Abstract
Objective
To evaluate the efficacy of repetitive transcranial magnetic stimulation (rTMS) on cognitive function, depression, and walking ability in patients with Parkinson’s disease.
Methods
A comprehensive search was conducted in PubMed, Web of Science, Embase, Cochrane Library, China National Knowledge Infrastructure (CNKI), Chinese Biomedical Literature Database (CBM), VIP Database, and Wanfang Database. Randomized controlled trials (RCTs) on rTMS treatment in Parkinson’s disease patients were retrieved, covering the period from the inception of each database to July 2024. The quality of the included studies was assessed using the Cochrane risk of bias tool. Two researchers independently screened the literature, extracted data, and assessed the risk of bias in the studies. Data synthesis and analysis were performed using RevMan 5.4 and Stata 17.0 software.
Results
A total of 15 studies were included. The meta-analysis revealed that rTMS significantly improved the MOCA score (MD = 2.98, 95% CI 2.08, 3.88, P = 0.000), TUGT score (SMD=-0.72, 95% CI -1.43, 0.00, P = 0.048), FOG-Q score (SMD=-0.54, 95% CI -0.97, -0.11, P = 0.01), and UPDRS-III score (SMD=-0.66, 95% CI -0.84, -0.47, P = 0.000) in Parkinson’s disease patients, and also alleviated depressive symptoms as measured by the HAMD (SMD=-0.43, 95% CI -0.72, -0.13, P = 0.004).
Conclusions
rTMS can improve cognitive function, depressive symptoms, and walking ability in patients with Parkinson’s disease.
Keywords: Walking ability, Cognitive function, Repetitive transcranial magnetic stimulation, Gait, Meta-analysis
Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder primarily affecting middle-aged individuals, characterized by the gradual loss of dopaminergic neurons in the substantia nigra pars compacta. The motor symptoms of PD include bradykinesia, tremor, rigidity, and gait disturbances, with freezing of gait (FOG) being one of the most debilitating gait disturbances associated with the disease. A study involving 990 PD patients reported a FOG incidence of 32% [1], which severely impairs mobility, increases the risk of falls [2], and leads to a decline in quality of life [3]. Non-motor symptoms of PD manifest as cognitive impairment, depressive symptoms, visual dysfunction, and even cardiovascular autonomic dysfunction [4–9], all of which significantly exacerbate the suffering of PD patients. Various pharmacological treatments are available for both motor and non-motor symptoms of PD, primarily involving levodopa therapy [4, 10]to alleviate symptoms. However, these medications sometimes fail to provide the desired effects and may even induce motor complications, particularly levodopa-induced dyskinesias during long-term treatment [11, 12]. Therefore, exploring alternative and promising therapeutic approaches for PD, such as non-invasive brain stimulation (NIBS) [13], is essential. Current evidence has identified various neuroimaging markers and their related neuropathological mechanisms in PD, which appear to contribute to both motor and non-motor dysfunctions [14–16].
rTMS, a form of NIBS, has demonstrated neuromodulatory effects [13]. During rTMS intervention, a coil generates a magnetic field that penetrates the scalp and skull, thereby altering cortical excitability. Different rTMS frequencies elicit distinct effects: high-frequency rTMS (≥ 5 Hz) induces cortical excitation, while low-frequency rTMS (≤ 1 Hz) produces inhibitory effects; longer stimulation durations may result in prolonged effects [17, 18]. Additionally, various target sites are available for rTMS intervention, including the primary motor cortex (M1) for motor symptoms, as well as the dorsolateral prefrontal cortex (DLPFC), supplementary motor area (SMA), and, in some cases, the cerebellum [19, 20].
Gait disturbances are typically characterised by slow movements, an unstable gait and an increased risk of falls, which have a significant impact on patients’ functional independence and quality of life. With regard to gait disturbances in PD, some studies have indicated that rTMS may represent a potential therapeutic approach [21], Although PD is a neurodegenerative disease with predominantly motor symptoms, its non-motor symptoms, such as cognitive deficits and depressive symptoms, also have a significant impact on patients’ quality of life. In recent years, rTMS has been identified as a potential treatment for alleviating these non-motor symptoms. A number of studies have demonstrated that rTMS has a substantial ameliorative effect on depressive symptoms in patients with Parkinson’s disease [22]. For example, a meta-analysis conducted by Lesenskyj et al. [23] on the treatment of depression associated with Parkinson’s disease demonstrated that rTMS significantly reduced depression scores and positively affected patients’ mood states. Furthermore, a study by Chen. et al. demonstrated that rTMS was capable of enhancing mood-related cognitive performance, which subsequently led to an improvement in patients’ overall mental health [24]. With regard to cognitive impairment, a recent meta-analysis indicated that rTMS exhibited some efficacy in improving executive function and working memory [25]. This evidence provides support for the hypothesis that rTMS may represent an effective strategy for the treatment of non-motor symptoms in Parkinson’s disease.
However, there are still some shortcomings in the current research on the use of rTMS in the treatment of cognition, depression and walking ability in PD. First, the results of existing studies on the effects of rTMS on walking ability vary widely [21, 26, 27], with some studies finding that rTMS did not have significant effects on improving gait imbalance and reducing the risk of falling [28]. Second, studies have varied widely in treatment parameters (e.g., frequency, stimulation site, and duration), limiting the comparability and consistency of results. In addition, findings on the specific effects of rTMS on cognitive function have been inconsistent [29, 30], with some studies failing to establish significant causal relationships, suggesting that our understanding of the cognitive benefits of rTMS is incomplete. Therefore, there is a need to comprehensively synthesise and analyse the efficacy of transcranial magnetic stimulation on cognitive function, depression and walking ability by assessing multiple scales.
The current study synthesises the latest research findings and comprehensively examines the different effects of rTMS in improving cognitive impairment, depressive symptoms and walking ability and identifies potential influencing factors through heterogeneity analysis. In addition, this analysis will use a systematic quality assessment method to ensure the scientific validity and reliability of the included studies. This will provide deeper insights for future clinical applications and lay the foundation for the development of personalised treatment plans.
Methods
This study has been registered with PROSPERO, registration number No. CRD42023410954.
Literature search
A comprehensive search was conducted in both English databases (PubMed, Embase, Cochrane Library, Web of Science) and Chinese databases (Wanfang, CNKI, VIP, CBM) up until July 1, 2024, to identify randomized controlled trials (RCTs) related to rTMS intervention in Parkinson’s disease.
English search strategy: (transcranial magnetic stimulation[MeSH Terms]) AND (Parkinson’s disease[MeSH Terms]) OR (idiopathic Parkinson’s disease).
Chinese search strategy: (pajinsenbing OR pajinsenzonghezheng) AND (chongfujingluciciji).
Inclusion criteria
Study Type: Randomized controlled trials (RCTs) examining the effects of rTMS on patients with PD.
Study Population: Patients who meet the international diagnostic criteria for Parkinson’s disease, have a confirmed PD diagnosis with stable disease progression (e.g., stabilized by levodopa or other anti-Parkinson medications or stable after drug withdrawal with no other interventions), are aged 18 years or older, and are of any gender.
Intervention: The experimental group received rTMS either alone or combined with other basic treatments (e.g., conventional training, pharmacotherapy). The control group received sham rTMS or was combined with other basic treatments.
Outcomes: Motor improvement was measured by the motor sections of the Unified Parkinson’s Disease Rating Scale (UPDRS Part III, also known as UPDRS-III) and the Movement Disorder Society-Sponsored Revision of the UPDRS (MDS-UPDRS Part III). The primary assessment indicators for walking ability included the FOG-Q and the TUGT.
