Abstract
Purpose
Atrial fibrillation (AF) being a prevalent cardiovascular condition globally, has an increased risk of stroke and other complications. The effective management of AF often involves the use of oral anticoagulants (OACs) to prevent thromboembolic events. This study aimed to evaluate anticoagulation knowledge and medication adherence in AF patients on OACs at a tertiary care center in Nepal.
Patients and Methods
A descriptive cross-sectional study involving patients diagnosed with AF who were prescribed OACs at the Cardiology Department of Dhulikhel Hospital, Nepal, was conducted from March to June 2024. Data were collected using questionnaires, including the Oral Anticoagulation Knowledge Tool (AKT) and the Adherence in Chronic Diseases Scale (ACDS). The study included patients from the Dhulikhel Atrial Fibrillation (DAF) Registry database along with other AF patients visiting the cardiac department. Descriptive statistics were used to summarize patient demographics, knowledge scores, and adherence levels. Inferential statistics were used to observe the associations.
Results
Among the 114 AF patients enrolled in the study, 93 were receiving OAC therapy and were interviewed. The mean age of the participants was 66.84 ± 12.3 years, with the majority being female (57%). The study revealed that a significant portion of patients lacked adequate knowledge about their OAC therapy, with only 48% having adequate knowledge as per the AKT. Additionally, 83.9% of the patients demonstrated high adherence to their medication regimen, whereas 16.1% showed medium adherence. The duration of use of OACs was found to be significantly associated with adequate anticoagulation knowledge.
Conclusion
The study findings indicate that a significant proportion of AF patients in Nepal lack adequate anticoagulation knowledge, highlighting an opportunity for improved educational interventions.
Keywords: anticoagulation knowledge, knowledge gaps, adherence, stroke prevention
Introduction
Atrial fibrillation (AF), the most prevalent cardiac arrhythmia, occurs when abnormal electrical activity in the atria causes rapid and irregular heartbeats.1 It is the most common sustained arrhythmia requiring hospital admission.2 AF has a significant effect on public health, including decreased quality of life, increased hospitalization rates, stroke incidence, and increased medical costs.3 It has been projected that 6 to 12 million individuals globally are expected to experience this ailment in the United States by the year 2050, with an estimated 17.9 million cases in Europe by 2060.4,5 The currently estimated prevalence of AF in adults is between 2% and 4%, and a 2.3-fold increase is expected owing to extended longevity in the general population and an intensified search for undiagnosed AF.6 Australia, Europe, and the USA have the highest reported prevalence of AF (1% in the adult population), but the prevalence of AF in low-income and middle-income countries is probably underestimated.7 In 2010, there were an estimated 33.5 million cases of AF globally (20.9 million men and 12.6 million women), with significant regional variations and heterogeneity and approximately 5 million new cases annually.8 The overall prevalence of AF in Asia is less than 1%.9 The prevalence of AF in Nepal was 13.8%, which was higher than that in Western countries, mainly because of endemic rheumatic heart disease.10 Another study done in Sindhupalchowk, Nepal found AF prevalence to be 11% among the elderly.11
Patients with AF in low- or middle-income countries (LMICs) have higher morbidity and mortality rates than do those with AF in high-income countries, with limited data on the economic burden in LMICs. Various studies from different countries, such as Algeria, China, Brazil, and India, have highlighted the economic burden of AF, with costs per patient-year ranging from 1003 USD to 8020 USD.12
Oral anticoagulation (OAC) is the most effective treatment for preventing ischemic stroke and systemic embolism associated with AF.13 Vitamin K antagonists (VKA: Warfarin, Acenocoumarole, Phenindione) and NOAC (Dabigatran, Rivaroxaban, Apixaban) comes under OAC group.14 NOACs provide advantages over traditional vitamin K antagonists such as warfarin, including reduced bleeding risk, the necessity for less monitoring, and more predictable pharmacological effects.15 Patients’ understanding of their medication and medical condition can influence the effectiveness of their treatment.16 This becomes especially crucial for patients on oral anticoagulants, given the narrow therapeutic range of these medications and the severe consequences that can result from either undertreatment or excessive anticoagulation.17 This is why information with respect to medicine is imperative for patients. Better drug-taking practices are influenced by knowledge of the disease, an understanding of why the drug is needed, and positive expectations or attitudes toward the course of therapy.18 Likewise, adherence to medication is vital for successful treatment, as noncompliance can worsen health conditions, increase healthcare costs, and even lead to fatal outcomes.19
There have been few studies on AF, including those specializing in warfarin therapy. A cross-sectional study performed at Shahid Gangalal National Heart Centre (SGNHC) in Kathmandu reported moderate knowledge of anticoagulation and good compliance with medication.20 Similarly, another study conducted at Manmohan Cardiothoracic Vascular and Transplant Center, Institute of Medicine, reported insufficient use of anticoagulants for both valvular and nonvalvular atrial fibrillation, which may be attributed to economic limitations and geographical challenges.21 Another study concluded that despite the increasing use of NOACs in patients with a higher risk of stroke, anticoagulants are still underutilized in most cases.22 In addition to some studies that focused on the prevalence and utilization of AF, knowledge and adherence have not been assessed in AF patients prescribed OACs. Therefore, this study aimed to assess knowledge and adherence among AF patients.
