.10 By comparing the IGF-1 and GH levels between critically and non-critically ill COVID-19 patients, a study in adult COVID-19 patients suggested that there may be an association between low IGF-1 (and possibly GH) and poor outcome.11,12 Another study compared COVID-19 patients with and without lung involvement and found that COVID-19 progressed worse with GH and IGF-1 deficiency, regardless of age and gender.13–15
GH is an important driver of somatic growth and height increase in children. Common pediatric indications for GH treatment include GHD, Turner syndrome, Prader-Willi syndrome, small for gestational age, chronic renal insufficiency, and idiopathic short stature (ISS). It is hypothesized that short stature children might exhibit a higher COVID-19 risk and that GH treatment may have a protective role against the risk. However, few studies have been conducted to examine the role of GH treatment in COVID-19 in short stature children. In a recent study in Israel,16 the authors investigated the association between GH treatment in children with GH issues and COVID-19 susceptibility and showed the protective role of GH treatment. The study was conducted before the vaccination campaign and did not address the effects of vaccines. Besides, it did not assess COVID-19 symptoms. We conducted a case-control survey study to evaluate the association between GH treatment and COVID-19 risk in short stature children, including susceptibility and severity.
Mendelian randomization (MR) analysis is an epidemiological approach that strengthens causal inference by using genetic variants as instruments to mimic the biological effects of related biomarkers. MR provides a more robust understanding of the influence of exposures on outcomes because germline genetic variants are randomly inherited from parents to offspring. This random inheritance reduces the likelihood that these variants are associated with potential confounding factors that can distort exposure–outcome associations. Consequently, genetic variants can serve as instrumental variables, effectively linking the proposed exposure to the outcome. This approach allows for the estimation of causal effects with reduced confounding and bias compared to conventional epidemiological methods, thereby enhancing the reliability of findings and offering clearer insights into the underlying causal relationship. To further evaluate the causal relationship of the association results, we conducted a two-sample MR study to further explore the causal associations between GH levels and COVID-19 risk (susceptibility, hospitalization, and severity) using genome-wide association study (GWAS) summary level data from the COVID-19 Host Genetics Initiative (COVID-19 HGI).
Methods
Study Design
The main study is a single-centre, case-control survey study conducted in Shanghai, China. The study period was from 20 January 2023 to 20 March 2023, right after the first peak of the COVID-19 outbreak in Shanghai. During the study period, all school-age children (7 to 18 years old) with short stature, defined as height SDS (HT-SDS) of two standard deviations (SD) below the average height of normal children of the same age and gender based on the Chinese general population at the time of screening, were included in the study. Upon a child’s hospital visit for short stature, the parents were invited to fill out an electronic questionnaire in the outpatient room. Thirty-two questions about the child were included in the questionnaire, covering demographic information, medical conditions of short stature, use and efficacy of GH, COVID-19 vaccination, history, and symptoms. COVID-19 history was required to be supported by a positive SARS-CoV-2 test or a physician’s diagnosis. Interviewers were trained to answer queries while parents filled out the form. Multiple participations were not allowed.
Analysis Sets
In total, 217 questionnaires were collected. The primary endpoint is COVID-19 susceptibility. The aim of the primary analysis was to determine whether GH treatment is associated with COVID-19 susceptibility, and the analysis set included 201 children with clear answers about COVID-19 history (yes or no). The secondary endpoint is the COVID-19 severity, including the incidence, duration, and severity of symptoms (fever, cough, and sore throat). The aim of the secondary analysis was to determine whether GH treatment is associated with COVID-19 severity in children with COVID-19 history, and the analysis set included 113 children. The exploratory endpoint is the frequency of common colds. The aim of the exploratory analysis was to determine whether GH treatment is associated with fewer common colds in children on GH treatment, and the analysis set included 159 children. For the primary and secondary analyses, two groups were defined: the GH Treated group, comprised of children with at least one injection of GH, and the GH Untreated group, comprised of children with no prior GH injection. For the exploratory analysis, only one group of GH Treated children was included.
