Abstract
Musculoskeletal disorders are a series of diseases involving bone, muscle, cartilage, and tendon, mainly caused by chronic strain, degenerative changes, and structural damage due to trauma. The disorders limit the function of patients due to pain and significantly reduce their quality of life. In recent years, adipose-derived mesenchymal stem cells have been extensively applied in regeneration medicine research due to their particular abilities of self-renewal, differentiation, and targeted homing and are more easily accessed compared with other sources. The paracrine effect of ADSCs plays a crucial role in intercellular communication by releasing mass mediators, including cytokines and growth factors, particularly the exosomes they secrete. Not only do these exosomes possess low immunogenicity, low toxicity, and an enhanced ability to penetrate a bio-barrier, but they also inherit their parent cells’ characteristics and carry various bioactive molecules to release to targeted cells, modulating their biological process. Meanwhile, these characteristics also make exosomes a natural nanocarrier capable of targeted drug delivery to specific sites, enhancing the bioavailability of drugs within the body and achieving precision therapy with fewer toxic side effects. Furthermore, the integration of exosomes with tissue engineering and chemical modification strategies can also significantly enhance their efficacy in facilitating tissue repair. However, the current research on ADSC-Exos for improving MSDs remains at an early stage and needs further exploration. Therefore, this review summarized the ADSC-Exo as a nanodrug carrier characteristics and mechanism in the treatment of fracture, osteoporosis, osteoarthritis, intervertebral disc degeneration, and tendon injury, which push forward the research progress of ADSC-Exo therapy for MSDs.
Keywords: exosomes, adipose mesenchymal stem cells, musculoskeletal disorders, regenerative medicine
Introduction
Musculoskeletal disorders (MSDs) are pain and function limitations in bones, joints, cartilage, muscles, and ligaments that cause a lower quality of life, including osteoporosis (OP), osteoarthritis (OA), rotator cuff tears, and sports injuries.1 Globally, the number of cases of MSDs is about 1.3 billion, and the female prevalence rates are higher than those of males and increase with age. Low back pain (36.8%) makes up the greatest proportion of MSD cases, while osteoarthritis (19.3%) is the second most prevalent.2 The major function of the skeleton is to act as mechanical levers for muscles to maintain normal locomotion, an operation that also involves the internal interactions between tendons, ligaments, and cartilage, reflecting a holistic musculoskeletal system.3 Therefore, patients with MSDs usually have limitations in mobility in different parts of their body with much pain, and typically undergo various treatments, such as pharmacological treatment, sports therapy, complementary medicine, and surgery, which can ease the symptoms. However, in addition to the risks of surgery, the curative effect of medicine is still unclear and inconsistent, and may have side-effects that induce other adverse reactions.4 Currently, as one of the rising fields in regenerative medicine, cell therapy has become an efficacious and practical method to improve MSDs.5
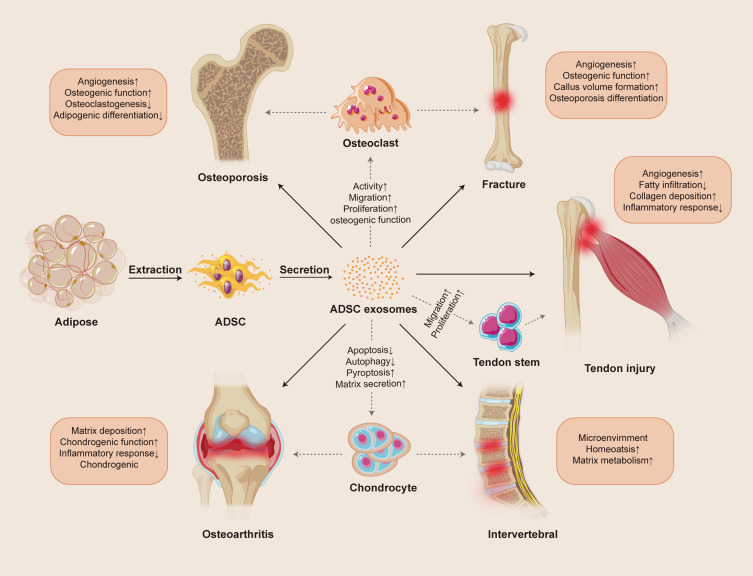
Mesenchymal stem cells (MSCs), also called multipotent mesenchymal stromal cells, are plastic adherent cells with a fibroblast-like morphology, extracted from multiple tissues in the human body, and have the potential to self-renew and differentiate into adipose, cartilage, and bone.6,7 MSCs leverage their low immunogenicity and anti-inflammatory properties to execute critical functions in tissue regeneration, including directed migration to damaged areas, promotion of angiogenesis for blood supply restoration, and suppression of immune reactions to foster a healing environment.8 Moreover, the unique differentiation capacity of MSCs enables the rapid replacement of damaged cell and remodels the dynamic cycles of degeneration and regeneration, thereby maintaining tissue function and integrity, approving injury repair.9 So far, MSCs have been instrumental in research pertaining to tissue reconstruction in the musculoskeletal, nervous, hepatic, myocardial, and dermal realms.10 Recent research indicated that the pleiotropic functions are attributed to the paracrine effect, to a large extent, and the major mediators secreted from MSCs contain cytokines and growth factors, particularly exosomes.11,12 As extracellular vesicles, exosomes (Exos) can be detected in most biological fluids and tissues, such as blood, urine, amniotic fluid, and malignant ascites.13 Of these sources, due to rich reserves, easy access, and minimally invasive extraction, adipose mesenchymal stem cells (ADSCs) have become one of the primary sources of exosomes in cell therapy.14 In addition to lipids and protein, Exos have been found to carry various nucleic acids, such as mRNA, microRNAs, and noncoding RNA, which are released into the intracellular space after fusion with the recipient cells to modulate many biological activities.15 However, the low toxicity, high targeting efficiency, favorable bio-barrier penetration, and environmental adaptability of exosomes themselves hold the potential to make them an excellent nanoscale drug delivery vehicle.16 The ADSC-Exos have been shown to improve the repair of musculoskeletal diseases through autoplastic transplantation or in combination with tissue engineering, showing their significant promise in the field of regenerative medicine.17–19 In this review, we summarize the functional features of ADSC-Exos and the current studies in musculoskeletal disease treatments and discuss the development of ADSC-Exo-based therapies for clinical setting (Figure 1).
