Abstract
Trichomonas tenax is predominant in dental caries and is commonly observed in patients with oral diseases; however, its presence in patients with pleural effusion remains rare. Pleural effusion can arise from various causes, including malignant tumors, tuberculosis and bacterial infections. Concurrent infections involving bacteria, fungi and Trichomonas are infrequent. This scenario is particularly rare in patients with tumor-associated Trichomonas tenax infection. The current study presents a case of Trichomonas tenax infection in a patient with a lung tumor. The patient, a 71-year-old male, experienced symptoms of chest tightness, shortness of breath, coughing and expectoration following surgery for a right lung tumor. The expectorated sputum was white and sticky, making coughing difficult. The patient had a history of a prior right lung tumor resection and was subsequently admitted to Heping Hospital Affiliated to Changzhi Medical College (Changzhi, China). Routine examination of the pleural effusion fluid revealed the presence of Trichomonas tenax under a wet-film microscope. Molecular sequencing confirmed that the isolate was Trichomonas tenax. This case highlights Trichomonas tenax as a potential opportunistic pathogen in patients with lung cancer, underscoring the need for heightened clinical awareness. This study offers valuable insights for the diagnosis and prevention of infectious diseases among patients with cancer in the future.
Keywords: Trichomonas tenax, molecular identification, lung tumor, pleural effusion
Introduction
Parasites play significant roles in human diseases worldwide. The clinical severity and outcomes of parasitic diseases typically hinge on the immune status of the host (1). As society evolves and lifestyles and dietary patterns change, opportunistic parasitic diseases have increased among individuals with compromised immune systems, along with an increase in imported parasitic diseases (2). This trend is particularly pronounced among cancer patients, whose primary treatment modalities, namely surgery, radiotherapy and chemotherapy, can weaken immune function and predispose them to pathogenic infections (3).
There are three types of trichomonads that parasitize the human body based on their location in the oral cavity, the intestinal tract and the vagina (4). Trichomonas vaginalis is a flagellate parasite in the human vagina and urinary tract that causes mainly trichomonal vaginitis and urinary tract inflammation. The condition is an infectious disease caused mainly by sexual transmission (5). Trichomonas hominis is a flagellate parasite of the intestinal tract. Trichomonas hominis also has only a trophoblast stage and no cyst stage, and its shape is similar to that of Trichomonas vaginalis. Parasites in the human cecum and colon are more common in the ileocecum, so they are also commonly known as Trichomonas hominis or simply called Pentarichomonas hominis (6). At present, whether Trichomonas hominis is pathogenic to the human body is still inconclusive. It has been reported that the parasite can cause diarrhea, especially in infants and people with low immunity, and it may cause trichomonal enteritis. Trichomonas tenax is a flagellated protozoan (7) characterized by an inverted pear shape, averaging 6-10 µm in body length, with four anterior flagella and one posterior flagellum, along with a rhythmic undulating membrane. The parasite is commonly found in patients with inadequate oral hygiene and has been implicated in the progression of periodontal diseases (8). The current study presents a recent case in which Trichomonas tenax was detected in the pleural fluid of a patient with a lung tumor, offering insights for the future diagnosis and prevention of infectious diseases.
Case report
Patient
A 71-year-old man with a history of postoperative lung tumors presented with symptoms of chest tightness, shortness of breath and discomfort when coughing upon admission to Heping Hospital Affiliated to Changzhi Medical College (Changzhi, China) in March 2024. Due to a malignant tumor of the right lung, the patient had undergone a thoracoscopic resection of the lower lobe of the right lung, a systematic lymph node dissection and a bullous ligation of the middle lobe of the right lung. The patient recovered well after the operation and was discharged from the hospital. A total of 16 days after the surgery, the patient suffered from chest tightness and shortness of breath. The expectorated sputum was white and sticky, which made it more difficult for the patient to cough. No obvious relief was experienced after resting. The patient's medical history revealed that the patient coughed uncomfortably when eating outside the hospital, and oral hygiene was poor. A laboratory test revealed an increase in white blood cells (10.1x109/l; normal range, 3.5-9.5x109/l; neutrophils, 91.8%; normal range, 40-75%) and a significant decrease in lymphocytes (0.52x109/l and 4.7%; normal range, 1.1-3.2x109/l and 20-50%) and hemoglobin (111 g/l; normal range, 130-175 g/l). A marked increase in BNP (1,728 ng/l; normal range, 0-125 ng/l) was also observed. The serum level of C-reactive protein (CRP) was increased (317.4 mg/l; normal range, 0-5 mg/l) and the level of procalcitonin (PCT) was slightly increased (0.16 ng/ml; normal range, 0-0.05 ng/ml). The results of the pleural effusion culture revealed Gemella haemolysans and anaerobes, including Prevotella intermedia, Solobacterium moorei and Streptococcus oralis. A chest radiograph and subsequent computed tomography (CT) revealed a pneumothorax in the right pleural cavity and a hydrothorax in the abdominal cavity, as shown in Fig. S1.