Statistical Analysis
Variables included in the study were all based on self-reported data. Missing data was rare. Only the vaccination question contains two missing values. Complete case analyses were conducted for the subgroup analysis of vaccination. For the primary analysis, two-proportion Z-test was used to compare the proportion of children with COVID-19 history between the GH Treated and GH Untreated groups. Multivariate logistic regression was conducted to adjust for confounders including age, sex, and vaccination. For the secondary analysis, two-proportion Z-test was used to compare the proportion of children reporting each symptom between the GH Treated and GH Untreated groups. Mann–Whitney U-test was conducted to compare the severity of symptoms. Multivariate ordinal logistic regression was conducted to adjust for confounders including age, sex, and vaccination.
Statistical analyses were performed using R (version 4.1.2). Categorical data were expressed as rates. Continuous variables with normal distributions were expressed as mean and SD. Student’s t-test and Fischer’s exact χ2 test were used as appropriate for continuous and categorical variables, respectively, to compare the normal distribution and variable characteristics. All tests were two-sided, with significance set at 0.05.
Mendelian Randomization
The study also incorporates MR analysis to explore the potential causal relationship. Two-sample MR analysis was conducted using the R package “TwoSampleMR”.17
The exposure was GH levels. In a whole genome sequencing study of 1329 individuals of European ancestry, the authors assessed the genetic architecture of 257 circulating protein biomarkers of cardiometabolic relevance, including GH.18 In the study, protein levels were determined using Olink’s proximity extension assay technology. Associated single-nucleotide polymorphisms (SNPs) were identified. Instruments were extracted at a significance threshold of 10−5. Linkage disequilibrium (LD) clumping was performed at a threshold of 10−5 with an r2 of 0.001 within 10,000 kb to return only independent significant associations. Fourteen SNPs were used as instrumental variables for GH.
To be used as outcomes, the summary level data of COVID-19 susceptibility, hospitalization, and severity from the COVID-19-HGI GWAS19 meta-analyses of data across 60 studies from 25 countries (Release 5, European ancestry) were obtained. The HGI dataset included 1,348,701 participants (32,494 laboratory-confirmed cases of SARS CoV-2 infection and 1,316,207 population controls) for COVID-19 susceptibility, 16,645 participants (4829 hospitalized and 11,816 not hospitalized) for COVID-19 hospitalization, and 1,059,456 participants (4792 very severe respiratory-confirmed cases and 1,054,664 population controls) for COVID-19 severity. Very severe respiratory-confirmed COVID-19 cases were defined as patients hospitalized for laboratory-confirmed SARS-CoV-2 infection who died or were given respiratory support.
For each outcome, MR was performed with the following steps. First, instruments from the exposure dataset were extracted from the outcome dataset, with the minimum LD r2 set to 0.8 and aligning tag alleles to target alleles enabled. Palindromic SNPs were allowed, with a minor allele frequency threshold for inference of 0.3. Second, the effects were harmonized, and duplicate summary sets were pruned on the basis of sample size. Third, we conducted the MR analysis. Random-effects inverse variance weighted (IVW) MR was used as the primary MR method, and MR Egger, weighted median, simple mode, and weighted mode were conducted as sensitivity analyses. The IVW estimates were calculated as the average of instrument ratio coefficients weighted by the inverse variance. For IVW and MR Egger, we also tested the heterogeneity across variant-level MR estimates using the Cochrane Q method. For MR Egger, we also tested the horizontal pleiotropy.
One SNP (rs116286137) was excluded from the MR analysis (for details, see Supplementary Figures 1-3), resulting in 9, 7, and 10 SNPs in the MR analysis for COVID-19 susceptibility, hospitalization, and severity, respectively.
Ethical Considerations
This is a blind survey which was fully anonymous; no personally identifiable information was collected. Prior to the initiation of the study, the research protocol was reviewed and approved by the Institutional Review Board (IRB) at Xinhua Hospital, Shanghai Jiao Tong University (Approval number: XHEC-C-2023-118-1).
Reporting
The survey analysis part was reported in accordance with the CROSS guideline.20 The MR analysis part was reported in accordance with the STROBE-MR guideline.21
Results
Survey Study
The study population included 201 short stature children aged 7 to 18 years with clear answers about COVID-19 history. Of the population, 113 (56.2%) reported COVID-19 history, and 149 (74.1%) used GH. The types of GH used were reported for 148 participants, including 20 (13.5%) who used powdered formulations, 111 (75.0%) who used aqueous solutions, and 17 (11.5%) who used polyethylene glycol recombinant long-acting preparation. The mean age was 11.0 ± 2.1 years, and 100 (49.8%) were male. The baseline characteristics of the study population are shown in Table 1, which were similar between the GH Treated and the GH Untreated groups.
