RESUME
Introduction: La radiothérapie stéréotaxique (SBRT) a révolutionné les soins oncologiques en offrant un traitement précis. Objectif: Cette étude a évalué l'expérience à l'Institut Salah Azaiez (SAI) chez les patients atteints de cancer du poumon. Méthodologie: nous avons mené une étude rétrospective sur les patients traités par SBRT entre 2019-2022. La planification de traitement a été faite sur des scanners quadridimensionnels en délimitant les volumes cibles et les organes-à-risque. Les doses étaient adaptées en fonction de la localisation tumorale. Résultats: Au total, 10 cas ont été inclus. Le sex-ratio homme/femme était de 4:1, avec un âge médian de 69,5 ans. Trois avaient des tumeurs pulmonaires primitives non confirmées histologiquement. Cinq avaient des adénocarcinomes inopérables de stade I-II, en raison d'une fonction respiratoire altérée. Deux présentaient des maladies pulmonaires oligométastatiques. Le statut de performance de Karnofsky était de 70 à 90. La taille médiane des lésions était de 40 mm. Les D95%, D99%, Dmax étaient respectivement de 60 Gy, 56Gy, 73Gy pour la prescription de 8x7,5 Gy sur l'isodose 80%. Les critères dosimétriques des organes-à-risque ont été respectés. Les toxicités aiguës comprenaient une aggravation de la toux chez 2 patients et de la fatigue chez 6. Après un suivi médian de 23 mois, aucune fracture de côte ou hémoptysie n'a été observée, et aucune récidive locale n'a été signalée. Conclusion: La SBRT est une technique prometteuse, bien que des difficultés persistent dans le ciblage précis et la gestion des mouvements. Une collaboration multidisciplinaire et des protocoles internes sont essentiels pour une mise en œuvre réussie.
ABSTRACT
Introduction: Stereotactic Body Radiation Therapy (SBRT) has transformed lung cancer care, delivering precise treatment with minimal harm to healthy tissue. Aim: This study examined the experience at the Salah Azaiez Institute (SAI) in lung cancer patients. Methodology: we conducted a retrospective study on patients treated with SBRT from 2019 to 2022. Planification imaging included four dimensional CT scans and delineation of target volumes and organs-at-risk was done as per international guidelines. Treatment doses were tailored based on tumour location. Results: A total of 10 cases were included. The male-to-female sex ratio was 4:1, with a median age of 69.5 years. Three had unconfirmed primary lung tumours, while five had inoperable stage I-IIA adenocarcinomas primarily due to compromised respiratory function. Two had oligometastatic lung diseases. All underwent recent thoracic-CT and PET-CT evaluations to exclude pulmonary fibrosis. The median lesion size was 40mm. Karnofsky’s performance status ranged from 70 to 90, with no contraindications to the supine position. Eight out of 10 patients received 8 fractions of 7.5 Gy at the 80% isodose. For that regimen, the D95%, D99%, and Dmax were respectively, 60 Gy, 56 Gy, 73 Gy. All organs-at-risk dosimetric criteria were met. Acute toxicities included worsened coughs in 2 patients and fatigue in 6. After a mean follow-up of 23 months, no rib fractures or haemoptysis were observed, and no local recurrence was reported on the last chest CT scan. Conclusion: SBRT demonstrates promise for lung cancer treatment, though challenges persist in precise targeting and motion management. Effective multidisciplinary collaboration and local protocols are crucial for successful implementation.
Introduction
Stereotactic body radiotherapy (SBRT) is an innovative radiation therapy technique delivering a high dose per fraction.
SBRT integrates modern imaging, simulation, treatment planning and delivery technologies to achieve high gradient of dose, high conformality, and heterogeneous dose distribution within the target (1).
It is also referred to as “" stereotactic ablative radiotherapy” (SABR) since the convergence of the different beams results in creating a hotspot with a rapid falloff of dose outside of the target, which in turn creates a lethal ‘' ablative’ effect (2).