Routine examination of pleural effusion
The pleural effusion sample appeared yellow and turbid, and tested positive using the Fan Li method (9). A number of motile organisms resembling rotating worms were observed under a wet-film microscope, and numerous rotating worms were found via wet-film microscopy, as shown in Fig. 1. Some worms shook in place, with the flagella shaking quickly. Some swam directionally in the field of vision; some were slightly larger than white blood cells and flagella could be seen (Video S1).
Figure 1.
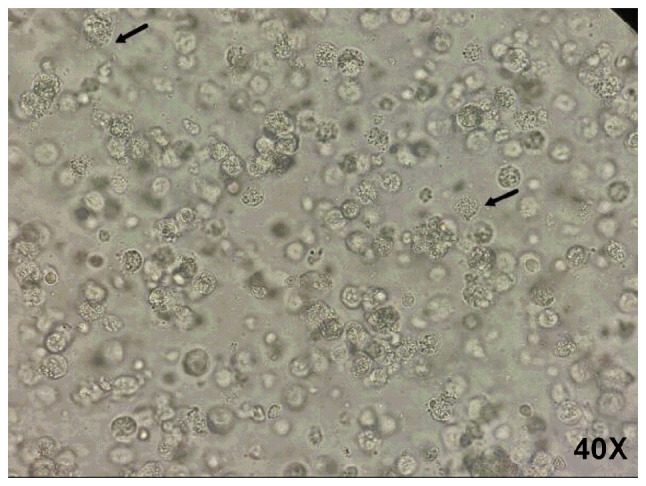
Wet-film microscopy. A number of rotating worms (arrows) were identified via wet-film microscopy.
Subsequently, the samples were centrifuged at x400 g for 5 min, stained with Wright-Giemsa for 10-15 min at 20-28˚C and examined under a microscope, as illustrated in Fig. 2.
Figure 2.
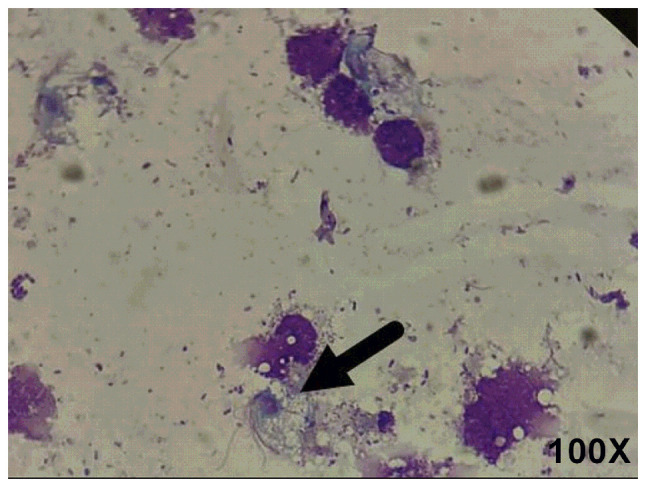
Giemsa staining. Samples were examined via light microscopy with Giemsa staining. The arrows indicate that a pear-shaped body with flagella was visible, containing a large dark purple long oval nucleus shaped like a mouse eye. There are four anterior flagella, and one posterior flagellum is as long as the undulatory membrane.