Materials and Methods
Recruitment
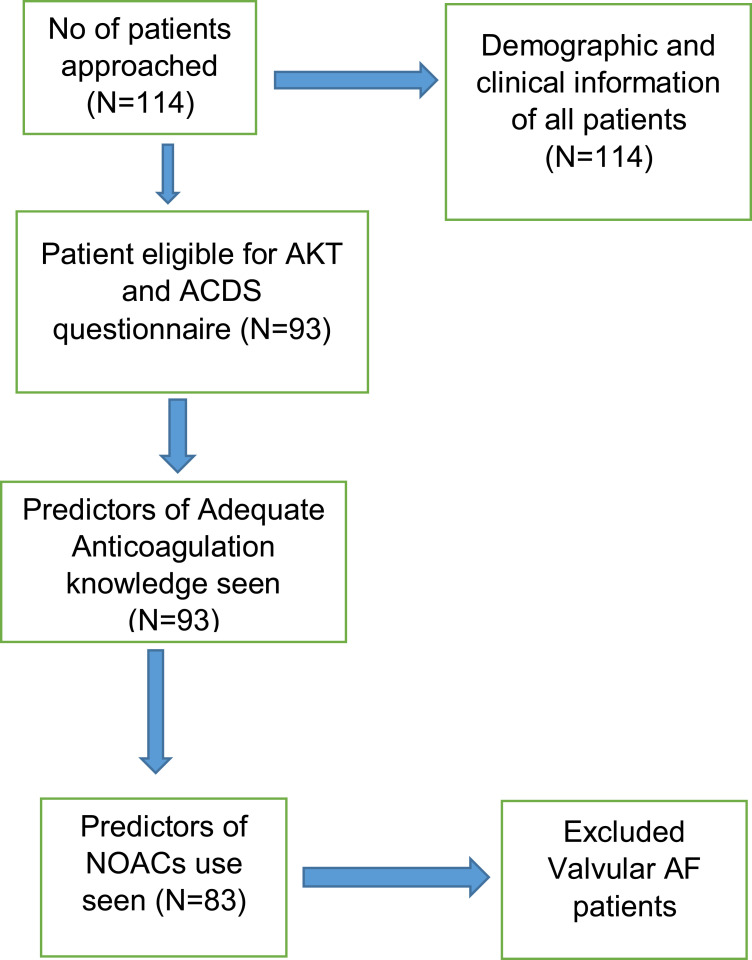
This quantitative, cross-sectional study was conducted in the cardiac outpatient department of Dhulikhel Hospital, Nepal, for four months (March to June 2024). The study adhered to the principles outlined in the Declaration of Helsinki and ethical approval was obtained from the Institutional Review Committee of Kathmandu University School of Medical Sciences (IRC-KUSMS Approval Number: 73/24). Patients from the Dhulikhel AF registry and other patients visiting the cardiac outpatient department were included. Patients who had taken any OAC drugs for at least 1 month and had a diagnosis of AF were included in the study through purposive convenience sampling. Written informed consent was obtained from the patients, clearly outlining the study’s purpose and its potential impact, while ensuring the confidentiality of all patient information. The study included 114 patients diagnosed with AF. Among those, 93 patients were on OACs and were included in the study and interviewed for AKT and ACDS questionnaires. Patients who were not on OACs or had valvular AF were excluded from the final analysis, leading 83 patients to be analyzed for predictors of NOACs use (Figure 1).
Figure 1.
Flowchart of Study Design.
Abbreviations: AKT, Oral Anticoagulation Knowledge Tool; ACDS, Chronic Disease Adherence Scale; NOACs, Non-Vitamin-K Oral Anticoagulants; AF, Atrial Fibrillation.
Data Collection Tools
The data collection tool was divided into three parts. The first section focused on the patients’ sociodemographic information and clinical characteristics. The second section is the questionnaire on the AKT, and the third section is the ACDS. The participants were asked if they currently had a diagnosis of AF and then asked whether they were on OACs or not and how long they had been taking it. Face‒to-face interviews were conducted with patients via the Oral Anticoagulation Knowledge Tool (AKT) and Chronic Disease Adherence Scale (ACDS), which were shown to have acceptable validity and reliability in a previous study.23,24 The questionnaire was translated into the Nepali language and then back-translated to English to retain the previous concept of the questionnaire. The pilot study was performed among 15 patients at Dhulikhel Hospital, and the data were excluded from the final analysis. The reliability of the questionnaire was checked and found to be 0.780.
Oral Anticoagulation Knowledge Tool (AKT)
Anticoagulation knowledge was assessed via the Oral Anticoagulation Knowledge tool, which was developed and validated in patients taking either VKA (Warfarin) or NOACs.23 The AKT has two sections: general questions (Section A – 20 items) and warfarin-specific questions (Section B – 8 items). All participants were required to answer section A, but only those on warfarin needed to answer section B. Participants on NOACs could achieve a maximum score of 25 points, as they were only required to complete Section A. Those on warfarin could score up to 35 points, needing to complete both Sections A and B.23 Final scores were presented as a percentage of correct answers for all participants.
Adherence in Chronic Disease Scale (ACDS)
The ACDS is a 7-item questionnaire.24 The scale for chronic diseases comprises 7 questions, each offering 5 possible answer options. The first 5 questions focus on behaviors that directly influence adherence, whereas the last 2 questions address circumstances and perspectives that might indirectly impact adherence.24 Scores were calculated as stated in the original reports.24
Variables
Dependent Variables: Knowledge, Adherence
Independent variables: Age, gender, level of education, duration since OAC use, length of time since AF diagnosis, CHA2DS2VASC score, HASBLED score, smoking, alcohol
Data Analysis
SPSS version 20 was used to analyze the data. Continuous variables are expressed as the means ± SDs. Categorical variables are presented as percentages and definite values. Categorical variables were compared via the chi-square test and Fisher’s exact test. A p value less than 0.05 was considered statistically significant. Knowledge of OAC was assessed via total AKT scores, which were determined by counting the number of correct responses and converting them into percentages. A cutoff of > 50% was considered an adequate knowledge score. An adequate knowledge threshold was set at 50% or higher. Thus, participants achieving 50% or more on their respective total score (converted to a percentage) were considered to have adequate anticoagulation knowledge, regardless of whether they were on NOACs or warfarin. Medication adherence scores were calculated by summing the numbers assigned to each response. The overall mean and median scores were calculated for both sections, and the mean scores of both sections were also reported for all demographic groups. The Mann–Whitney U-test (for two groups) and the Kruskal–Wallis test (for more than two groups) were used to compare mean scores across different demographic characteristics. A p value of less than 0.05 was considered statistically significant. Binary logistic regression was conducted to identify the factors associated with adequate knowledge scores and predictors of NOACs. Both univariate and multivariate logistic regression analyses were performed. A p value of less than 0.05 was considered statistically significant.
Results
Table 1 presents the baseline characteristics of 114 AF patients. The majority of patients were aged between 18 and 64 years. The mean age was 66.84 years, with a standard deviation (SD) of 12.3. The majority of patients were aged 18--64 years (n=43, 37.7%), followed by those aged 65--74 years (n=38, 33.3%) and 75 years and above (n=33, 28.9%). More than half (57%) of the patients were females. In terms of social habits, the majority of patients were former smokers (67.5%) or former drinkers (56.1%). More than half (85.1%) of the patients were NVAF in origin.
Table 1.