Figure 1.
Overview of action mechanisms of ADSC-Exos therapy in musculoskeletal disease.
Exosomes
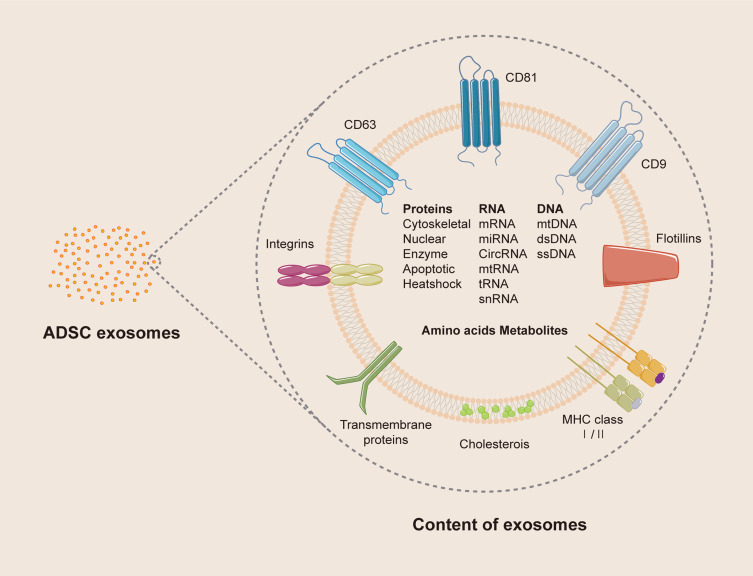
Exosomes are single membrane-bound nanovesicles with a topology resembling that of cells and usually participate in intercellular communication (Figure 2).20 As early as the late 1960s, extracellular vesicles, varying from ~100 nm in the maximum dimension, were first identified under electron microscopy by Anderson in the cartilage matrix of the tibial upper epiphyseal plate.21 With the deepening of research, Johnstone isolated the vesicles in sheep reticulocytes through centrifugation and named them “exosomes” in 1986.22 Today, exosomes are confirmed to be the smallest type of extracellular vesicles, about 40–120 nm in diameter, with the size differing significantly from different sources.23 The process of generating exosomes is primarily through a paracrine manner of the parent cell. First, the plasma membrane will invaginate inward and intraluminal vesicles (ILVs), formed by early endosomes, accumulate in the cell, constituting large multivesicular bodies (MBVs). After mature MBVs fuse with the cell membrane, the internal ILVs are secreted into the extracellular space, which are then defined as exosomes.24,25 However, there is evidence demonstrating that exosomes are also directly released by ILVs budding from the plasma membrane, indicating that endosomal budding is not the only means by which exosomes arise.26 Furthermore, the exosomes inherit the difficult-to-change characteristics and particular RNA expression of the parent cells.27,28 A recent study found that MSCs cultured in an adipocyte extracellular matrix with osteoblast-derived exosomes expressed osteogenic differentiation potential, reflecting that the pedigree-specific exosomes may have more of an impact on MSC differentiation than did the extracellular matrix.29 For isolating exosomes in different extracellular matrices, the size-based ultrafiltration technique is the gold standard, but other alternative methods have been developed, such as immunoaffinity capture, exosomal precipitation, zonal rate centrifugation, and microfluidic isolation techniques.30 Moreover, extracted exosomes were initially characterized through their expression of protein, especially ESCRT proteins, TSG101, Alix, and chaperones (Hcs70, Hsp90).31 As the research of exosomes became deeper, physical analyses for determining the particle size or concentration have also been performed by electron microscopy, nanoparticle tracking analysis, and dynamic light scattering.32 In addition, adipose-derived exosomes were discovered to contain adipogenesis-related gene transcripts (leptin, adiponectin, PPARγ2), and the RNA from special sources also became unique biomarkers.33
Figure 2.
The structure and content of exosomes.