SBRT is also shown to induce vascular damage and to have an immunological effect, both of which indirectly cause cell death (3).
Hypo-fractionated radiotherapy, such as SBRT, offers advantages including a reduced treatment duration with fewer sessions compared to conventional radiotherapy, and minimized exposure of surrounding healthy tissues to radiation.
Since 2019 our patients have started to benefit from this innovative technique in Tunisia at the Salah Azaiez Institute (SAI).
The purpose of this study is to present our results of SBRT lung cancer patients in terms of local control and tolerance.
Methods
We conducted a retrospective, descriptive study from 2019 to 2022.
This study enrolled lung cancer patients treated with SBRT.
We included inoperable patients with primary lung tumours, stage I-IIA, up to 5 cm in greatest size.
An FDG PET-CT was mandatory for staging. For metastatic patients, SBRT was indicated for patients presenting with oligometastatic lung disease ( 1–5 metastatic sites, in up to 3 organs, excluding serous lesions), measuring less than 5 cm in size, with slow growth and whose primary tumour is controlled (4).
We didn’t include patients with ultra-central lesions.
Data collection
We collected clinical data including smoking habit, cardiopulmonary history, tumour size and localisation, number of lesions, TNM classification, Karnofsky performance status (KPS), and respiratory function.
Dosimetric data were collected from the treatment planning system (TPS) Eclipse (Varian Medical System Inc., version 13.7).
The conformity index (CI) and the homogeneity index (HI) were calculated to evaluate the quality of the treatment plan.
The CI was defined as the ratio of the volume of the PTV covered by the prescription dose to the total volume of the PTV, while HI was defined as the ratio of the maximum dose to the prescription dose (5).
Treatment setup
A complete and recent radiological workup (≤ 4 weeks) with a thoracic CT and a PET-CT was required for optimum disease staging.
Patients need to be fit to tolerate the supine position.
Adequate pulmonary function was needed (FEV1 higher than 40% predicted and DLCO higher than 40% predicted).
In case of altered pulmonary function or pre-existing pulmonary fibrosis, the decision was left up to be made during the RT doctors’ board meeting for a less hypofractionated SBRT and consequent adaptation of dosimetric objectives and constraints.(appendix 1)
We performed a four-dimensional (4D) acquisition on 10 respiratory phases (with non-forced inhalation, nonforced exhalation and free breathing) with diaphragmatic compression to reduce motion (Fig .1).
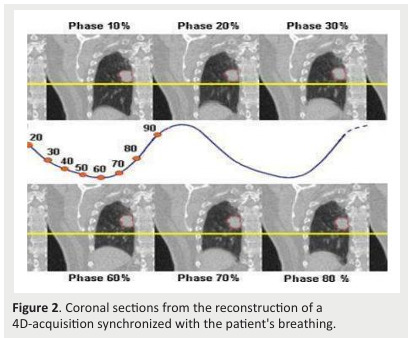
The scan series are then exported to the Eclipse TPS where we generate the “" average” CT to calculate the radiotherapy dose and the Maximum Intensity Projected CT (MIP) to define the treated volumes. (Fig .2)
Figure 1. Immobilization setup: A. Long base plate with cushions for arms, knees, and feet. B. Abdominal compression plate .
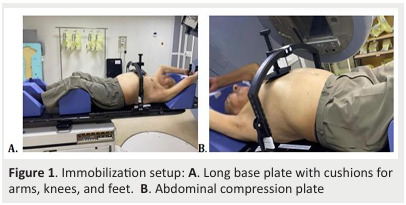
Figure 2. Coronal sections from the reconstruction of a 4D-acquisition synchronized with the patient's breathing.
Target volumes and prescriptions
We used the Eclipse software to import images and to register the PET-CT with the planning-CT.
The definitions of target volumes are summarized in table 1.
The organs at risks (OAR) were outlined on the average CT according to the RTOG recommendations and contouring atlases(Radiation Therapy Oncology Group). (appendix 2)
Treatment planning was performed using volumetricmodulated arc-therapy (VMAT), Treatment delivery used coplanar or non-coplanar 6 MV photon arcs from a Varian iX Linac with an MLC of 80 pairs of 0.5 cm thickness projected at the isocentre. ( Fig.3).