Baseline Characteristics of AF Patients (N=114)
| Parameter | Overall Sample (n=114) | |
|---|---|---|
| Frequency (n) | Percentage (%) | |
| Gender | ||
| Female | 65 | 57 |
| Male | 49 | 43 |
| Age group of patients | ||
| Age, years, Mean (SD) | 66.84 (12.3) | |
| 18 to 64 | 43 | 37.7 |
| 65 to 74 | 38 | 33.3 |
| ≥75 | 33 | 28.9 |
| Highest education level | ||
| No formal Education | 85 | 74.6 |
| Below SLC | 20 | 17.5 |
| SLC and above | 9 | 7.9 |
| Duration since AF diagnosis | ||
| Less than 2 years | 46 | 40.4 |
| More than 2 years | 68 | 59.6 |
| Employment status | ||
| Employed | 7 | 6.1 |
| Unemployed | 107 | 93.9 |
| Smoking | ||
| Yes | 3 | 2.6 |
| No | 34 | 29.8 |
| Ex-smoker | 77 | 67.5 |
| Alcohol | ||
| Yes | 2 | 1.8 |
| No | 48 | 42.1 |
| Exdrinker | 64 | 56.1 |
| CHA2DS2VASC RISK | ||
| CHA2 DS2 VASC score Mean(SD) | 2.58 (1.42) | |
| Low risk (0) | 6 | 5.3 |
| Intermediate risk (1) | 24 | 21.1 |
| High risk (≥2) | 84 | 73.7 |
| HASBLED RISK | ||
| HASBLED score Mean (SD) | 0.96 (0.775) | |
| Low risk (0) | 34 | 29.8 |
| Moderate risk (1–2) | 78 | 68.4 |
| High risk (≥3) | 2 | 1.8 |
| BMI of patients | ||
| Severely underweight (< 16.5 kg/m^2) | 3 | 2.6 |
| Underweight (16.5–18.5 kg/m^2) | 6 | 5.3 |
| Normal weight (18.5 to 24.9 kg/m^2) | 58 | 50.9 |
| Obesity (≥30 kg/m^2) | 47 | 41.2 |
| AF Type | ||
| Valvular AF | 17 | 14.9 |
| Non Valvular AF | 97 | 85.1 |
| AF Type | ||
| Unknown | 94 | 82.5 |
| Paroxysmal AF | 17 | 14.9 |
| Permanent AF | 1 | 0.9 |
| Persistent AF | 2 | 1.8 |
| Category of drug used | ||
| No antithrombotic therapy | 4 | 3.5 |
| OAC therapy | 93 | 81.6 |
| Antiplatelet therapy | 17 | 14.9 |
| Comorbidities | ||
| Rheumatic heart Disease | 26 | 22.8 |
| COPD | 29 | 25.4 |
| Acute Kidney Injury | 8 | 7.0 |
| Other comorbidities | 63 | 55.3 |
| Congestive heart failure(Signs/symptoms of heart failure or objective evidence of reduced left ventricular ejection fraction) | 35 | 30.7 |
| Dyslipidemia | 4 | 3.5 |
| Carotid Artery Disease | 6 | 5.3 |
| Hypothyroidism | 14 | 12.3 |
| Hypertension(Resting BP >140/90 mmHg on at least two occasions or current antihypertensive treatment) fraction | 45 | 39.5 |
| Age (65–74 yrs) | 38 | 33.3 |
| Age ≥ 75yrs | 33 | 28.9 |
| Diabetes mellitus(Fasting glucose >125 mg/dL (7 mmol/L) or treatment with oral hypoglycemic agent and/or insulin) | 16 | 14.0 |
| Prior stroke, transient ischemic attack, or Thromboembolism | 14 | 12.3 |
| Stroke | 12 | 10.5 |
| Bleeding history/Predisposition(anemia) | 9 | 7.9 |
| Elderly (>65yr) | 71 | 62.3 |
| Antiplatelet therapy | 15 | 13.2 |
Abbreviations: AF, atrial fibrillation; CHA2DS2VASC, congestive heart failure, hypertension, age ≥75 years (doubled), diabetes mellitus, prior stroke or transient ischemic attack or thromboembolism (doubled), vascular disease, age 65 to 74 years, sex category; HASBLED, Hypertension, Abnormal renal/liver function, Stroke, Bleeding history or predisposition, Labile international normalized ratio, Elderly (.65 years), Drugs/alcohol concomitantly; BMI, Body Mass Index; OAC, Oral Anticoagulants; COPD, Chronic Obstructive Pulmonary Disease.
The most common comorbidity was hypertension (n=45, 39.5%), followed by congestive heart failure (n=35, 30.7%), COPD (n= 29, 25.4%), RHD (n=26, 22.8%), and DM (n=16. 14%), hypothyroidism (n=14, 12.3%), stroke (n=12, 10.5%), and CAD (n=6, 5.3%).
The mean CHA2DS2VASC score was 2.58 (±1.42). Most of the patients had a CHA2DS2VASC score of 3 (n=31, 27.2%), followed by 2 (n=25, 21.9%), 1 (n=24, 21.1%), 5 (n=14, 12.3%), 4 (n=13, 11.4%) and 0 (n=6, 5.3%). The mean HASBLED value without the labile INR was 0.96 (±0.775). Most patients had high CHA2DS2VASc scores, indicating a high risk of stroke, and moderate HASBLED scores, indicating a moderate risk of bleeding. The antithrombotic treatment distribution of 114 patients revealed that the majority of patients received anticoagulants (n=93, 81.6%), followed by antiplatelet therapy (n=17, 14.9%) and no therapy (n=4, 3.5%). A total of 74.6% had no formal education, 17.5% had education below SLC, and 7.9% had done SLC or above.
Most of the participants used rivaroxaban (n=51, 54.8%), more than one quarter used warfarin (n=34, 36.6%), and few used apixaban (n=8, 8.6%) (Table 2).
Table 2.
Types of OACs Used (N=93)
| OACs Type | n | % |
|---|---|---|
| Warfarin | 34 | 36.6 |
| Rivaroxaban | 51 | 54.81 |
| Apixaban | 8 | 8.6 |
Abbreviation: OACs, Oral Anticoagulants.
From Table 3, there was significant difference in age distribution between VKA and NOAC users (p=0.001). There was a significant difference in the proportion of patients aged >75 years (p=0.003), with more in the NOAC group (32.2%) than the VKA group (5.9%). Education levels differed significantly between groups (p=0.008). The NOAC group had a higher proportion of patients with no formal education (86.4%) compared to the VKA group (58.8%). Likewise, there was a significant difference in the length of time since AF diagnosis (p<0.001). The majority of VKA users (94.1%) had been diagnosed for more than 2 years, compared to only 49.2% of NOAC users. CHA2DS2VASC scores differed significantly between groups (p<0.001). A higher proportion of NOAC users (86.4%) were categorized as high risk compared to VKA users (52.9%). The duration of OAC use differed significantly among the groups (p<0.001). While on comorbidities, RHD (p<0.001) and Hypertension (p=0.001) were found to have significant difference between the groups.
Table 3.