Table 1.
Baseline Characteristics of the Study Population
| Overall | GH Treated | GH Untreated | P-value | |
|---|---|---|---|---|
| N | 201 | 149 | 52 | |
| Age, years | 11.0±2.1 | 11.2±2.0 | 10.5±2.3 | 0.051 |
| Age group, N (%) | 0.217 | |||
| 7 to 12 years | 139 (69.2) | 99 (66.4) | 40 (76.9) | |
| 13 to 18 years | 62 (30.8) | 50 (33.6) | 12 (23.1) | |
| Gender, N (%) | 0.600 | |||
| Male | 100 (49.8) | 72 (48.3) | 28 (53.8) | |
| Female | 101 (50.2) | 77 (51.7) | 24 (46.2) | |
| Height, cm | 142.7±15.4 | 143.4±16.1 | 140.8±13.0 | 0.282 |
| Weight, kg | 38.4±13.1 | 39.0±13.4 | 36.6±12.4 | 0.270 |
| Short stature type, N (%) | ||||
| GHD | 86 (42.8) | 86 (57.7) | 0 (0.0) | <0.001 |
| ISS | 24 (11.9) | 24 (16.1) | 0 (0.0) | |
| Others | 91 (45.3) | 39 (26.2) | 52 (100.0) | |
| Vaccination, N (%) | 0.256 | |||
| < 2 | 56 (28.1) | 38 (25.7) | 18 (35.3) | |
| 2+ | 143 (71.9) | 110 (74.3) | 33 (64.7) |
Note: Continuous variables were expressed with the mean and standard deviation.
Abbreviations: GH, growth hormone; GHD, growth hormone deficiency; ISS, idiopathic short stature.
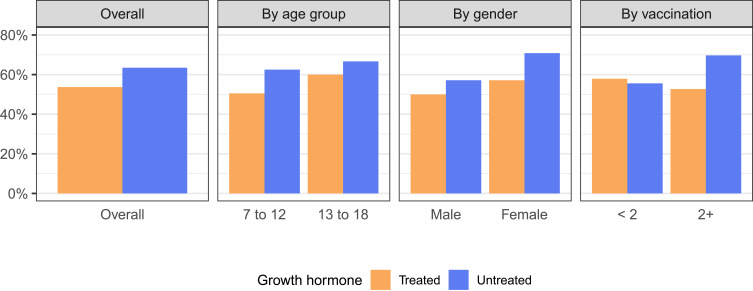
The proportions of children with COVID-19 history in GH Treated and GH Untreated groups were compared in the overall population and subgroups (Figure 1 and Table 2) using the primary analysis set. GH treatment was associated with a somewhat lower proportion of COVID-19 history (−9.77%, 95% confidence interval [CI] −26.41% to 6.87%). However, the difference was not statistically significant (P = 0.289). After adjusting for confounders using multivariate logistic regression, the odds ratio (OR) for GH treatment is 0.58 (95% CI 0.29 to 1.14, P = 0.120), which shows a protective association against COVID-19 but is not statistically significant. This finding is consistent across the subgroups, especially in children aged 7 to 12 years, females, and children with 2 or more vaccinations, where the differences in proportions were larger than 10%. In our post-hoc power calculation, in the overall population, at an alpha level of 0.05, the statistical power is only 19.8%, which implicates a lack of power and an insufficient sample size for the current test.
Figure 1.
Proportions of children with COVID-19 history in different growth hormone treatment groups in the overall population and subgroups.
Table 2.
