Abstract
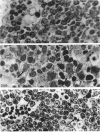
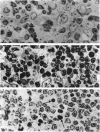
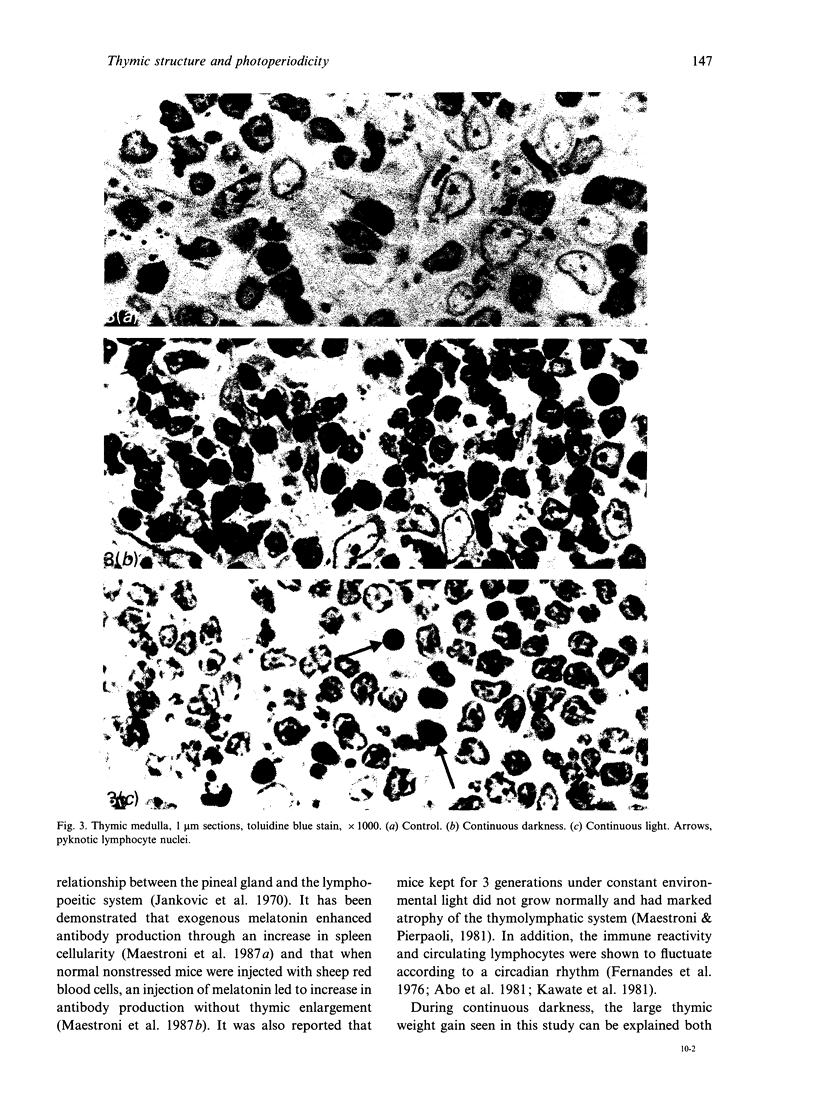
It is known that neuroendocrine responses to environmental stimuli, such as light, can influence immune responses through the pineal gland. It is also known that periods of constant darkness and constant light cause stimulation and inhibition of melatonin secretion from the pineal gland, successively. In this study, we provide experimental evidence that changes in the rhythm of the photoperiod have considerable effects on thymic structure of the rat. Male albino Wistar rats were divided into 3 groups. Group 1 rats were kept in a dark room, group 2 in a room under a bright artificial light and group 3 (control) animals were exposed to a 12:12 h light: dark cycle. All animals were killed after 4 wk. In group 1, thymus weight increased by 315%, the increase in volume affecting the medulla (cortex 190%, medulla 655%). The absolute number of epithelial cells and lymphocytes increased both in the cortex and medulla. Thymic cortical epithelial cells were hypertrophied and contained numerous large clear vesicles. Perivascular spaces were enlarged. In group 2 thymus weight decreased by 53%, the reduction in volume affecting mainly the cortex (cortex 61%, medulla 27%). The absolute numbers of cortical epithelial cells and lymphocytes were decreased, and pyknotic lymphocyte nuclei were frequent both in the cortex and medulla. It is concluded that constant darkness causes hypertrophy and increased cellularity of the thymus, while constant light causes involution of the thymus and death of lymphocytes. These changes possibly reflect the well known immunostimulatory effects of melatonin acting directly or indirectly, on the thymic lymphocytes and epithelial cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo T., Kawate T., Itoh K., Kumagai K. Studies on the bioperiodicity of the immune response. I. Circadian rhythms of human T, B, and K cell traffic in the peripheral blood. J Immunol. 1981 Apr;126(4):1360–1363. [PubMed] [Google Scholar]
- Bennett G. Synthesis and migration of glycoproteins in cells of the rat thymus, as shown by radioautography after 3H-fucose injection. Am J Anat. 1978 Jun;152(2):223–255. doi: 10.1002/aja.1001520205. [DOI] [PubMed] [Google Scholar]
- Bloodworth J. M., Jr, Hiratsuka H., Hickey R. C., Wu J. Ultrastructure of the human thymus, thymic tumors, and myasthenia gravis. Pathol Annu. 1975;10:329–391. [PubMed] [Google Scholar]
- Clark S. L., Jr Incorporation of sulfate by the mouse thymus: its relation to secretion by medullary epithelial cells and to thymic lymphopoiesis. J Exp Med. 1968 Nov 1;128(5):927–957. doi: 10.1084/jem.128.5.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGHERTY T. F. Effect of hormones on lympatic tissue. Physiol Rev. 1952 Oct;32(4):379–401. doi: 10.1152/physrev.1952.32.4.379. [DOI] [PubMed] [Google Scholar]
