Abstract
Infection prevention and control (IPC) programs form the basis of minimizing spread of pathogens in the healthcare setting and beyond. The COVID-19 pandemic amplified the demand for IPC. However, the environmental impact of IPC practices has yet to be addressed and attempts to quantify its climate implications have been sparse. We performed a scoping review to identify current evidence regarding the environmental footprint of IPC measures and to highlight existing gaps in the literature. We included 30 articles, with 23 quantifying the environmental impact by mass of waste generated, six via carbon emissions, and one reporting on the concentration of volatile organic compounds. The mass of infectious waste ranged from 0.16 to 3.95 kg/bed/day, with large variability between countries. In general, higher-income countries produced more waste than lower-income countries. Significant carbon emission savings resulted from substituting reusable gowns and sharps containers, compared to single use items. The most significant gaps are the overall lack of standardisation in quantifying the environmental footprint of IPC-related practices, and a lack of studies on carbon emissions stemming from low and lower-middle income countries. We quantify the environmental impact of IPC practices, suggest areas of infection control that warrant further evaluation, and an approach to standardising environmental metrics in an attempt to better map out the climate implications of adopted IPC measures.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13756-024-01507-0.
Keywords: Infection prevention, Infection control practices, Sustainability, Climate change, Environmental impact
Introduction
Climate change has evolved into the greatest global health threat of the twenty-first century [1]. Rising temperatures beyond target thresholds of 2 °C are likely to negatively impact every facet of human life.
Dedicated measures within health care settings to contain and reduce the risk of transmission of infectious pathogens and hospital acquired multi-drug resistant organisms (MDROs), otherwise known as infection prevention and control (IPC), have clear benefits in reducing morbidity and mortality from healthcare-acquired infections (HAIs). However, IPC practices require substantial personal protective equipment (PPE), including gowns, gloves and masks which are disposed after one time use. Other single use items such as IVs, IV tubing, single-dose vials, etc., and those needed for cleaning and disinfecting are needed almost daily in patient care. Single-use items reduce the risk of transmission of pathogens between patients whether it be by person to person transfer (hands), environmental transfer (fomites) or transmission via contaminated fluids including body fluids. The use of PPE and other single use items for IPC not only requires large amounts of energy for manufacturing but can also create infectious or chemical waste, all of which are detrimental to the environment. A single surgical mask is estimated to release 0.059 kg carbon dioxide equivalents (CO2eq) into the atmosphere and contribute to 12–13 g of waste per unit [2, 3]. Plastic debris from improper face mask disposal is expected to result in 150–390 thousand tons of marine pollution annually worldwide [4]. There is a need to balance good IPC practices with sustainability; the recent COVID-19 pandemic has highlighted the environmental impact of IPC measures, especially with the surge in demand for PPE and disposable products [5–8].
The principles governing IPC are well established, and evidence supports the benefits and cost-savings of IPC programmes, though low- and lower-middle income countries continue to be underrepresented [9]. In contrast, there is a paucity of studies assessing the environmental implications of IPC practices [9]. Some studies have explored the impact of eye-health [10], anaesthesia [11], and surgery [12–14], on the climate, highlighting the evolving need for healthcare to not only serve its apparent medical purpose, but to also be environmentally sustainable in the face of a volatile climate. We therefore undertook this systematic scoping review in an attempt to quantify the available evidence on the environmental impact of various forms of infection control, how such an impact translates to sustainability in the long-term, and to identify gaps in the literature.
Methods
Search strategy
We performed a systematic search while adhering to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [15], and registered the study with PROSPERO (CRD42023456805). The PRISMA Extension for Scoping Reviews (PRISMA-ScR) can be found in Table S1 [16]. We searched Medline (Ovid) and Embase databases from inception through 18 September 2024 using the keywords “infection”, “transmission”, “climate change”, “global warming” and relevant infection control terms such as “mask”, “PPE” and “gloves” (Table S2). References were imported into EndNote X9 for the initial sieve with the removal of duplicates. References of related reviews and included articles were also hand-screened to ensure a comprehensive search.
Study selection
Eligibility for inclusion was determined by two authors (OL and WYC) who screened articles independently from the initial sieve, with a third independent author involved in the resolution of conflicts (AW). We included studies which quantitatively measured the environmental impact of infection control practice found in standard, contact, droplet or airborne precautions [17], using measures including mass of waste (usually in kg or tons), carbon dioxide equivalents (CO2eq), energy expenditure (kWh), and air or marine pollution, in a hospital setting and/or health-care facility. Studies were excluded if they were reviews, meta-analyses, editorials, commentaries or non-Human studies. Conference abstracts were included if they contained relevant information. We sought translation for non-English language studies if the need arose. With previous reviews having analysed the environmental cost of surgery [13], we excluded studies with a surgical focus and those performed in the setting of an operating theatre. Studies which reported data that did not have direct environmental implications or lacked suitable conversion formulae to a measurable metric of interest (i.e. economic cost of waste management, number/incidence of new infections, number of hospital admissions) were also excluded.
Data extraction
Data from the included articles were extracted independently by two authors who were blinded in the process. The data collection template can be accessed in Table S3. Briefly, we extracted data on study characteristics (country, year of study, setting of study, COVID-19 vs non COVID-19, income-level of country in the year of publication (as defined by the World Bank classification), infection-control related data (specific type of precaution being studied, main findings), and environmental impact (mass of waste, number of beds and patients, carbon dioxide emissions, and other relevant environmental matrices if reported by the authors). Any discrepancies which arose post-extraction were brought up for discussion and resolved, with involvement of a third independent author where deemed necessary. Data on infectious waste and emissions were reported in differing units across the included studies, and were standardised wherever possible.
Data synthesis
We had initially planned to perform a systematic review. However, recommendations have been made for scoping reviews over systematic reviews when the scope of a topic remains poorly defined, and when broad research questions have yet to be answered [18, 19]. The lack of literature and overwhelming heterogeneity of data led to a switch in study type. Data from studies were thematically assessed, with both qualitative and quantitative data synthesised and presented narratively. We expanded on themes related to the environmental cost of each form of IPC, how cost was quantified across studies, and looked for possible reasons when discrepancies arose.
Role of the funding source
There was no funding source for this study.