Proportions of Children with COVID-19 history
| N | GH Treated | GH Untreated | Difference (95% CI) | P-value | ||
|---|---|---|---|---|---|---|
| Overall | 201 | 80 / 149 (53.7%) | 33 / 52 (63.5%) | −9.77% (−26.41%, 6.87%) | 0.289 | |
| Age group | 7 to 12 years | 139 | 50 / 99 (50.5%) | 25 / 40 (62.5%) | −11.99% (−31.70%, 7.71%) | 0.273 |
| 13 to 18 years | 62 | 30 / 50 (60.0%) | 8 / 12 (66.7%) | −6.67% (−41.76%, 28.43%) | 0.924 | |
| Gender | Male | 100 | 36 / 72 (50.0%) | 16 / 28 (57.1%) | −7.14% (−31.29%, 17.00%) | 0.675 |
| Female | 101 | 44 / 77 (57.1%) | 17 / 24 (70.8%) | −13.69% (−37.70%, 10.32%) | 0.338 | |
| Vaccination | < 2 | 56 | 22 / 38 (57.9%) | 10 / 18 (55.6%) | 2.34% (−27.81%, 32.49%) | 1 |
| 2+ | 143 | 58 / 110 (52.7%) | 23 / 33 (69.7%) | −16.97% (−37.19%, 3.25%) | 0.127 | |
Abbreviations: GH, growth hormone; CI, confidence interval.
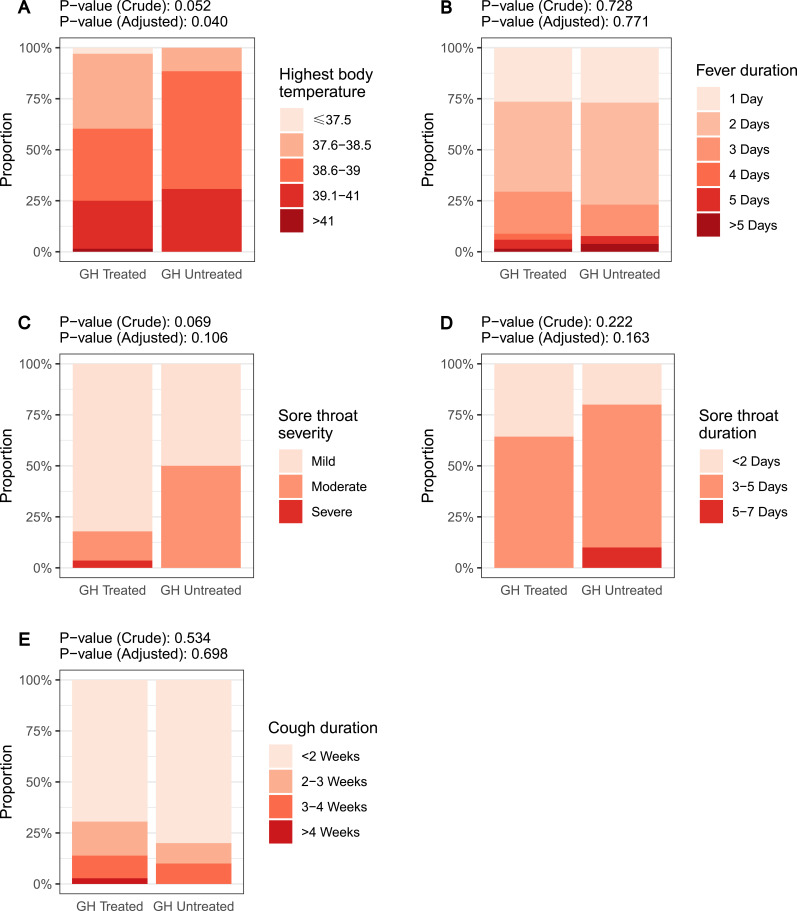
The secondary analysis set included 113 children with COVID-19 histories. Most of the children had mild or moderate disease, with no hospitalization, myocarditis, or encephalitis. Two (1.7%) children experienced pneumonia. The most frequent symptoms include fever (99 [87.6%]), cough (44 [38.9%]), and sore throat (39 [34.5%]). Comparison between groups showed that higher proportions of children in the GH Treated group experienced fever, cough, and sore throat, but the differences were not statistically significant (Table 3). When comparing the severity and duration of COVID-19 symptoms (Figure 2), the highest body temperature was significantly lower in the GH Treated group.
Table 3.
