Abstract
Objective
To analyse the parameters of shear wave elastography (SWE) and contrast-enhanced ultrasound (CEUS) in breast non-mass-like lesions (NMLs) and to evaluate the added diagnostic value of SWE and CEUS when combined with B-mode ultrasound (US) for differentiating NMLs.
Methods
A total of 118 NMLs from 115 patients underwent US, SWE, and CEUS examinations. The SWE parameter with the highest areas under the receiver operating characteristic (ROC) curves (Az) and independent variables of CEUS obtained by logistic regression were used to adjust the BI-RADS-US (Breast Imaging Reporting and Data System for Ultrasound) classification. The adjusted BI-RADS risk stratification was then compared with the original classification. Additionally, the diagnostic effectiveness of US+SWE, US+CEUS, and US+SWE+CEUS combinations was calculated and compared.
Results
The “stiff rim sign” was used as the optimal SWE indicator for BI-RADS adjustment. CEUS diagnostic criteria for adjustment included enhancement intensity, enhancement size, and the presence of radial or penetrating vessels. The Az values of US+SWE+CEUS and US+CEUS combinations were significantly higher than that of US alone (P<0.05). However, there was no significant difference in the Az value of US+SWE and US (P = 0.072). US+SWE+CEUS combination showed significantly higher Az values compared to other combinations (P<0.05), and achieved the highest sensitivity and specificity.
Conclusion
Adding SWE and CEUS to conventional US enhances diagnostic accuracy for NMLs, offering a meaningful incremental value for BI-RADS classification in the assessment of NMLs.
Keywords: breast, shear wave elastography, contrast-enhanced ultrasound, non-mass-like lesion
Introduction
Non-mass-like lesions (NMLs) are a type of breast lesion characterized by the absence of well-defined boundaries and morphological features on imaging, making it challenging to accurately distinguish between benign and malignant lesions using conventional imaging techniques.1 NMLs are relatively common in breast ultrasound (US) examinations, accounting for 5.3–9.2% of all breast lesions.2 Currently, the Breast Imaging Reporting and Data System (BI-RADS) lacks a clear diagnostic criterion for NMLs.3,4 Diagnosis of NMLs primarily relies on B-mode US. However, despite its high sensitivity for detecting breast lesions, B-mode US often fails to provide sufficient tissue information for accurate classification, resulting in frequent misdiagnosis and missed diagnoses.5 And invasive biopsy is often required for definitive diagnosis, leading to unnecessary physical and psychological distress for patients.6 Therefore, improving the diagnostic accuracy of NMLs remains a critical challenge in clinical practice.
Shear wave elastography (SWE) and contrast-enhanced ultrasound (CEUS) are two emerging techniques widely applied in breast lesion diagnosis, providing additional diagnostic information.7,8 SWE quantitatively assesses tissue stiffness, offering an objective indicator of tumor rigidity. It has been shown that malignant breast lesions often exhibit the “stiff rim sign”, which helps distinguish between benign and malignant lesions.9 On the other hand, CEUS uses microbubble contrast agents to visualize the microvascular structure of lesions, revealing tumor blood flow distribution. Malignant lesions are typically associated with irregular and abundant blood flow.10,11 Combining SWE, CEUS, and US results can enhance the diagnostic efficiency for NMLs in the breast, while reducing reliance on invasive biopsy procedures.8,12
Currently, research on the combined evaluation of NMLs using conventional US, SWE, and CEUS is limited. This study aims to assess the incremental value of SWE and CEUS in the differential diagnosis of breast NMLs and to optimize the BI-RADS classification for NMLs. By integrating the quantitative parameters of SWE and CEUS with the imaging features of conventional US, the study seeks to provide a more precise diagnostic approach for clinical practice, ultimately improving the clinical management of NMLs.
Materials and Methods
Patients
From October 2021 to December 2022, a total of 873 patients underwent breast US examinations in our department, among whom 140 were identified with NMLs. Exclusion criteria included allergy to contrast agents, prior radiotherapy or chemotherapy, and the presence of breast implants (n=15). Additionally, patients lacking CEUS or SWE data or pathological results were excluded (n=10). Ultimately, 115 eligible patients with a total of 118 NMLs were included in the study. Informed consent was obtained from all patients undergoing CEUS before contrast injection, with information provided regarding potential adverse reactions. This study was approved by the Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University (Approval No.: KY2022-R169) and complies with the Declaration of Helsinki. Given the retrospective nature of the data collection, the Ethics Committee waived the requirement for additional consent, and all patient data were anonymized and kept strictly confidential.
Ultrasound Examination
The US, SWE, and CEUS were all performed using the Resona 8 ultrasound system (Mindray Medical International, Shenzhen, China). For conventional US and SWE, the L14-5 probe (3–15 MHz) was used, while the L9-3U probe (3–11 MHz) was used for CEUS. The contrast agent used was SonoVue (Bracco Suisse SA). All US images were independently analyzed by two sonographers with over 10 years of experience in breast disease US diagnostics, and the relevant parameters of NMLs were recorded.