Results
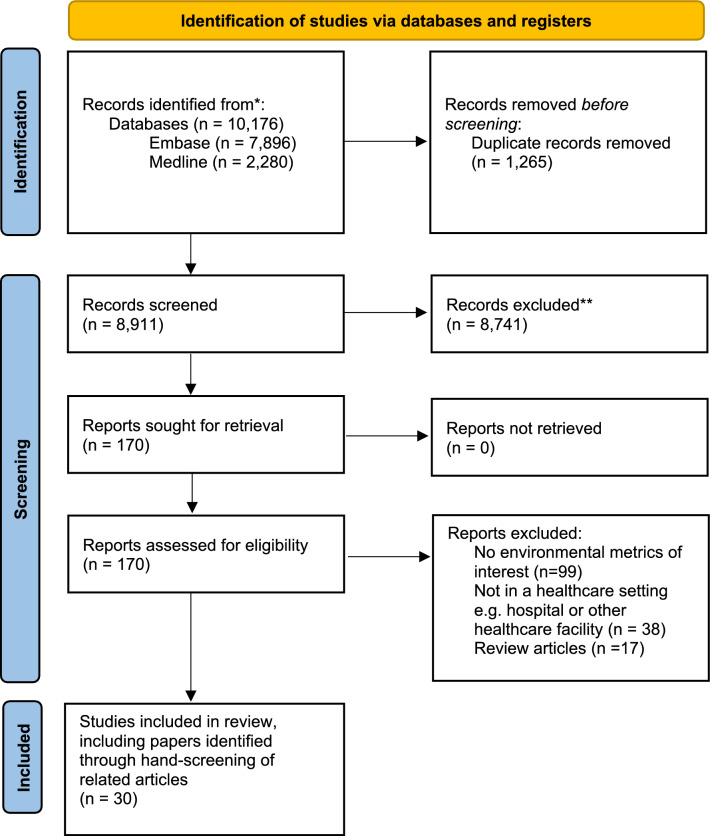
Summary of included studies
Of 8,911 articles, we excluded 8,741 articles and shortlisted 170 full texts for review. A total of 30 studies were included. [20–49] (Fig. 1). Fourteen of the studies [22, 23, 25, 27, 30, 31, 34–36, 39, 42, 44, 48, 49] were identified via hand-screening of included articles and citation-searching. 23 studies reported on the mass of infectious waste produced [20–29, 32–34, 36–40, 42–44, 47, 48], six on carbon emissions, [31, 35, 41, 45, 46, 49] and one on the concentration of volatile organic compounds (VOCs) emitted. [30] The summary of included articles can be found in Table 1.
Fig. 1.
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram
Table 1.
Summary of included studies
| Title; Author | Country, year | Income-level* | Summary of study design | Type of precaution studied | Main findings | Variable used to quantify environmental impact |
|---|---|---|---|---|---|---|
|
Characteristics of the medical waste generated at the Jordanian hospitals Abu-Qdais et al. [19] |
Jordan, 2006 | Lower middle |
Cross-sectional study Weighing, sorting of waste and surveys were conducted in 5 hospitals in Jordan |
Standard precaution | The weighted mean infectious medical waste by the hospitals covered by the survey is 0·61 kg/bed/day | Mass of infectious waste (kg/bed/day) |
|
Statistical analysis and characteristics of hospital medical waste under novel Coronavirus outbreak Abu-Qdais et al. [20] |
Jordan, 2020 | Upper middle |
Cross-sectional study The composition of medical waste generated was analysed during the COVID-19 pandemic in a major tertiary care hospital in Jordan |
Standard precaution | The mean amount of infectious medical waste generated from coronavirus treatment was 3·95 kg/bed/day, which are more than tenfold higher than the average generation rate of 0·41 kg/bed/day before the pandemic | Mass of infectious waste (kg/bed/day) |
|
Estimation of COVID-19 generated medical waste in the Kingdom of Bahrain Al-Omran et al. [21]† |
Bahrain, 2021 | High |
Cross-sectional study The amount of PPE waste generated during COVID-19 among healthcare facilities in the Kingdom of Bahrain was studied. PPE waste generation per healthcare worker (HCW) per day was estimated |
Contact precaution | PPEs used by medical staff in 5 healthcare facilities was 2·62 kg/HCW/day | Mass of PPE waste (kg/HCW/day) |
|
Clinical laboratory waste management in Shiraz, Iran Askarian et al. [22] |
Iran, 2012 | Upper middle |
Cross-sectional study Waste across 109 clinical laboratories were collated over a period of 1 month |
Standard precaution | Infectious waste amounted to 0.4 ± 0.35 kg/patient/day across the 109 laboratories | Mass of infectious waste (kg/patient/day) |
|
Characterization and management of solid medical wastes in the Federal Capital Territory, Abuja Nigeria Bassey et al. [23] |
Nigeria, 2006 | Low |
Cross-sectional study Management of solid medical wastes in five selected hospitals was studied |
Standard precaution | The mean infectious waste produced was 0·35 kg/bed/day | Mass of infectious waste (kg/bed/day) |
|
Qualitative and quantitative evaluation of medical waste products in Côte d'Ivoire Bitty et al. [24]† |
Ivory Coast, 2013 | Lower middle |
Cross-sectional study Medical waste across both the public and private healthcare systems were monitored |
Standard precaution | Taking the total proportion of infectious waste to be 59.39% of total medical waste as reported by the authors, the national average of infectious waste is estimated at 0.37 kg/bed/day | Mass of infectious waste (kg/bed/day) |
|
Medical waste production at hospitals and associated factors Cheng et al. [25] |
Taiwan, 2008 | High |
Cross-sectional study This study was conducted to evaluate the quantities of medical waste generated of 150 healthcare establishments in Taiwan |
Standard precaution |
Mean infectious waste generated by type of medical establishment Medical centres: 0.60 kg/bed/day Regional hospitals: 0.44 kg/bed/day Local hospitals: 0.88 kg/bed/day Clinics and others: 0.19 kg/bed/day |
Mass of infectious waste (kg/bed/day) |
|
Thinking green: Modelling respirator reuse strategies to reduce cost and waste Chu et al. [26]† |
USA, 2021 | High |
Cost-analysis study The authors assumed a model with universal masking of all healthcare workers across 6 months of the COVID-19 pandemic. Waste generated per patient was estimated by dividing the total amount of waste by the total number of hospitalised patients with COVID-19 during the first 6 months of the pandemic. Multiple respirator strategies were analysed |
Airborne precaution |
Assuming 6 months to be 182.5 days, the estimated environmental impact of N95 respirators with various strategies are outlined 1 per patient encounter: 1.16 kg/patient/day 1 per day: 0.515 kg/patient/day Ultraviolet germicidal irradiation (UVGI) decontaminated 3 M 1860 N95 respirators: 0.257 kg/patient/day H2O2 decontaminated 3 M 1860 N95 respirators: 0.180 kg/patient/day Reusable respirator and disposable filters: 0.217 kg/patient/day Reusable respirator and decontaminated filters: 0.022 kg/patient/day 1 surgical mask per day: 0.386 kg/patient/day |
Mass of N95/respirator waste (kg/patient/day) |
|
Medical waste management in Ibadan, Nigeria: Obstacles and prospects Coker et al. [27] |
Nigeria, 2008 | Lower middle |
Cross-sectional study 52 healthcare facilities in Ibadan, Nigeria were studied, of which only 1 was a tertiary care hospital |
Standard precaution | In the tertiary hospital, the mean amount of infectious waste produced was 20·1 kg/day | Mass of infectious waste (kg/day) |
|
Assessment of the health care waste generation rates and its management system in hospitals of Addis Ababa, Ethiopia, 2011 Debere et al. [28] |
Ethiopia, 2011 | Low |
Cross-sectional study 6 hospitals in Addis Ababa in Ethiopia was recruited to assess the health care waste generation rate and its management system across a period of two months |
Standard precaution | The amount of infectious waste generated varied from 0·037 to 0·116 kg/patient/day |
Mass of infectious waste (kg/patient/day) |
|
The safety of non-incineration waste disposal devices in four hospitals of Tehran Farshad et al. [29] |
Iran, 2014 | Upper middle |
Cross-sectional study The concentration of volatile organic compounds (VOCs) emitted from four non-incinerator waste disposal methods were analysed in four hospitals across a 10-week period |
Standard precaution |
Among 40 VOCs tested, benzene, toluene, ethyl benzene, and xylene, collectively BTEX, were detected. Mean concentration of VOCs produced Autoclave without shredder:1.78 ppm Dry-heat system: 5.47 ppm Autoclave with shredder: 9.3 ppm Hydroclave: 5.5 ppm |
Concentration of VOCs (ppm) |
|
Before/after intervention study to determine impact on life-cycle carbon footprint of converting from single-use to reusable sharps containers in 40 UK NHS trusts Grimmond et al. [30] |
UK, 2020 | High |
Life-cycle assessment Across 40 acute care hospitals in the UK, the carbon footprint of utilising single-use sharps containers and reusable sharps containers were compared across a 12 month period |