Proportions of Children with COVID-19 Symptoms
| GH Treated | GH Untreated | Difference (95% CI) | OR (95% CI) | |
|---|---|---|---|---|
| Fever | 72 / 80 (90.0%) | 27 / 33 (81.2%) | 8.18% (−8.67%, 25.03%) | 1.99 (0.60, 6.37) |
| Cough | 34 / 80 (42.5%) | 10 / 33 (30.3%) | 12.20% (−9.00%, 33.39%) | 2.13 (0.87, 5.56) |
| Sore throat | 29 / 80 (36.2%) | 10 / 33 (30.3%) | 5.95% (−15.08%, 26.98%) | 1.28 (0.53, 3.22) |
Abbreviations: GH, growth hormone; OR, odds ratio; CI, confidence interval.
Figure 2.
Severity and duration of COVID-19 symptoms in different growth hormone treatment groups: highest body temperature (A), fever duration (B), sore throat severity (C), sore throat duration (D), and cough duration (E). The crude and adjusted P-values for each comparison were shown in each figure.
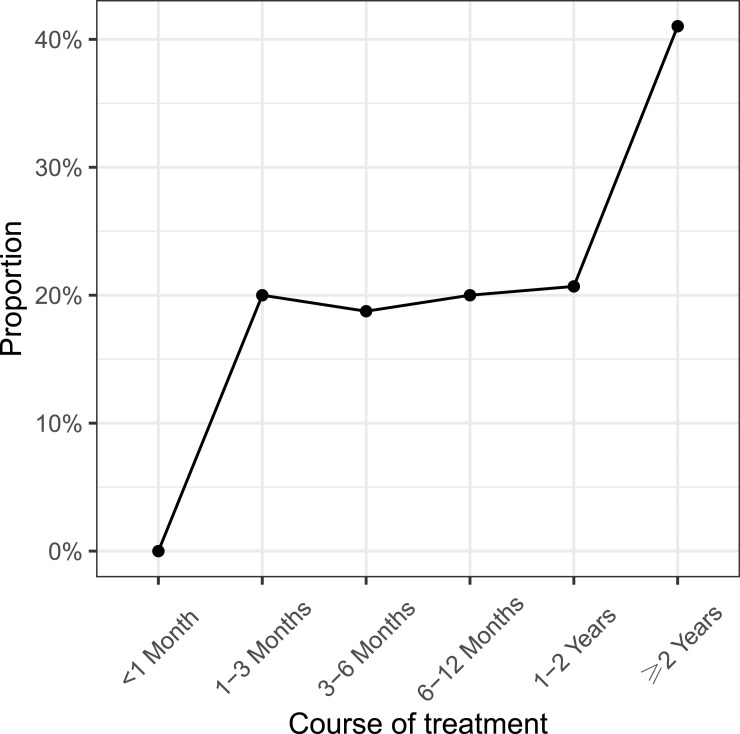
The exploratory analysis set included 159 children on GH treatment with or without COVID-19 histories. Overall, 30 (18.9%) of the children reported fewer, 79 (49.7%) reported similar, and 3 (1.9%) reported more common colds after GH treatment. The reduction was reported for children with courses of treatment longer than one month and was more obvious for children whose courses of treatment were longer than two years (Figure 3).
Figure 3.
Proportion of children reporting fewer common colds with the course of growth hormone treatment.
Mendelian Randomization Analyses
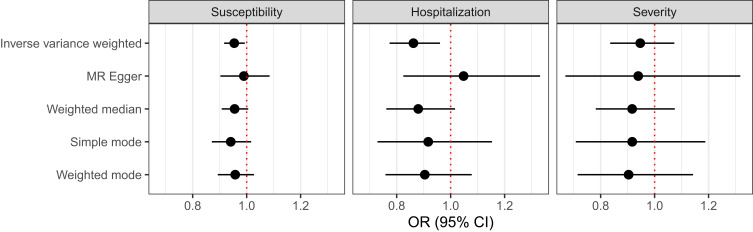
As the association between GH treatment and COVID-19 susceptibility and severity in children with short stature shown in the survey study may be explained by an increase in GH levels, we further explored the causal relationship between GH levels and COVID-19 using MR analyses. The GH level was used as the exposure, and the COVID-19 susceptibility, hospitalization, and severity were used as outcomes using GWAS summary level data. IVM MR was used as the primary MR method. The results showed that a higher genetically predicted GH level has significant causal associations with reduced COVID-19 susceptibility and hospitalization (Table 4 and Figure 4). For one unit increase in GH level, the OR was 0.95 (95% CI 0.92 to 0.99, P = 0.022) for COVID-19 susceptibility, 0.86 (95% CI 0.77 to 0.96, P = 0.007) for COVID-19 hospitalization, and 0.95 (95% CI 0.84 to 1.07, P = 0.392) for COVID-19 severity. Estimates from the other methods were mostly in the same direction as those from the IVW method.