Non-motor outcomes included cognitive improvement and antidepressant effects, with cognitive improvement measured by the Montreal Cognitive Assessment (MoCA) scale and antidepressant effects measured by the Hamilton Depression Rating Scale (HDRS, also sometimes abbreviated as HRSD or HAMD in other articles).
Studies should include the mean values and standard deviations (SD) of the aforementioned scales before and after the intervention in both the rTMS intervention group and the sham control group.
Exclusion criteria
Duplicate studies;
Non-randomized controlled trials;
Studies in which the subjects were not Parkinson’s disease patients or the intervention in the treatment group was not rTMS;
Studies where the full text could not be obtained, data could not be extracted, or data were missing.
Data extraction
The process included the following steps: Two researchers independently conducted the initial screening by reviewing the titles, abstracts, and study types to remove duplicates. A second round of screening was performed based on full-text information and inclusion criteria. If discrepancies arose between the two researchers’ screening results, a third researcher would adjudicate the final inclusion. The final selected studies were included in the meta-analysis.
Extracted variables included: (1) Study design; (2) Demographic characteristics (including the number of patients, country, age, disease duration); (3) Mean scores and standard deviations of the following scales: (I) Cognitive scales, including MoCA; (II) Depression scales, including HAMD; (III) Walking ability scales, including FOG-Q, TUGT, and UPDRS-III; (4) rTMS parameters (frequency, intensity, site, and treatment duration). If a study had multiple rTMS intervention groups with different frequencies, these groups were treated as separate studies in our analysis. Summary data were independently extracted by two researchers from these RCTs.
Quality assessment of included studies
The quality of the included studies was evaluated using the Cochrane Handbook 5.1.0 criteria [31], which include the generation of random sequences, allocation concealment, blinding of researchers and subjects, blind assessment of outcomes, completeness of outcome data, selective reporting of results, and other biases. According to the quality assessment criteria, the risk of bias was categorized into three levels: “unclear risk,” “low risk,” and “high risk.” Two researchers independently assessed the quality of the studies and cross-checked their results. In case of disagreement, a third researcher determined the final risk level.
Statistical analysis
Meta-analysis was performed using RevMan 5.4 and Stata 17.0 statistical software. Data for continuous variables were expressed as mean differences (MD) or standardized mean differences (SMD) with 95% confidence intervals. Heterogeneity among studies was assessed using the I² statistic and P-value: If I²<50% and P > 0.1, heterogeneity among studies was considered low, and a fixed-effects model was used; If I²≥50% and P ≤ 0.1, heterogeneity was considered high, and a random-effects model was adopted. Subgroup analysis and sensitivity analysis were conducted using Stata 14.0 software to explore sources of heterogeneity in outcome indicators. Egger’s test was used to assess publication bias for studies with five or more included studies; Funnel plots were used for bias assessment in studies with ten or more included studies. Statistical significance was indicated by a P-value of less than 0.05, with P ≥ 0.05 indicating no significant difference.
GRADE evidence quality assessment
The evidence quality for outcome indicators with three or more studies was assessed using the GRADE method. The quality of evidence was rated considering risk of bias, inconsistency, imprecision, indirectness, and publication bias, and was categorized into four levels: high, moderate, low, and very low.
Results
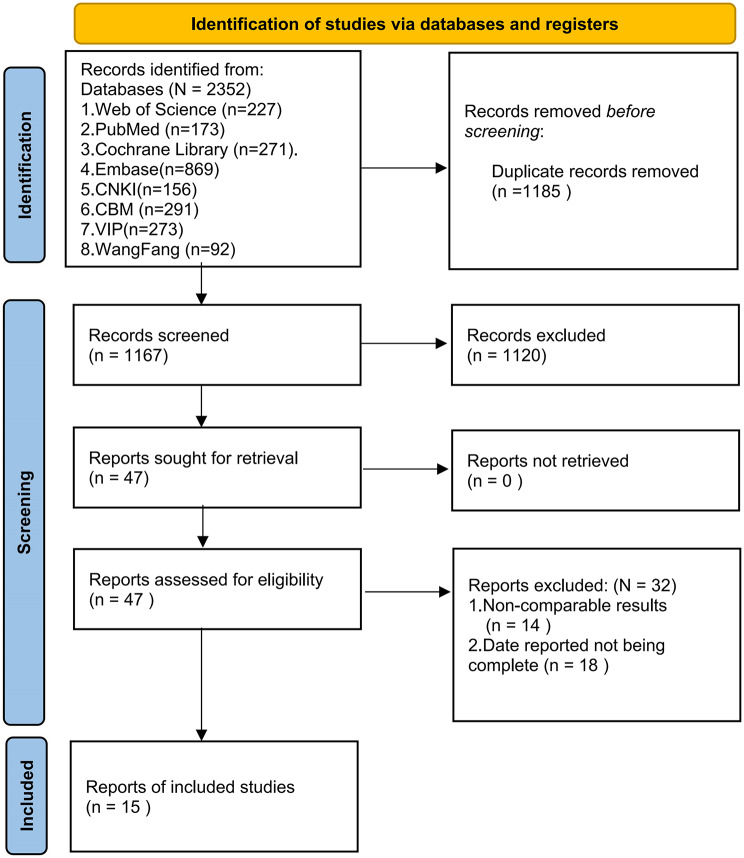
A total of 2,352 related studies were retrieved. After preliminary screening, 1,185 duplicate studies were excluded. Subsequently, 1,120 studies were further excluded based on their titles and abstracts. After reviewing the full texts according to the inclusion and exclusion criteria, an additional 32 studies were excluded. Ultimately, 15 studies were included. The detailed process and results of literature screening are presented in Fig. 1.
Fig. 1.
Literature screening flowchart
Characteristics of included studies
A total of 15 studies were included, with 9 studies in Chinese [32–40] and 6 in English [41–46], involving a total of 789 PD patients. In most studies, the average age of patients was over 50 years, and the average disease duration was over 3 years. Cognitive function analysis was performed on 175 patients, FOG-Q analysis on 90 patients, TUGT analysis on 178 patients, HAMD analysis on 201 patients, and UPDRS III analysis on 489 patients. Among these studies, 11 [32, 35–39, 41, 43–46] used high-frequency (≥ 5 Hz) rTMS to treat Parkinson’s disease, 1 study [34] used low-frequency (≤ 1 Hz) rTMS, and 3 studies [33, 40, 42] used both low and high-frequency rTMS. Nine studies targeted the DLPFC, six targeted M1, one targeted both M1 and DLPFC, and one study did not provide stimulation intensity information. Most patients in these studies had stable medication regimens before and during treatment. Detailed information on patient and rTMS variables used in these studies can be found in Table 1.
Table 1.