Standard precaution | The use of single-use sharps containers produced 3896.4 tonnes of CO2 across the 12-month period, compared to 628.9 tonnes of CO2 after switching to reusable containers, a 83.9% decrease. This further eliminated incineration of 900.8 tonnes of plastic and 132.5 tonnes of cardboard | Metric tonnes carbon dioxide equivalent (MTCO2e) |
|
Variations in Hospital Waste Quantities and Generation Rates Hamoda et al. [31]† |
Kuwait, 2005 | High |
Cross-sectional study The authors quantified waste generation from the 2 largest hospitals in Kuwait |
Standard precaution | The mean infectious waste produced was 1·04 kg/bed/day and 1·09 kg/bed/day for Amiri Hospital & Mubarak Hospital respectively | Mass of infectious waste (kg/bed/day) |
|
Pattern of medical waste management: existing scenario in Dhaka City, Bangladesh Hassan et al. [32] |
Bangladesh, 2008 | Low |
Cross-sectional study Health care establishments in Dhaka City, amounting to 2884 beds, were surveyed on waste production and management |
Standard precaution | DMCH, BMCH and General Hospitals produced 0·29, 0·24 and 0·22 kg/bed/day of infectious waste respectively | Mass of infectious waste (kg/bed/day) |
|
Characteristics and management of infectious industrial waste in Taiwan Huang et al. [33] |
Taiwan, 2008 | High |
Cross-sectional study Data from Taiwan’s Department of Health and EPA were retrieved to survey the production of infectious waste |
Standard precaution | Assuming a bed-occupancy of 100% and a total number of 95,810 beds across Taiwan’s general hospitals, infectious waste was 2.5 kg/bed/day | Mass of infectious waste (kg/bed/day) |
|
Sustainability and shared smart and mutual green growth (SSaM-GG) in Korean medical waste management Koo et al. [34] |
South Korea, 2015 | High |
Life-cycle assessment Four available treatment systems of medical waste (incineration, incineration with heat recovery, steam sterilisation, and microwave disinfection) were studied to treat infectious waste prior to disposal. A functional unit of 1,000 kg of regulated medical waste (RMW) was chosen |
Standard precaution |
Incineration: 1213 kg-CO2/t Incineration with heat recovery: 455 kg-CO2/t Steam sterilisation: 490 kg-CO2/t Microwave disinfection: 99 kg-CO2/t |
Mass of CO2 emission per ton of waste (kg-CO2/t) |
|
Auditing an intensive care unit recycling program Kubicki et al. [35]† |
Australia, 2013 | High |
Cross-sectional study The weight and proportion of ICU waste and recyclables were studied across 7 non-consecutive days in a 11-bed ICU |
Standard precaution | Mean infectious waste produced was 1.78 kg/bed/day | Mass of infectious waste (kg/bed/day) |
|
Characteristics of Medical Waste in Taiwan Kuo et al. [36] |
Taiwan, 1998 | High |
Cross-sectional study Twenty-eight public hospitals in Taiwan were surveyed and records on general and infectious waste production were kept for one year |
Standard precaution | The mean infectious waste produced was 0·39 kg/bed/day | Mass of infectious waste (kg/bed/day) |
|
Analyses of the recycling potential of medical plastic wastes Lee et al. [37]† |
USA, 2002 | High |
Cross-sectional study Site visits to five typical city hospitals were conducted. Plastic waste was physically examined, and data regarding each hospital’s waste stream and disposal was conducted |
Standard precaution | The mean amount of infectious waste produced was 1·49 kg/bed/day | Mass of infectious waste (kg/bed/day) |
|
Healthcare waste management status in Lagos State, Nigeria: a case study from selected healthcare facilities in Ikorodu and Lagos metropolis Longe et al. [38] |
Nigeria, 2011 | Lower middle |
Cross-sectional study 20 healthcare institutions comprising of diagnostic centres, clinics, health centres, and hospitals (both public and private) were visited across 12 weeks |
Standard precaution | We excluded the four diagnostic centres because information on bed number was not available. Across the rest of the 16 sites totalling 1,243 beds, the mean amount of infectious waste was 0.22 kg/bed//day | Mass of infectious waste (kg/bed/day) |
|
Medical Waste Management: A Case Study of the Souss-Massa-Drâa Region, Morocco Mbarki et al. [39]† |
Morocco, 2013 | Lower middle |
Cross-sectional study The authors conducted a study regarding medical waste generation, separation, collection, storage, transportation, and disposal across seven hospitals |
Standard precaution | The mean infectious waste produced was 0·16 kg/bed/day | Mass of infectious waste (kg/bed/day) |
|
Influence of COVID-19 on the 10-year carbon footprint of the Nagoya University Hospital and medical research centre Morooka et al. [40] |
Japan, 2020 | High |
Longitudinal study Data on electricity, gas, and water usage, pharmaceutical and medical supply costs, and waste amounts were recorded for Nagoya University Hospital from April 2010 to March 2021. The effect of the COVID-19 pandemic on the carbon footprint was then compared for three types of emission sources |
Standard precaution |
Total emission from infectious medical waste: 2019 (Pre-COVID): 114,470 kg-CO2/ year 2020 (COVID):147,620 kg-CO2/ year |
Mass of CO2 emission per year (kg-CO2/ year) |
|
Bio-Medical Waste Managment in a Tertiary Care Hospital: An Overview Pandey et al. [41] |
India, 2016 | Lower middle |
Cross-sectional study The observational study was carried out over a period of five months in Chhatrapati Shivaji Subharti Hospital, Meerut |
Standard precaution | The mean infectious waste generated was 0·34 kg/bed/day | Mass of infectious waste (kg/bed/day) |
|
Assessment and selection of the best treatment alternative for infectious waste by modified Sustainability Assessment of Technologies methodology Rafiee et al. [42] |
Iran, 2016 | Upper middle |
Cross-sectional study Across a period of three months, infectious waste generated in the Iman Khomeini hospital complex was measured |
Standard precaution | The mean amount of infectious waste produced was 1·15 kg/bed/day | Mass of infectious waste (kg/bed/day) |
|
Assessment and selection of the best treatment alternative for infectious waste by Sustainability Assessment of Technologies (SAT) methodology Rahmani et al. [43] |
Iran, 2020 | Lower middle |
Cross-sectional study Four hospitals in Ardabil formed the setting of this study, with in-person and field visits performed. Mass of waste generated was tabulated |
Standard precaution | The mean amount of infectious waste produced was amounted to 2·42 kg/bed/day | Mass of infectious waste (kg/bed/day) |
|
The carbon footprint of waste streams in a UK hospital Rizan et al. [44] |
UK, 2020 | High |
Cross-sectional study The carbon footprint of different waste streams based on disposal methods in 3 UK hospitals were measured |
Standard precaution |
Decontamination of hospital waste, including electricity, gas/oil, and water supplies 338 kg CO2e/t Infectious waste Autoclave decontamination: 569 kg CO2e/t Clinical, sharps, anatomical and medicinal waste High temperature incineration: 1074 kg CO2e/t |
Mass of CO2 emission per ton of waste (kg-CO2/t) |
|
Environmental impact of personal protective equipment distributed for use by health and social care services in England in the first six months of the COVID-19 pandemic Rizan et al. [45] |
UK, 2021 | High |