In comparison to traditional treatments such as drugs, exercise and surgery, exosome therapy has certain advantages due to its biological nature, and can be classified by five aspects. First, exosomes have low immunogenicity. Human bone marrow mesenchymal stem cells (BMSCs)-derived exosomes can promote osteogenesis without any adverse reactions during animal experiments; in contrast, the expression of the surface marker MHC I from hBMSCs will lead to immune rejection.34 Second, the toxicity of exosomes is relatively low. Compared to the inevitable toxic effect of most nanoscale drug carriers in lipid membrane synthesis, exosomes have not shown to be toxic either in vivo or in vitro, greatly pushing forward the application of nanomedicine.35,36 Third, exosomes are highly targeting and transfer signals to recipient cells mainly through direct membrane fusion, phagocytosis, or ligand-receptor interactions. Exosomes not only target specific organs due to their inheriting the homing ability of parent cells but also directly participate in cell-to-cell communication by surface receptors.37 Fourth, exosomes have an enhanced ability to penetrate bio-barriers, and therefore they can act as effective medicine carriers to improve modulation of target tissue.38 Fifth, exosomes remain stable in circulation and retain their integrity in a pathological environment, such as mildly hyperthermic, acidic, high-glucose, and systemic disease environments, in which various environment factors also modify exosomes to regulate positive or negative effects.28 In conclusion, exosomes have a strong potential to treat musculoskeletal disorders through targeted carrier transport.
ADSC-Exos
MSCs have been widely applied in the related research of tissue repair due to their self-renewability and the capacity of differentiating into multiple cell types. The potential remains to further exploit exosomes in genomic manipulation and combination therapy of MSCs with scaffolds or drugs and the use of secreted exosomes.10 Currently, the research on exosomes from BMSCs is comparatively mature. BMSC-Exos have been demonstrated to improve OA through the regulation of immune homeostasis, the dampening of inflammatory reactions, and the stimulation of cellular proliferation and differentiation.39 Meanwhile, BMSC-Exos encapsulate a plethora of bioactive molecules, including proteins, miRNA and transcription factors, which are capable of modulating the microenvironment in a transorgan manner, thus fostering the reparative mechanisms within compromised tissues.40 In a study by Zhenyu Li et al, the miR-23a-3p in BMSC-Exos improved the polarization of macrophages from M1 to M2 and decreased the early inflammatory response in the knee joint by inhibiting the IRF1 and NF-κB pathways, thus promoting bone-tendon repair, showing the potential of BMSC-Exos in the treatment of MSDs.41 Furthermore, human umbilical-derived MSC exosomes (UCMSC-Exos) improved osteoporosis by promoting the proliferation and differentiation of osteoblasts, an effect that was similar to estrogen supplementation with less adverse reactions.42 Despite the benefits, both the bone marrow and human umbilical cord are difficult to access, have limited reserves and can be easily damaged during isolation, limiting their exosome clinical use.42 In contrast, adipose tissue is extensively present in our bodies, with renewability and no ethical concerns, and its use greatly reduces the complexity and cost of isolation in a sustainable manner, which is a more desirable source of exosome.32 In addition, the ADSCs from subcutaneous adipose tissue are an optimal reservoir of exosomes that show a higher osteogenic tendency and increased stemness properties compared to ADSCs from deep regions.43 These attribute position ADSC-Exos as a promising therapeutic agent for MSDs, offering a more viable solution in the future clinical therapy.
According to the research of Han et al, most ADSC-Exos have a circular shape with concave sides ranging from 20 to 300 nm, larger than the 30–120 nm range noted in earlier studies.44 This observation with extensive research highlighting the diversity of exosome characteristics among various cellular sources.45 It was established that ADSC-Exos participate in cell-to-cell exchange of signaling and metabolism altered in vivo by the protein, DNA, miRNA, and lipid molecules enclosed in the exosomes.46 A horizontal bioinformatics analysis of diversely sourced exosomes found 457, 771, and 431 proteins in ADSC-Exos, BMSC-Exos, and UCMSC-Exos, respectively. Regarding biological function, ADSC-Exo and UCMSC-Exo proteins are both mainly involved in the immune response of leukocyte activation and adhesion molecule binding, whereas BMSC-Exos are enriched in regulating cell migration and activating granulocytes.47 Moreover, the RNA in MSC-Exos, generally ranging from 200 to 400 nt, is too short to carry information for coding proteins, while miRNAs containing pri-miRNA and pre-miRNA have increasingly shown an intercellular transfer role by loading onto the RNA-induced silencing complex (RISCs).48,49 Interestingly, the five common miRNAs (miR-10a-5p, miR-486-5p, miR-222-3p, miR-10b-5p, and miR-191-5) account for 43–59% of all ADSC-Exo miRNAs reads, which is completely different from BMSC-Exos.27 Table 1 shows distinctive features of Exos from familiar MSC sources (Table 1).
Table 1.