Table 4.
Mendelian Randomization Results
| Outcome | SNPs | Method | OR (95% CI) | P (Effect) | P (Heterogeneity) | P (Intercept) |
|---|---|---|---|---|---|---|
| Susceptibility | 9 | IVW | 0.95 (0.92, 0.99) | 0.022 | 0.951 | |
| MR Egger | 0.99 (0.90, 1.08) | 0.831 | 0.962 | 0.413 | ||
| Weighted median | 0.96 (0.91, 1.00) | 0.082 | ||||
| Simple mode | 0.94 (0.87, 1.02) | 0.163 | ||||
| Weighted mode | 0.96 (0.89, 1.03) | 0.260 | ||||
| Hospitalization | 7 | IVW | 0.86 (0.77, 0.96) | 0.007 | 0.467 | |
| MR Egger | 1.05 (0.82, 1.33) | 0.717 | 0.789 | 0.134 | ||
| Weighted median | 0.88 (0.76, 1.02) | 0.082 | ||||
| Simple mode | 0.92 (0.73, 1.15) | 0.487 | ||||
| Weighted mode | 0.90 (0.76, 1.08) | 0.305 | ||||
| Severity | 10 | IVW | 0.95 (0.83, 1.07) | 0.392 | 0.352 | |
| MR Egger | 0.94 (0.67, 1.32) | 0.726 | 0.266 | 0.961 | ||
| Weighted median | 0.92 (0.78, 1.07) | 0.284 | ||||
| Simple mode | 0.92 (0.71, 1.19) | 0.528 | ||||
| Weighted mode | 0.90 (0.71, 1.14) | 0.419 |
Abbreviations: SNP, single-nucleotide polymorphism; IVW, inverse variance weighted; MR, Mendelian randomization; OR, odds ratio; CI, confidence interval.
Figure 4.
Mendelian randomization result.
Discussions
The present study investigates the association between GH treatment and COVID-19 susceptibility and severity in children with short stature. Our findings indicate that GH treatment may be associated with a somewhat reduced risk of COVID-19. Additionally, MR analyses indicate a possible causal relationship between GH levels and COVID-19 susceptibility and hospitalization. These results contribute to the growing body of literature examining the potential immunomodulatory effects of GH, particularly in pediatric populations.
Previous studies have indicated that GH has complex interactions with immune system functions, potentially enhancing protective responses against infections. Our study aligns with earlier research suggesting that GH treatment could somewhat mitigate COVID-19 risk among children, particularly those with underlying conditions that require GH therapy. In a previous study of 2382 children with GH issues in Israel, somatotropin treatment was associated with a significantly reduced risk of SARS-CoV-2 positivity.16 However, in our study, the association was not statistically significant before or after adjusting for confounders. A post-hoc power calculation showed that our statistical power is 19.8%, which implicates insufficient power and therefore insufficient sample size for the current test. This highlights the need for larger, more definitive studies to confirm these associations. Subgroup analysis showed that the association was consistent across the subgroups, especially in children aged 7 to 12 years, females, and children with 2 or more vaccinations. Although we adjusted for confounders such as age, sex, and vaccination status, there may still be residual confounding factors that were not collected in the survey, including underlying health conditions, immune status, household dynamics, and lifestyle factors such as physical activity.
Most of the children had mild or moderate symptoms, and no hospitalizations were reported. We also explored the association between GH treatment and the presence, duration, and severity of COVID-19 symptoms. Consistently, the proportions of children with fever, cough, and sore throat were similar in the GH Treated group and in the GH Untreated group, but the symptoms were less severe and had shorter durations in the GH Treated group. However, statistical significance was only reached for the highest body temperature. This finding suggests that GH treatment may influence the severity of viral infections, potentially by modulating inflammatory responses. While the differences in symptom severity were not universally significant, the trend is noteworthy and warrants further investigation.
In the exploratory analysis, children reported less frequent common colds after GH treatment, which is more obvious with prolonged treatment. This observation raises intriguing questions about the long-term benefits of GH in enhancing overall immune function and reducing the frequency of the common cold.