On the basis of the morphological description, the organism was identified as Trichomonas.
PCR amplification and Sanger sequencing
To confirm that the pathogen was Trichomonas tenax, pleural effusion samples were sent to Wuhan University (Wuhan, China) for molecular detection. DNA purification from the samples was conducted via an Animal DNA Extraction kit (cat. no. BL1043A; Biosharp Life Sciences) following the manufacturer's protocol. For PCR detection of Trichomonas, the primers used were the PT3 forward primer (5'-AGTTCCATCGATGCCATTC-3') and the PT7 reverse primer (5'-GCATCTAAGGACTTAGACG-3'), which target a 776-base pair region of the 18S ribosomal RNA, as previously described by Kikuta et al (1997) (10). For PCR amplification, 0.1 µg DNA template was mixed with 25 µl Super PCR Mix [2X Taq PCR MasterMix (with dyestuff); Beijing Solarbio Science & Technology Co., Ltd.] and 2 µl of each primer. The steps of PCR were as follows: Initial denaturation at 94˚C for 3 min, followed by 30 cycles of 94˚C for 30 sec, 55˚C for 30 sec and 72˚C for 1 min, with a final extension at 72˚C for 5 min. Subsequently, confirmation of the sequence as Trichomonas tenax was achieved via agarose gel electrophoresis. The amplicons were analyzed on 2% agarose gels (Fig. 3).
Figure 3.
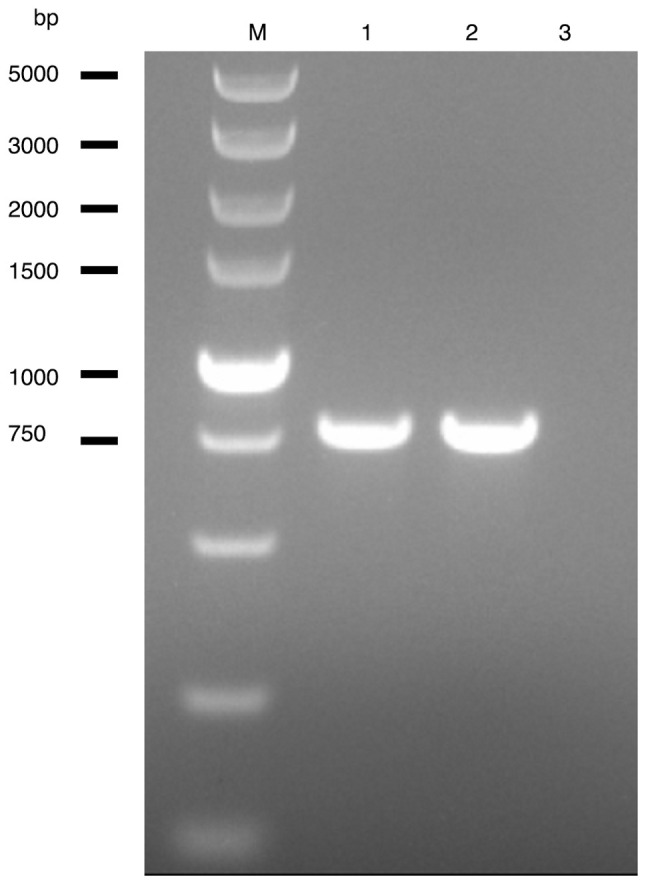
Gel electrophoresis of the PCR products. The PCR products were separated by gel electrophoresis of 2%. M, ladder (size marker), 100-5,000 bp; 1, PCR amplification products from the pleural effusion sample (concentration: 100 ng/µl; A260/280=1.78); 2, positive controls; 3, negative controls.