Following the ESTRO/ACROP recommendations for dose prescription, We aimed to set up risk-adapted fractionation regimens according to the location of the tumour, with 48h in-between fractions (4).
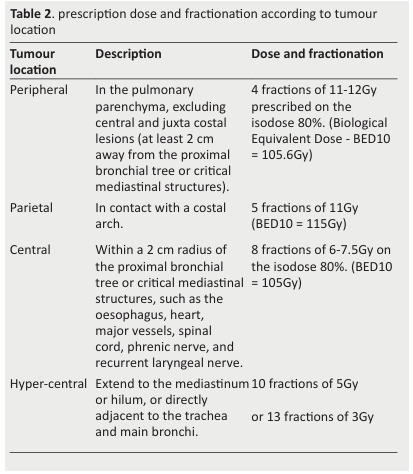
The main locations were defined as follows (Table 2).
Table 1. definitions of target volumes in SBRT .
Figure 3. A. Linear Accelerator, B. LINAC Treatment Head .
Table 2. prescription dose and fractionation according to tumour location .
The control of positioning was performed using portal imaging, low energy on board two-dimensional imaging(kV orthogonal pair), and three-dimensional repositioning by cone beam computed tomography (CBCT).
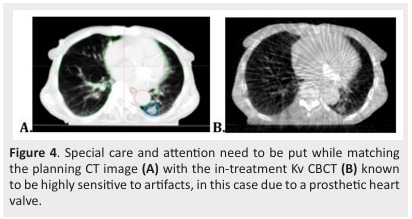
A CBCT is performed before each treatment arc under the direct supervision of the radiation oncologist (Fig.4).
Figure 4. Special care and attention need to be put while matching the planning CT image (A) with the in-treatment Kv CBCT (B) known to be highly sensitive to artifacts, in this case due to a prosthetic heart valve.
Results
From 2019 to 2022, the SAI radiotherapy department experienced an interest in SBRT.
Among the first 20 patients who consulted our department to benefit from SBRT, a selection process based on established indications was applied.
For each patient meeting the criteria for SBRT was reviewed at the radiotherapy board meeting,where the indication for treatment was discussed.
Ultimately, only 10 patients were eligible for SBRT.
The cohort included 8 men and 2 women, giving a sex ratio of 4:1.
The median age was 69.5 years, with a range from 60 to 81 years.
Out of the 10 patients, seven were smokers and all had a medical history involving heart or lung disease.
Three had primary lung tumours without histological confirmation, while five were diagnosed with inoperable stage I-IIA (T1-T2a N0, according to the 8th TNM edition) adenocarcinomas due to compromised respiratory function or comorbidities.
Additionally, two patients had oligometastatic lung diseases.
Notably, the maximum selected tumor size was 5 cm and these lesions displayed slow growth and the primary tumours (located contralaterally in the lung and breast) were under control.
The tumor was peripheral in 7 cases and central in 3 cases.
They all had to perform a recent chest CT scan and a PET-CT scan as part of the disease evaluation.
We identified and evaluated targeted lesions, with a maximum accepted size of 50 mm.
Clinical evaluation based on the KPS varied between 70 and 90.
The forced expiratory volume in the first second of expiration (FEV) was less than 70% in 9 of 10 cases [50-80%].
Eight patients with a central tumour, received 8 fractions of 7,5 Gy, while the 2 others with a parietal tumour received, respectively, 8 fractions of 6 Gy and 6 fractions of 7,5 Gy at the 80% isodose.
For all patients, 100% of the prescribed isodose covering the PTV between 95% and 100%.
In 8 out of the 10 cases, the maximum dose within the PTV did not exceed 125% of the prescribed dose.
Furthermore, all D99% values were at least 90% of the prescribed dose.