A previous MR study22 has provided evidence for the causal relationship between high IGF-1 levels and decreased COVID-19 susceptibility and hospitalization. In our MR analyses, we confirmed the causal relationship between high GH levels and decreased COVID-19 susceptibility and hospitalization.
Our study has several advantages. First, we evaluated both the susceptibility and symptomatology of COVID-19. Second, we combined a survey study with MR analyses to explore both the association and causation relationships, and the results were consistent. Our results suggest that GH treatment may play a role in reducing COVID-19 susceptibility and hospitalization rates in children with short stature. Clinically, this indicates that healthcare providers may consider the potential immunomodulatory effects of GH when managing children at risk for COVID-19, particularly those with conditions that necessitate GH treatment. By understanding the relationship between GH levels and COVID-19 outcomes, clinicians can better inform treatment plans and risk assessments for pediatric patients.
Our study also has limitations. First, we suffer from insufficient power due to the limited sample size. More samples are needed to increase the statistical power and confirm our results. Second, it is an observational study, which is vulnerable to bias and confounding factors. Prospective cohort studies with a more representative sample are required to draw a conclusion. Third, the study is a survey study where both COVID-19 histories and GH treatment histories were based on self-report, where inaccuracies may exist. Additionally, detailed information about GH treatment, such as dosages and brand names, was not collected, which may impact the interpretation of the results. Fourth, previously reported risk factors for COVID-19, like attention deficit hyperactivity disorder, 25-OH vitamin D levels, asthma, and influenza vaccination,16 were not collected and may thus not be fully adjusted in the analysis.
Conclusions
Our study showed that in children with short stature, GH treatment was associated with somewhat decreased COVID-19 susceptibility, which was consistent across the subgroups. However, the association was not statistically significant before or after adjusting for confounders. We further confirmed the possible causal relationship between GH levels and COVID-19 susceptibility and hospitalization using MR analyses.
Funding Statement
This work was financially supported by the National Key R&D Program of China (No. 2022YFC2703400), Shanghai Healthcare Commission Project (202340103), Clinical Research Centre for Congenital Adrenal Insufficiency, Pediatrics College, Shanghai Jiao Tong University School of Medicine (ELYZX202106), and Clinical Innovation Project of Xinhua Hospital, School of Medicine, Shanghai Jiao Tong University (23XHCR12B).
Data Sharing Statement
The datasets generated and analyzed during the current study are not publicly available because we are not allowed to share individual level data. However, additional information about the data is available from the corresponding author on reasonable request.
Ethics Approval
Prior to the initiation of the study, the research protocol was reviewed and approved by the IRB at Xinhua Hospital, Shanghai Jiao Tong University (Approval number: XHEC-C-2023-118-1).
Consent for Publication
Written informed consent was obtained from all the participants.
Disclosure
No potential conflicts of interest are disclosed.
References
- 1.World Health Organization. Coronavirus COVID-19 Dashboard. 2023. Available from: https://covid19.who.int. Accessed December 11, 2024.
- 2.Centers for Disease Control and Prevention. Risk for COVID-19 Infection, Hospitalization, and Death by Age Group. 2023. Available from: https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-age.html. Accessed December 11, 2024.
- 3.World Health Organization. COVID-19 Disease in Children and Adolescents: Scientific Brief, 29 September 2021. 2021. Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-Sci_Brief-Children_and_adolescents-2021.1. Accessed December 11, 2024.