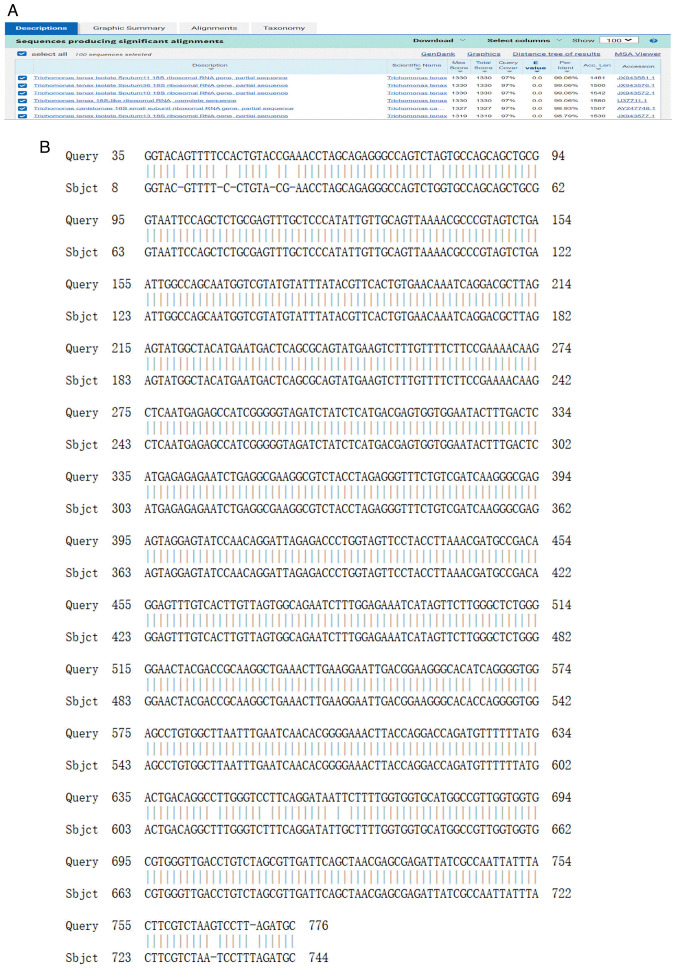
Finally, the samples were confirmed by Sanger sequencing, which identified the pathogen as Trichomonas tenax. A comparison of the nucleotide sequences from the samples with those in the GenBank database (http://www.ncbi.nlm.nih.gov/genbank/) revealed 100% homology with Trichomonas tenax, and a comparison of the 18S rRNA gene sequences of Trichomonas tenax (accession number: D49495) was performed (Fig. 4).
Figure 4.
Comparisons of the nucleotide sequences of the samples with the GenBank database. (A) Comparisons of the nucleotide sequences of the study samples with those in the GenBank database. (B) ‘Query’ represents 18S rRNA gene sequences of Trichomonas tenax (Gene Bank accession number: D49495). ‘Sbjct’ represents the PCR amplification products from the pleural effusion sample.
The patient was administered metronidazole (at a dosage of 2 g per day, intravenously) four times a day for 7 days. The presence of trichomonads was negative from day 3 of metronidazole therapy. However, the patient's mixed infection status was serious, with bacterial infection in the early stage and fungal infection in the later stage, and the prognosis was poor.
Discussion
The average size of Trichomonas tenax is 7.1x4.7 µm. The parasite possesses four anterior flagella, which are often divided into two groups. The posterior flagellum is as long as the undulatory membrane. The undulatory membrane is shorter than the body, and the axial column is slender; most of the nuclei contain stained plasmids with a dark particle color (11). Parasites in the oral cavity, dental plaque and dental cavities, as well as trophozoites, spread through direct or indirect contact. Parasites often coexist with periodontal diseases such as gingivitis (12).
Trichomonas tenax parasitizes the oral cavity, dental plaque and cavities of the teeth. The parasites feed on bacteria, live in a slightly aerobic environment and are often accompanied by a large number of Clostridium and spirochetes. At present, the infection of lower respiratory tract caused by Trichomonas tenax is considered to be by inhalation via the oropharynx (13).
To the best of our knowledge, a report on oral diseases caused by oral Trichomonas infection exists (14), but few reports discuss infections affecting the lungs or other organs. According to the literature, Trichomonas tenax has been detected in the pleural effusion fluid in only 9 reported cases since 1966 (11,13,15-21). These patients included patients with various systemic diseases requiring immunosuppressive therapy, as well as patients with malignant tumors, as detailed in Table I. In the lungs, the proliferation of Trichomonas tenax appears to be facilitated by the presence of bacteria (13). As indicated in Table I, all 9 patients had concurrent infections of Trichomonas tenax with bacterial or fungal species, suggesting that Trichomonas tenax may utilize aerobic or anaerobic bacteria as a food source (11). Treatment with metronidazole was administered to all patients, resulting in mostly favorable outcomes; however, metronidazole is not without potential side effects (15-17) in humans.