Characteristics of AF Patients Taking VKA and NOACs (N=93)
| S.N. | Variables | Total OAC Sample (n=93) | VKA (n=34) |
NOAC (n=59) |
P value |
|---|---|---|---|---|---|
| 1 | Age, n (%) | ||||
| 18 to 64 years | 37(39.8) | 18(52.9) | 19(32.2) | ||
| 65 to 74 years | 34(36.6) | 14(41.2) | 20(33.9) | 0.007* | |
| ≥ 75 years | 22(23.7) | 2(5.9) | 20(33.9) | ||
| 2 | Gender, n (%) | ||||
| Female | 58(62.4) | 24(70.6) | 34(57.6) | 0.214 | |
| Male | 35(37.6) | 10(29.4) | 25(42.4) | ||
| 3 | Education level, n (%) | ||||
| No formal education | 71(76.3) | 20(58.8) | 51(86.4) | ||
| Below SLC | 15(16.1) | 9(26.5) | 6(10.2) | 0.008* | |
| SLC and above | 7(7.5) | 5(14.7) | 2(3.4) | ||
| 4 | Employment, n (%) | ||||
| Employed | 5(5.4) | 3(8.8) | 2(3.4) | 0.263 | |
| Unemployed | 88(94.6) | 31(91.2) | 57(96.6) | ||
| 5 | Duration since diagnosis of AF, n (%) | ||||
| Less than 2 years | 32(34.4) | 2(5.9) | 30(50.8) | <0.001* | |
| More than 2 years | 61(65.6) | 32(94.1) | 29(49.2) | ||
| 6 | Smoking, n (%) | ||||
| Yes | 3(3.2) | 1(2.9) | 2(3.4) | 0.666 | |
| No | 31(33.3) | 9(26.5) | 22(37.3) | ||
| Ex-smoker | 59(63.4) | 24(70.6) | 35(59.3) | ||
| 7 | Alcohol, n (%) | ||||
| Yes | 2(2.2) | 1(2.9) | 1(1.7) | 1.000 | |
| No | 39(41.9) | 14(41.2) | 25(42.4) | ||
| Ex-drinker | 52(55.9) | 19(55.9) | 33(55.9) | ||
| 8 | CHA2DS2VASC risk, n (%) | ||||
| Low risk (0) | 5(5.4) | 4(11.8) | 1(1.7) | ||
| Intermediate risk (1) | 19(20.4) | 12(35.3) | 7(11.9) | <0.001* | |
| High risk (≥2) | 69(74.2) | 18(52.9) | 51(86.4) | ||
| 9 | HASBLED risk, n (%) | ||||
| Low risk (0) | 32(34.4) | 15(44.1) | 17(28.8) | 0.245 | |
| Moderate risk (1–2) | 60(64.5) | 19(55.9) | 41(69.5) | ||
| High risk (≥3) | 1(1.1) | - | 1(1.7) | ||
| 10 | Duration since OAC use, n (%) | ||||
| Less than 2 years | 36(38.7) | 2(5.9) | 34(57.6) | ||
| More than 2 years | 57(61.3) | 32(94.1) | 25(42.4) | <0.001* | |
| 11 | RHD | ||||
| No | 69(74.2) | 12(35.3) | 57(96.6) | <0.001* | |
| Yes | 24(25.8) | 22(64.7) | 2(3.4) | ||
| 12 | COPD | ||||
| No | 74(79.6) | 29(85.3) | 45(76.3) | 0.299 | |
| Yes | 19(20.4) | 5(14.7) | 14(23.7) | ||
| 13 | Hypertension | ||||
| No | 58(62.4) | 31(91.2) | 27(45.8) | <0.001* | |
| Yes | 35(37.6) | 3(8.8) | 32(54.2) | ||
| 14 | Age (65–74 years) | ||||
| No | 41(44.1) | 19(55.9) | 22(37.3) | 0.082 | |
| Yes | 52(55.9) | 15(44.1) | 37(62.7) | ||
| 15 | Age (>75 years) | ||||
| No | 72(77.4) | 32(94.1) | 40(67.8) | 0.003* | |
| Yes | 21(22.6) | 2(5.9) | 19(32.2) | ||
| 16 | Diabetes Mellitus | ||||
| No | 81(87.1) | 32(94.1) | 49(83.1) | 0.125 | |
| Yes | 12(12.9) | 2(5.9) | 10(16.1) | ||
| 17 | Prior Stroke | ||||
| No | 82(88.2) | 29(85.3) | 53(89.8) | 0.514 | |
| Yes | 11(11.8) | 5(14.7) | 6(10.2) | ||
| 18 | Bleeding History | ||||
| No | 86(92.5) | 30(88.2) | 56(94.9) | 0.240 | |
| Yes | 7(7.5) | 4(11.8) | 3(5.1) |
Note: (*) indicates statistical significance with p<0.05 (95% confidence interval).
Abbreviations: AF, atrial fibrillation; VKA, Vitamin K antagonists; NOACs, Non Vitamin K Antagonist Oral Anticoagulants; CHA2DS2VASC, congestive heart failure, hypertension, age ≥75 years (doubled), diabetes mellitus, prior stroke or transient ischemic attack or thromboembolism (doubled), vascular disease, age 65 to 74 years, sex category; HASBLED, Hypertension, Abnormal renal/liver function, Stroke, Bleeding history or predisposition, Labile international normalized ratio, Elderly (. 65 years), Drugs/alcohol concomitantly; BMI, Body Mass Index; OAC, Oral Anticoagulants; RHD, Rheumatic Heart Disease; COPD, Chronic Obstructive Pulmonary Disease.
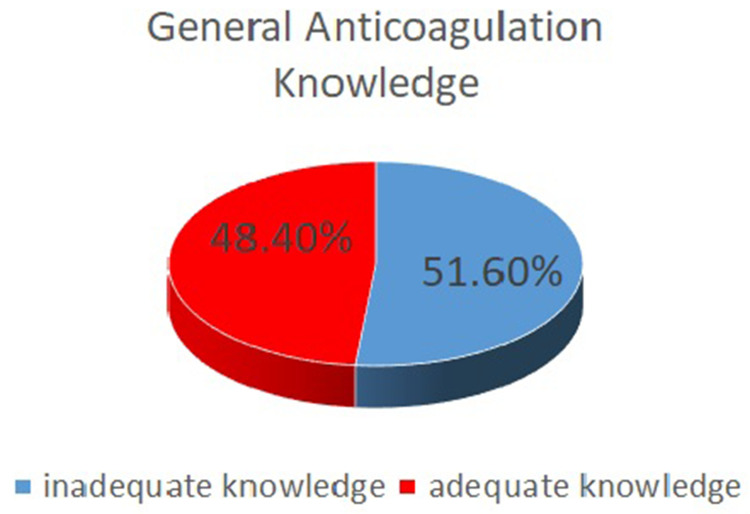
In the knowledge assessment, patients on VKA had higher median knowledge scores than those on NOACs did (p=0.001) (Table 4). Overall anticoagulation knowledge was inadequate (51.6%) (Figure 2).