A Comparison of MSC-Exos Derived from Different Sources
| Exosome type | Sources | Morphology | Markers | Advantages | Common applications | Ref |
|---|---|---|---|---|---|---|
| ADSC-Exos | Adipose tissue and inguinal fat pads | A circular shape with concave sides ranging from 20–300 nm diameter. | CD9, CD63, CD81, TSG101, Hsp70, Hsp90, PPARγ2, leptin, adiponectin | This source is rich in reserves and easy to access. The proteins of ADSC-Exos mainly involve immune responses of leukocyte activation. | Wound healing, inflammation suppression, and musculoskeletal disorder treatment; eg, osteoporosis, osteoarthritis, and tendon injury. | [44,47] |
| BMSC-Exos | Bone marrow | A round or elliptical shape ranging from 50–150 nm diameter. | CD9, CD44, CD63, CD81, Alix, TSG101, Hsp70, flotillin-1, sntenin1 | BMSC-Exos have good osteogenesis and chondrogenicity potential and excellent biocompatibility (B7). | Organ tissue regeneration and bone metabolism-related disease; eg, osteoporosis, fracture, and incomplete bone healing. | [50,51] |
| HUCMSC-Exos | Umbilical cord | A spherical morphology ranging from 40–100 nm diameter. | CD9, CD63, CD81, Alix, TSG101 | HucMSC-Exos have high proliferation and self-renewal rates. They alleviate oxidative stress and strengthen vascular remodeling. | Brain injury and kidney disease repair and wound healing; eg, photodamage, burns, skin injury, and scar formation. | [52,53] |
| SFMSCs-Exos | Synovial fluid in arthritis | Diameters ranging from 60–120 nm. | CD9, CD63, CD81, TSG101 | Local MSC-Exos may have priority in promoting remodeling. They express the hyaluronan receptor CD44 and synthesize enzymes. | Mainly applied to localized joints for disease treatment; eg, rheumatoid arthritis, gout, and osteoarthritis. | [14,54] |
ADSC-Exo therapeutic effects have been demonstrated in diabetic complications,55,56 abdominal aortic aneurysms,57 myocardial infarctions,58 hepatic fibrosis,59 Parkinson’s disease,60 Alzheimer’s disease,61 lung infection injuries,62 and musculoskeletal diseases. ADSC-Exos mediate these effects by alleviating inflammation, reducing immune responses, accelerating angiogenesis, regulating metabolism, and promoting tissue regeneration.14,63 In addition, exosomes also modulate the proliferation and differentiation of key immune cell (B and T cells) in autoimmune diseases, suppressing inflammation and regulating cytotoxicity, thereby mitigating pathological immune responses.16 In the bone tissue engineering field, ADSC-Exos also reportedly enhance the efficacy of biomaterials as an osteoinductive factor.64 The combination between ADSC-Exo treatments and magnesium-organic nanocomposite scaffolds promoted the formation and osseointegration of new bone by stabilizing the bone microenvironment and improving osteogenic differentiation, which showed the potential therapeutic value of MSDs.65
ADSC-Exos Therapy in MSDs
Bone Repair
The skeletal system is composed of hard cortical bone and sponge-like cancellous bone, serving functions such as body support, protection of internal organs, facilitation of movement, mineral storage, and blood cell production. As a dynamic tissue, bone maintains favorable strength and the correct mineral balance via continual self-remodeling; various cells, such as osteoblasts, osteoclasts, MSCs, and endothelial cells, promote this complex process.66 The osteoblasts play an important role in bone matrix synthesis and mineralization, and osteoclasts are the predominant bone resorption cells, both of which maintain bone microenvironment homeostasis.67 The precise bone regeneration cycle is comprised of quiescence, activation, resorption, reversal, formation, mineralization, and termination, seven sequential phases in total. While osteoclasts are resorbing irregular bone callus, osteoblasts are depositing new lamellar bone, restoring it to a standard cortical structure68 (Y72). In addition to the familiar systemic hormones, namely calcitonin, parathyroid hormone, vitamin D3, and estrogen, the manipulation of growth factor pathways has been used to improve bone regeneration.69 Diverse growth factor pathways, such as the BMP/TGF-β, MAPK, P13K/Akt/mTOR, and Wnt/β-catenin pathways, stimulate intracellular biological processes to mediate bone embryogenesis development and fracture repair.70 The Wnt proteins activate β-catenin in a downstream pathway after binding with their seven-pass transmembrane frizzled receptors, and the signaling participates throughout the bone remodeling as an osteoinductive factor, improving the adult bone mass.71,72 In the fracture nonunion model of a type 2 diabetes mellitus rat, ADSC-Exos enhanced the expression of Wnt/β-catenin protein to promote osteogenic differentiation in BMSCs after being injected around the fracture sites, reinforcing the ability of bone regeneration in vivo.73 Furthermore, after ADSCs were preconditioned for 3 days with tumor necrosis factor-alpha (TNF-α), the Wnt-3a content was elevated in secreted exosomes and human primary osteoblastic cells (HOBs) internalizing the TNF-ADSC-Exos, thus promoting migration, proliferation, and osteogenic functions more than that of HOBs grown in ADSC-Exos without preconditioning.74
The vascular system is critical for osteoblast differentiation with an increased oxygen supply in the local regions, which is necessary for hard callus formation during bone regeneration.75 Recent studies have shown that ADSC-Exos may stimulate bone vessel formation by promoting endothelial cell migration, proliferation, and tube formation, thereby regulating osteogenesis and further preventing medication-related osteonecrosis of the jaw.76,77 Furthermore, in osteonecrosis of the femoral head (ONFH), ADSC-Exos with miR-378 overexpression increased the angiogenic capacity of human vein endothelial cells and promoted BMSC osteogenic differentiation in vitro by negatively regulating Sufu and activating the Shh signaling pathways.78 It has also been discovered that miR-378 can compete with the same seed region of the vascular endothelial growth factor (VEGF) 3′-UTR to affect VEGF expression and mediate endochondral bone formation.79 Moreover, under a pathological environment of hypoxia and ischemia, osteocyte apoptosis increased, inducing an elevation in the receptor activator of nuclear factor kappa-B ligand (RANKL) and a reduction in osteoprotegerin (OPG), which activated high expression of osteoclasts.80,81 However, under the same conditions, ADSC-Exos lowered the levels of reactive oxygen species to reduce osteocyte apoptosis by promoting the expression of anti-apoptotic Bcl-2 mRNA and decreasing pro-apoptotic Bax mRNA, cytochrome c protein, and cleaved caspase-3 and caspase-9 protein levels, as well as inhibiting osteoclastogenesis by controlling the ratio of RANKL/OPG.82 Furthermore, the exosomes from ADSCs with low-level laser irradiation treatment efficiently suppressed osteocyte apoptosis induced via a hypoxic and serum-deprived environment.83