Table I.
Clinical and microbiological characteristics of the 9 cases of Trichomonas tenax detected in pleural effusion published in the literature since 1968.
| First author, year | Age, years | Sex | Underlying disease(s) | Immunosuppressive therapy | Coinfection pathogen | Treatment | Outcome | (Refs.) |
|---|---|---|---|---|---|---|---|---|
| Memik, 1968 | 87 | M | Chronic pulmonary disease | No | Bacteria | MTZ and TET | Clinical improvement | (15) |
| Ohkura et al, 1985 | 70 | M | Alcohol abuse | No | Bacteria | MTZ, CEF | Clinical improvement | (16) |
| Shiota et al, 1998 | 53 | M | Acromegaly rectal adenocarcinoma | Chemotherapy, corticotherapy and cobalt irradiation | Bacteria | MTZ | Clinical improvement | (17) |
| Porcheret et al, 2002 | 59 | M | Lung adenocarcinoma | Corticotherapy | Bacteria | MTZ, GEN and CIP | Death | (18) |
| Mallat et al, 2004 | 58 | M | Esophagus adenocarcinoma | No | Bacteria | MTZ, PTZ and GEN | Death | (19) |
| Bellanger et al, 2008 | 33 | F | Heart transplantation | Yes | Bacteria | MTZ and PTZ | Clinical improvement | (20) |
| Leterrier et al, 2012 | 67 | F | Glioblastoma high | Corticotherapy | Bacteria | MTZ | Death | (13) |
| Wu et al, 2021 | 16 | M | Cerebral palsy | No | Fungus | MTZ and VOR | Clinical improvement | (11) |
| Cai and Fang, 2022 | 69 | M | Cerebral infarction | No | Bacteria | MTZ and PTZ | Clinical improvement | (21) |
M, male; F, female; MTZ, metronidazole; TET, tetracycline; CEF, cephalotin; GEN, gentamicin; CIP, ciprofloxacin; PTZ, piperacillin-tazobactam; VOR, voriconazole.
The present study shares one case in which Trichomonas tenax was detected in a patient with pleural effusion. Pathogen metagenomics sequencing (PMseq) provides a direct and high-throughput method for sequencing infected samples, allowing comprehensive detection of microorganisms in clinical samples through detailed reports. Initially, PMseq (BGI Group) was conducted on pleural effusion samples, but Trichomonas tenax was not identified in the initial results. Some oral bacteria, including Prevotella multiformis, Parvimonas micra, Fusobacterium nucleatum, Porphyromonas endodontalis, Dialister pneumosintes, Olsenella uli and Mogibacterium timidum, were detected. At the same time, a large pneumothorax in the right pleural cavity was detected. Therefore, it is speculated that the Trichomonas in the patient's pleural effusion was likely inhaled into the lower respiratory tract from the oropharynx, which led to the aforementioned clinical symptoms. Clinically, there are also reports that most cases of pulmonary infection caused by Trichomonas are complicated with bacterial infection, which further supports the speculation that the Trichomonas tenax is most likely to come from the pharynx.
Upon consultation with technical support, it was determined that the sequencing database (Shenzhen Huada Medical Inspection Laboratory) did not include Trichomonas tenax. Subsequent reanalysis of the data revealed 306 reads corresponding to Trichomonas tenax in the non-human database. This underscores the importance of selecting an appropriate database, as the choice of database can significantly impact the accuracy of the final results.
The patients in the 9 literature cases were administered metronidazole (2 g intravenously per day) four times a day for 7 days. In some cases, metronidazole (1,000 mg) was administered orally twice a day for 5 days. In the present study, the presence of trichomonads was negative from day 3 of the institution's metronidazole therapy. However, this patient's mixed infection status was serious, with bacterial infection in the early stage and fungal infection in the later stage, and the prognosis was poor.