Characteristics of included literatures
| Researcher and Year | Country | Sample Size (C/T) | Age (C/T) | Disease Duration (C/T) | Interventions (C/T) | Stimulation Frequency | Stimulation Intensity | Stimulation Site | Treatment Duration | Outcome Measures | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cognition | Depression | Walking Ability | ||||||||||||
|
Zhuang et al. 2020 [34] |
China | 14/19 |
61.57 ± 13.25/ 60.58 ± 9.21 |
68.57 ± 45.29 M 70.37 ± 52.26 M |
①/rTMS | 1 Hz | 110%RMT |
Right DLPFC |
20 min/d,10d | MOCA | HAMD | - | UPDRSIII | - |
|
Hu et al. 2023 [32] |
China | 47/47 |
68.13 ± 5.41/ 68.13 ± 5.41 |
4.21 ± 0.69 4.30 ± 0.71 |
②/②rTMS | 5 Hz | 80%MT |
Bilatera DLPFC |
5times/week,2week | - | - | - | UPDRSIII | - |
|
Chen et al. 2014 [40] |
China | 18/21 |
66.61 ± 8.00/ 65.81 ± 9.38、63.19 ± 8.16 |
6.22 ± 3.96 5.88 ± 5.29 |
①/rTMS | 1 Hz/5Hz | 100%RMT | M1 | 1times/d,10d | - | HAMD | - | UPDRSIII | - |
|
Yu et al. 2017 [39] |
China | 30/31 |
60.16 ± 10.14/ 60.32 ± 9.63 |
2.62 ± 0.86 2.33 ± 0.74 |
①②/rTMS | 5 Hz | 100%MEP |
Bilatera DLPFC |
1times/d,10d | - | - | - | UPDRSIII | - |
|
Li et al. 2019 [36] |
China | 41/41 |
54.54 ± 3. 51/ 54. 51 ± 3. 49 |
3.12 ± 0.53 3.13 ± 0.55 |
②③/②③rTMS | 5 Hz | 80%MT |
Bilatera DLPFC |
1times/d, 5 d /w,4w |
MoCA | - | - | UPDRSIII | - |
|
Dong et al. 2018 [38] |
China | 30/30 | 65.83 ± 11.57 | 7.3 ± 2.4 | ②/②rTMS | 5 Hz | 80%MT | BilateraDLPFC |
1times/d, 5d /w,4w |
MoCA | - | - | - | - |
|
Li et al. 2020 [35] |
China | 24/24 |
61.46 ± 8.40/ 61.67 ± 6.92 |
6.46 ± 5.17 5.48 ± 3.69 |
①/rTMS | 20 Hz | 80%RMT | M1 | 20 min/d | - | HAMD | - | - | - |
|
Chung et al. 2020 [33] |
Hong Kong | 16/17 |
62.1 ± 5.7/ 62.1 ± 5.7 62.7 ± 6.8 |
6.9 ± 3.3 7.5 ± 4.9 |
①/rTMS | 1 Hz/25Hz | 80%RMT | M1 | 20 min/times, 5times/d | - | - | TUGT | UPDRSIII | - |
|
Mi et al. 2019 [37] |
China | 10/20 |
65.60 ± 8.68/ 62.65 ± 10.56 |
7.40 ± 4.83 9.15 ± 5.82 |
①/rTMS | 10 Hz |
46.0 ± 6.8%/ 45.6%±6.7% |
SMA |
1times/d, 5d/w,2w |
- | - | TUGT | UPDRSIII | FOG-Q |
|
Khedr et al. 2019 [41] |
United Kingdom | 11/19 |
57.4 ± 10.0/ 60.7 ± 8.8 |
6.5 ± 3.7 5.7 ± 3.9 |
①/rTMS | 20 Hz | 90%RMT | M1 |
10times/d, 5d/w |
- | - | - | UPDRSIII | - |
|
Cohen et al. 2018 [42] |
United Kingdom | 21/21 |
66.8 ± 8.1/ 64.4 ± 6.8 |
5.6 ± 3.7 4.7 ± 3.4 |
①/rTMS | 1/10Hz |
110%/ 100%RMT |
M1/DLPFC | 1times/d, 12w | - | - | TUGT | - | - |
|
Brys et al. 2016 [43] |
United Kingdom | 15/12 |
64.0 ± 7.4/ 64.6 ± 12.3 |
4.5 ± 2.2 7.7 ± 4.2 |
①/rTMS | 10 Hz | - | Left DLPFC | 2,000times/d, 10d | - | HAMD | - | UPDRSIII | - |
|
Kim et al. 2015 [44] |
South Korea | 17 | 64.5 ± 8.4 | 7.8 ± 4.9 | ①/rTMS | 10 Hz | 90%RMT | M1 | 5times/w | - | - | TUGT | UPDRSIII | FOG-Q |
|
Pal et al. 2010 [46] |
United Kingdom | 10/12 |
67.5(57.0,72.0)/ 68.5(59.5,70.0) |
6.5(3.75–10.5) 6.0(3.0–9.5) |
①/rTMS | 5 Hz | 90%RMT | Left DLPFC | 600times/d,10 d | - | - | TUGT | UPDRSIII | - |
|
Benninger et al. 2012 [45] |
United States | 13/13 |
63.7 ± 8.3/ 64.5 ± 9.1 |
9.3 ± 6.8 8.6 ± 4.1 |
①/rTMS | 50 Hz | 80%AMT | M1 | 1times/d, 4d/w, 2w | - | - | - | - | FOG-Q |
Notes: T represents the Experimental Group; C represents the Control Group; M1 refers to the Primary Motor Cortex; SMA stands for the Supplementary Motor Area; PFC indicates the Prefrontal Cortex; MT refers to the Motor Threshold; AMT denotes the Active Motor Threshold; RMT is the Resting Motor Threshold. M: month The interventions are categorized as follows: ① Sham Stimulation, ② Conventional Anti-Parkinson Medication ③ Cognitive Training
Quality assessment of included studies
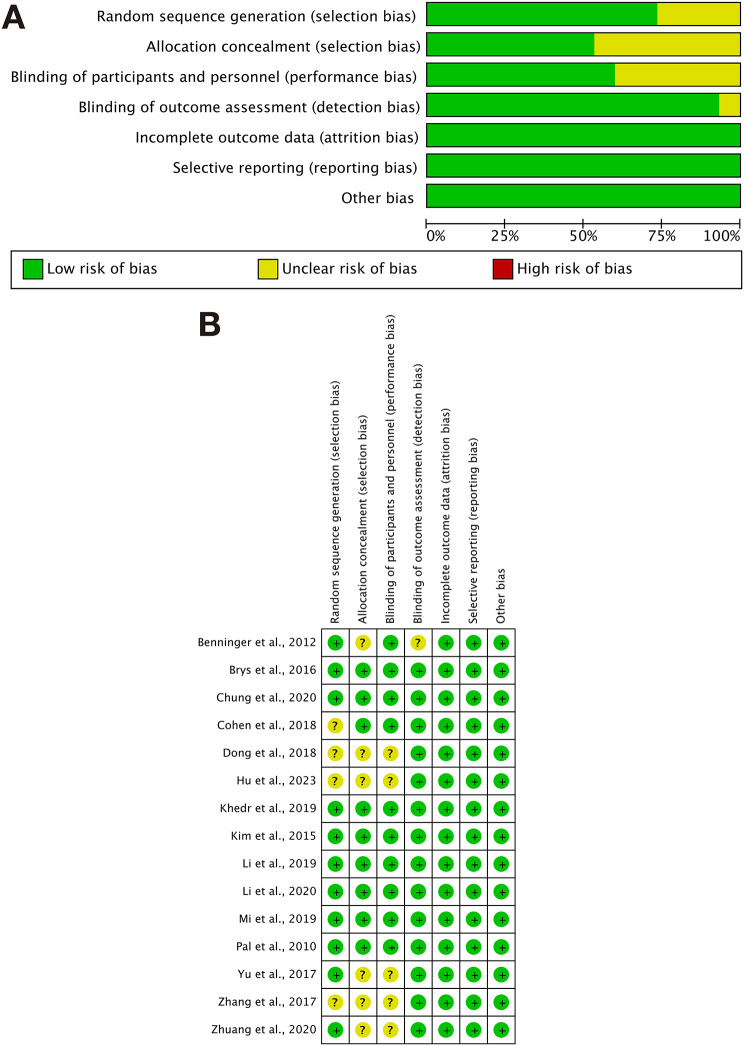
Among the 15 included studies, 10 studies [33–35, 37, 41–46] mentioned randomization and provided specific methods, while 5 studies [36, 38–40] did not specify the methods used, and one study [32] had a un risk in the randomization process. Four studies [33, 35, 37, 41] provided detailed descriptions of allocation concealment, while the remaining 9 did not mention it. 9 studies [33, 35, 37, 41–46] blinded both researchers and subjects, one study [34] blinded only the researchers, and the remaining five studies [32, 36, 38–40] did not mention blinding methods. All 15 studies reported complete data, and all 15 studies reported their results without mentioning any other biases. Details are shown in Fig. 2.