Life-cycle assessment The environmental impact of commonly used PPE were individually assessed. The impact was then extrapolated to health and social care services in England during the COVID-19 pandemic across a 6-month period. Besides global warming potential, other metrics including ionising radiation, water consumption, and marine, air, and land pollution were analysed |
Contact precaution |
Carbon footprint of individual PPE items: Single-use gown: 905 g CO2e Face shield: 231 g CO2e Cup fit FFP respirator: 125 g CO2e Duckbill FFP respirator: 76 g CO2e Apron: 65 g CO2e Glove: 26 g CO2e Surgical mask (type IIR): 20 g CO2e Surgical mask (type II): 13 g CO2e Total 6 month carbon footprint of all PPE items: 106,477,990 kg CO2e |
Mass of CO2 emission (g CO2e) |
|
Healthcare waste generation and management practice in government health centers of Addis Ababa, Ethiopia Tadesse et al. [46] |
Ethiopia, 2014 | Low |
Cross-sectional study Ten health centres were chosen, with seven consecutive days of waste collection performed. Total waste/day was measured using a weighing scale. On site visits and interviews were also conducted |
Standard precaution | The mean amount of infectious waste was 2·29 kg/day | Mass of infectious waste (kg/day) |
|
The challenge of medical waste management: a case study in northwest Iran-Tabriz Taghipour et al. [47] |
Iran, 2009 | Upper middle |
Cross-sectional study The amount of infectious waste produced in 10 of the 25 active hospitals in Tabriz (Iran’s fourth largest city) were collated. Further observations and figures regarding the disposal of waste was made |
Standard precaution | The mean amount of infectious waste produced across the ten hospitals was 1·04 kg/bed/day | Mass of infectious waste (kg/bed/day) |
|
Environmental considerations in the selection of isolation gowns: A life cycle assessment of reusable and disposable alternatives Vozzola et al. [48] |
USA, 2021 | High |
Life-cycle assessment The environmental impact of reusable and disposable isolation gowns were compared on the basis of energy consumption, greenhouse gas emissions, blue water consumption and solid waste generation |
Contact precaution |
Metrics are presented per 1,000 uses Reusable vs disposable Global warming potential: 218 vs 310 kg CO2eq Natural resource energy: 3,712 vs 5,150 MJ Blue water: 43.8 vs 74.6 kg Solid waste at hospital site: 0.4 vs 63.4 kg |
Mass of CO2 emission (kg CO2eq) Natural resource energy (MJ) Blue water (kg) Mass of waste (kg) |
*Based-off the World Bank classification for economies. Studies were performed across different time periods, and the income-level of the country in that particular year was used. Income levels ranged from low, lower middle, upper middle and high
Wdi—the world by income and region [Internet]. [cited 2023 Oct 30]. Available from: https://datatopics.worldbank.org/world-development-indicators/the-world-by-income-and-region.html
†Based off manual calculation from reported data in articles
Mass of waste
The majority of our articles reported on mass of waste, of which a large proportion (21 studies) was infectious waste. Other specific waste types included PPE waste and N95 or respirator generated waste (one study each). Studies were evenly spread across income brackets, with 12 and 11 studies originating from upper-middle to high income countries and low to lower-middle income countries respectively. We attempted to standardise the units for waste generated, ideally reporting it in kg/bed/day (or kg/patient/day) to allow for some degree of inter-study comparison. Across all studies, infectious waste ranged from 0.16 to 3.95 kg/bed/day [21, 40]. The study responsible for the largest amount of waste was conducted at the height of the COVID-19 outbreak [21]. Excluding this study, all other studies were non-COVID-19 in nature, with infectious waste ranging from 0.16 to 2.5 kg/bed/day [34, 40]. Four articles reported on infectious waste using other units, ranging from 2.3 to 20.1 kg/day and 0.04 kg/patient/day to 0.4 kg/patient/day.
A cost-analysis study by Chu et al. reported on the mass of N95 waste with different respirator strategies. We assumed a period of 6 months to be approximately 182.5 days, and estimated the environmental impact to range from 0.022 kg/patient/day when using reusable respirator and decontaminated filters to 1.16 kg/patient/day if one N95 respirator was used per patient encounter. Across five healthcare facilities in Bahrain, we calculated that the average amount of PPE utilised per healthcare worker was 2.62 kg/day, although this included facilities dealing with both suspected and confirmed cases of COVID-19 infection [22].
Carbon emissions
Six studies quantified environmental impact through carbon emissions. Two studies looked at the environmental impact of sterilisation methods. Rizan et al. examined the carbon footprint of waste streams in a UK hospital, with high-temperature incineration having the greatest environmental impact producing 1074 kg CO2e/t of waste. This method of sterilisation was utilised for clinical waste, clinical sharps, anatomical waste and medicinal waste, as mandated by national guidelines [45]. Specific to infectious waste, autoclave decontamination produced 569 kg CO2e/t. The carbon footprint from electricity, gas/oil, and water supplies was 338 kg CO2e/t. A similar study on treatment systems for infectious waste found the highest and lowest global warming potentials with incineration and microwave disinfection respectively (1213 kg CO2e/t vs. 99 kg CO2e/t).
Articles performing life-cycle assessments were also included. A study of PPE use during the first six months of the COVID-19 pandemic in the UK was performed. The carbon footprint of individual PPE items were: Single-use gown (905 g CO2e), face shield (231 g CO2e), respirators (76–125 g CO2e), apron (65 g CO2e), gloves (26 g CO2e), surgical masks (13–20 g CO2e). The total carbon footprint produced over 6 months from all PPE items was 106,477,990 kg CO2e. Two studies compared reusable vs disposable IPC strategies related to isolation gowning and sharps container use. Per 1,000 uses, reusable gowns emitted 30% less greenhouse gases (218 vs. 310 kg CO2eq). Sensitivity analyses with different products showed consistent environmental benefits with a reusable gown strategy. Adopting a reusable strategy with sharps containers generated 628.9 tonnes of CO2 compared to 3896.4 tonnes over a 12 month period, representing a 83.9% decrease in CO2 emissions.
In the sole longitudinal study across a 10 year period, the total annual carbon footprint produced by the Nagoya University Hospital steadily increased. This was accompanied by a spike in infectious waste from 2019 to 2020 and a significant increase in yearly infectious waste related emissions from 114.47 to 147.62 tCO2eq, although the overall carbon footprint had dropped during the pandemic owing to confinement measures and lower patient load [41]. A positive correlation between the monthly average temperature and monthly carbon emissions across a 7-year period was also seen, with a significant increase in the carbon footprint per admission between 2018 and 2020 owing to ‘more intensive medical care’ provided per-admission during the early part of the COVID-19 pandemic, and longer average hospital stays in 2020 as compared to 2018 (12.2 days vs. 11.9 days).