Conventional US examination: First, all lesions underwent conventional US evaluation, documenting their location, size, internal echogenicity, margins, shape, calcification, and blood flow. Based on the US image feature classification method proposed by Ko et al,1 NMLs were categorized as follows: Type I (ductal hypoechoic srea): Ia: No calcification, classified as BI-RADS 4B. Ib: With calcification, higher malignancy risk, classified as BI-RADS 4C. Type II (non-ductal hypoechoic area): IIa: No calcification, with a vague shape and lower malignancy risk, classified as BI-RADS 4A. IIb: With calcification, higher malignancy risk, classified as BI-RADS 4C. Type III: Features structural distortion with a vague area, classified as BI-RADS 4B. Type IV: Margins unclear with hypoechoic areas accompanied by posterior acoustic attenuation, classified as BI-RADS 4B. The classification of all NMLs was based on their US features (unclear margins, irregular shape, and not parallel to the skin),13 with consideration of malignancy risk, ensuring that all lesions were classified as at least BI-RADS 4A.
SWE examination: In SWE mode, the largest cross-section of the lesion was positioned at the center of the sampling box, ensuring that the lesion was at least 3 mm away from the sampling box borders. Patients were instructed to hold their breath, and the dual dynamic mode for SWE quality and speed was selected. A high-quality image was obtained when the quality image displayed a uniform green background without obvious artifacts. The mode was then switched to grayscale SWE speed mode (where red indicates hard tissue and blue indicates soft tissue) to observe for the presence of a “stiff rim sign” (indicated by red or orange around the lesion).14 Elasticity parameters were recorded, including the lesion’s mean, maximum, minimum elasticity values, and standard deviation (Emean, Emax, Emin, and Esd); the surrounding area (2 mm around the lesion) (Esmean, Esmax, Esmin, and Essd); and the lesion plus the surrounding area (Elsmean, Elsmax, Elsmin, and Elssd), with elasticity modulus measurements ranging from 0 to 140 kPa.
CEUS examination: The transducer was switched, and CEUS mode was activated, selecting the plane that best displayed the lesion’s surrounding normal breast tissue, irregular morphology, or areas of rich vascularity. After the intravenous injection of 4.8 mL of contrast agent, 5 mL of saline was immediately administered, and the timer and storage function were started to observe the lesion in real time for three minutes while recording the images. CEUS parameters of the lesion were recorded, including enhancement timing, enhancement intensity, enhancement sharpness, enhancement boundary, enhancement distribution, enhancement direction, enhancement size (whether enlarged), radiating or penetrating vessels, perfusion defect, and wash-out timing.15
Statistical Analysis
Statistical analysis was performed using SPSS software version 27.0. Differences between quantitative variables were analyzed using the t-test, while differences between categorical variables were analyzed using the chi-square test. The area under the ROC curve (Az) was calculated, and Az values were compared using the Z-test. The optimal cutoff values for SWE parameters were determined according to the Youden index, and a logistic regression model was used to evaluate the effectiveness of CEUS in the differential diagnosis of NMLs. Based on the optimal Az values for SWE parameters and the logistic regression results for CEUS, the BI-RADS classification of NMLs was reassessed to calculate the diagnostic efficacy of US+SWE, US+CEUS, and US+SWE+CEUS. For each method, Az, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were obtained. Statistical significance was defined as P < 0.05.
Result
Participants and Pathology
This study included 118 NMLs from 115 patients, with a mean lesion size of 20.8 mm (range, 4–64 mm) and a mean patient age of 47 years (range, 19–76 years). Among these lesions, 58 (49.1%) were benign and 60 (50.8%) were malignant. Of the 115 female patients, 50 presented with a palpable breast mass, 17 had symptoms of nipple discharge, and 14 experienced breast pain. Detailed pathological results are shown in Table 1. The most common benign lesion was adenosis (24.1%), while the most common malignant lesion was ductal carcinoma in situ (DCIS) (33.3%).
Table 1.
Pathological Diagnosis of 118 Breast Non-Mass-Like Lesions
| Pathological Result | No.of Lesions |
|---|---|
| Benign | 58 |
| Adenosis | 14 |
| Adenosis with fibroadenoma | 8 |
| Adenosis with duct ectasia | 8 |
| Intraductal papilloma | 5 |
| Fibroadenoma | 7 |
| Inflammation | 5 |
| Adenosis with ductal epithelial hyperplasia | 3 |
| Intraductal papilloma with ductal epithelial hyperplasia | 3 |
| Atypical ductal or lobular hyperplasia | 2 |
| Fibroadenosis | 2 |
| Malignant | 60 |
| Ductal carcinoma in situ | 20 |
| Ductal carcinoma in situ with invasive ductal carcinoma | 19 |
| Invasive ductal carcinoma | 18 |
| Invasive lobular carcinoma | 2 |
| Paget’s disease | 1 |
Diagnostic Performance of SWE Parameters
With the exception of Emin, Esmin, and Elsmin, the SWE parameters for malignant NMLs were significantly higher than those for benign NMLs (Table 2). The stiff rim sign, having the highest Az value (0.822), was selected as the optimal criterion for adjusting BI-RADS categorization, with a sensitivity of 88.3% and a specificity of 81.0%. BI-RADS categorization was upgraded by one level if the stiff rim sign was present and downgraded by one level if it was absent.