Fig. 2.
Risk assessment chart. A Risk of bias graph. B Risk of bias summary
Meta-analysis
Baseline consistency test
In this paper, all outcome indicators were tested for consistency at baseline, i.e. there was no significant difference between the baseline scores of the control and baseline groups before the effect sizes were combined post-intervention (See Table 2).
Table 2.
Baseline test score table
| Outcome measures | Study (Patient) | SMD(95%CI) | P-value of the intervention effect | Heterogeneity | |
|---|---|---|---|---|---|
| Cognitive function | MOCA | 3(n = 175) | 0.103,(-0.195;0.401) | P = 0.496 | I2 = 0%, P = 0.544 |
| Depressive symptoms | HAMD | 4(n = 168) | 0.071,(-0.219;0.362) | P = 0.631 | I2 = 2.6%, P = 0.392 |
| Walk ability | FOG-Q | 3(n = 90) | 0.023,(-0.398;0.444) | P = 0.915 | I2 = 0%, P = 0.967 |
| TUGT | 5(n = 178) | -0.182,(-0.470; 0.106) | P = 0.216 | I2 = 4%, P = 0.108; | |
| UPDRSIII | 11(n = 489) | -0.03,(-0.203;0.142) | P = 0.730 | I2 = 0%, P = 0.993 |
Effect of rTMS on MOCA in PD patients
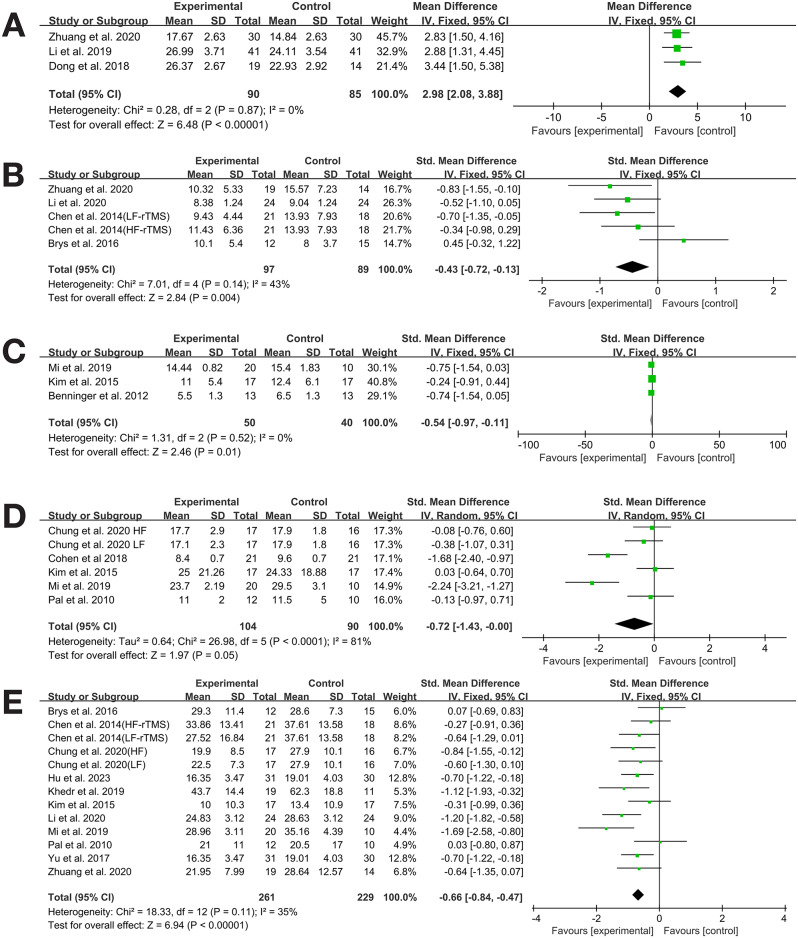
Three studies [34, 36, 38] reported MOCA as an outcome measure. The study by Zhuang et al. used low-frequency (1 Hz) stimulation, focusing on the right DLPFC, for a shorter treatment course (10 days), while the study by Li and Dong et al. used high-frequency (5 Hz) stimulation and was performed on both DLPFCs for a longer treatment course (4 weeks)。The heterogeneity test indicated no heterogeneity (I²=0%, P = 0.544), so a fixed-effects model was used for analysis. Meta-analysis showed that transcranial magnetic stimulation significantly improved cognitive function in patients with Parkinson’s disease after the intervention (MD = 2.98, 95% CI 2.08 to 3.88, P = 0.000) (See Table 3; Fig. 3A).
Table 3.
Meta-analysis results
| Outcome measures | Study (Patient) | SMD/MD (95%CI) | P-value of the intervention effect | Heterogeneity | Publication bias | |
|---|---|---|---|---|---|---|
| Cognitive function | MOCA | 3(n = 175) | 2.98,(2.08;3.88) | P = 0.000 | I2 = 0%, P = 0.87 | |
| Depressive symptoms | HAMD | 4(n = 186) | -0.43,(-0.72;-0.13) | P = 0.004 | I2 = 43%%, P = 0.14 | |
| Intervention Frequency | ||||||
| ≥ 5 Hz | 2(n = 114) | -0.19,(-0.73; 0.35) | P = 0.50 | I2 = 51%, P = 0.13 | ||
| ≤ 1 Hz | 2(n = 72) | -0.88,(-1.37; -0.39) | P = 0.0005 | I2 = 0%, P = 0.42 | ||
| Stimulation Site | P = 0.361 | |||||
| DLPFC | 2(n = 60) | -0.23,(-0.76;0.30) | P = 0.39 | I2 = 82%, P = 0.02 | ||
| M1 | 2(n = 126) | -0.52,(-0.88;-0.16) | P = 0.004 | I2 = 0%, P = 0.74 | ||
| Walk ability | FOG-Q | 3(n = 90) | -0.54,(-0.97;-0.11) | P = 0.01 | I2 = 0%, P = 0.01; | |
| TUGT | 5(n = 178) | -0.72,(-1.43; 0.00) | P = 0.048 | I2 = 81%, P = 0.000 | Egger’s test, P = 0.243 | |
| Stimulation Site | ||||||
| M1 | 3(n = 83) | -0.54,(-1.60; 0.52) | P = 0.32 | I2 = 0%, P = 0.85 | ||
| DLPFC | 2(n = 64) | 1.19,(0.77; 1.61) | P<0.00001 | I2 = 0%, P = 0.68 | ||
| SMA | 1(n = 30) | -5.8,(-7.95;-3.65) | P<0.00001 | - | ||
| UPDRSIII | 11(n = 489) | -0.66,(-0.84; -0.47) | P = 0.000 | I2 = 35%, P = 0.083 | Egger’s test, P = 0.976 |
Fig. 3.