Table 4.
Median(IQR) Knowledge Scores in Patients (N=93)
| Interquartile Range of Knowledge Scores | p valuea | ||
|---|---|---|---|
| VKA (n=34) | NOAC (n=59) | ||
| General Knowledge (Section A) | 14(11–17) | 12(9–14) | 0.001 |
| Warfarin related knowledge (Section B)b | 3.50(2.75–5) | – | – |
| General plus specific knowledge (Section A + B) | 18(13–30) | 12(9–14) | <0.001 |
Notes: amann–Whitney U-test between VKA and NOAC groups. bSection B is only applicable to VKA group.
Abbreviations: IQR, Inter-quartile range; NOAC, Non-Vitamin-K-Antagonist Oral Anticoagulants; VKA, Vitamin K antagonist.
Figure 2.
Distribution of Anticoagulation Knowledge.
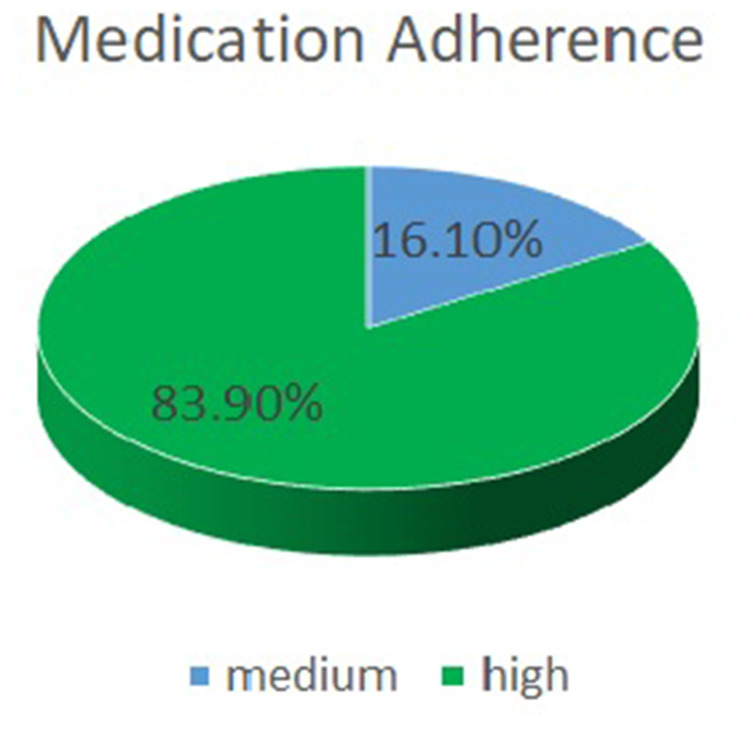
When medication adherence was assessed, there were no significant differences in the median scores (p=0.193) between the two groups (Table 5). Medium (83.90%) and high adherence (16.10%) rates were reported by most patients (Figure 3).
Table 5.
Medication Adherence Median(IQR) Scores in Patients (N=93)
| Interquartile Range of Adherence Scores | P value | ||
|---|---|---|---|
| VKA (n=34) | NOAC (n=59) | ||
| Adherence score | 29(26.75–30) | 29(28–30) | 0.193 |
Abbreviations: IQR, Inter-quartile range; NOAC, Non-Vitamin-K-Antagonist Oral Anticoagulants; VKA, Vitamin K antagonist.
Figure 3.
Distribution of Medication Adherence.
Abbreviations: AF, Atrial fibrillation; OACs, Oral Anticoagulants; LMICs, Low or middle income country; AKT, Oral Anticoagulation Knowledge Tool; ACDS, Chronic Disease Adherence Scale; NOACs, Non-Vitamin-K-Antagonist Oral Anticoagulants; VKA, Vitamin K Antagonists.
From Table 6, while assessing for association between level of knowledge and adherence, there was no significant association seen between them.
Table 6.
Association Between Knowledge and Adherence
| Variables | Level of Knowledge | Chi-square | p value | ||
|---|---|---|---|---|---|
| Adequate Knowledge | Inadequate Knowledge | ||||
| Level of Adherence | Medium adherence | 6 | 9 | 0.504 | 0.478 |
| High adherence | 39 | 39 | |||
According to the binary logistic regression, the duration since the use of oral anticoagulants was found to be associated with adequate knowledge (OR, 1.018; 95% CI, 1.002--1.033; p=0.026) (Table 7).
Table 7.
Factors Associated with Adequate Knowledge
| S.N. | Odds of Adequate Anticoagulation Knowledge | Univariate Odds Ratio (95% CI) | p value | Multivariate Odds Ratio (95% CI) | p value |
|---|---|---|---|---|---|
| 1 | Age | 0.95(0.91–0.99) | 0.013* | 0.98(0.92–1.03) | 0.389 |
| 2 | Gender (Female*/Male) | 1.22(0.53–2.82) | 0.649 | – | – |
| 3 | Total Adherence | 1.11(0.93–1.31) | 0.253 | – | – |
| 4 | Duration since use of Oral anticoagulants | 1.02(1.01–1.03) | 0.001* | 1.018(1.002–1.033) | 0.026* |
| 5 | Level of Education | ||||
| No formal education | |||||
| Below SLC | 0.29(0.05–1.61) | 0.158 | 0.47(0.06–3.78) | 0.478 | |
| SLC and Above* | 0.80(0.11–5.68) | 0.823 | 0.99(0.10–9.56) | 0.991 | |
| 6 | Employment status | – | – | ||
| (Employed*/Unemployed) | 4.59(0.49–42.69) | 0.181 | |||
| 7 | Current Oral Anticoagulant taken by patient | ||||
| Warfarin* | |||||
| Rivaroxaban | 0.31(0.12–0.77) | 0.012* | 1.48(0.24–9.06) | 0.670 | |
| Apixaban | 0.16(0.03–0.92) | 0.040* | 1.22(0.09–15.48) | 0.877 | |
| 8 | Rheumatic Heart Disease | ||||
| Yes*/No | 0.36(0.14–0.96) | 0.041* | 0.87(0.17–4.42) | 0.867 | |
| 9 | Chronic Obstructive Pulmonary Disease | ||||
| Yes*/No | 2.41(0.83–7.04) | 0.106 | 1.86(0.48–7.29) | 0.373 | |
| 10 | Age>75 years | – | – | ||
| Yes*/No | 5.62(1.72–18.38) | 0.004* | |||
| 11 | Duration since AF diagnosis | 1.01(1.00–1.02) | 0.002* | – | – |
| 12 | Diabetes mellitus | ||||
| Yes*/No | 2.38(0.66–8.53) | 0.184 | 0.26(0.06–1.19) | 0.083 | |
| 13 | Bleeding History | ||||
| Yes*/No | 0.35(0.06–1.89) | 0.222 | 0.30(0.04–2.32) | 0.250 | |
| 14 | Body Mass Index | ||||
| Yes*/No | 1.11(1.00–1.23) | 0.043* | – | – | |
| 15 | CHA2DS2VASC risk | – | – | ||
| Low risk | 1.95(0.31–12.42) | 0.480 | |||
| Intermediate risk | 2.23(0.78–6.35) | 0.133 | |||
| High risk* |
Notes: (*) indicates odds ratio and adjusted odds ratio with 95%confidence interval is significant at p<0.05.