Table 2.
Correlation Between SWE Parameters and Pathology in Non-Mass-Like Lesions
| Variables | Benign (n=58) | Malignant (n=60) | P value | Az value | Cutoff point | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|---|
| Stiff rim sign(yes/no) | 10/58 | 50/60 | <0.001 | 0.822 | Yes | 83.3% | 81% |
| Emean(kPa) | 34.78±14.65 | 45.94±12.63 | <0.001 | 0.722 | 34.23 | 88.3% | 56.9% |
| Emax(kPa) | 65.64[44.28,84.30] | 109.31[81.73,152.33] | <0.001 | 0.796 | 79.47 | 76.7% | 74.1% |
| Emin(kPa) | 15.57[8.68,21.11] | 16.65[11.26,24.40] | 0.162 | 0.575 | 22.02 | 35.0% | 81.0% |
| Esd | 9.38[6.70,12.38] | 13.64[10.46,19.22] | <0.001 | 0.724 | 11.18 | 70.0% | 70.7% |
| Esmean(kPa) | 33.45[24.24,43.92] | 49.91[41.95,56.08] | <0.001 | 0.754 | 44.32 | 73.3% | 75.9% |
| Esmax(kPa) | 66.64[52.06,92.36] | 127.12[100.92,163.41] | <0.001 | 0.807 | 98.01 | 78.3% | 81.0% |
| Esmin(kPa) | 12.47[6.47,18.09] | 14.31[7.22,18.89] | 0.403 | 0.545 | 16.38 | 43.3% | 70.7% |
| Essd | 11.52[8.05,15.34] | 18.61[13.18,23.37] | <0.001 | 0.773 | 12.90 | 80.0% | 67.2% |
| Elsmean(kPa) | 33.12[24.34,44.34] | 48.20[41.24,54.40] | <0.001 | 0.748 | 34.27 | 90.0% | 55.2% |
| Elsmax(kPa) | 70.72[52.06,92.97] | 135.05[106.84,175.50] | <0.001 | 0.820 | 98.56 | 80.0% | 79.3% |
| Elsmin(kPa) | 12.15[6.47,17.39] | 14.00[6.69,18.70] | 0.527 | 0.534 | 18.07 | 35.0% | 79.3% |
| Elssd | 10.52[7.94,15.08] | 15.43[12.84,21.49] | <0.001 | 0.751 | 11.69 | 86.7% | 62.1% |
Abbreviations: Emean, mean elastic modulus for lesion; Emax, maximum elastic modulus for lesion; Emin, minimum elastic modulus for lesion; Esd, elastic modulus standard deviation for lesion; Esmean, mean elastic modulus for shell; Esmax, maximum elastic modulus for shell; Esmin, minimum elastic modulus for shell; Essd, elastic modulus standard deviation for shell; Elsmean, mean elastic modulus for lesion plus shell; Elsmax, maximum elastic modulus for lesion plus shell; Elsmin, minimum elastic modulus for lesion plus shell; Elssd, standard deviation of elastic modulus for lesion plus shell.
Of the 35 initial BI-RADS 4A lesions, 27 were downgraded to BI-RADS 3, and 8 were upgraded to BI-RADS 4B. For the 29 initial BI-RADS 4B lesions, 19 were downgraded to BI-RADS 4A, and 10 were upgraded to BI-RADS 4C. For the 54 initial BI-RADS 4C lesions, 11 were downgraded to category 4B, and 43 were upgraded to category 5. As a result of this adjustment, 26 (44.8%) benign NMLs could be managed with follow-up instead of biopsy. Meanwhile, 98.3% of malignant NMLs (59 out of 60) could be accurately diagnosed and managed with this adjustment.
Diagnostic Performance of CEUS Parameters
As shown in Table 3, Chi-square tests revealed significant statistical differences in five enhancement characteristics: enhancement time, enhancement intensity, perfusion defects, enhancement size, and radial or penetrating vessels. The final step of logistic regression analysis identified three independent variables, with the following formula:
Table 3.