Meta-analysis results. A MOCA. B HAMD. C TUGT. D FOG-Q. E UPDRSIII
Effect of rTMS on HAMD in PD patients
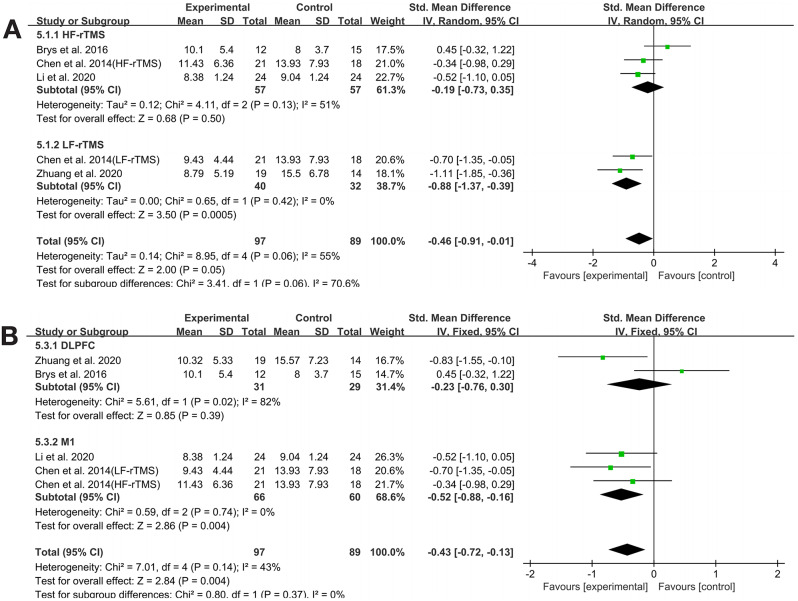
Four studies [34, 35, 40, 43] provided HAMD outcome data. The meta-analysis indicated that rTMS significantly alleviated depressive symptoms in PD patients (SMD=-0.43, 95% CI -0.72 to -0.13, P = 0.004). The heterogeneity test showed low heterogeneity (P = 0.14, I²=43%), so a fixed-effects model was used for analysis. Subgroup analysis was conducted by rTMS frequency and stimulation site to explore the sources of heterogeneity in HAMD outcomes. The results indicated that the effect of rTMS on HAMD in PD patients was not influenced by stimulation frequency or site, and no sources of heterogeneity were found in either subgroup (See Table 3. Figs. 3B. and 4)..
Fig. 4.
HAMD subgroup analysis
Walking ability
Effect of rTMS on TUGT in PD patients
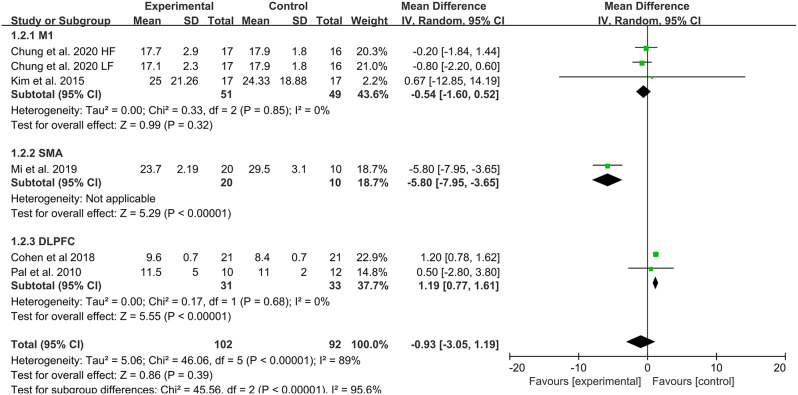
Five studies [33, 37, 42, 44, 46] evaluated TUGT as an outcome measure. The meta-analysis showed that there was a statistically significant difference between the experimental group and the control group in TUGT (SMD=-0.72, 95% CI -1.43 to 0.00, P = 0.048). The heterogeneity test indicated high heterogeneity (I²=81%, P = 0.000); to further explore the sources of heterogeneity, subgroup analysis was conducted by rTMS frequency, stimulation site, and intervention measures. The results indicated that the effect of rTMS on TUGT in PD patients was related to the stimulation site (See Table 3; Figs. 3C and 5).
Fig. 5.
TUGT subgroup analysis
Effect of rTMS on FOG-Q in PD patients
Three studies [37, 44, 45] evaluated FOG-Q as an outcome measure.The heterogeneity test indicated no heterogeneity (P = 0.497, I²=0%), so a fixed-effects model was used for analysis. The meta-analysis showed that rTMS significantly improved freezing of gait in PD patients (SMD=-0.54, 95% CI -0.97 to -0.11, P = 0.01) (See Table 3; Fig. 3D).
Effect of rTMS on UPDRS III in PD patients
Eleven studies [32–34, 36, 37, 39–41, 43, 44, 46] provided UPDRS III outcome data. The meta-analysis indicated that rTMS significantly improved UPDRS III scores in PD patients (SMD=-0.66, 95% CI -0.84 to -0.47, P = 0.000). The heterogeneity test showed low heterogeneity (I²=35%, P = 0.083), and a fixed-effects model was used for analysis (See Table 3; Fig. 3E).
Publication bias
Egger’s test was used to assess publication bias for outcome indicators with five or more studies, and funnel plots were used for bias assessment for outcome indicators with ten or more studies. The results showed no publication bias for cognitive function (MOCA, P = 0.070 > 0.05), TUGT (P = 0.243 > 0.05), and UPDRS III (P = 0.976 > 0.05), indicating negative results (See Fig. 6).
Fig. 6.
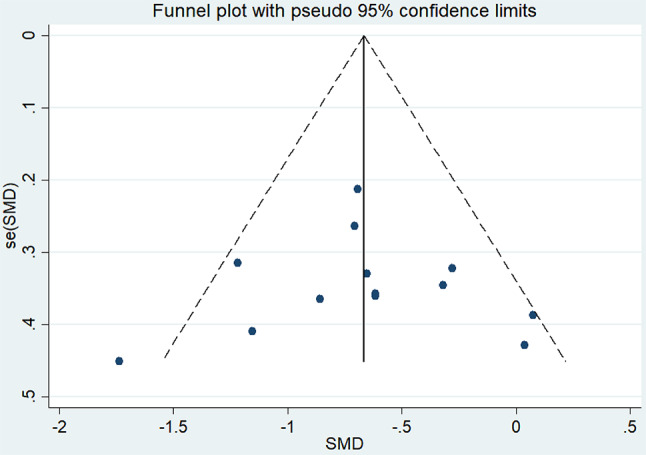
UPDRSIII funnel plot
Evidence credibility
The GRADE quality of evidence was assessed for studies that included MOCA scores, HAMD scores, TUGT scores, FOG-Q scores, and UPDRS III scores (See Table 4).
Table 4.
GRADE quality of evidence assessment of included studies
| Outcome Measures | Number of Studies | Sample Size | Limitations | Inconsistency | Indirectness | Imprecision | Publication Bias | Evidence Quality |
|---|---|---|---|---|---|---|---|---|
| MOCA Score | 3 | 175 | 0 | 0 | 0 | Downgraded by One Grade (b) | 0 | Moderate |
| HAMD Score | 4 | 186 | 0 | 0 | 0 | Downgraded by One Grade (b) | 0 | Moderate |
| TUGT Score | 5 | 178 | 0 | Downgraded by One Grade (a) | 0 | Downgraded by One Grade (b) | 0 | Low |
| FOG-Q Score | 3 | 90 | 0 | 0 | 0 | Downgraded by One Grade (b) | 0 | Moderate |
| UPDRS III Score | 11 | 489 | 0 | Downgraded by One Grade (a) | 0 | 0 | 0 | Moderate |
Note: a: The confidence intervals of different studies have poor overlap, with a high I² value and a low P-value in the heterogeneity test; b: The sample size is too small, resulting in a wide confidence interval and poor overlap of confidence intervals; c: Publication bias is present
Discussion
The risk of cognitive impairment in PD increases as the disease progresses. This study assessed the efficacy of rTMS on cognitive function in PD patients by examining the differences in MOCA scores before and after rTMS intervention. Overall, the effects of rTMS were positive. The effective mechanisms of rTMS in improving cognitive function in PD patients lie mainly in promoting neuroplasticity and reorganisation of functional networks in the brain. rTMS is able to improve neural conduction and promote synaptic plasticity by modulating the neural excitability of specific brain regions, which can lead to improvements in cognitive domains such as executive function, attention and working memory [47]. Studies have shown that rTMS stimulation targeting the frontal and motor cortex improves neural activity related to attention and decision making and promotes functional connectivity of the prefrontal cortex with other brain regions [48]. In addition, the application of high-frequency (e.g., 10–20 Hz) rTMS, which is commonly used to activate cortical functions and strengthen neural networks, especially in tasks related to motor control and cognitive processing, can significantly improve patients’ cognitive performance [49]. In contrast, low frequency (e.g.1 Hz) rTMS is used to inhibit overactive brain regions and help balance the relationship between inhibition and activation [50], which also plays an important role in cognitive interventions in PD.