Quick and accurate identification is crucial for effective infection control. Trichomoniasis, which has long been neglected in scientific research, is now gaining renewed attention regarding its pathogenesis (22). Variations in the host-parasite interaction may involve human polymorphisms and environmental factors (23).
There is limited research available on Trichomonas tenax. In the present study, the parasite was identified via molecular methods. Among the diagnostic techniques available in laboratories, culture and molecular assays are considered the most effective for detecting parasites (24). Culture is typically the gold standard for identifying pathogenic microbes in infections (25), despite its drawbacks, such as low sensitivity and specificity, and time-consuming processes. By contrast, PCR has greatly advanced microbial identification due to its high sensitivity and specificity. PCR allows for quick determination of a microbial presence in small samples (12). It is recommended that future studies employ molecular methods, as relying solely on microscopy can lead to underestimation of the true prevalence of oral parasites. Further research is needed to explore the molecular epidemiology of these oral parasites.
Oral Trichomonas species predominantly inhabit anaerobic environments, such as gingival crevicular spaces (26). When the host's immunity decreases, it creates a environment that is conducive to the proliferation of pathogenic bacteria, facilitating the growth and reproduction of Trichomonas tenax. The protozoa metabolize host epithelial cell glycogen through glycolysis to obtain energy, further promoting the growth of pathogenic bacteria. Consequently, patients, especially those undergoing chemotherapy or radiotherapy for tumors, may experience concurrent oral Trichomonas infections alongside various pathogenic bacteria. Chemotherapy and radiotherapy can induce side effects such as neutropenia and compromised humoral and cellular immunity, which increase susceptibility to severe bacterial, viral and parasitic infections (12,27).
The potential pathways for Trichomonas tenax infection can be defined on the basis of published literature on pulmonary Trichomonas tenax (13,28) as follows: i) Primary or secondary immunocompromised conditions (29), including but not limited to solid tumors, hematological malignancies, rheumatic diseases, use of immunosuppressive agents, long-term systemic corticosteroid use, and transplantation of solid organs and hematopoietic stem cells. ii) Oral/periodontal diseases, risk factors for aspiration and any procedure of a therapeutic or diagnostic nature related to the upper thorax or esophagus. The third risk factor is with regard to Trichomonas tenax infection in the lungs, given the residence of Trichomonas tenax in the human oral cavity.
The results of the study by Kooshki et al revealed a high frequency of oral cavity parasites in children who were diagnosed with malignancies or who were receiving treatment with chemotherapy in Lorestan Province, Iran (3). Renal disorders increase the susceptibility of patients to infections, including those caused by oral cavity infections (28).
The present case involved a postoperative patient with lung cancer, and in association with the immune status of the patient, the patient had poor immunity, which provides an opportunity for pathogens, including oral Trichomonas and other potential co-infectious pathogens (such as bacteria, fungi and viruses), to escape immunity. In addition, it is worth considering whether lung cancer surgery provides opportunities for oral Trichomonas to enter the lower respiratory tract, and whether there is the possibility of open communication between the digestive system and chest cavity of patients with lung cancer. Finally, chest CT on admission revealed a large pneumothorax in the right pleural cavity, which provided an opportunity for the oral Trichomonas to enter the lungs.
As a result, oncologists and dental practitioners need to be vigilant in managing oral health issues in patients susceptible to various infections, with particular emphasis on those undergoing cancer treatment. Laboratory professionals should maintain vigilance and careful observation to avoid overlooking positive indicators of infections.