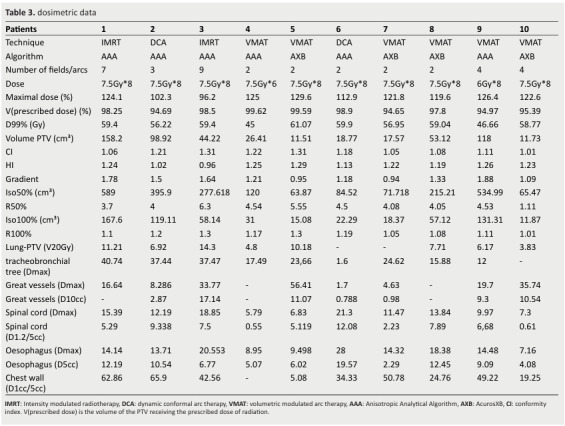
For OAR constraints, V20Gy for lung-PTV was < 0% for 5 patients and D10cc for great vessels was < 44Gy in all patients (Table 3).
In terms of acute toxicity, 2 patients experienced an exacerbation of cough, while 6 reported temporary fatigue.
Importantly, there were no occurrences of grade 3 or 4 adverse events, according to the criteria set out in CTCAE V5.0.
After a median follow-up of 23 months, no incidents of rib fractures or hemoptysis were detected.
Additionally, it is important to highlight that 50% of patients had ground glass opacities on their CT scan, suggesting the presence of fibrous changes in the irradiated tissue.
The CT scans were performed every 4 to 6 months, our findings revealed a median progressionfree survival of 11 months, with a 1-year progressionfree survival rate of 70%.
Median overall survival was 12 months (4-36).
It is regrettable to note that one patient's death, occurring four months post-treatment, was linked to complications arising from COVID-19.
Table 3. dosimetric data .
Discussion
The primary treatment for early-stage NSCLC has been for a long time anatomical surgical resection by lobectomy, segmentectomy, or wedge resection (6).
Other treatment options have been applied including conventional radiation therapy or radiofrequency ablation.
In recent decades there has been growing interest in SBRT for early primary NSCLC.
Compared to surgery, SBRT is a non-invasive, organ-sparing outpatient treatment that typically lasts 1-2 weeks without requiring anaesthesia and allowing immediate return to activities.
Furthermore, while segmentectomy offers good local control (LC) for small lesions less than 20 mm, achieving 98.1%, this result is not maintained for larger lesions, with LC falling to 62.9% (7).
Compared with wedge resection, there was a trend toward reduced local recurrence with SBRT (4% vs. 20%; p = 0.07).
SBRT also achieves high LC rates reaching 97%, regardless of tumour size (8,9).
Radiofrequency ablation, on the other hand, provides a significantly lower 5-year LC rate compared to SBRT (42% vs. 86%; p < 0.001)(110 ).
The CHISEL trial highlighted the superiority of SBRT over conventional radiotherapy, showing a decrease in local failure (31% vs. 14%) and an increase in survival(median overall survival of 3 years vs. 5 years; p = 0.027)compared to conventional radiotherapy (11).
SBRT is an option for the management of stage I-II (T1-T2a N0) NSCLC, measuring up to 5 cm, for inoperable patients(major medical comorbidity, severely limited lung function) or patients refusing surgery (4).
It is also indicated for primary lung tumours without histological evidence, particularly those increasing in size on 2 consecutive CTs performed 8-12 weeks interval, hypermetabolic on PETCT and without other proven etiology (12).
Note that systematic screening for tuberculosis is necessary in our institute to exclude it in cases of suspicion, as our country has intermediate endemicity.
For lung metastases in oligometastatic patients, two randomized phase II trials suggest that patients with synchronous oligometastatic or oligo persistent cancer can benefit from curative treatment once the disease has stabilized with initial systemic therapy (13,14).
Eligibility for SBRT requires certain conditions.
A complete and recent radiological workup (≤ 4 weeks), including thoracic CT and PET-CT, is required for optimal disease staging.
Targeted lesions should not exceed 5 cm in size (12 ).