Depression has the highest prevalence among non-motor symptoms in PD, affecting 20–50% of patients, and most antidepressants have limited efficacy [51]. In this study, four studies showed significant effect sizes on depression scales (SMD=-0.43), supporting the potential antidepressant effects of active rTMS over sham rTMS in PD patients. However, subgroup analysis by rTMS frequency and stimulation site showed no differences. Multiple reviews and meta-analyses have indicated that rTMS intervention can reduce depression scale scores, suggesting a potential antidepressant effect [48, 52]. Studies have shown that high-frequency stimulation of the left prefrontal lobe increases its neural activity, thereby increasing the release of neurotransmitters such as dopamine and serotonin, which has been directly linked to improvements in depressive symptoms [53]. In addition, low-frequency stimulation can be used to inhibit hyperactivity in the right prefrontal lobe, a mechanism that is also important in regulating mood and suppressing negative thinking [54]. Other studies have also found that TMS can help remodel patients’ functional brain networks by promoting neuroplasticity and strengthening synaptic connections [55]. Depressed patients often show abnormalities in functional brain connectivity, such as dysregulation between the default mode network and the emotion regulation network. The use of TMS may help to restore the coordination of these networks and improve the brain’s ability to regulate emotions, thereby improving the patient’s overall mental health. Additionally, some studies have shown that rTMS intervention exhibits antidepressant-like effects similar to oral medications such as selective serotonin reuptake inhibitors (SSRIs) used in clinical antidepressant treatment [56]. To our knowledge, rTMS intervention may be used not only for treating PD-related depression but also for other types of depression. Therefore, we believe that rTMS is effective in treating depression, with the DLPFC being a potential target. However, the appropriate stimulation site (left or right DLPFC, unilateral or bilateral) and frequency require further study.
The TUGT is a more sensitive method for assessing functional mobility and gait stability in the elderly, and it is clinically significant for the diagnosis and treatment of gait disturbances in PD. Five studies [33, 37, 42, 44, 46] in this paper used TUGT as an outcome measure, showing a significant difference between the rTMS group and the sham stimulation group, with the rTMS group performing better. This suggests that rTMS can improve TUGT ability in PD patients. However, the heterogeneity among studies was high for this measure (P = 0.000, I²=81%). To further investigate the source of heterogeneity, subgroup analysis was conducted, which indicated that different stimulation sites might affect TUGT outcomes. Treatment effects were better when M1 was selected as the stimulation site compared to DLPFC and SMA. Freezing of gait (FOG) is one of the common symptoms of PD, and its improvement helps reduce the risk of falls, enhance walking ability, and improve quality of life. Based on data from three studies [37, 44, 45] using FOG-Q as an outcome measure, the results showed a significant reduction in FOG-Q scores, indicating that rTMS significantly improved FOG in PD patients. Mi [37] reported that HF-rTMS of the SMA could improve FOG in PD, while Kim [44] reported that HF-rTMS of the leg primary motor cortex (M1-LL) in the dominant hemisphere also improved FOG. Due to the small number of studies in our research, we did not further analyze the stimulation sites. Therefore, future research is needed to determine whether different stimulation sites for HF-rTMS can better improve FOG. Eleven studies [32–34, 36, 37, 39–41, 43, 44, 46] used UPDRS III as an outcome measure, and the results showed that the rTMS group was significantly better than the control group in improving motor function in PD patients, consistent with the conclusions of Li et al. [57]. Overall, rTMS can effectively improve walking function in PD patients compared to the control group.By stimulating specific regions of the motor cortex, TMS can effectively enhance the brain’s control of lower limb movement while improving coordination and stability during movement. Studies have shown that high-frequency rTMS stimulation of the primary motor cortex increases neural excitability in this region, which in turn improves stride length and gait smoothness [58], and that gait deficits are mainly due to basal ganglia dysfunction and incoordination of the motor control network [59]. In addition, TMS may promote neuroplasticity by improving cortical functional connectivity. Compared to normal gait, patients with PD often show abnormalities in motor network connectivity, which can lead to gait instability and reduced stride length. TMS stimulation can reintegrate functional networks between motor-related brain regions, thereby improving brain motor control and gait performance [60]. In particular, coordinated stimulation of different brain regions (e.g. combined stimulation of the cerebellum and motor cortex) can synchronise the improvement of gait and static balance. Although TMS has been shown to be effective in improving gait disorders in PD patients, the optimisation of stimulation parameters (e.g. stimulation frequency, intensity and number of repetitions) and their effects on long-term outcomes need to be thoroughly investigated. In addition, combining TMS with other rehabilitation tools (e.g., physical therapy or medication) may further enhance the effect of gait improvement.
This study has some limitations. First, this meta-analysis did not further analyze the specific stimulation sites for rTMS intervention on cognition. We acknowledge that the lack of this separate evaluation is one of the limitations of our study, and future clinical research and meta-analyses should consider this. Second, due to the lack of high-quality randomized controlled trials on non-motor symptoms, we could not further evaluate the efficacy of rTMS on various aspects of cognition. Therefore, unlike other reviews and meta-analyses, we did not further analyze changes in various cognitive domains. Third, we did not assess other non-motor symptoms that might be early PD symptoms, such as sleep disorders, although Babiloni et al. [61] demonstrated the potential positive effects of rTMS intervention on sleep disorders. Fourth, although this study focused mainly on rTMS intervention, several new TMS protocols, such as theta burst stimulation (TBS), paired associative stimulation (PAS), and other types of NIBS, including transcranial direct current stimulation (tDCS), are rapidly developing and deserve attention. These new protocols introduce innovative methods for modulating cortical excitability, and future network meta-analyses should further compare the efficacy of these methods in improving PD symptoms to select the optimal protocol.
Conclusion
rTMS significantly improves cognitive function, depressive symptoms, and walking ability in Parkinson’s disease patients. Different stimulation sites may influence TUGT outcomes, while the improvement of depressive symptoms is not affected by stimulation frequency or site.
Abbreviations
- PD
Parkinson’s Disease
- FOG
Freezing of Gait
- rTMS
Repetitive Transcranial Magnetic Stimulation
- M1
Primary Motor Cortex
- DLPFC
Dorsolateral Prefrontal Cortex
- SMA
Supplementary Motor Area
- UPDRS
Unified Parkinson’s Disease Rating Scale
- MDS-UPDRS
Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale
- FOG-Q
Freezing of Gait Questionnaire
- TUGT
Timed Up and Go Test
- MoCA
Montreal Cognitive Assessment
- HAMD (or HDRS, HRSD)
Hamilton Depression Rating Scale
- SMD
Standardized Mean Difference
- MD
Mean Difference
- I²
Inconsistency Index (for heterogeneity in meta-analysis)
- GRADE
Grading of Recommendations, Assessment, Development, and Evaluations
Author contributions
M.W. and W.Z. contributed to data analysis and wrote the original draft. W.Z. collected the data and conducted the literature review. M.W. and W.Z. contributed to reviewing and editing the manuscript. W.Z. supervised the project, and W.Z. handled project administration. All authors reviewed and approved the final manuscript.