Supplementary Material
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
JW, QZ, YN and AJ conceptualized the study. JW, QZ, LD and YY performed the investigation. JW, QZ and DL were responsible for the wet-film microscopy, Giemsa staining and gel electrophoresis of the PCR products. JW performed the formal analysis. JW wrote the initial draft. JW, QZ, YN, LD, JZ and AJ reviewed and edited the manuscript. All authors have read and approved the manuscript. JW, QZ, DL and AJ confirm the authenticity of all the raw data.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
The patient provided written informed consent for publication.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Theel ES, Pritt BS. doi: 10.1128/microbiolspec.DMIH2-0013-2015. Parasites. Microbiol Spectr 4: DMIH2-0013-2015, 2016. [DOI] [PubMed] [Google Scholar]
- 2.Hsu SL, Fan CK. Emerging and reemerging parasitic diseases in Taiwan: A retrospective study of clinical case reports in 2001~2018. Pathogens. 2024;13(383) doi: 10.3390/pathogens13050383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kooshki F, Khalaf AK, Mahmoudvand H, Baharvand P, Gandomi Rouzbahani F, Selahbarzin B. Molecular epidemiology and associated risk factors of parasites in oral cavity of children with malignancies in western Iran. Iran J Parasitol. 2023;18:324–330. doi: 10.18502/ijpa.v18i3.13755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maritz JM, Land KM, Carlton JM, Hirt RP. What is the importance of zoonotic trichomonads for human health? Trends Parasitol. 2014;30:333–341. doi: 10.1016/j.pt.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kochanowsky JA, Mira PM, Elikaee S, Muratore K, Rai AK, Riestra AM, Johnson PJ : Trichomonas vaginalis extracellular vesicles up-regulate and directly transfer adherence factors promoting host cell colonization. Proc Natl Acad Sci USA. 2024;121(e2401159121) doi: 10.1073/pnas.2401159121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao HD, Zhang S, Lv YH, Zhang ZD, Su N, Li LL, Zhu XQ, Xie SC, Gao WW. First molecular detection and genetic characterization of Tetratrichomonas buttreyi and Pentatrichomonas hominis in Donkeys in Shanxi Province, China. Animals (Basel) 2024;14(2651) doi: 10.3390/ani14182651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang SE, Brooks AES, Poole AM, Simoes-Barbosa A. Determinants of translation efficiency in the evolutionarily-divergent protist Trichomonas vaginalis. BMC Mol Cell Biol. 2020;21(54) doi: 10.1186/s12860-020-00297-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rashidi Maybodi F, Haerian Ardakani A, Fattahi Bafghi A, Haerian Ardakani A, Zafarbakhsh A. The effect of nonsurgical periodontal therapy on Trichomonas tenax and Entamoeba gingivalis in patients with chronic periodontitis. J Dent (Shiraz) 2016;17:171–176. [PMC free article] [PubMed] [Google Scholar]
- 9.Chen CP. Fetal therapy and cytogenetic testing: Prenatal detection of chromosome aberration during thoracocentesis for congenital chylothorax by karyotyping from pleural effusion fluid and review of the literature. Genet Couns. 2005;16:301–305. [PubMed] [Google Scholar]
- 10.Kikuta N, Yamamoto A, Fukura K, Goto N. Specific and sensitive detection of Trichomonas tenax by the polymerase chain reaction. Lett Appl Microbiol. 1997;24:193–197. doi: 10.1046/j.1472-765x.1997.00379.x. [DOI] [PubMed] [Google Scholar]
- 11.Wu Y, Ye Y, Yang Y, Yang W, Lin J, Cao K. Pyopneumothorax from coinfection by Trichomonas tenax and Geotrichum capitatum in a child from China: A case report. BMC Infect Dis. 2021;21(842) doi: 10.1186/s12879-021-06539-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehr AK, Zarandi A, Anush K. Prevalence of oral Trichomonas tenax in periodontal lesions of down syndrome in Tabriz, Iran. J Clin Diagn Res. 2015;9:Zc88–Zc90. doi: 10.7860/JCDR/2015/14725.6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leterrier M, Morio F, Renard BT, Poirier AS, Miegeville M, Chambreuil G. Trichomonads in pleural effusion: Case report, literature review and utility of PCR for species identification. New Microbiol. 2012;35:83–87. [PubMed] [Google Scholar]