Abbreviations: AF, atrial fibrillation; CHA2DS2VASC, congestive heart failure, hypertension, age ≥75 years (doubled), diabetes mellitus, prior stroke or transient ischemic attack or thromboembolism (doubled), vascular disease, age 65 to 74 years, sex category.
Similarly, for predictors of NOAC use, duration since the use of oral anticoagulants (OR, 1.01; 95% CI, 0.99–1.03; p=0.007) and hypertension (OR, 0.00; 95% CI, 0.00–0.44; p=0.023) were found to be associated with NOAC use (Table 8).
Table 8.
Predictors of NOAC Use Over Warfarin
| S.N. | Odds of NOACs Use | Univariate Odds Ratio (95% CI) | p value | Multivariate Odds Ratio (95% CI) | p value |
|---|---|---|---|---|---|
| 1 | Age of the patient | 1.09(1.04–1.15) | 0.001* | 1.14(0.96–1.35) | 0.146 |
| 2 | Gender | 1.76(0.72–4.34) | 0.216 | – | – |
| Female*/Male | |||||
| 3 | Duration since AF diagnosis | 0.99(0.98–0.99) | 0.001* | 1.01(0.99–1.03) | 0.487 |
| 4 | Duration since use of Oral anticoagulants | 0.94(0.91–0.99) | 0.000* | 0.86(0.77–0.96) | 0.007* |
| 5 | Level of Education | ||||
| No formal education* | – | – | – | – | |
| Below SLC | 0.19(0.06–0.66) | 0.009* | 0.05(0.00–3.02) | 0.150 | |
| SLC and Above | 0.139(0.025–0.789) | 0.026* | 0.02(0.00–2.68) | 0.118 | |
| 6 | Hypertension | ||||
| Yes*/No | 0.05(0.01–0.25) | 0.000* | 0.00(0.00–0.44) | 0.023* | |
| 7 | Diabetes mellitus | ||||
| Yes/No* | 3.26(0.67–15.89) | 0.143 | – | – | |
| 8 | Rheumatic Heart Disease | – | – | ||
| Yes/No* | 0.02(0.00–0.09) | 0.000* | |||
| 9 | CHA2DS2VASc risk | ||||
| Low risk | 0.08(0.01–0.75) | 0.028* | 13.56(0.19–986.83) | 0.233 | |
| Intermediate risk | 0.18(0.060–0.55) | 0.003* | 1.43(0.04–45.13) | 0.838 | |
| High risk* | |||||
| 10 | HASBLED risk | – | – | ||
| Low risk | 0 | 1.000 | |||
| Intermediate risk | 0 | 1.000 | |||
| High risk* | |||||
| 11 | Body Mass Index | 1.11(0.99–1.24) | 0.055 | – | – |
| 12 | Bleeding History | 0.40(0.08–1.91) | 0.252 | – | – |
| Yes/No* |
Note: (*) indicates odds ratio and adjusted odds ratio with 95% confidence interval is significant at p<0.05.
Abbreviations: AF, atrial fibrillation; NOACs, Non Vitamin K Antagonist Oral Anticoagulants; CHA2DS2VASC, congestive heart failure, hypertension, age ≥75 years (doubled), diabetes mellitus, prior stroke or transient ischemic attack or thromboembolism (doubled), vascular disease, age 65 to 74 years, sex category; HASBLED, Hypertension, Abnormal renal/liver function, Stroke, Bleeding history or predisposition, Labile international normalized ratio, Elderly (. 65 years), Drugs/alcohol concomitantly.
Discussion
This study aimed to assess oral anticoagulation knowledge and medication adherence among AF patients prescribed OACs in a cardiac outpatient department of a tertiary care hospital in Nepal. The demographic analysis revealed that the patient population had a high burden of comorbidities, with significant rates of hypertension (39.5%), COPD (25.4%), and congestive heart failure (30.7%). This demographic profile aligns with the literature, which indicates that AF predominantly affects older adults with multiple comorbidities.25–27
A total of 45 (48.4%) patients had overall adequate knowledge of anticoagulation, accounting for more than half of the patients who had inadequate knowledge. Considering the demographics, since most of the patients had no formal education, this might have resulted in gaps in their knowledge. The median anticoagulation knowledge score of those on warfarin was significantly higher than that of those on NOACs. Several possible explanations could account for this finding. Since warfarin requires frequent monitoring after the International Normalized Ratio (INR) test and dose adjustments if needed, these patients might have received comprehensive counseling. In contrast, those taking NOACs required less monitoring and had fewer visits to the hospital. These findings align with those of other studies conducted in various populations, where suboptimal knowledge of oral anticoagulants has been consistently reported. Our findings align with global studies highlighting gaps in anticoagulation knowledge among AF patients, yet reveal unique aspects specific to the Nepalese context. Similar to studies conducted in Australia and the United States, our research found suboptimal knowledge levels among AF patients, particularly those on NOACs, suggesting a need for consistent patient education across all anticoagulant types.17,28–30
A significant finding of this study was the association between the duration of OAC use and the level of anticoagulation knowledge. Patients with a longer duration of OAC use were more likely to have adequate knowledge about their oral anticoagulant therapy.31 These findings suggest that ongoing education and reinforcement over time may improve patients’ understanding of their treatment regimens.
Participants who are well versed in anticoagulation can make more informed decisions and manage their illness on their own. This study revealed that patients receiving NOACs have limited awareness of oral anticoagulants. It is critical to incorporate knowledge assessment into counseling programs and deliver it to patients with atrial fibrillation at the start of their oral anticoagulant therapy and on an ongoing basis to investigate and address an awareness gap. In the absence of frequent coagulation monitoring for NOACs, a comparable follow-up session could be developed for NOAC users to assess their understanding of oral anticoagulants and other patient-related outcomes.