- Duncan M. J., Goldman B. D., Di Pinto M. N., Stetson M. H. Testicular function and pelage color have different critical daylengths in the Djungarian hamster, Phodopus sungorus sungorus. Endocrinology. 1985 Jan;116(1):424–430. doi: 10.1210/endo-116-1-424. [DOI] [PubMed] [Google Scholar]
- Erlich S. S., Apuzzo M. L. The pineal gland: anatomy, physiology, and clinical significance. J Neurosurg. 1985 Sep;63(3):321–341. doi: 10.3171/jns.1985.63.3.0321. [DOI] [PubMed] [Google Scholar]
- Fernandes G., Halberg F., Yunis E. J., Good R. A. Circadian rhythmic plaque-forming cell response of spleens from mice immunized with SRBC. J Immunol. 1976 Sep;117(3):962–966. [PubMed] [Google Scholar]
- Gilman S. C., Schwartz J. M., Milner R. J., Bloom F. E., Feldman J. D. beta-Endorphin enhances lymphocyte proliferative responses. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4226–4230. doi: 10.1073/pnas.79.13.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijnen C. J., Croiset G., Zijlstra J., Ballieux R. E. Modulation of lymphocyte function by endorphins. Ann N Y Acad Sci. 1987;496:161–165. doi: 10.1111/j.1749-6632.1987.tb35760.x. [DOI] [PubMed] [Google Scholar]
- Janković B. D., Isaković K., Petrović S. Effect of pinealectomy on immune reactions in the rat. Immunology. 1970 Jan;18(1):1–6. [PMC free article] [PubMed] [Google Scholar]
- Kalland T., Fossberg T. M., Forsberg J. G. Effect of estrogen and corticosterone on the lymphoid system in neonatal mice. Exp Mol Pathol. 1978 Feb;28(1):76–95. doi: 10.1016/0014-4800(78)90066-7. [DOI] [PubMed] [Google Scholar]
- Kawate T., Abo T., Hinuma S., Kumagai K. Studies of the bioperiodicity of the immune response. II. Co-variations of murine T and B cells and a role of corticosteroid. J Immunol. 1981 Apr;126(4):1364–1367. [PubMed] [Google Scholar]
- Kinson G. A., Peat F. The influences of illumination, melatonin and pinealectomy on testicular function in the rat. Life Sci I. 1971 Mar 1;10(5):259–269. doi: 10.1016/0024-3205(71)90313-4. [DOI] [PubMed] [Google Scholar]
- Maestroni G. J., Conti A., Pierpaoli W. Role of the pineal gland in immunity. III. Melatonin antagonizes the immunosuppressive effect of acute stress via an opiatergic mechanism. Immunology. 1988 Mar;63(3):465–469. [PMC free article] [PubMed] [Google Scholar]
- Maestroni G. J., Conti A., Pierpaoli W. Role of the pineal gland in immunity: II. Melatonin enhances the antibody response via an opiatergic mechanism. Clin Exp Immunol. 1987 May;68(2):384–391. [PMC free article] [PubMed] [Google Scholar]
- Maestroni G. J., Conti A., Pierpaoli W. The pineal gland and the circadian, opiatergic, immunoregulatory role of melatonin. Ann N Y Acad Sci. 1987;496:67–77. doi: 10.1111/j.1749-6632.1987.tb35747.x. [DOI] [PubMed] [Google Scholar]