While SBRT is feasible for tumours larger than 5 cm, low-quality evidence limits its use in these cases (15).
Patients need to have a KPS of at least 70 and with no contraindication to a supine position.
Special considerations are taken into account for patients with cardiac implantable electronic devices (16).
In case of altered pulmonary function or pre-existing pulmonary fibrosis, the decision is made during the RT board meeting for a less hypo-fractionated SBRT and adaptation of dosimetric constraint (17).
There is no single standard scheme for all tumour presentations.
Published data generally reflect the experience of individual institutions, which to some extent explains interinstitutional differences in total dose, fractionation schedule, total treatment time, and dose delivery technique.
These differences make it difficult to standardize dosing regimens and the feasibility of SBRT.
However, the recommended fractionation regimens are all equivalent to a minimum Biological Effective Dose (BED) of 100 Gy (17).
LC is significantly improved with a BED> 100Gy, and BED at the peripheral tumour margin was found to be the strongest predictor of LC.
As per the ESTRO/ACRO recommendations, riskadapted fractionation regimens were set up according to the location of the tumour, with 48 hours in-between fractions (4).
Some tumours might be “" too central” to be safely treated with SBRT when applying a BED10> 100 Gy (3).
These are called ‘hyper-central’ tumors, and they extend directly to the bronchial tree or critical mediastinal structures.
In this case, an 8-fraction regimen results in a high risk of grade 3 to 5 toxicities as well as treatmentrelated death, and thus a more fractionated schedule is recommended (18).
When planning treatment, dose inhomogeneity within the PTV does not pose a problem; it is sought after in the case of SBRT to obtain a steep dose profile (3).
Additionally, as we are dealing with small fields and high doses per fraction, appropriate dose calculation algorithms must be used.
Another important dosimetric indicator to be considered is ensuring rapid dose fall-off.
Depending on the PTV volume, RTOG metrics for limiting dose spillage are R50% (the ratio of 50% isodose volume to the PTV) and D2cm [%] (the maximum dose at 2 cm from PTV in any direction) (19) (appendix 3).
Concerning OAR, dose-volume limits are well established for conventional radiotherapy (1.8 to 2.0 Gy per fraction) and moderately fractionated radiotherapy.
Although dose equivalence can be established using linear quadratic models, there is uncertainty about when the extreme hypofractionation doses (≥6 Gy) used in SBRT are applied to small volumes,particularly to serial OARs ( 15).
Therefore, dose-volume constraints specific to different dose fractionation schemes have been systematically defined in prospective clinical trials and correlated with toxicity rates.
Several guidelines have been published by national and international bodies aimed at standardizing the practice of SBRT for early-stage NSCLC.
In our practice, we refer to the RTOG, JCOG and EORTC recommendations for dose constraints per number of fractions (20).
Patients are informed of the risks of radiation before starting treatment.
Monitoring is crucial for early detection and treatment of side effects.
Acute toxicities may manifest as asthenia, cough, dyspnoea, subacute radiation pneumonitis and esophagitis (21).
Late toxicity includes pulmonary fibrosis, rib fracture, oesophageal stenosis, radiation plexitis, and rarely massive haemorrhage or tracheoesophageal fistula(21).
Risk factors for such toxicities include proximal tumour topography or contact with the chest wall or a BED > 120 Gy.
It is important to note that radiation-induced fibrotic changes are difficult to distinguish from residual tumours and in this situation, PET-CT can prove very useful.
The 1-year progression-free survival rate of 70% observed in our cohort is encouraging.
However, our preliminary findings regarding survival and loco-regional control outcomes should be interpreted within the context of our study’s limitations, namely the small sample size, the inclusion of both metastatic and not metastatic patients, and the relatively short follow-up period.
To substantiate our findings and enable meaningful comparisons with the literature, future research should prioritize larger patient cohorts and longer follow-up periods.
Conclusion
SBRT has emerged as a promising and non-invasive paradigm in the management of lung cancer.
Its potential for better outcomes in lung cancer treatment is evident.