During the assessment of medication adherence, most patients had high adherence scores. These findings are similar to studies performed across the world and in Nepal.20,32 In contrast to studies where AF patients seem to be nonadherent to their medication, the context here is different.33 The reason behind this might be the regular follow-up of the patients due to the health insurance policy of the hospital. The patients were provided medication for one month, and they had to revisit the doctor to prescribe their medication. Though the patients showed good adherence, since their knowledge seemed to be inadequate, they might lack essential information. This comparison shows that adopting international practices, such as providing consistent education for all patients, could improve safe and effective anticoagulation in Nepal. Tailored education programs could help close knowledge gaps and better support AF patients in managing their treatment.
To the best of our knowledge, this study is the first to compare anticoagulation knowledge between patients taking warfarin and those taking NOACs among AF patients in Nepal. This offers insights that could guide local clinical practice and inform future guidelines. The findings of this study contribute to the existing limited research on anticoagulation knowledge among AF patients prescribed OACs and further focus on interventions to improve this knowledge. Our study suggests that NOAC users often receive less education about their medication compared to warfarin users, possibly due to NOACs’ simpler dosing and monitoring. This knowledge gap indicates that clinical practice could be improved by providing consistent education to all AF patients, regardless of the anticoagulant they use. Standardized educational protocols for all AF patients on medication risks and adherence, including routine sessions for NOAC users, could improve understanding and adherence, ultimately lowering risks of stroke and bleeding complications. Similarly, the findings of NOAC predictors of use among OAC patients enabled us to identify subjects who are more likely to be prescribed a NOAC, allowing us to focus interventions on promoting proper NOAC use.
This study underscores the importance of accessible and consistent patient education on anticoagulant therapy, regardless of medication type. Policymakers could use these findings to support initiatives that prioritize standardized patient education as a routine part of care for AF patients. Integrating such education into public health guidelines could improve patient outcomes, reduce preventable complications, and potentially lower healthcare costs associated with inadequate anticoagulation management. Our study highlights a knowledge gap between warfarin and NOAC users, adding evidence from Nepal to support the need for consistent anticoagulant education across settings.
Limitations
Our study has several limitations. First, the number of patients was small compared with that in other studies, probably due to the lower prevalence of AF. Second, as this was a single-center study, the findings could be generalizable. Third, among the patients who were found in adequate numbers, those who were not receiving OAC therapy, for whom questionnaire administration could not be performed, had to be excluded.
Future Directions
Future research should focus on testing different educational methods to find the most effective approaches. This could lead to guidelines that improve patient knowledge, support self-management, and reduce health risks across anticoagulant therapies. Future research should continue to explore the factors influencing anticoagulant use and the effectiveness of various educational strategies in improving patient knowledge and adherence.
Conclusion
Our findings reveal that AF patients on warfarin demonstrate greater knowledge of anticoagulation therapy compared to those on NOACs, likely due to the increased monitoring and counseling typically provided with warfarin. This knowledge gap highlights the need for consistent education across all anticoagulant types to improve patient understanding for better outcome. Enhanced education, especially for NOAC users, may support safer and more effective anticoagulation therapy. These conclusions are directly supported by the data, which showed significant differences in knowledge levels based on anticoagulant type.
Our study suggests that all AF patients, including those on NOACs, would benefit from standardized education on their medication. Clinicians could improve patient outcomes by ensuring consistent guidance on the risks and adherence needs for both NOAC and warfarin users.
In conclusion, addressing knowledge gaps and enhancing adherence through tailored educational interventions such as one-on-one counselling sessions, visual aids and pamphlets, mobile apps and reminders, follow-up calls, group education sessions are critical in optimizing anticoagulation therapy for atrial fibrillation patients.
Acknowledgments
The author(s) acknowledge all the participants in the study. The author(s) would also like to acknowledge the healthcare professionals and staffs from Dhulikhel Hospital for their contribution to the research.
Data Sharing Statement
The data supporting the findings of this study are available upon request from the corresponding author. However, the data are not publicly accessible, as they contain information that could compromise the privacy of the research participants.
Ethical Approval and Informed Consent
Ethical approval was obtained from the Institutional Review Committee of Kathmandu University School of Medical Sciences (IRC-KUSMS Approval Number: 73/24) which was in accordance with the Declaration of Helsinki. The participants in the study were informed about the purpose of the research and the significance of their involvement. Written informed consent was obtained from every participant before the survey was conducted.
Author Contributions
All authors had significant contribution in the work from conception, study design, execution, and acquisition of data, data analysis, interpretation and manuscript writing. They all took part in revising and critically reviewing the article and approved the final version of the paper before submission to journal and agree to be accountable for all aspects of the work.
Disclosure
The author(s) report no conflicts of interest in this work. The author(s) have no relevant financial or nonfinancial interests to disclose. This study was conducted without any financial support or sponsorship from any organization or entity that could influence the results or interpretation of the findings.
References
- 1.Nesheiwat Z, Goyal A, Jagtap M. Atrial Fibrillation. StatPearls. StatPearls Publishing; 2024. Available from: http://www.ncbi.nlm.nih.gov/books/NBK526072/. Accessed February 6, 2024. [PubMed] [Google Scholar]
- 2.Caldarola P, De Iaco F, Pugliese FR, et al. ANMCO-SIMEU consensus document: appropriate management of atrial fibrillation in the emergency department. G Ital Cardiol. 2023;24(2):136–159. doi: 10.1714/3963.39422 [DOI] [PubMed] [Google Scholar]
- 3.Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res. 2014;114(9):1453–1468. doi: 10.1161/CIRCRESAHA.114.303211 [DOI] [PubMed] [Google Scholar]
- 4.Krijthe BP, Kunst A, Benjamin EJ, et al. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J. 2013;34(35):2746–2751. doi: 10.1093/eurheartj/eht280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel NJ, Deshmukh A, Pant S, et al. Contemporary trends of hospitalization for atrial fibrillation in the United States, 2000 through 2010: implications for healthcare planning. Circulation. 2014;129(23):2371–2379. doi: 10.1161/CIRCULATIONAHA.114.008201 [DOI] [PubMed] [Google Scholar]
- 6.Hindricks G, Potpara T, Dagres N, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373–498. doi: 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 7.Rahman F, Kwan GF, Benjamin EJ. Global epidemiology of atrial fibrillation. Nat Rev Cardiol. 2014;11(11):639–654. doi: 10.1038/nrcardio.2014.118 [DOI] [PubMed] [Google Scholar]
- 8.Worldwide epidemiology of atrial fibrillation | circulation. Available from: https://www.ahajournals.org/doi/full/10.1161/CIRCULATIONAHA.113.005119. Accessed January 4, 2024.