- McCain H. W., Lamster I. B., Bozzone J. M., Grbic J. T. Beta-endorphin modulates human immune activity via non-opiate receptor mechanisms. Life Sci. 1982 Oct 11;31(15):1619–1624. doi: 10.1016/0024-3205(82)90054-6. [DOI] [PubMed] [Google Scholar]
- Plotnikoff N. P., Miller G. C. Enkephalins as immunomodulators. Int J Immunopharmacol. 1983;5(5):437–441. doi: 10.1016/0192-0561(83)90020-6. [DOI] [PubMed] [Google Scholar]
- Scheiff J. M., Cordier A. C., Haumont S. Epithelial cell proliferation in thymic hyperplasia induced by triiodothyronine. Clin Exp Immunol. 1977 Mar;27(3):516–521. [PMC free article] [PubMed] [Google Scholar]
- Shirama K., Furuya T., Takeo Y., Shimizu K., Maekawa K. Direct effect of melatonin on the accessory sexual organs in pinealectomized male rats kept in constant darkness. J Endocrinol. 1982 Oct;95(1):87–94. doi: 10.1677/joe.0.0950087. [DOI] [PubMed] [Google Scholar]
- Simpson J. G., Gray E. S., Beck J. S. Age involution in the normal human adult thymus. Clin Exp Immunol. 1975 Feb;19(2):261–265. [PMC free article] [PubMed] [Google Scholar]
- Sobhon P., Jirasattham C. Effect of sex hormones on the thymus and lymphoid tissue of ovariectomized rats. Acta Anat (Basel) 1974;89(2):211–225. doi: 10.1159/000144285. [DOI] [PubMed] [Google Scholar]
- Sterio D. C. The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc. 1984 May;134(Pt 2):127–136. doi: 10.1111/j.1365-2818.1984.tb02501.x. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Choi K., Suzuki Y. Light activation of gonadal function restrained by continuous dark treatment in male rats. Endocrinol Jpn. 1971 Apr;18(2):195–203. doi: 10.1507/endocrj1954.18.195. [DOI] [PubMed] [Google Scholar]
- Vitale P. M., Darrow J. M., Duncan M. J., Shustak C. A., Goldman B. D. Effects of photoperiod, pinealectomy and castration on body weight and daily torpor in Djungarian hamsters (Phodopus sungorus). J Endocrinol. 1985 Sep;106(3):367–375. doi: 10.1677/joe.0.1060367. [DOI] [PubMed] [Google Scholar]
- WEAVER J. A. Changes induced in the thymus and lymph nodes of the rat by the administration of cortisone and sex hormones and by other procedures. J Pathol Bacteriol. 1955 Jan-Apr;69(1-2):133–139. doi: 10.1002/path.1700690119. [DOI] [PubMed] [Google Scholar]
- Weibel E. R., Kistler G. S., Scherle W. F. Practical stereological methods for morphometric cytology. J Cell Biol. 1966 Jul;30(1):23–38. doi: 10.1083/jcb.30.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellon S. M., Goldman B. D. Photoperiod control of reproductive development in the male Djungarian hamster (Phodopus sungorus). Endocrinology. 1984 Feb;114(2):664–670. doi: 10.1210/endo-114-2-664. [DOI] [PubMed] [Google Scholar]