Funding
Not applicable.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethical approval and consent to participate
This article contains no studies with human participants or animals performed by any authors.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Giladi N, McMahon D, Przedborski S, et al. Motor blocks in Parkinson’s disease[J]. Neurology. 1992;42(2):333–9. [DOI] [PubMed] [Google Scholar]
- 2.Kerr GK, Worringham CJ, Cole MH, et al. Predictors of future falls in Parkinson disease[J]. Neurology. 2010;75(2):116–24. [DOI] [PubMed] [Google Scholar]
- 3.Rahman S, Griffin HJ, Quinn NP, et al. Quality of life in Parkinson’s disease: the relative importance of the symptoms[J]. Mov Disord. 2008;23(10):1428–34. [DOI] [PubMed] [Google Scholar]
- 4.Nemade D, Subramanian T, Shivkumar V. An Update on Medical and Surgical Treatments of Parkinson’s Disease[J]. Aging Dis. 2021;12(4):1021–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batool F, Kirmani L A S A. Neurological and Neurodegenerative Disorders: Novel Concepts and Treatment[Z]. 2021: 12, 950–953. [DOI] [PMC free article] [PubMed]
- 6.Hendricks RM, Khasawneh MT. A Systematic Review of Parkinson’s Disease Cluster Analysis Research[J]. Aging Dis. 2021;12(7):1567–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu S, Li H, Xu X, et al. The Pathogenesis and Treatment of Cardiovascular Autonomic Dysfunction in Parkinson’s Disease: What We Know and Where to Go[J]. Aging Dis. 2021;12(7):1675–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilat M, Ginis P, Zoetewei D, et al. A systematic review on exercise and training-based interventions for freezing of gait in Parkinson’s disease[J]. NPJ Parkinsons Dis. 2021;7(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu Z, Hu W, Liao H, et al. Association of visual impairment with risk for future Parkinson’s disease[J]. EClinicalMedicine. 2021;42:101189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connolly BS, Lang AE. Pharmacological treatment of Parkinson disease: a review[J]. JAMA. 2014;311(16):1670–83. [DOI] [PubMed] [Google Scholar]
- 11.Fahn S. The history of dopamine and levodopa in the treatment of Parkinson’s disease[J]. Mov Disord. 2008;23(Suppl 3):S497–508. [DOI] [PubMed] [Google Scholar]
- 12.Espay AJ, Morgante F, Merola A, et al. Levodopa-induced dyskinesia in Parkinson disease: Current and evolving concepts[J]. Ann Neurol. 2018;84(6):797–811. [DOI] [PubMed] [Google Scholar]
- 13.Sanches C, Stengel C, Godard J, et al. Past, Present, and Future of Non-invasive Brain Stimulation Approaches to Treat Cognitive Impairment in Neurodegenerative Diseases: Time for a Comprehensive Critical Review[J]. Front Aging Neurosci. 2020;12:578339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu S, Deng B, Huang Z, et al. Hot cross bun is a potential imaging marker for the severity of cerebellar ataxia in MSA-C[J]. NPJ Parkinsons Dis. 2021;7(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu S, Li H, Deng B, et al. Various Diseases and Clinical Heterogeneity Are Associated With Hot Cross Bun[J]. Front Aging Neurosci. 2020;12:592212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin A, Zheng W, He Y, et al. Gut microbiota in patients with Parkinson’s disease in southern China[J]. Parkinsonism Relat Disord. 2018;53:82–8. [DOI] [PubMed] [Google Scholar]
- 17.Brunoni AR, Chaimani A, Moffa AH, et al. Repetitive Transcranial Magnetic Stimulation for the Acute Treatment of Major Depressive Episodes: A Systematic Review With Network Meta-analysis[J]. JAMA Psychiatry. 2017;74(2):143–52. [DOI] [PubMed] [Google Scholar]
- 18.Kennedy SH, Lam RW, McIntyre RS, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder: Sect. 3. Pharmacological Treatments[J]. Can J Psychiatry. 2016;61(9):540–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jankovic J. Parkinson’s disease: clinical features and diagnosis[J]. J Neurol Neurosurg Psychiatry. 2008;79(4):368–76. [DOI] [PubMed] [Google Scholar]
- 20.Shin HW, Hallett M, Sohn YH. Cerebellar repetitive transcranial magnetic stimulation for patients with essential tremor[J]. Parkinsonism Relat Disord. 2019;64:304–7. [DOI] [PubMed] [Google Scholar]
- 21.Deng S, Dong Z, Pan L, et al. Effects of repetitive transcranial magnetic stimulation on gait disorders and cognitive dysfunction in Parkinson’s disease: A systematic review with meta-analysis[J]. Brain Behav. 2022;12(8):e2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang W, Deng B, Xie F, et al. Efficacy of repetitive transcranial magnetic stimulation in Parkinson’s disease: A systematic review and meta-analysis of randomised controlled trials[J]. EClinicalMedicine. 2022;52:101589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lesenskyj AM, Samples MP, Farmer JM, et al. Treating refractory depression in Parkinson’s disease: a meta-analysis of transcranial magnetic stimulation[J]. Transl Neurodegener. 2018;7:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen X, Zhang T, Shan X, et al. High-frequency repetitive transcranial magnetic stimulation alleviates the cognitive side effects of electroconvulsive therapy in major depression[J]. Front Psychiatry. 2022;13:1002809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng TC, Huang SF, Wu SY, et al. Integration of Virtual Reality into Transcranial Magnetic Stimulation Improves Cognitive Function in Patients with Parkinson’s Disease with Cognitive Impairment: A Proof-of-Concept Study[J]. J Parkinsons Dis. 2022;12(2):723–36. [DOI] [PubMed] [Google Scholar]
- 26.Xie YJ, Gao Q, He CQ, et al. Effect of Repetitive Transcranial Magnetic Stimulation on Gait and Freezing of Gait in Parkinson Disease: A Systematic Review and Meta-analysis[J]. Arch Phys Med Rehabil. 2020;101(1):130–40. [DOI] [PubMed] [Google Scholar]
- 27.Chen KS, Chen R. Invasive and Noninvasive Brain Stimulation in Parkinson’s Disease: Clinical Effects and Future Perspectives[J]. Clin Pharmacol Ther. 2019;106(4):763–75. [DOI] [PubMed] [Google Scholar]
- 28.Hvingelby VS, Glud AN, Sørensen J, et al. Interventions to improve gait in Parkinson’s disease: a systematic review of randomized controlled trials and network meta-analysis[J]. J Neurol. 2022;269(8):4068–79. [DOI] [PubMed] [Google Scholar]
- 29.Martin DM, McClintock SM, Forster J, et al. Does Therapeutic Repetitive Transcranial Magnetic Stimulation Cause Cognitive Enhancing Effects in Patients with Neuropsychiatric Conditions? A Systematic Review and Meta-Analysis of Randomised Controlled Trials[J]. Neuropsychol Rev. 2016;26(3):295–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Begemann MJ, Brand BA, Ćurčić-Blake B, et al. Efficacy of non-invasive brain stimulation on cognitive functioning in brain disorders: a meta-analysis[J]. Psychol Med. 2020;50(15):2465–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higgins JP, Savović J, Page MJ et al. Assessing risk of bias in a randomized trial[M]//2019:205–28.