- 9.Kodani E, Atarashi H. Prevalence of atrial fibrillation in Asia and the world. J Arrhythmia. 2012;28(6):330–337. doi: 10.1016/j.joa.2012.07.001 [DOI] [Google Scholar]
- 10.Adhikari K, Malla R, Limbu D, et al. Prevalence of atrial fibrillation in patients attending emergency department of Shahid Gangalal National Heart Centre, Kathmandu, Nepal. Nepalese Heart J. 2016;13(1):1–4. doi: 10.3126/njh.v13i1.14536 [DOI] [Google Scholar]
- 11.Adrega T, Ribeiro J, Santos L, Santos JA. Prevalence of cardiovascular disease risk factors, health behaviours and atrial fibrillation in a Nepalese post-seismic population: a cross-sectional screening during a humanitarian medical mission. Nepalese Heart J. 2018;15(2):9–14. doi: 10.3126/njh.v15i2.21470 [DOI] [Google Scholar]
- 12.Santos IS, Goulart AC, Olmos RD, et al. Atrial fibrillation in low- and middle-income countries: a narrative review. Eur Heart J Suppl. 2020;22(Suppl O):O61–O77. doi: 10.1093/eurheartj/suaa181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galea R, Seiffge D, Räber L. Atrial fibrillation and ischemic stroke despite oral anticoagulation. J Clin Med. 2023;12(18):5784. doi: 10.3390/jcm12185784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary. Circulation. 2014;130(23):2071–2104. doi: 10.1161/CIR.0000000000000040 [DOI] [PubMed] [Google Scholar]
- 15.Aktan A, Güzel T, Aslan B, et al. Comparison of the real-life clinical outcomes of warfarin with effective time in therapeutic range and non-vitamin K antagonist oral anticoagulants: insight from the AFTER-2 trial. Polish Heart J. 2023;81(2):132–140. doi: 10.33963/KP.a2022.0287 [DOI] [PubMed] [Google Scholar]
- 16.Li SC. Factors affecting therapeutic compliance: a review from the patient’s perspective. TCRM. 2008;4:269–286. doi: 10.2147/TCRM.S1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryals CA, Pierce KL, Baker JW. INR goal attainment and oral anticoagulation knowledge of patients enrolled in an anticoagulation clinic in a veterans affairs medical center. J Manag Care Pharm. 2011;17(2):133–142. doi: 10.18553/jmcp.2011.17.2.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Awwad O, Akour A, Al-Muhaissen S, Morisky D. The influence of patients’ knowledge on adherence to their chronic medications: a cross-sectional study in Jordan. Int J Clin Pharm. 2015;37(3):504–510. doi: 10.1007/s11096-015-0086-3 [DOI] [PubMed] [Google Scholar]
- 19.Jimmy B, Jose J. Patient medication adherence: measures in daily practice. Oman Med J. 2011;26(3):155–159. doi: 10.5001/omj.2011.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shakya R, Maharjan M, Karki S, K.c T. Knowledge and compliance of oral anticoagulation therapy at warfarin clinic in Kathmandu: a cross-sectional study. J Karnali Acad Health Sci. 2023;6(3):3–7. [Google Scholar]
- 21.Shakya S, Sayami A, Gajurel RM, et al. Variance in clinical profile and use of anticoagulants in valvular and non valvular atrial fibrillation. World J Cardiovasc Dis. 2020;10(7):488–499. doi: 10.4236/wjcd.2020.107049 [DOI] [Google Scholar]
- 22.Dhungana S, Sherpa K. Antithrombotic agents and risk profile of patients with atrial fibrillation from Rural Part of Nepal. J Inst Med. 2015;37(2):16–21. doi: 10.59779/jiomnepal.672 [DOI] [Google Scholar]
- 23.Obamiro KO, Chalmers L, Bereznicki LRE. Development and validation of an oral Anticoagulation Knowledge Tool (AKT). PLoS One. 2016;11(6):e0158071. doi: 10.1371/journal.pone.0158071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kubica A, Kosobucka A, Michalski P, et al. The adherence in chronic diseases scale — a new tool to monitor implementation of a treatment plan. Folia Cardiologica. 2017;12(1):19–26. [Google Scholar]
- 25.Zathar Z, Karunatilleke A, Fawzy AM, Lip GYH. Atrial fibrillation in older people: concepts and controversies. Front Med Lausanne. 2019;6:175. doi: 10.3389/fmed.2019.00175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wasmer K, Eckardt L, Breithardt G. Predisposing factors for atrial fibrillation in the elderly. J Geriatr Cardiol. 2017;14(3):179–184. doi: 10.11909/j.issn.1671-5411.2017.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jahangir A, Lee V, Friedman PA, et al. Long-term progression and outcomes with aging in patients with lone atrial fibrillation. Circulation. 2007;115(24):3050–3056. doi: 10.1161/CIRCULATIONAHA.106.644484 [DOI] [PubMed] [Google Scholar]
- 28.Winans ARM, Rudd KM, Triller D. Assessing anticoagulation knowledge in patients new to warfarin therapy. Ann Pharmacother. 2010;44(7–8):1152–1157. doi: 10.1345/aph.1P092 [DOI] [PubMed] [Google Scholar]
- 29.Joshua JK, Kakkar N. Lacunae in patient knowledge about oral anticoagulant treatment: results of a questionnaire survey. Indian J Hematol Blood Transfus. 2015;31(2):275–280. doi: 10.1007/s12288-014-0415-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Obamiro KO, Chalmers L, Lee K, Bereznicki BJ, Bereznicki LRE. Anticoagulation knowledge in patients with atrial fibrillation: an Australian survey. Int J Clin Pract. 2018;72(3):e13072. doi: 10.1111/ijcp.13072 [DOI] [PubMed] [Google Scholar]
- 31.Wang M, Swinton M, Troyan S, et al. Perceptions of patients and healthcare providers on patient education to improve oral anticoagulant management. Evaluation Clin Pract. 2022;28(6):1027–1036. doi: 10.1111/jep.13665 [DOI] [PubMed] [Google Scholar]
- 32.Mayet AY. Patient adherence to warfarin therapy and its impact on anticoagulation control. Saudi Pharm J. 2016;24(1):29–34. doi: 10.1016/j.jsps.2015.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salmasi S, Loewen PS, Tandun R, Andrade JG, De Vera MA. Adherence to oral anticoagulants among patients with atrial fibrillation: a systematic review and meta-analysis of observational studies. BMJ Open. 2020;10(4):e034778. doi: 10.1136/bmjopen-2019-034778 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of this study are available upon request from the corresponding author. However, the data are not publicly accessible, as they contain information that could compromise the privacy of the research participants.