- 14.Yaseen A, Mahafzah A, Dababseh D, Taim D, Hamdan AA, Al-Fraihat E, Hassona Y, Şahin GÖ, Santi-Rocca J, Sallam M. Oral colonization by Entamoeba gingivalis and Trichomonas tenax: A PCR-based study in health, gingivitis, and periodontitis. Front Cell Infect Microbiol. 2021;11(782805) doi: 10.3389/fcimb.2021.782805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Memik F. Trichomonads in pleural effusion. JAMA. 1968;204:1145–1146. [PubMed] [Google Scholar]
- 16.Ohkura T, Suzuki N, Hashiguchi Y. Invasion of the human respiratory tracts by trichomonads. Am J Trop Med Hyg. 1985;34(823) [PubMed] [Google Scholar]
- 17.Shiota T, Arizono N, Morimoto T, Shimatsu A, Nakao K : Trichomonas tenax empyema in an immunocompromised patient with advanced cancer. Parasite. 1998;5:375–377. doi: 10.1051/parasite/1998054375. [DOI] [PubMed] [Google Scholar]
- 18.Porcheret H, Maisonneuve L, Estève V, Jagot JL, Le Pennec MP. Pleural trichomoniasis due to Trichomonas tenax. Rev Mal Respir. 2002;19:97–99. (In French) [PubMed] [Google Scholar]
- 19.Mallat H, Podglajen I, Lavarde V, Mainardi JL, Frappier J, Cornet M. Molecular characterization of Trichomonas tenax causing pulmonary infection. J Clin Microbiol. 2004;42:3886–3887. doi: 10.1128/JCM.42.8.3886-3887.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bellanger AP, Cabaret O, Costa JM, Foulet F, Bretagne S, Botterel F. Two unusual occurrences of trichomoniasis: Rapid species identification by PCR. J Clin Microbiol. 2008;46:3159–3161. doi: 10.1128/JCM.00322-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cai DH, Fang XL. Pyopneumothorax caused by Trichomonas tenax and Porphyromonas endodontalis coinfection in a patient with previous cerebral infarction: A case report and literature review. Infect Drug Resist. 2022;15:6101–6108. doi: 10.2147/IDR.S381859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hinderfeld AS, Simoes-Barbosa A. Vaginal dysbiotic bacteria act as pathobionts of the protozoal pathogen Trichomonas vaginalis. Microb Pathog. 2020;138(103820) doi: 10.1016/j.micpath.2019.103820. [DOI] [PubMed] [Google Scholar]
- 23.Milner DA Jr. Malaria pathogenesis. Cold Spring Harb Perspect Med. 2018;8(a025569) doi: 10.1101/cshperspect.a025569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santos JO, Roldán WH. Entamoeba gingivalis and Trichomonas tenax: Protozoa parasites living in the mouth. Arch Oral Biol. 2023;147(105631) doi: 10.1016/j.archoralbio.2023.105631. [DOI] [PubMed] [Google Scholar]
- 25.Ahmed NH. Cultivation of parasites. Trop Parasitol. 2014;4:80–89. doi: 10.4103/2229-5070.138534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duboucher C, Mogenet M, Perie G. Salivary trichomoniasis. A case report of infestation of a submaxillary gland by Trichomonas tenax. Arch Pathol Lab Med. 1995;119:277–279. [PubMed] [Google Scholar]
- 27.Azadbakht K, Baharvand P, Al-Abodi HR, Yari Y, Hadian B, Fani M, Niazi M, Mahmoudvand H. Molecular epidemiology and associated risk factors of oral cavity parasites in hemodialysis patients in western Iran. J Parasit Dis. 2023;47:146–151. doi: 10.1007/s12639-022-01551-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dybicz M, Perkowski K, Sędzikowska A, Baltaza W, Chomicz L. Studies on prevalence of infection with Trichomonas tenax identified by molecular techniques-in respect to oral health of patients with various systemic disease requiring immunosuppressive therapy. Ann Parasitol. 2018;64:193–197. doi: 10.17420/ap6403.151. [DOI] [PubMed] [Google Scholar]
- 29.Jiang J, Li Y, Wang Q, Zeng H, Yang W, Wu Y, Peng W, Pan P, Hu C, Deng P. Clinical implications of trichomonads detected in bronchoalveolar fluid by metagenomic next-generation sequencing: A multicenter retrospective study. Front Cell Infect Microbiol. 2024;14(1289231) doi: 10.3389/fcimb.2024.1289231. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in the present study may be requested from the corresponding author.