- 4.Hersch EC, Merriam GR. Growth hormone (GH)-releasing hormone and GH secretagogues in normal aging: fountain of Youth or Pool of Tantalus? Clin Interventions Aging. 2008;3(1):121–129. doi: 10.2147/CIA.S3247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Witkowska-Sędek E, Pyrżak B. Chronic inflammation and the growth hormone/insulin-like growth factor-1 axis. Central Eur J Immunol. 2020;45(4):469–475. doi: 10.5114/ceji.2020.103422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meazza C, Pagani S, Travaglino P, Bozzola M. Effect of growth hormone (GH) on the immune system. Pediatr Endocrinol Rev. 2004;1((Suppl 3)):490–495. [PubMed] [Google Scholar]
- 7.Bozzola M, De Benedetti F, De Amici M, et al. Stimulating effect of growth hormone on cytokine release in children. Eur J Endocrinol. 2003;149(5):397–401. doi: 10.1530/eje.0.1490397 [DOI] [PubMed] [Google Scholar]
- 8.Pagani S, Meazza C, Travaglino P, De Benedetti F, Tinelli C, Bozzola M. Serum cytokine levels in GH-deficient children during substitutive GH therapy. Eur J Endocrinol. 2005;152(2):207–210. doi: 10.1530/eje.1.01827 [DOI] [PubMed] [Google Scholar]
- 9.Zhu Z, Zhao Z, Chen X, et al. Effects of growth hormone/estrogen/androgen on COVID-19-type proinflammatory responses in normal human lung epithelial BEAS-2B cells. BMC Mol Cell Biol. 2022;23(1):42. doi: 10.1186/s12860-022-00442-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lubrano C, Masi D, Risi R, et al. Is growth hormone insufficiency the missing link between obesity, male gender, age, and COVID‐19 severity? Obesity. 2020;28(11):2038–2039. doi: 10.1002/oby.23000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu S, Jiang S, Qi X, Bai R, Ye X, Xie T. Races of small molecule clinical trials for the treatment of COVID-19: an up-to-date comprehensive review. Drug Dev Res. 2022;83(1):16–54. doi: 10.1002/ddr.21895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ilias I, Diamantopoulos A, Botoula E, et al. Covid-19 and growth hormone/insulin-like growth factor 1: study in critically and non-critically ill patients. Front Endocrinol. 2021;12. doi: 10.3389/fendo.2021.644055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou Y, Li L, Yu Z, et al. Dermatophagoides pteronyssinus allergen Der p 22: cloning, expression, IgE-binding in asthmatic children, and immunogenicity. Pediatric Allergy and Immunology. 2022;33(8):e13835. doi: 10.1111/pai.13835 [DOI] [PubMed] [Google Scholar]
- 14.Jiang Y, Chen R, Xu S, et al. Endocrine and metabolic factors and the risk of idiopathic pulmonary fibrosis: a Mendelian randomization study. Front Endocrinol. 2024;14:1321576. doi: 10.3389/fendo.2023.1321576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baykan EK, Baykan AR, Utlu M, et al. Growth hormone level in COVID-19 patients. North Clin Istanb. 2022;9(5):470–475. doi: 10.14744/nci.2021.90094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brill G, Manor I, Bril Paroz R, et al. The association between somatotropin therapy and the risk of SARS-CoV-2 infection in children with short stature: a population-based cross-sectional study. Children. 2022;9(12):1844. doi: 10.3390/children9121844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hemani G, Zheng J, Elsworth B, et al. The MR-base platform supports systematic causal inference across the human phenome. ELife. 2018;7:e34408. doi: 10.7554/eLife.34408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilly A, Park Y-C, Png G, et al. Whole-genome sequencing analysis of the cardiometabolic proteome. Nat Commun. 2020;11(1):6336. doi: 10.1038/s41467-020-20079-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.COVID-19 host Genetics Initiative. The COVID-19 host genetics initiative, a global initiative to elucidate the role of host genetic factors in susceptibility and severity of the SARS-CoV-2 virus pandemic. Eur J Hum Genet. 2020;28(6):715–718. doi: 10.1038/s41431-020-0636-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma A, Minh Duc NT, Luu Lam Thang T, et al. A consensus-based checklist for reporting of survey studies (CROSS). J Gen Intern Med. 2021;36(10):3179–3187. doi: 10.1007/s11606-021-06737-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith GD, Davies NM, Dimou N, et al. STROBE-MR: guidelines for strengthening the reporting of Mendelian randomization studies. PeerJ. 2019;7:e27857v1. doi: 10.7287/peerj.preprints.27857v1 [DOI] [Google Scholar]
- 22.Li X, Zhou Y, Yuan S, et al. Genetically predicted high IGF-1 levels showed protective effects on COVID-19 susceptibility and hospitalization: a Mendelian randomisation study with data from 60 studies across 25 countries. ELife. 2022;11:e79720. doi: 10.7554/eLife.79720 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are not publicly available because we are not allowed to share individual level data. However, additional information about the data is available from the corresponding author on reasonable request.