Correlation Between CEUS Parameters and Pathology in Non-Mass-Like Lesions
| Parameter | Benign n(%) | Malignant n(%) | P |
|---|---|---|---|
| Enhancement time | 0.025 | ||
| Earlier | 31 (53.4) | 44 (73.7) | |
| Synchronous or later | 27 (46.6) | 16 (26.7) | |
| Enhancement intensity | <0.001 | ||
| Hyperenhancement | 17 (29.3) | 51 (85.0) | |
| Iso-/hypo-enhancement | 41 (70.7) | 9 (15.0) | |
| Enhancement margin | 0.077 | ||
| Clear | 20 (34.5) | 12 (20.0) | |
| Unclear | 38 (65.5) | 48 (80.0) | |
| Enhancement sharpness | 0.102 | ||
| Regular | 17 (29.3) | 10 (16.7) | |
| Irregular | 41 (70.7) | 50 (83.3) | |
| Enhancement distribution | 0.061 | ||
| Homogenous | 25 (43.1) | 16 (26.7) | |
| Heterogeneous | 33 (56.9) | 44 (73.3) | |
| Enhanced direction | 0.099 | ||
| Centripetal | 30 (51.7) | 40 (66.7) | |
| Centrifugal/diffuse | 28 (48.3) | 20 (33.3) | |
| Perfusion defect | 0.040 | ||
| Present | 13 (22.4) | 24 (40.0) | |
| Absent | 45 (77.6) | 36 (60.0) | |
| Enhancement size | <0.001 | ||
| Enlarged | 9 (15.5) | 45 (75.0) | |
| Nonenlarged | 49 (84.5) | 15 (25.0) | |
| Radial or penetrating vessels | <0.001 | ||
| Present | 6 (10.3) | 34 (56.7) | |
| Absent | 52 (89.7) | 26 (43.3) | |
| Regression time | 0.062 | ||
| Earlier | 21 (36.2) | 32 (53.3) | |
| Synchronous or later | 37 (63.8) | 28 (46.7) |
Abbreviation: CEUS, Contrast-enhanced ultrasound.
Logit (P) = −1.704 + (1.537 ×enlarged enhancement size) + (1.239 × radial or penetrating vessels) + (1.198× hyperenhancement). Based on these three independent variables (hyperenhancement, enlarged enhancement size, and radial or penetrating vessels), we established a diagnostic criterion for NMLs combining conventional US with CEUS: if CEUS shows two or three risk factors, the BI-RADS category is upgraded by one level; otherwise, it is downgraded by one level.
Among the 35 NMLs initially classified as BI-RADS 4A, 9 were upgraded to BI-RADS 4B, and 26 were downgraded to BI-RADS 3. Of the 29 NMLs initially classified as BI-RADS 4B, 9 were upgraded to BI-RADS 4C, and 20 were downgraded to BI-RADS 4A. For the 54 NMLs classified as BI-RADS 4C, 39 were upgraded to BI-RADS 5, while 15 were downgraded to BI-RADS 4B. A total of 26 (44.8%) benign NMLs could have been switched from biopsy to follow-up management. All 60 malignant NMLs (100%) could be accurately diagnosed.
Re-Evaluated BI-RADS-US Combined with Both SWE and CEUS
Based on the optimal cutoff point for SWE (presence of a stiff rim sign) and the diagnostic criteria for CEUS (≥ 2 independent variables), the adjustment method for combining conventional US with SWE and CEUS is outlined in Table 4. The method includes downgrading, upgrading, or maintaining the BI-RADS categorization. We also provide the corresponding imaging features based on the combined analysis of conventional US, SWE, and CEUS as the basis for the downgrading (Figure 1), upgrading (Figure 2), or no change (Figure 3) in the BI-RADS classification.
Table 4.
Adjusted BI-RADS Grading Standard
| BI-RADS Category | SWE-The stiff rim Sign | CEUS-Independent Variables | Adjusted BI-RADS Category |
|---|---|---|---|
| 4A | Yes | ≥2 | 4B |
| Yes | <2 | 4A | |
| No | ≥2 | 4A | |
| No | <2 | 3 | |
| 4B | Yes | ≥2 | 4C |
| Yes | <2 | 4B | |
| No | ≥2 | 4B | |
| No | <2 | 4A | |
| 4C | Yes | ≥2 | 5 |
| Yes | <2 | 4C | |
| No | ≥2 | 4C | |
| No | <2 | 4B |
Abbreviations: BI-RADS, Breast imaging reporting and data system; SWE, Shear wave elastography; CEUS, Contrast-enhanced ultrasound.
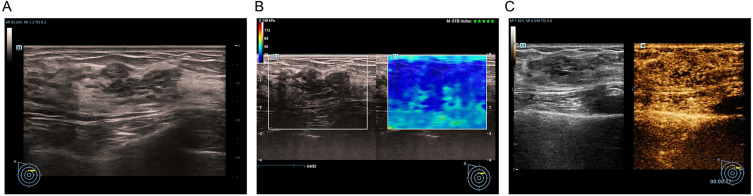
Figure 1.