Other environmental metrics
The concentration of volatile organic compounds (VOCs) emitted via four non-incinerator waste disposal methods (autoclave with and without shredder, dry-heat system, and hydroclave) were studied by Farshad et al. Briefly, VOCs have been linked to a wide range of environmental and health implications, including respiratory, neurological and carcinogenic effects [50]. The concentration of VOCs ranged from 1.78 to 9.3 ppm when using an autoclave without and with a shredder respectively. The comparative study on reusable vs disposable isolation gowns also reported on other environmental metrics besides carbon emissions, with a reusable strategy consuming 28% less energy (3712 vs. 5150 MJ), 41% less blue water (43.8 vs. 74.6 kg, with blue water defined as all water that is removed from the supply chain, including water lost to evaporation and incorporated into the product), and large savings in waste generation (0.4 kg vs. 63.4 kg).
Trends across income level
Economic fluctuations in the countries were adjusted for based on year-specific data provided by the World Bank [51]. An assessment of the studies which had reported waste in kg/bed/day revealed that nine were conducted in countries belonging to low and lower-middle income brackets, of which eight generated less than 1 kg/bed/day of infectious waste. In contrast, all studies conducted in upper-middle and high income countries generated more than 1 kg/bed/day of infectious waste, with the exception of two Taiwanese studies producing 0.19 kg/bed/day to 0.39 kg/bed/day of infectious waste [26, 37].
Discussion
In its 2020 guidance report, the World Health Organisation (WHO) underlined the need for a sustainable approach to healthcare given a rapidly changing climate [52]. At the same time, the recent WHO global report on IPC reveals a worrying lack of progress, especially with “respect to the proportion of countries with an active national IPC programme, evidence-based and standardised national guidelines [9]”. However, there has been little mention in guidelines of the environmental impacts of current well-established IPC programs and the impact that the scaling up of programs globally will have on increasing carbon emissions from healthcare in general. Regardless, some countries have made concerted efforts to meet the aims of introducing sustainability in healthcare. The National Healthcare System (NHS) launched the ‘Greener NHS’ campaign to decarbonise itself, and move toward being a ‘net zero’ service by 2040 for emissions directly under its purview. A recent analysis across 49 regions demonstrated an increase of resource footprints in healthcare systems in the last 20 years, and is expected to grow as more energy intensive treatments continue to be implemented [53]. The need for greener healthcare services has clearly gained traction on the international stage. Yet, our scoping review highlighted the paucity of data measuring the environmental impact of the numerous IPC practices undertaken, rightfully, for patient safety. In contrast, the impact of volatile anaesthetic gases, such as desflurane, has received major attention throughout the years, due to its substantial global warming potential, and is gradually being phased out from use internationally [11, 54, 55]. The ubiquity of infection control is perhaps many times greater than the use of volatile anaesthetics, as evidenced by the unprecedented COVID-19 pandemic, and is poised to grow with the WHO’s recent call to increase IPC programs world-wide. Infection control practices are already being included as part of larger carbon footprint assessments, albeit as a constituent of other sectors such as medical and non-medical equipment, as well as water and waste [55, 56]. Isolating its impact from the larger umbrella of healthcare sectors would provide greater clarity on emissions attributable to infection control. This is critical for IPC programs so as to direct available resources at practices that are most likely to reduce the carbon footprint, while also preventing health care associated infections.
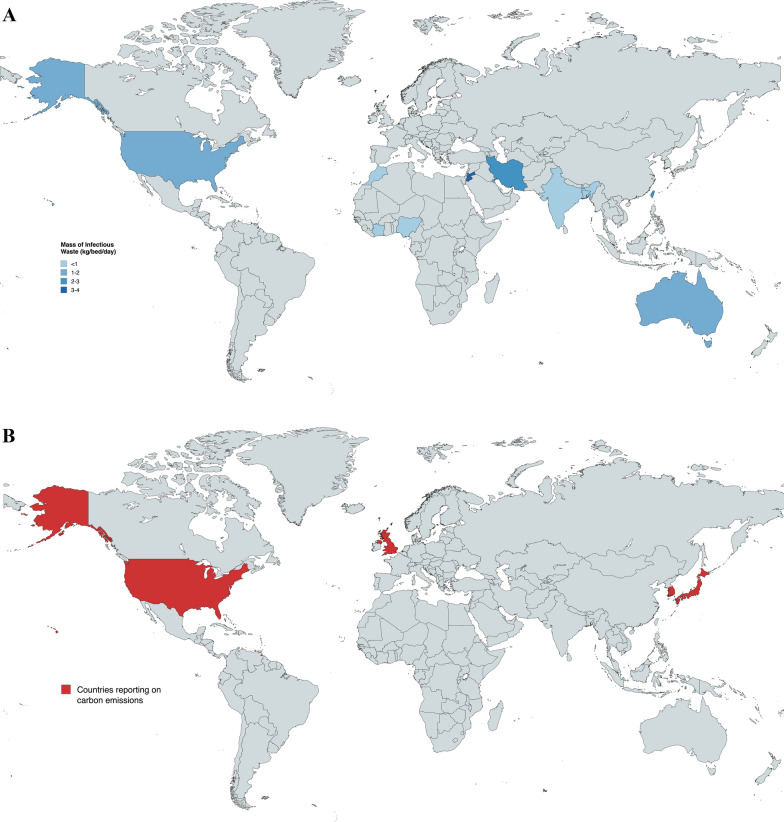
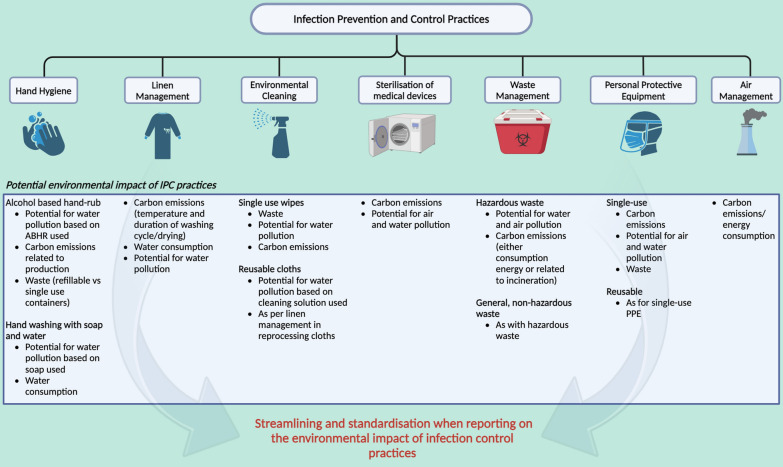
Most published studies focused on the mass of waste generated, in particular infectious waste, but demonstrated great variability between studies ranging from 0.16 kg/bed/day in a Moroccan study to 2.5 kg/bed/day in Taiwan. However, the Taiwanese study assumed a 100% occupancy rate of 95,810 beds across the nation, possibly overestimating the waste generated. We noted a general pattern of higher waste production with high and upper-middle income countries as opposed to those in lower-income brackets (Fig. 2A). This finding mirrors that of previous literature, including WHO’s fact sheet on healthcare waste [57, 58]. An important caveat lies with higher rates of improper waste segregation in lower-income countries, which we found to approach 30–50% in our studies [20, 42, 47], perhaps underestimating the true extent of infectious waste output in these countries. While mass of waste serves as a valuable research metric, its utility may fail to extend much further. Management of infectious waste differs depending on local policies and waste management strategies, ultimately generating varying amounts of carbon emissions even with the same amount of waste. Nonetheless, we propose that mass of waste be reported in kg/bed/day whenever feasible for future studies, instead of raw mass, enabling greater standardisation and comparison across both time and region. There was limited data on how generated waste further translated to greenhouse gas emissions, which failed to capture the potential carbon footprint of included IPC practices. The Japanese study was unique in the manner in which it broke down its carbon footprint in a detailed and practical way with measurements of electricity, gas, and waste, which were then coupled with country-specific emission factors to derive its carbon footprint [41]. However, the logistical and financial constraints of being able to amass such data should not be underestimated. Japan mandates that its institutions report their annual carbon footprint [59]. Additional costs incurred by round-the-year monitoring and out-sourcing of waste are not realistic on a global scale, particularly in less developed countries. Future studies should aim to quantify—ideally using standardised units—the carbon emissions, energy and water consumption, and risk for environmental pollution, from both the materials used for IPC, and those resulting from waste of the products, while accounting for any recycling versus disposal of IPC products (Fig. 3, Table S4).