- 32.Xiaoying H, Hongyan L, Tong Z, et al. Clinical observation of bilateral dorsolateral prefrontal rTMS stimulation in patients with Parkinson’s disease[J]. Chin J Practical Nerv Dis. 2023;26(04):443–7. [Google Scholar]
- 33.Chung CL, Mak MK, Hallett M. Transcranial Magnetic Stimulation Promotes Gait Training in Parkinson Disease[J]. Ann Neurol. 2020;88(5):933–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhuang S, Wang FY, Gu X et al. Low-Frequency Repetitive Transcranial Magnetic Stimulation over Right Dorsolateral Prefrontal Cortex in Parkinson’s Disease[J]. Parkinsons Dis. 2020;2020:7295414. [DOI] [PMC free article] [PubMed]
- 35.Li J, Mi TM, Zhu BF, et al. High-frequency repetitive transcranial magnetic stimulation over the primary motor cortex relieves musculoskeletal pain in patients with Parkinson’s disease: A randomized controlled trial[J]. Parkinsonism Relat Disord. 2020;80:113–9. [DOI] [PubMed] [Google Scholar]
- 36.Yu L, Yanhong G, Lijuan Y. Effects of Repetitive Transcranial Magnetic Stimulation on Gait in Parkinson’s Patients[J]. World Chin Med. 2019;14(12):3389–93. [Google Scholar]
- 37.Mi TM, Garg S, Ba F, et al. High-frequency rTMS over the supplementary motor area improves freezing of gait in Parkinson’s disease: a randomized controlled trial[J]. Parkinsonism Relat Disord. 2019;68:85–90. [DOI] [PubMed] [Google Scholar]
- 38.Wei D, Wenming Y. Clinical Study on Repetitive Transcranial Magnetic Stimulation in the Treatment of Parkinson’s Disease[J]. Clin J Traditional Chin Med. 2018;30(12):2269–71. [Google Scholar]
- 39.Wen-wen Y, Zhen-guang L, Hai-rong S, et al. Effects of High Frequency Repetitive Transcranial Magnetic Stimulation on Motor Fuction, Cognitivefunction and Autonomic Disorder in Patients with Early Parkinson’s Disease[J]. Acta Academiae Medicinae Weifang. 2017;39(04):288–90. [Google Scholar]
- 40.Jing C, Changguo Z, Hongbo Z, et al. Clinical observation of high-frequency versus low-frequency repetitive transcranial magnetic stimulation in the treatment of Parkinson’s disease[J]. Chin J Rehabilitation Med. 2014;29(05):464–7. [Google Scholar]
- 41.Khedr EM, Mohamed KO, Soliman RK, et al. The Effect of High-Frequency Repetitive Transcranial Magnetic Stimulation on Advancing Parkinson’s Disease With Dysphagia: Double Blind Randomized Clinical Trial[J]. Neurorehabil Neural Repair. 2019;33(6):442–52. [DOI] [PubMed] [Google Scholar]
- 42.Cohen OS, Rigbi A, Yahalom G, et al. Repetitive Deep TMS for Parkinson Disease: A 3-Month Double-Blind, Randomized Sham-Controlled Study[J]. J Clin Neurophysiol. 2018;35(2):159–65. [DOI] [PubMed] [Google Scholar]
- 43.Brys M, Fox MD, Agarwal S, et al. Multifocal repetitive TMS for motor and mood symptoms of Parkinson disease: A randomized trial[J]. Neurology. 2016;87(18):1907–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim MS, Chang WH, Cho JW, et al. Efficacy of cumulative high-frequency rTMS on freezing of gait in Parkinson’s disease[J]. Restor Neurol Neurosci. 2015;33(4):521–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Benninger DH, Iseki K, Kranick S, et al. Controlled study of 50-Hz repetitive transcranial magnetic stimulation for the treatment of Parkinson disease[J]. Neurorehabil Neural Repair. 2012;26(9):1096–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pal E, Nagy F, Aschermann Z, et al. The impact of left prefrontal repetitive transcranial magnetic stimulation on depression in Parkinson’s disease: a randomized, double-blind, placebo-controlled study[J]. Mov Disord. 2010;25(14):2311–7. [DOI] [PubMed] [Google Scholar]
- 47.Wang Y, Ding Y, Guo C. Assessment of noninvasive brain stimulation interventions in Parkinson’s disease: a systematic review and network meta-analysis[J]. Sci Rep. 2024;14(1):14219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lefaucheur JP, Aleman A, Baeken C, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014–2018)[J]. Clin Neurophysiol. 2020;131(2):474–528. [DOI] [PubMed] [Google Scholar]
- 49.Zhou D, Xie H, Chen L, et al. The cognitive improvement in patients with schizophrenia following low-intensity repetitive transcranial magnetic stimulation could last for 6 months: A randomized controlled trial[J]. Psychiatry Res. 2024;332:115672. [DOI] [PubMed] [Google Scholar]
- 50.Xie Y, He Y, Guan M, et al. Low-frequency rTMS treatment alters the topographical organization of functional brain networks in schizophrenia patients with auditory verbal hallucination[J]. Psychiatry Res. 2022;309:114393. [DOI] [PubMed] [Google Scholar]
- 51.Su W, Liu H, Jiang Y, et al. Correlation between depression and quality of life in patients with Parkinson’s disease[J]. Clin Neurol Neurosurg. 2021;202:106523. [DOI] [PubMed] [Google Scholar]
- 52.Yu T, Chen W, Huo L, et al. Association between daily dose and efficacy of rTMS over the left dorsolateral prefrontal cortex in depression: A meta-analysis[J]. Psychiatry Res. 2023;325:115260. [DOI] [PubMed] [Google Scholar]
- 53.Croarkin PE, Elmaadawi AZ, Aaronson ST, et al. Left prefrontal transcranial magnetic stimulation for treatment-resistant depression in adolescents: a double-blind, randomized, sham-controlled trial[J]. Neuropsychopharmacology. 2021;46(2):462–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guo B, Zhang M, Hao W, et al. Neuroinflammation mechanisms of neuromodulation therapies for anxiety and depression[J]. Transl Psychiatry. 2023;13(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rossini PM, Ferilli MA, Rossini L, et al. Clinical neurophysiology of brain plasticity in aging brain[J]. Curr Pharm Des. 2013;19(36):6426–39. [DOI] [PubMed] [Google Scholar]
- 56.Tao Y, Liang Q, Zhang F, et al. Efficacy of non-invasive brain stimulation combined with antidepressant medications for depression: a systematic review and meta-analysis of randomized controlled trials[J]. Syst Rev. 2024;13(1):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li R, He Y, Qin W, et al. Effects of Repetitive Transcranial Magnetic Stimulation on Motor Symptoms in Parkinson’s Disease: A Meta-Analysis[J]. Neurorehabil Neural Repair. 2022;36(7):395–404. [DOI] [PubMed] [Google Scholar]
- 58.Dong L, Ma W, Wang Q, et al. The Effect of Repetitive Transcranial Magnetic Stimulation of Cerebellar Swallowing Cortex on Brain Neural Activities: A Resting-State fMRI Study[J]. Front Hum Neurosci. 2022;16:802996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Camargo C, Ferreira-Peruzzo SA, Ribas D, et al. Imbalance and gait impairment in Parkinson’s disease: discussing postural instability and ataxia[J]. Neurol Sci. 2024;45(4):1377–88. [DOI] [PubMed] [Google Scholar]
- 60.Lench DH, DeVries W, Kearney-Ramos TE, et al. Paired inhibitory stimulation and gait training modulates supplemental motor area connectivity in freezing of gait[J]. Parkinsonism Relat Disord. 2021;88:28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Herrero BA, Bellemare A, Beetz G, et al. The effects of non-invasive brain stimulation on sleep disturbances among different neurological and neuropsychiatric conditions: A systematic review[J]. Sleep Med Rev. 2021;55:101381. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.