Adenosis with duct ectasia in a 31-year-old female. Conventional US revealed a ductal hypoechoic area without calcification, initially categorized as BI-RADS 4B (A). Shear wave elastography did not show the stiff rim sign (B). CEUS of the lesion revealed iso- and synchronous enhancement without tumor size enlargement (C). The risk categorization was adjusted to BI-RADS 4A.
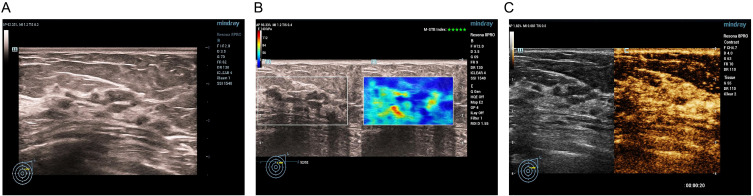
Figure 2.
Ductal carcinoma in situ in a 76-year-old female. Conventional US revealed a ductal hypoechoic area with microcalcification, initially categorized as BI-RADS 4C (A). SWE showed the stiff rim sign (B). CEUS of the lesion revealed earlier and hyperenhancement with tumor size enlargement and radial vessels (C). The risk categorization was upgraded to BI-RADS 5.
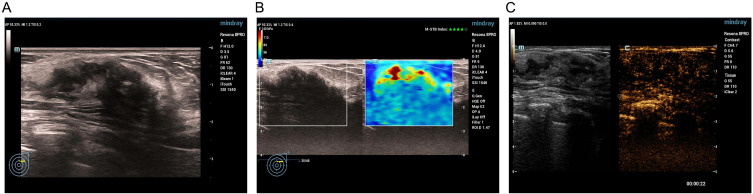
Figure 3.
Adenosis in a 67-year-old female. Conventional US revealed a nonductal hypoechoic area without microcalcification, initially categorized as BI-RADS 4A (A). SWE showed the stiff rim sign (B). CEUS of the lesion revealed iso- and synchronous enhancement without tumor size enlargement (C). The risk categorization remained BI-RADS 4A.
Among the 35 NMLs initially classified as BI-RADS 4A, 4 were upgraded to BI-RADS 4B, 9 remained unchanged, and 22 were downgraded to BI-RADS 3. For the 29 NMLs classified as BI-RADS 4B, 4 were upgraded to BI-RADS 4C, 11 remained unchanged, and 14 were downgraded to BI-RADS 4A. Of the 54 NMLs classified as BI-RADS 4C, 33 were upgraded to BI-RADS 5, 5 were downgraded to BI-RADS 4B, and 16 remained unchanged. A total of 22 (37.9%) benign NMLs could be switched from biopsy to follow-up management, while 100% of the malignant NMLs (60 out of 60) were accurately diagnosed.
Comparison of Diagnostic Performances
The initial BI-RADS categorization of 118 NMLs and the re-evaluated BI-RADS categorization using different methods are shown in Table 5, with diagnostic efficiencies of the methods presented in Table 6. The sensitivities of US+SWE (91.7%), US+CEUS (93.3%), and US+SWE+CEUS (96.7%) were similar to that of US (93.3%). However, the specificities of the different combinations (US+SWE, 70.7%; US+CEUS, 72.4%; US+SWE+CEUS, 82.8%) were significantly higher than that of US (53.4%, P < 0.05 for all). Additionally, the combination of US+SWE+CEUS demonstrated the highest accuracy, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV).
Table 5.
Comparison of Preliminary and Re-Evaluated BI-RADS Categorization for Diagnosis of NMLs
| Pathology | BI-RADS-US categorization | Total | ||||
|---|---|---|---|---|---|---|
| 3 | 4A | 4B | 4C | 5 | ||
| Benign | 0 | 31 | 17 | 10 | 0 | 58 |
| Malignant | 0 | 4 | 12 | 44 | 0 | 60 |
| BI-RADS-US categorization+SWE | ||||||
| 3 | 4A | 4B | 4C | 5 | ||
| Benign | 26 | 15 | 11 | 2 | 4 | 58 |
| Malignant | 1 | 4 | 8 | 8 | 39 | 60 |
| BI-RADS-US categorization+CEUS | ||||||
| 3 | 4A | 4B | 4C | 5 | ||
| Benign | 26 | 16 | 12 | 1 | 3 | 58 |
| Malignant | 0 | 4 | 12 | 8 | 36 | 60 |
| BI-RADS-US categorization+SWE+CEUS | ||||||
| 3 | 4A | 4B | 4C | 5 | ||
| Benign | 22 | 26 | 3 | 6 | 1 | 58 |
| Malignant | 0 | 2 | 11 | 15 | 32 | 60 |
Abbreviations: BI-RADS, Breast imaging reporting and data system; SWE,Shear wave elastography; CEUS, Contrast-enhanced ultrasound; US, Ultrasonography.