Fig. 2.
A: world map illustrating the geographic distribution of studies reporting on mass of waste. World map illustrating the geographic distribution of studies reporting on mass of waste. Countries reporting on mass of infectious waste (kg/bed/day) are shaded blue, with darkening of the colour gradient as mass of infectious waste produced increases. Countries shaded are Bangladesh (< 1), Morocco (< 1), Nigeria (< 1), India (< 1), Ivory Coast (< 1), Kuwait (1–2), Australia (1–2), USA (1–2), Taiwan (2–3), Iran (2–3) and Jordan (3–4). Several studies conducted in Ethiopia, Iran, and Nigeria reported on infectious waste using units other than kg/bed/day (e.g. kg/patient/day or kg/day) and are not shaded in the diagram. B: world map illustrating the geographic distribution of studies reporting on carbon emissions. World map illustrating the geographic distribution of studies reporting on carbon emissions. All countries reporting on carbon emissions are high-income, and include the UK (3 studies), South Korea, Japan and the USA
Fig. 3.
Standardising reporting of environmental impact of infection control practices
The studies on carbon emissions provided valuable input on the environmental impact of various IPC measures. Notably, we found large savings in carbon emissions when switching to reusable isolation gowning and sharps disposal from single-use. Of note, transport distances may play a substantial role in overall carbon savings with reusable containers, having accounted for 67.1% of its life-cycle global warming potential. The vast reduction in CO2 emissions were made possible with “relatively short UK transport distances”, and may suggest attenuated environmental savings with different healthcare settings that are more geographically sparse. This finding underlines one of many differences that exist between countries and health systems, limiting the ability to extrapolate such data to a global context. Importantly, we noted that all six studies reporting on carbon emissions stemmed from high-income countries (Fig. 2B), highlighting yet another discrepancy in data across income brackets. High income countries remain the main drivers of greenhouse gas emissions, while lower income countries experience a disproportionate burden from the climate crisis [60]. Improvements in quantifying the environmental impact of IPCs could, and should, begin with institutions in high income countries where resources are ample, before being implemented in lower income countries where the need for proper infection control continues to grow. This is in line with the Sustainable Development Goals (SDGs) by the United Nations, SDG 13— “Climate Action” and SDG 17—“Partnerships For The Goals”, where collective action to tackle the global threat of climate change is paramount [61].
Fundamentally, any change from current IPC practices for an environmental benefit will need to retain efficacy and patient safety in order to justify the switch. Limited data exist which adequately address environmental costs of current versus alternative IPC practices and the climate benefits (or drawbacks) of each option, especially in terms of showing longitudinal safety outcomes. We had initially set out with the aim of categorising studies into the various types of precautions for ease of data organisation and presentation. However, we found that no studies had a pre-specified focus on a particular set of precautions, and papers had to be independently screened and manually categorised where possible. Future studies, focusing on singular aspects of infection control, be it a single method, or Infection Control bundles, are likely to be more useful. This is especially since focusing on a particular aspect over the full life-cycle, will allow for an easier calculation of the corresponding carbon emissions data from said practice. In our drive to be sustainable, identification of “carbon hotspots” is a necessary step to determine the highest impact practices to focus on. Various countries such as the UK [55], USA [62], China [63], and Australia [64], have broadly identified the supply chain (62%), overall hospital care (36%), and public hospitals (47% and 34%) as their biggest contributors to healthcare emissions respectively. Scaling it down to IPC-specific components, and using standardized metrics for measurements, would allow infection control specialists, policymakers and governmental organisations to focus efforts on IPC “hotspots” that contribute most heavily to greenhouse gas emissions.
We hope that our study will help to inform the type of future research needed in this field, and act as a precursor for future systematic reviews where specific elements of infection control are more comprehensively and systematically addressed. The sheer scale of IPC programs across hospitals of all sizes suggests the urgent need for dedicated studies. Studies comparing the impact of various aspects of in-hospital infection control practices, using the WHO breakdown of the 7 aspects of an IPC program would be a good first step, and includes components that are frequently overlooked, but of everyday importance. These components are outlined by the WHO Minimum Requirements for infection prevention and control programmes, and include hand hygiene, linen management, environmental cleaning, sterilization of medical devices, waste management, personal protective equipment (PPE) and air management [17]. We summarise these components in Fig. 3, and provide specific suggestions on environmental metrics that may be useful in quantifying environmental impact based-off each IPC measure.
Our study had some important limitations. First, the cross-sectional nature of most included studies inherently carry limitations when attempting to establish causality, and associations should be interpreted with caution. Second, we had to make manual calculations in an effort to standardise units and allow comparison. However, we were careful to make these calculations only if sufficient data was made available by the original article, and assumptions, if any, were clearly stated, as in the case of time conversions made (e.g. months to days). Third, besides life-cycle assessment studies, other articles, especially those on waste production, did not provide sufficient granularity when assessing the environmental impact of each stage of a product’s lifecycle (e.g. production vs transport vs disposal). Identification of IPC-specific activities in some articles also proved challenging. Nonetheless, we acknowledge that the field of IPC sustainability appears relatively nascent, with no scoping reviews performed to date, which led us to adopt a broad criteria for inclusion so long as useful environmental metrics were presented. Fourth, we would have liked to assess for trends across time, but were limited by substantial inter-study heterogeneity in waste and data collection methodology, along with variations in healthcare setting. It would have been difficult to pin-point any conclusions drawn on variations in waste solely to the effect of time. We had also planned to compare the environmental impact across different healthcare settings, such as hospital-based care vs primary care. Unfortunately, most of the studies reported solely on hospital-generated waste, with an insufficient number of articles available for inter-setting comparisons to be made. Finally, we were unable to expand on all forms of environmental metrics included in our scoping review, notably on concentration of VOCs emitted. Given the broad scope of the topic, we decided to focus our discussion on the more ubiquitous metrics reported in healthcare settings globally. Nevertheless, these metrics represent important markers of environmental impact, and will be better characterized once more relevant studies are performed.
In conclusion, our scoping review found only 30 articles attempting to quantify the environmental impact of IPC measures, with most reporting on mass of waste generated. The few studies reporting on carbon emissions were all conducted in high-income countries, highlighting a marked discrepancy in studies being performed across countries of varying incomes. Overall, the quality and scope of the available evidence on IPC appears relatively limited considering its importance, warranting an urgent need to invest in IPC environmental impact research to strengthen the evidence base that must be considered in order to move toward a more sustainable IPC agenda. The largest survey on IPC in healthcare facilities covering 81 countries was concluded in 2022, and reflected the WHO’s resolution to rapidly understand the interplay between IPC preparedness and pathogen transmission on a global scale [65]. The utility of IPC programs will gradually increase as lower-income countries continue to refine healthcare standards to meet minimum requirements. To achieve sustainability moving forward, collective action from every rung of the ladder needs to be initiated in addressing the environmental implications that infection control practices will likely precipitate in our natural environment.