Autologous and allogeneic bone grafts, the current gold standard therapy for bone regeneration, may result in various complications, including bone non-union, infections, and graft-versus-host disease.76 However, bone tissue engineering can provide alternative traditional bone grafts by combining biomaterials, signaling factors, and cells, with a lower incidence of complications; exosomes as a new cargo has also attracted attention in this field.84 Recent research shows that ADSC-Exos and their included miRNA not only have significant osteoinductive potential but also regulate their release to promote bone regeneration when added to tissue engineered constructs as biological factors.85 In calvarial defect models, hydrogel mixed with miRNA-375 overexpressing ADSC-Exos (50 μg/mL) enhanced the bone repair capacity through the targeted release of the miRNA to inhibit IGFBP3 in vivo.86 Furthermore, miRNA-451a in ADSC-Exos in a gelatin nanoparticle hydrogel promoted the polarization of macrophages towards the M1 phenotype, reducing the inflammatory response resulting from biomaterial implantation and accelerating bone healing.87 Of the frequently used BMSCs in cell therapy, ADSC-Exos play an osteoinductive role in the differentiation process and recruited host MSCs to newly formed bone tissue by immobilizing on polydopamine-coating PLGA scaffolds.17 Meanwhile, a gelatin sponge polydopamine scaffold after ADSC-Exo modification also enhanced the migration and proliferation of BMSCs to improve bone repair.88 Additionally, exosomes can induce the activation of regulatory T cells, promote tissue repair, and secrete anti-inflammatory cytokines (IDO, PGE2, and TGF-β) to regulate immune tolerance in the body, suppress lymphocyte proliferation, and thereby alleviate graft-versus-host disease (GVHD).89,90 In the fat grafting model, ADSC-Exos that have undergone hypoxia treatment can effectively promote the survival of transplanted grafts, enhance blood vessel formation, and reduce the infiltration of local inflammatory cells, demonstrating their substantial potential in the field of allogeneic transplantation.44
Osteoporosis is a common chronic skeletal disease characterized by bone mineral density decreases, microarchitectural destruction, and an increased risk of fracture, and results from a dynamic bone turnover imbalance between bone resorption and formation.91 In osteoporosis patients, post-menopausal women are the largest proportion due to estrogen deficiency, in whom fragility fracture of hip, vertebrae, or distal radius easily occur.92 During the osteoporosis repair process, osteoblasts secrete osteoids to fill the resorption cavity formed by osteoclasts and ultimately become mineralized, preserving normal strength.93 Evidence has indicated that IncRNA-KCNQ1OT1-modified ADSC-Exos sponge the expression of the target-binding site and miRNA-141-5p and attenuate TNF-α-induced cytotoxicity and apoptosis of primary osteoblasts to improve osteoporosis.94 Moreover, both ADSCs and BMSCs have the ability to differentiate into osteoblast cells via the osteogenic lineage, and the balance with adipogenic and osteogenic differentiation directly affects the bone remodeling process.95,96 ADSC-Exos overexpressing miRNA-130a-3p downregulate SIRT7 expression and activate the Wnt signaling pathway while promoting the osteogenic differentiation of ADSCs.97 Under Wen-Shen-Tong-Luo-Zhi-Tong decoction induction, ADSC-Exos also improved the bone-fat balance in osteoporosis mice via their miRNA-122-5p cargo that targeted SPRY2 and MAPK pathways to regulate osteogenesis and adipogenic differentiation.98 In diabetic osteoporosis patients, inflammation caused by hyperglycemia, oxidative stress, and advanced glycation products is the primary contributor to bone mineral loss.99 However, a recent study has confirmed that ADSC-Exos alleviated diabetic osteoporosis in rat models by suppressing NLRP3 inflammasome activation and the expression levels of related proteins, such as ASC, Caspase-1, IL-1β, and IL-18.100 In addition, miR-146a overexpression by ADSC-Exos effectually exerted an anti-inflammation effect in osteoclasts and inhibited the production of pro-inflammatory cytokines, thereby reversing streptozotocin-induced bone loss.101 Table 2 shows new research in which ADSC-Exos improved bone repair through treatment (Table 2).
Table 2.
Studies of ADSC-Exos in Bone Defect Models
| Disease | Related pathway | Mechanism | Dose and delivery | Subject | Ref |
|---|---|---|---|---|---|
| ONFH | Overexpression of miR-378, Sufu, Shh | Promoting proliferation, migration, and osteogenic differentiation; improving angiogenesis and osteogenesis. | 50 μg/mL in vitro; 100 μg via intravenous tail injection in vivo. | Rat | [78] |
| Fracture | Wnt3a/β-catenin | Promoting osteogenic differentiation; improving femur fracture healing. | 200 μg/mL in vitro; 600 μL (200 μg/mL) via local injection in vitro. | Rat | [73] |
| OP | MIR-146a | Inhibiting inflammatory responses and bone resorption in osteoclasts. | 1.6 mg/kg via intravenous tail injection in vivo. | Rat | [101] |
| OP | MIR-122-5p, SPRY2, MAPK | WSTLZT pre-treatment; regulating differentiation; improving bone microarchitecture and bone-fat balance | 30 μg via intravenous tail injection in vitro. | Mice | [98] |
| OP | NLRP3 | Suppressing pro-inflammatory cytokine secretions of osteoclasts; reversing bone mineral loss. | 0.1, 0.2, 0.5, and 1.0 μg/μL in vitro; 1.6 mg/kg via intravenous tail injection in vivo. | Rat | [100] |
Abbreviations: ONFH: osteonecrosis of the femoral head, OP: osteoporosis.