Nevertheless, implementing SBRT presents significant challenges related to accurate target delineation, efficient motion management, and optimized dosimetry to spare OAR.
We emphasize the importance of implementing local departmental procedures and efficient multidisciplinary communication to carry out SBRT in the safest and best conditions.
This collaborative approach reinforces our commitment to providing high-quality care and underscores our dedication to advancing the field of radiation oncology in pulmonary cancer treatment.
References
- Ricardi U, Badellino S, Filippi AR. Stereotactic body radiotherapy for early stage lung cancer: History and updated role. Lung Cancer Amst Neth. 2015 Dec;90(3):388–396. doi: 10.1016/j.lungcan.2015.10.016. [DOI] [PubMed] [Google Scholar]
- Timmerman R, Galvin J, Michalski J, Straube W, Ibbott G, Martin E, et al. Accreditation and quality assurance for Radiation Therapy Oncology Group: Multicenter clinical trials using Stereotactic Body Radiation Therapy in lung cancer. Acta Oncol Stockh Swed. 2006;45(7):779–786. doi: 10.1080/02841860600902213. [DOI] [PubMed] [Google Scholar]
- Song CW, Glatstein E, Marks LB, Emami B, Grimm J, Sperduto PW, et al. Biological Principles of Stereotactic Body Radiation Therapy (SBRT) and Stereotactic Radiation Surgery (SRS): Indirect Cell Death. Int J Radiat Oncol Biol Phys. 2021 May;110(1):21–34. doi: 10.1016/j.ijrobp.2019.02.047. [DOI] [PubMed] [Google Scholar]
- Guckenberger M, Andratschke N, Dieckmann K, Hoogeman MS, Hoyer M, Hurkmans C, et al. ESTRO ACROP consensus guideline on implementation and practice of stereotactic body radiotherapy for peripherally located early stage non-small cell lung cancer. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2017 Jul;124(1):11–17. doi: 10.1016/j.radonc.2017.05.012. [DOI] [PubMed] [Google Scholar]
- ICRU ICRU Report 83, Prescribing, Recording, and Reporting Intensity Modulated Photon-Beam Therapy (IMRT) – ICRU. 2023. [cited 2023 Oct 3]. https://www.icru.org/report/prescribing-recording-and-reporting-intensity-modulated-photon-beam-therapy-imrticru-report-83/
- Asamura H. Treatment of choice for stage I non-small cell lung cancer: surgery or radiotherapy? J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. 2006 Oct;1(8):766–767. [PubMed] [Google Scholar]
- Okada M, Nishio W, Sakamoto T, Uchino K, Yuki T, Nakagawa A, et al. Effect of tumor size on prognosis in patients with non-small cell lung cancer: the role of segmentectomy as a type of lesser resection. J Thorac Cardiovasc Surg. 2005 janv;129(1):87–93. doi: 10.1016/j.jtcvs.2004.04.030. [DOI] [PubMed] [Google Scholar]
- Grills IS, Mangona VS, Welsh R, Chmielewski G, McInerney E, Martin S, et al. Outcomes after stereotactic lung radiotherapy or wedge resection for stage I non-small-cell lung cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2010 févr 20;28(6):928–935. doi: 10.1200/JCO.2009.25.0928. [DOI] [PubMed] [Google Scholar]