Table 6.
Diagnostic Performance of US, US+SWE, US+CEUS and US+SWE+CEUS
| Method | Accuracy (%) | Sensitivity(%) | Specificity (%) (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|
| US | 73.7 | 93.3 | 53.4 | 67.5 | 88.6 |
| US + SWE | 81.4 | 91.7 | 70.7 | 76.4 | 89.1 |
| US + CEUS | 83.0 | 93.3 | 72.4 | 77.8 | 91.3 |
| US+SWE+CEUS | 89.8 | 96.7 | 82.8 | 85.3 | 96.0 |
Abbreviations: US, Ultrasonography; SWE, shear wave elastography; CEUS, contrast-enhanced ultrasound; NPV, negative predictive value; PPV, positive predictive value.
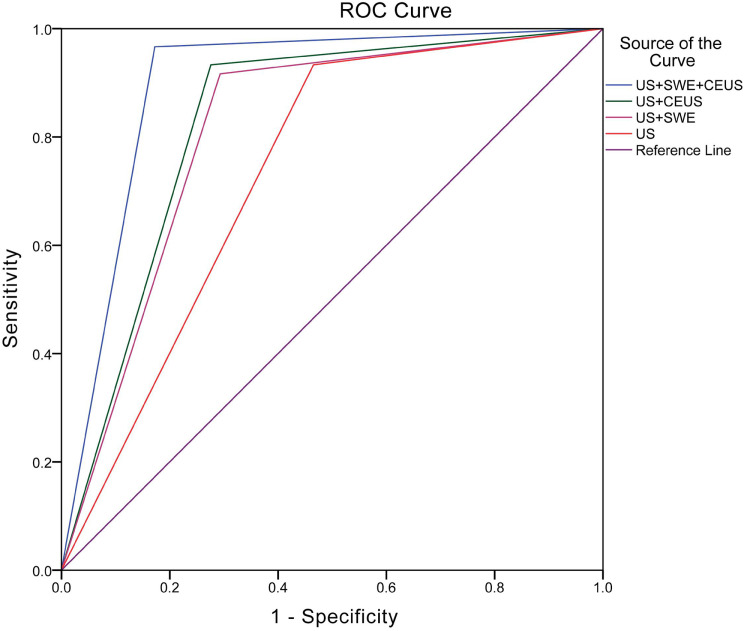
The Az values for US, US+SWE, US+CEUS, and US+SWE+CEUS were 0.734, 0.812, 0.829, and 0.897, respectively (Figure 4). The Az values for US+CEUS (Z = 2.123, P = 0.034) and US+SWE+CEUS (Z = 4.463, P < 0.001) were significantly higher than that of US. There was no significant difference in the Az value between US and US+SWE (Z = 1.798, P = 0.072) or between US+CEUS and US+SWE (Z = 0.443, P = 0.658). The Az value of US+SWE+CEUS was significantly higher than that of the other combinations, including US+SWE (Z = 2.756, P = 0.006) and US+CEUS (Z = 2.036, P = 0.042).
Figure 4.
Receiver operating characteristic curves of US, US+SWE, US+CEUS and US+SWE+CEUS. The Az value was 0.734 for US, 0.812 for US+SWE, 0.829 for US+CEUS, and 0.897 for US+SWE+CEUS. SWE, shear wave elastography; CEUS, contrast-enhanced ultrasound.
Discussion
Breast NMLs present significant diagnostic challenges due to their unclear boundaries and ambiguous morphological features.16 Conventional US often fails to provide sufficient diagnostic information, particularly in distinguishing benign from malignant lesions.8 Our study demonstrates that combining SWE and CEUS with conventional US can significantly improve the diagnostic accuracy for NMLs.
Among various ultrasound elastography (USE) techniques, SWE is considered highly reproducible and less operator-dependent. Unlike other USE methods, such as strain elastography, which require manual compression and release to induce tissue deformation, SWE minimizes operator involvement, thus enhancing consistency in results.16,17 SWE has shown notable potential in differentiating malignant lesions, particularly through the “stiff rim sign”, which manifests as a rigid outer edge at the tumor boundary.14 This characteristic likely arises from fibroblast proliferation at the tumor edge in malignant lesions, creating a stiff peripheral halo visible in SWE imaging.9,18 This feature is especially valuable in the differential diagnosis of NMLs. In our study, the stiff rim sign demonstrated the highest area under the the ROC curve (Az value) among SWE parameters (0.822), making it the most accurate indicator for malignancy. Notably, the stiff rim sign showed superior diagnostic accuracy, particularly when other quantitative SWE parameters, such as Emin and Esmax, exhibited lower sensitivity and specificity. Although SWE sensitivity is comparable to conventional US, it significantly improves specificity. This increase in specificity reduces unnecessary biopsies, offering substantial clinical value, especially in managing benign lesions.