Cartilage Regeneration
Cartilage is primarily composed of chondrocytes, a matrix rich in collagen and proteoglycans, and may contain elastic fibers or collagen fibers, forming a connective tissue with elasticity and resilience. During joint movement, cartilage provides a gliding motion as a smooth surface, and the lubricin and hyaluronic acid produced by chondrocytes and synovial cells provides a boundary layer of lubricants that lower the friction coefficient.102 However, the intrinsic reparative ability of cartilage tissues is limited due to the lack of direct nutrition supplied by blood and the distribution of nerves or lymphatics, which are finally replaced by scar tissue or fibrocartilage with poor function after damage, easily leading to post-traumatic osteoarthritis.103 As the solo constituent cells of cartilage, chondrocytes play a primary role in the response to chemical or mechanical extracellular perturbations, and regulate the cartilage composition, although making up only 1–5% by volume of the articular cartilage.104,105 In recent decades, the paracrine effect of MSCs has become highlighted in cartilage engineering, as the Exos carrying miRNA have the ability to promote chondrocyte proliferation and matrix regeneration and reduced inflammation to improve cartilage repair.106 The miRNA-486-5p-modified ADSC-Exos have been demonstrated to alleviate chondrocyte apoptosis and accelerate matrix secretion via inhibiting endoplasmic reticulum stress in OA progression.107 Based on ADSC chondrogenesis characteristics, ADSC-Exos with karyogenic pretreatment also significantly promoted ADSC differentiation to chondrocytes by enhancing cell proliferation and activity while enhancing the expression of chondrogenic-related genes and inhibiting chondrolysis-related genes.108 Furthermore, in a 3D culture of porous Gelma hydrogel, overexpressed exosomes, isolated from passage 4 ADSCs with miR-23a-3p transfection, enhanced microtia chondrocyte proliferation and attenuated apoptosis in vivo and in vitro, improving cartilage regeneration.109 Interestingly, after RNA sequence analysis, the Exos from differentiated chondrogenic ADSCs had 23 upregulated and 163 downregulated lncRNA compared with the Exos from the undifferentiated ADSC. Among the results, the largest changes were in PDLIM5 and CRADD expression, which was similar to the expression trend during chondrogenesis, and reflected the potential of Exos and associated lncRNA in the cartilage regeneration domain.110
Of all the cartilage diseases, OA is the most familiar chronic arthritis form and is caused by long-term joint wear and aberrant remodeling of cartilage tissue under the influence of inflammatory mediators within the impaired joint.111 In addition to risk factors such as sex, obesity, heredity, and mechanical damage, OA is strongly associated with age, affecting about 18% of women and 9.6% of men over 60 years ago.112 The characteristics of OA joints include the inflammation and degradation of articular cartilage, osteophyte formation, sclerosis of the subchondral bone, and a series of pathological changes of periarticular tissues, which most commonly occur in the hip or knee.113 However, existing treatments for patients with osteoarthritis, such as drugs, surgery and exercise therapy, mainly tend to decrease pain and manage stiffness and slow down the progress of OA.114 Currently, therapy based on MSCs provides more chondroprotective and anti-inflammatory effects, especially via the exosomes of paracrine signaling to more efficiently regulate impaired joints.115 The most common MSC sources, involving bone marrow, adipose tissue, and placenta, have been shown to play an important part in treating the progression of OA through trophic effects, anti-apoptotic activity, chondrogenesis, immunomodulation, and other mechanisms.116 Among them, ADSCs exhibit greater activity of indoleamine 2.3-dioxygenase compared with BMSCs in the same environment and possess more immunoregulatory potential, which indicates that ADSCs may be an ideal exosome source for OA.117 In the arthritic mouse model, ADSC-derived exosomal miR-93-5p have been confirmed to reduce the release of pro-inflammatory factors (TNF-α, IL-1β, IL-6) by targeting ADAMTS9 and activating the PI3K/AKT/mTOR signaling pathway to inhibit autophagy and apoptosis in IL-1β-treated chondrocytes, preventing the further development of OA.118 In the same model, the exosomes derived from ADSCs transfected with miR-338-3p not only decreased the expression of related inflammatory factors, such as MMP3 and MMP13, but also inhibited RUNX2 expression to promote the repair of impaired chondrocytes.119 Furthermore, miR-376c-3p of the ADSC-Exos suppressed the Wnt-β-catenin signaling pathway by targeting Wnt3 and