- Kestin L, Grills I, Guckenberger M, Belderbos J, Hope AJ, Werner-Wasik M, et al. Dose-response relationship with clinical outcome for lung stereotactic body radiotherapy (SBRT) delivered via online image guidance. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2014 mars;110(3):499–504. doi: 10.1016/j.radonc.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Bi N, Shedden K, Zheng X, Kong FMS. Comparison of the Effectiveness of Radiofrequency Ablation With Stereotactic Body Radiation Therapy in Inoperable Stage I Non-Small Cell Lung Cancer: A Systemic Review and Pooled Analysis. Int J Radiat Oncol Biol Phys. 2016 août 1;95(5):1378–1390. doi: 10.1016/j.ijrobp.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball D, Mai GT, Vinod S, Babington S, Ruben J, Kron T, et al. Stereotactic ablative radiotherapy versus standard radiotherapy in stage 1 non-small-cell lung cancer (TROG 09.02 CHISEL): a phase 3, open-label, randomised controlled trial. Lancet Oncol. 2019 avr;20(4):494–503. doi: 10.1016/S1470-2045(18)30896-9. [DOI] [PubMed] [Google Scholar]
- Khalifa J, Lerouge D, Le Péchoux C, Pourel N, Darréon J, Mornex F, et al. Radiotherapy for primary lung cancer. Cancer Radiother J Soc Francaise Radiother Oncol. 2022;26(1-2):231–243. doi: 10.1016/j.canrad.2021.11.005. [DOI] [PubMed] [Google Scholar]
- Iyengar P, Wardak Z, Gerber DE, Tumati V, Ahn C, Hughes RS, et al. Consolidative Radiotherapy for Limited Metastatic Non-Small-Cell Lung Cancer: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2018 janv 11;4(1):e173501. doi: 10.1001/jamaoncol.2017.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, et al. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial. J Clin Oncol Off J Am Soc Clin Oncol. 2020 Sept;38(25):2830–2838. doi: 10.1200/JCO.20.00818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videtic GMM, Donington J, Giuliani M, Heinzerling J, Karas TZ, Kelsey CR, et al. Stereotactic body radiation therapy for early stage non-small cell lung cancer: Executive Summary of an ASTRO Evidence-Based Guideline. Pract Radiat Oncol. 2017;7(5):295–301. doi: 10.1016/j.prro.2017.04.014. [DOI] [PubMed] [Google Scholar]
- Escande A, Frey P, Lacornerie T, Mervoyer E, Chargari C, Laurans M, et al. Radiotherapy for patient with cardiac implantable electronic device, consensus from French radiation oncology society. Cancer Radiother J Soc Francaise Radiother Oncol. 2022;26(1-2):404–410. doi: 10.1016/j.canrad.2021.11.003. [DOI] [PubMed] [Google Scholar]
- Onishi H, Araki T, Shirato H, Nagata Y, Hiraoka M, Gomi K, et al. Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma: clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer. 2004 Oct;101(7):1623–1631. doi: 10.1002/cncr.20539. [DOI] [PubMed] [Google Scholar]
- Lindberg K, Grozman V, Karlsson K, Lindberg S, Lax I, Wersäll P, et al. The HILUS-Trial-a Prospective Nordic Multicenter Phase 2 Study of Ultracentral Lung Tumors Treated With Stereotactic Body Radiotherapy. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. 2021 July;16(7):1200–1210. doi: 10.1016/j.jtho.2021.03.019. [DOI] [PubMed] [Google Scholar]
- Manyam BV, Verdecchia K, Videtic GMM, Zhuang T, Woody NM, Wei W, et al. Validation of RTOG 0813 Proximal Bronchial Tree Constraints for Pulmonary Toxicity With Stereotactic Body Radiation Therapy for Central Non-small Cell Lung Cancer. Int J Radiat Oncol Biol Phys. 2020 May;107(1):72–78. doi: 10.1016/j.ijrobp.2020.01.009. [DOI] [PubMed] [Google Scholar]
- Nagata Y, Matsuo Y, Takayama K, Norihisa Y, Mizowaki T, Mitsumori M, et al. Current status of stereotactic body radiotherapy for lung cancer. Int J Clin Oncol. 2007 Feb;12(1):3–7. doi: 10.1007/s10147-006-0646-6. [DOI] [PubMed] [Google Scholar]
- Bucknell NW, Belderbos J, Palma DA, Iyengar P, Samson P, Chua K, et al. Avoiding Toxicity With Lung Radiation Therapy: An IASLC Perspective. J Thorac Oncol. 2022 Aug;17(8):961–973. doi: 10.1016/j.jtho.2022.05.003. [DOI] [PubMed] [Google Scholar]