Supplementary Information
Acknowledgements
Not applicable
Abbreviations
- IPC
Infection prevention and control
- CO2eq
Carbon dioxide equivalents
- PPE
Personal protective equipment
- WHO
World Health Organisation
- NHS
National Healthcare System
- UN
United Nations
Author contributions
All authors were involved in the initial planning of the study. OL, WYC, AW, and RRL worked on the study design and methodology, search strategy, and screening of articles. OL, WYC, and AW collected, analysed and interpreted data, and drafted the tables and figures. OL and WYC drafted the manuscript. RRL, HCC, SCQ, SW, and JW performed critical revisions of the manuscript for intellectually important content. All authors provided critical conceptual input, interpreted the data analysis, read, and approved the final draft. SW and JS have accessed and verified the data. SW and JS were responsible for the decision to submit the manuscript.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
RRL acknowledges research support from the Clinician Scientist Development Unit, Yong Loo Lin School of Medicine, National University of Singapore. JS received an honorarium from Sanofi-Aventis Singapore Pte. Ltd.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Costello A, Abbas M, Allen A, Ball S, Bell S, Bellamy R, et al. Managing the health effects of climate change: lancet and university college london institute for global health commission. Lancet. 2009;373(9676):1693–733. [DOI] [PubMed] [Google Scholar]
- 2.Selvaranjan K, Navaratnam S, Rajeev P, Ravintherakumaran N. Environmental challenges induced by extensive use of face masks during COVID-19: a review and potential solutions. Environ Chall. 2021;3:100039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klemeš JJ, Fan YV, Jiang P. The energy and environmental footprints of COVID-19 fighting measures - PPE, disinfection, supply chains. Energy. 2020;211:118701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chowdhury H, Chowdhury T, Sait SM. Estimating marine plastic pollution from COVID-19 face masks in coastal regions. Mar Pollut Bull. 2021;168:112419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu M, Cheng SZ, Xu KW, Yang Y, Zhu QT, Zhang H, et al. Use of personal protective equipment against coronavirus disease 2019 by healthcare professionals in Wuhan, China: cross sectional study. BMJ. 2020;369:m2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng S, Shen C, Xia N, Song W, Fan M, Cowling BJ. Rational use of face masks in the COVID-19 pandemic. Lancet Respir Med. 2020;8(5):434–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenhalgh T, Schmid MB, Czypionka T, Bassler D, Gruer L. Face masks for the public during the covid-19 crisis. BMJ. 2020;369:m1435. [DOI] [PubMed] [Google Scholar]
- 8.Livingston E, Desai A, Berkwits M. Sourcing Personal Protective Equipment During the COVID-19 Pandemic. JAMA. 2020;323(19):1912–4. [DOI] [PubMed] [Google Scholar]
- 9.World Health O. Global report on infection prevention and control; 2022 Report No.: ISBN: 978–92–4–005116–4.
- 10.Buchan JC, Thiel CL, Steyn A, Somner J, Venkatesh R, Burton MJ, et al. Addressing the environmental sustainability of eye health-care delivery: a scoping review. Lancet Planet Health. 2022;6(6):e524–34. [DOI] [PubMed] [Google Scholar]
- 11.McGain F, Muret J, Lawson C, Sherman JD. Environmental sustainability in anaesthesia and critical care. Br J Anaesth. 2020;125(5):680–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacNeill AJ, Lillywhite R, Brown CJ. The impact of surgery on global climate: a carbon footprinting study of operating theatres in three health systems. Lancet Planet Health. 2017;1(9):e381–8. [DOI] [PubMed] [Google Scholar]
- 13.Lam K, Gadi N, Acharya A, Winter Beatty J, Darzi A, Purkayastha S. Interventions for sustainable surgery: a systematic review. Int J Surg. 2023;109(5):1447–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dettenkofer M, Scherrer M, Hoch V, Glaser H, Schwarzer G, Zentner J, et al. Shutting down operating theater ventilation when the theater is not in use: infection control and environmental aspects. Infect Control Hosp Epidemiol. 2003;24(8):596–600. [DOI] [PubMed] [Google Scholar]
- 15.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–73. [DOI] [PubMed] [Google Scholar]
- 17.World Health O. Minimum requirements for infection prevention and control programmes. Geneva: World Health Organization; 2019. p. 2019. [Google Scholar]
- 18.Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32. [Google Scholar]
- 19.Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018;18(1):143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abu Qdais H, Rabi A, Abdulla F. Characteristics of the medical waste generated at the Jordanian hospitals. Clean Technol Environ Policy. 2007;9(2):147–52. [Google Scholar]
- 21.Abu-Qdais HA, Al-Ghazo MA, Al-Ghazo EM. Statistical analysis and characteristics of hospital medical waste under novel coronavirus outbreak. Global J Environ Sci Manag. 2020;6:21–30. [Google Scholar]
- 22.Al-Omran K, Khan E, Ali N, Bilal M. Estimation of COVID-19 generated medical waste in the Kingdom of Bahrain. Sci Total Environ. 2021;801:149642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Askarian M, Motazedian N, Palenik CJ. Clinical laboratory waste management in Shiraz. Iran Waste Manag Res. 2012;30(6):631–4. [DOI] [PubMed] [Google Scholar]
- 24.Bassey BE, Benka-Coker MO, Aluyi HS. Characterization and management of solid medical wastes in the Federal Capital Territory. Abuja Nigeria Afr Health Sci. 2006;6(1):58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bitty M, Kamelan O, Coha Y, et al. Qualitative and quantitative evaluation of medical waste products in Côte d’Ivoire. Antimicrob Resist Infect Control. 2013;2:363. [Google Scholar]
- 26.Cheng YW, Sung FC, Yang Y, Lo YH, Chung YT, Li KC. Medical waste production at hospitals and associated factors. Waste Manag. 2009;29(1):440–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chu J, Ghenand O, Collins J, Byrne J, Wentworth A, Chai PR, et al. Thinking green: modelling respirator reuse strategies to reduce cost and waste. BMJ Open. 2021;11(7):e048687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coker A, Sangodoyin A, Sridhar M, Booth C, Olomolaiye P, Hammond F. Medical waste management in Ibadan, Nigeria: obstacles and prospects. Waste Manag. 2009;29(2):804–11. [DOI] [PubMed] [Google Scholar]
- 29.Debere MK, Gelaye KA, Alamdo AG, Trifa ZM. Assessment of the health care waste generation rates and its management system in hospitals of Addis Ababa, Ethiopia, 2011. BMC Public Health. 2013;13:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farshad A, Gholami H, Farzadkia M, Mirkazemi R, Kermani M. The safety of non-incineration waste disposal devices in four hospitals of Tehran. Int J Occup Environ Health. 2014;20(3):258–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grimmond TR, Bright A, Cadman J, Dixon J, Ludditt S, Robinson C, et al. Before/after intervention study to determine impact on life-cycle carbon footprint of converting from single-use to reusable sharps containers in 40 UK NHS trusts. BMJ Open. 2021;11(9):e046200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamoda HM, El-Tomi HN, Bahman QY. Variations in hospital waste quantities and generation rates. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2005;40(2):467–76. [DOI] [PubMed] [Google Scholar]