Wnt9, and then regulating chondrocyte and synovial fibroblast function, improving OA.120 To optimize the ADSC-Exos regenerative potential, we can also modulate its biological function by changing environment factors. ADSC-Exos pretreated with tropoelastin for 48 h enhanced the COL II and ACAN expression of chondrocytes more remarkably than untreated ADSC-Exos and promoted regeneration and cartilage extracellular matrix (ECM) deposition in vitro. Furthermore, the TE-ADSC-Exo intro-articular injection also alleviated cartilage impairment of rats with knee osteoarthritis in vivo, which may be related to differentially upregulated miR-451-5p.121 Moreover, the inducement of a pulsed electromagnetic field (PEMF) enhanced the inhibitory effect of ADSC-Exos on chondrocyte inflammation and matrix degeneration, significantly alleviating OA in the same rat models.122 In addition to familiar MSCs, many nonclassical sources of MSC-Exos have been demonstrated to have treatment potential for OA, including the infrapatellar fat pad (IPFP) and synovial and platelet-rich plasma.54 As a branch of adipose tissue, IPFP-MSC-derived exosomes heightened chondrocyte catabolism to alleviate the cartilage destruction of OA, improving gait patterns and promoting the mTOR autophagy pathway through miR-100-5p mediation, providing a potential strategy for OA therapy.123
Intervertebral discs between the vertebrae provide sufficient flexibility to the spine and are composed of three anatomical parts: the fibrous annulus, the central nucleus pulposus (NP), and the cartilaginous endplates.124 With aging and ongoing degeneration, disc height decreases, endplates become damaged, the fibrous nucleus dehydrates, and the loss of proteoglycans and other lesions result in intervertebral disc degeneration (IVDD), which is strongly connected to low back pain.125 During the process of IVDD, the proinflammatory cytokines (IL‑1α, IL‑1β, IL‑6, IL‑17, and TNF) secreted by intervertebral disc cells are higher than normal levels, and these changes promote ECM degradation and an imbalance of catabolic and anabolic responses in intervertebral disc, inducing functional cells to undergo autophagy, senescence, or apoptosis.126,127 Today, MSC-EVs, including exosomes, have been a new focus of cell therapy for IVDD, which avoids the peripheral inflammatory response caused by dead MSCs under the ischemic and inflammatory microenvironment of IVDD.128 In an in vitro experiment, ADSC-EVs were found to promote proliferation and the expression of chondrocyte RNA (Sox‑9, collagen‑II, and aggrecan) in human NP cells, and significantly inhibit the release of associated inflammatory cytokines.129 The latest research showed that pyroptosis of NP cells was activated by NLRP3 in an IVDD mouse model induced by lipopolysaccharide. At the same time, miR-410 of ADSC-Exos directly bound to NLRP3 to suppress the pyroptosis response in NP cells, offering a new opportunity to treat IVDD.130 Furthermore, ADSC-Exos transfected with miR-155-5p decreased the level of pyroptosis and increased the expression of autophagy markers (LC3, p62) by targeting the TGFβR2 signaling pathway in NP cells, which alleviated IVDD in vivo.131 In addition to traditional exosome transplants, injection of hydrogel coupled with ADSC-Exos into the ECM provided sustained release of exosomes into each disc to regulate the synthesis and degradation of the matrix and control NP cell pyroptosis, simultaneously maintaining microenvironment homeostasis in early IVDD via this novel drug delivery system.19 Table 3 shows new research in which ADSC-Exos improved cartilage damage through treatment (Table 3).
Table 3.
Studies of ADSC-Exos in Cartilage Damage Models
| Disease | Related pathway | Mechanism | Dose and delivery | Subject | Ref |
|---|---|---|---|---|---|
| OA | miR-376c-3p, Wnt/β-catenin | Regulating chondrocyte and synovial fibroblast function; mitigating OA processes. | 10 μg/mL in vitro; 100 μg via intra-articular injection in vivo. | Mice | [120] |
| OA | COL2A1, SOX9, ACAN | PEMF pre-treatment; suppressing the degeneration and inflammation of chondrocytes; reversing cartilage damage. | 1×108 particles/mL in vitro; 10 μL (1×109 particles/mL) via intra-articular injection in vivo. | Rat | [122] |
| OA | miR-451-5p | TE pre-treatment; maintaining the chondrocyte phenotype; promoting cartilage repair. | 5×108 particles/mL in vitro; 10 μL (1×1010 particles/mL) via intra-articular injection in vivo. | Rat | [121] |
| IVDD | miR-155-5p, TGFβR2 | Promoting autophagy and inhibiting pyroptosis in NPCs; alleviating IVDD symptoms. | 100 μg/mL in vitro; 1.5×106 particles/mL via local injection in vivo. | Rat | [131] |
Abbreviations: OA: osteoarthritis, IVDD: intervertebral disc degeneration, NPC: nucleus pulposus cell.