Malignant lesions typically exhibit irregular and abundant blood flow patterns. CEUS provides additional diagnostic information by visualizing the microvascular features of NMLs.11,19 In our study, enhancement intensity, enhancement size, and radial or penetrating vessels were identified as key CEUS indicators for diagnosing NMLs. Combining these features significantly improved the diagnostic performance of CEUS. Moreover, compared to conventional US, the US+CEUS combination demonstrated significantly higher sensitivity and specificity, underscoring the added value of CEUS in evaluating NMLs.
Further analysis revealed that the combination of conventional US, SWE, and CEUS (US+SWE+CEUS) yielded the highest Az value of 0.897, significantly surpassing other single imaging modalities or dual imaging combinations, indicating its superior diagnostic performance for NMLs. After re-evaluating the BI-RADS classification, incorporating SWE and CEUS led to significant adjustments in the BI-RADS categorization of benign lesions. Approximately 37.9% of benign NMLs were downgraded from BI-RADS 4A, which originally required biopsy, to BI-RADS 3. In contrast, the diagnosis of malignant lesions was completely accurate, with 100% of malignant NMLs being correctly diagnosed. These results further validate the critical role of SWE and CEUS in enhancing diagnostic accuracy and adjusting the BI-RADS classification of NMLs. In studies similar to ours,10 malignant NMLs on CEUS images typically exhibit characteristics such as hyperenhancement, early enhancement, increased tumor size, and penetrating vessels. These features provide clear criteria for the clinical identification of malignant lesions. The combination of CEUS with SWE and conventional B-mode US has also been used to establish a more comprehensive evaluation framework, offering higher accuracy and reliability in the risk stratification of breast NMLs.
However, this study has several limitations. First, it is a single-center retrospective analysis with a relatively small sample size, particularly for certain types of NMLs, which may limit the generalizability of the results. Second, although we analyzed SWE quantitative parameters, we only assessed the elasticity values within a 2-mm region around the lesion, without including 1-mm or 3-mm ranges. Finally, for lesions downgraded to BI-RADS 3, the long-term clinical outcomes could not be fully confirmed without biopsy or follow-up, and this limitation should be addressed in future studies.
Conclusion
This study combined SWE and CEUS with conventional B-mode US, improving the accuracy of BI-RADS risk stratification for breast NMLs and enhancing diagnostic efficiency. This multimodal imaging approach provided more comprehensive lesion information, effectively reducing unnecessary biopsies while ensuring the timely diagnosis of malignant lesions, thereby improving clinical diagnostic efficiency and enhancing patients’ quality of life.
Funding Statement
This work was supported by the Wenzhou Municipal Science and Technology Plan Project (Grant No. Y20220451), The Key Research and Development Program of Zhejiang Province (No. 2021C03003) and The Basic Scientific Research Project of Wenzhou (2024Y0436).
Abbreviations
SWE, shear wave elastography; CEUS, contrast-enhanced ultrasound; NMLs, non-mass-like lesions; US, ultrasound; Az,areas under the receiver operating characteristic curves; BI-RADS-US, Imaging Reporting and Data System for Ultrasound; PPV, positive predictive value; NPV, negative predictive value; DCIS, ductal carcinoma in situ; IDC, invasive ductal carcinoma; Emean, mean elastic modulus for lesion; Emax, maximum elastic modulus for lesion; Emin, minimum elastic modulus for lesion; Esd, elastic modulus standard deviation for lesion; Esmean, mean elastic modulus for shell; Esmax, maximum elastic modulus for shell; Esmin, minimum elastic modulus for shell; Essd, elastic modulus standard deviation for shell; Elsmean, mean elastic modulus for lesion plus shell; Elsmax, maximum elastic modulus for lesion plus shell; Elsmin, minimum elastic modulus for lesion plus shell; Elssd, standard deviation of elastic modulus for lesion plus shell.
Ethics Approval and Informed Consent Statement
The study was approved by the Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University (Approval No.: KY2022-R169) and complies with the Declaration of Helsinki. Ethics Committee granted a waiver of consent for retrospective data analysis. All patient data were anonymized and kept confidential.
Data Sharing Statement
All data generated or analyzed during this study are included in the article.
Author Contributions
All authors made significant contributions to the work reported, whether in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all of these areas; participated in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; agreed to submit the article to this journal; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no commercial or financial relationships that could be perceived as a potential conflict of interest.