- 33.Hassan MM, Ahmed SA, Rahman KA, Biswas TK. Pattern of medical waste management: existing scenario in Dhaka City, Bangladesh. BMC Public Health. 2008;8(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang MC, Lin JJ. Characteristics and management of infectious industrial waste in Taiwan. Waste Manag. 2008;28(11):2220–8. [DOI] [PubMed] [Google Scholar]
- 35.Koo JK, Jeong SI. Sustainability and shared smart and mutual–green growth (SSaM-GG) in Korean medical waste management. Waste Manag Res. 2015;33(5):410–8. [DOI] [PubMed] [Google Scholar]
- 36.Kubicki MAM F, O'Shea CJ, Bates S. Auditing an intensive care unit recycling program. Critical Care Resusc 2015; 17:135 [PubMed]
- 37.Kuo H-W, Shu S-L, Wu C-C, Lai J-S. Characteristics of medical waste in Taiwan. Water Air Soil Pollut. 1999;114(3):413–21. [Google Scholar]
- 38.Lee BK, Ellenbecker MJ, Moure-Eraso R. Analyses of the recycling potential of medical plastic wastes. Waste Manag. 2002;22(5):461–70. [DOI] [PubMed] [Google Scholar]
- 39.Longe EO. Healthcare waste management status in Lagos State, Nigeria: a case study from selected healthcare facilities in Ikorodu and Lagos metropolis. Waste Manag Res. 2012;30(6):562–71. [DOI] [PubMed] [Google Scholar]
- 40.Mbarki A, Kabbachi B, Ezaidi A, Benssaou M. Medical waste management: a case study of the souss-massa-drâa region Morocco. J Environ Prot. 2013;04(09):914. [Google Scholar]
- 41.Morooka H, Yamamoto T, Tanaka A, Furuhashi K, Miyagawa Y, Maruyama S. Influence of COVID-19 on the 10-year carbon footprint of the Nagoya University Hospital and medical research centre. Global Health. 2022;18(1):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pandey A, Ahuja S, Madan M, Asthana AK. Bio-medical waste managment in a tertiary care hospital: an overview. J Clin Diagn Res. 2016;10(11):DC01-dc3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rafiee A, Yaghmaeian K, Hoseini M, Parmy S, Mahvi A, Yunesian M, Nabizadeh R. Assessment and selection of the best treatment alternative for infectious waste by modified sustainability assessment of technologies methodology. J Environ Health Sci Eng. 2016;14:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rahmani K, Alighadri M, Rafiee Z. Assessment and selection of the best treatment alternative for infectious waste by sustainability assessment of technologies (SAT) methodology. J Air Waste Manag Assoc. 2020;70(3):333–40. [DOI] [PubMed] [Google Scholar]
- 45.Rizan C, Bhutta MF, Reed M, Lillywhite R. The carbon footprint of waste streams in a UK hospital. J Clean Prod. 2021;286:125446. [Google Scholar]
- 46.Rizan C, Reed M, Bhutta MF. Environmental impact of personal protective equipment distributed for use by health and social care services in England in the first six months of the COVID-19 pandemic. J R Soc Med. 2021;114(5):250–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tadesse ML, Kumie A. Healthcare waste generation and management practice in government health centers of addis ababa Ethiopia. BMC Public Health. 2014;14:1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taghipour H, Mosaferi M. The challenge of medical waste management: a case study in northwest Iran-Tabriz. Waste Manag Res. 2009;27(4):328–35. [DOI] [PubMed] [Google Scholar]
- 49.Vozzola E, Overcash M, Griffing E. Environmental considerations in the selection of isolation gowns: a life cycle assessment of reusable and disposable alternatives. Am J Infect Control. 2018;46(8):881–6. [DOI] [PubMed] [Google Scholar]
- 50.David E, Niculescu VC. Volatile organic compounds (VOCs) as environmental pollutants: occurrence and mitigation using nanomaterials. Int J Environ Res Public Health. 2021;18(24):13147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.BANK W. The World By Income and Region THE WORLD BANK2022 Available from: https://datatopics.worldbank.org/world-development-indicators/the-world-by-income-and-region.html.
- 52.WHO guidance for climate resilient and environmentally sustainable health care facilities [Internet]. [cited 2023 Oct 22]. Available from: https://www.who.int/publications-detail-redirect/9789240012226.
- 53.Andrieu B, Marrauld L, Vidal O, Egnell M, Boyer L, Fond G. Health-care systems’ resource footprints and their access and quality in 49 regions between 1995 and 2015: an input-output analysis. Lancet Planet Health. 2023;7(9):e747–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sulbaek Andersen MP, Nielsen OJ, Karpichev B, Wallington TJ, Sander SP. Atmospheric chemistry of isoflurane, desflurane, and sevoflurane: kinetics and mechanisms of reactions with chlorine atoms and OH radicals and global warming potentials. J Phys Chem A. 2012;116(24):5806–20. [DOI] [PubMed] [Google Scholar]
- 55.Tennison I, Roschnik S, Ashby B, Boyd R, Hamilton I, Oreszczyn T, et al. Health care’s response to climate change: a carbon footprint assessment of the NHS in England. Lancet Planet Health. 2021;5(2):e84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lenzen M, Malik A, Li M, Fry J, Weisz H, Pichler PP, et al. The environmental footprint of health care: a global assessment. Lancet Planet Health. 2020;4(7):e271–9. [DOI] [PubMed] [Google Scholar]
- 57.Janik-Karpinska E, Brancaleoni R, Niemcewicz M, Wojtas W, Foco M, Podogrocki M, et al. Healthcare waste-a serious problem for global health. Healthcare. 2023;11(2):242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.World Health O. Health-care waste: World Health Organization; 2018 [updated 8 February 2018. Available from: https://www.who.int/news-room/fact-sheets/detail/health-care-waste.
- 59.Japan meteorological agency [Internet]. [cited 2023 Oct 22]. Available from: https://www.jma.go.jp/jma/indexe.html.
- 60.Rasheed FN, Baddley J, Prabhakaran P, De Barros EF, Reddy KS, Vianna NA, et al. Decarbonising healthcare in low and middle income countries: potential pathways to net zero emissions. BMJ. 2021;375:n1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.The 17 goals | sustainable development [Internet]. [cited 2023 Oct 30]. Available from: https://sdgs.un.org/goals.
- 62.Eckelman MJ, Sherman J. Environmental impacts of the US health care system and effects on public health. PLoS One. 2016;11(6):e0157014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu R. The carbon footprint of the Chinese health-care system: an environmentally extended input-output and structural path analysis study. Lancet Planet Health. 2019;3(10):e413–9. [DOI] [PubMed] [Google Scholar]
- 64.Malik A, Lenzen M, McAlister S, McGain F. The carbon footprint of Australian health care. Lancet Planet Health. 2018;2(1):e27–35. [DOI] [PubMed] [Google Scholar]
- 65.Tomczyk S, Twyman A, de Kraker MEA, Coutinho Rehse AP, Tartari E, Toledo JP, et al. The first WHO global survey on infection prevention and control in health-care facilities. Lancet Infect Dis. 2022;22(6):845–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.