References
- 1.Ko KH, Hsu HH, Yu JC, et al. Non-mass-like breast lesions at ultrasonography: feature analysis and BI-RADS assessment. Eur J Radiol. 2015;84(1):77–85. doi: 10.1016/j.ejrad.2014.10.010 [DOI] [PubMed] [Google Scholar]
- 2.Stoblen F, Landt S, Ishaq R, et al. High-frequency breast ultrasound for the detection of microcalcifications and associated masses in BI-RADS 4a patients. Anticancer Res. 2011;31(8):2575–2581. doi: 10.1245/s10434-011-1597-3 [DOI] [PubMed] [Google Scholar]
- 3.Li J, Liu Y, Li Y, et al. Comparison of diagnostic potential of shear wave elastography between breast mass lesions and non-mass-like lesions. Eur J Radiol. 2023;158:110609. doi: 10.1016/j.ejrad.2022.110609 [DOI] [PubMed] [Google Scholar]
- 4.Qu XX, Song Y, Zhang YH, et al. Value of ultrasonic elastography and conventional ultrasonography in the differential diagnosis of non-mass-like breast lesions. Ultrasound Med Biol. 2019;45(6):1358–1366. doi: 10.1016/j.ultrasmedbio.2019.01.020 [DOI] [PubMed] [Google Scholar]
- 5.Lin M, Wu S. Ultrasound classification of non-mass breast lesions following BI-RADS presents high positive predictive value. PLoS One. 2022;17(11):e0278299. doi: 10.1371/journal.pone.0278299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang ZL, Li N, Li M, et al. Non-mass-like lesions on breast ultrasound: classification and correlation with histology. Radiol Med. 2015;120(10):905–910. doi: 10.1007/s11547-014-0493-x [DOI] [PubMed] [Google Scholar]
- 7.Ko KH, Jung HK, Kim SJ, et al. Potential role of shear-wave ultrasound elastography for the differential diagnosis of breast non-mass lesions: preliminary report. Eur Radiol. 2014;24(2):305–311. doi: 10.1007/s00330-013-3034-4 [DOI] [PubMed] [Google Scholar]
- 8.Guo W, Wang T, Li F, et al. Non-mass breast lesions: could multimodal ultrasound imaging be helpful for their diagnosis? Diagnostics (Basel). 2022;12(12):2923. doi: 10.3390/diagnostics12122923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou J, Zhan W, Chang C, et al. Breast lesions: evaluation with shear wave elastography, with special emphasis on the “stiff rim” sign. Radiology. 2014;272(1):63–72. doi: 10.1148/radiol.14130818 [DOI] [PubMed] [Google Scholar]
- 10.Zhang W, Xiao X, Xu X, et al. Non-mass breast lesions on ultrasound: feature exploration and multimode ultrasonic diagnosis. Ultrasound Med Biol. 2018;44(8):1703–1711. doi: 10.1016/j.ultrasmedbio.2018.05.005 [DOI] [PubMed] [Google Scholar]
- 11.Xu P, Yang M, Liu Y, et al. Breast non-mass-like lesions on contrast-enhanced ultrasonography: feature analysis, breast image reporting and data system classification assessm ent. World J Clin Cases. 2020;8(4):700–712. doi: 10.12998/wjcc.v8.i4.700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uematsu T. Non-mass-like lesions on breast ultrasonography: a systematic review. Breast Cancer. 2012;19(4):295–301. doi: 10.1007/s12282-012-0364-z [DOI] [PubMed] [Google Scholar]
- 13.Lee J, Lee JH, Baik S, et al. Non-mass lesions on screening breast ultrasound. Medical Ultrasonography. 2016;18(4):446. doi: 10.3390/diagnostics12122923 [DOI] [PubMed] [Google Scholar]
- 14.Xu YJ, Gong HL, Hu B, et al. Role of “Stiff Rim” sign obtained by shear wave elastography in diagnosi s and guiding therapy of breast cancer. Int J Med Sci. 2021;18(15):3615–3623. doi: 10.7150/ijms.64243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao X, Ou B, Yang H, et al. Breast contrast-enhanced ultrasound: is a scoring system feasible? A preliminary study in China. PLoS One. 2014;9(8):e105517. doi: 10.1371/journal.pone.0105517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong S, Li W, Gao W, et al. Diagnostic performance of elastography for breast non-mass lesions: a systematic review and meta-analysis. Eur J Radiol. 2021;144:109991. doi: 10.1016/j.ejrad.2021.109991 [DOI] [PubMed] [Google Scholar]
- 17.Youk JH, Son EJ, Park AY, et al. Shear-wave elastography for breast masses: local shear wave speed (m/sec) versus Young modulus (kPa). Ultrasonography. 2014;33(1):34–39. doi: 10.14366/usg.13005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ece B, Aydin S, Kantarci M. Shear wave elastography-correlated dose modifying: can we reduce corticosteroid doses in idiopathic granulomatous mastitis treatment? Preliminary Results Journal of Clinical Medicine. 2023;12(6). doi: 10.3390/jcm12062265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li W, Zhou Q, Xia S, et al. Application of contrast‐enhanced ultrasound in the diagnosis of ductal carcinoma in situ: analysis of 127 cases. J Ultrasound Med. 2020;39(1):39–50. doi: 10.1002/jum.15069 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in the article.