Tendon Healing
Tendons, composed of collagen fibers arranged in parallel, also contain a small number of elastic fibers and proteoglycans, which provide them with a degree of elasticity and toughness. In our daily activities, tendons transmit the force generated by muscle to bone as a load bearing medium to bring about normal joint movements.132 During the process, tendon tissue is required to withstand and store substantial energy, while long-time overuse will induce abnormalities of the tendon microstructure and multiple overlapping pathological changes, resulting in pain and functional impairment.133 Tendon injury mainly occurs in athletes and workers in specific occupations who perform repetitive or high force activities and commonly involves the rotator cuff tendon, lateral and medial humerus condyles, gluteal tendons, patellar tendon, and the Achilles tendon.134 Today, cell-free therapy based on exosomes is widely applied to treat tendon injuries with a high safety profile and plays a significant role in the overlapping stages of repair, especially for the rotator cuff tear (RCT).18 In the first phase after injuries, the inflammatory stage, ADSC-Exos have been shown to regulate endothelial tip cells to promote angiogenesis with their miR-125a cargo, ultimately accelerating the efficiency of tendon healing.14,135 In order to restore the intracellular metabolic balance caused by inflammation, ADSC-Exos remarkably augmented M2 macrophage polarization, inhibited M1 polarization, and alleviated relevant inflammatory reactions to improve chronic rotator cuff tendinopathy.136 Although glucocorticoids (GCs), the most common conservative therapy, have potent analgesic and anti-inflammatory effects, they may also accelerate degeneration of the impaired tendon.137 Synergistic therapy using ADSC-Exos and GCs significantly decreased RAW cell senescence, apoptosis, the transcription of tenocytic degradative enzymes caused by GCs, and improved cellular activity, thus reducing inflammation, which overrode the GC-induced detrimental effects.138 During the subsequent fibroblastic phase, tendon stem cells (TSCs) are found to absorb inoculated ADSC-Exos to increase migration and proliferation in the damaged tendon through the activation of SMAD2/3 and SMAD1/5/9 and lessened the inflammatory response, promoting tendon healing.139 A local injection of hydrogel combined with ADSC-Exos upregulated the miRNA expression of tenogenesis markers (TNC, TNMD, Scx) and improved osteogenic and adipogenesis differentiation as well as biomechanical properties of a rotator cuff tendon injury.140 After granulation tissues have matured, a provisional scar is replaced by matrix- and organ-specific cells to restore the original properties; however, tendon tissue is deficient in full regeneration potential as it is difficult to effectively remodel type III into a type I collagen matrix.141 Zhang et al demonstrated that ADSC-Exos were able to promote the expression of type I and III collagen-encoding genes and increase the type I/III ratio to maintain metabolic homeostasis in torn human rotator cuff tendon in in vitro experiments, which may have been achieved via activating the AMPK pathway to suppress Wnt/β-catenin signaling.142 Moreover, a hypoxic environment triggered ADSCs and tenocyte-derived exosomes to release mass regenerative factors into the ECM and protected the tendons from damage. Among the mediators, the key proteins related to ECM homeostasis included MMP2, CTSD, TN-C, and COL6A from hypoxic ADSC-Exos; these proteins remain to be further explored for their association with signaling pathways in tendon remodeling.143
Rotator cuff tear patients typically have degenerative changes that commonly include rotator cuff atrophy and fatty infiltration, which significantly decrease the viability and elasticity of the injured tissues and increase the re-tear rates, which is a critical cause of repair failures and poor clinical outcomes.144 However, ADSC-Exos have been verified to stimulate myoblast proliferation, migration, and myogenic differentiation, and improve protein synthesis via multiple signaling pathways, including the Wnt, MAPK, PI3K/Akt, and JAK/STAT pathways, thus promoting muscle regeneration.145 Furthermore, Wang et al found that ADSC-Exos have the potential to prevent fatty infiltration and atrophy and improve biomechanical properties in rotator cuff tear rat models.146 In subsequent experiments using a rabbit model, the ADSC-Exo injection group produced more collagen I deposition and revealed markedly lower fatty infiltration rates and more continuous fibrous tissues at the tendon–bone interface than the saline group did.147 Table 4 shows new research in which ADSC-Exos improved tendon injury through treatment (Table 4).
Table 4.
Studies of ADSC-Exos in Tendon Injury Models
| Disease | Related pathway | Mechanism | Dose and delivery | Subject | Ref |
|---|---|---|---|---|---|
| Tendon injury | SMAD2/3, SMAD1/5/9 | Promoting proliferation, migration, and tenogenic differentiation of TSCs; regulating inflammation and improving tendon healing. | 50 μg/mL in vitro; 200 μg via local injection in vivo. | Rat | [139] |
| RCT | TNMD, TND, Scx | Promoting proliferation and differentiation of TSCs; improving tendon biomechanics and repair. | 0.3 mg/mg in vitro; 100 μL (0.3 mg/mL) via local injection in vivo. | Rat | [140] |
Abbreviations: RCT, rotator cuff tear, TSC, tendon stem cell.
Discussion
Exosomes, with their biocompatibility and low immunogenicity, are adept at delivering a variety of bioactive molecules such as proteins, nucleic acids, and lipids directly to target tissues to alleviate local inflammation and promote tissue repair. Moreover, these characteristics make exosomes an ideal nanoscale drug delivery vehicle, capable of enhancing the precision and efficiency of drug targeted delivery, providing effective strategies for the treatment of musculoskeletal diseases. Currently, the therapeutic efficiency of ADSC-Exos has been increased via diverse regulatory pathways. The fusion of exosomal surface proteins involving signaling peptides, the recruitment of specific proteins or RNAs by molecule sorting modules in exosomes, and small nucleic acid molecule loading all effectively demonstrate the potential of exosomes, such as for targeted drug delivery, gene treatment, and tissue regeneration.148 Recent studies have suggested that local MSC-derived exosomes may preferentially accelerate healing and microenvironment remodeling at injured sites using different means, which may make exosomes an ideal source in the future.54,149 In the tissue engineering field, the combination of biological scaffolds, including decellularized matrices, ceramics, natural polymers, and hybrid scaffolds with ADSC-Exos, showed better vascularization and integration and mediated local tissue regeneration, especially through the release of the carried miRNAs.85 Furthermore, pre-conditioning of ADSCs directly enhanced secreted exosome performance with cytokines, agonists, or pharmacological molecules, and the use of customized conditioned media with trophic factors also showed a beneficial effect in cell growth process.150,151
However, most of the exosome extraction methods still lack standardization, are complicated operations, require a high budget, and may include irrelevant components from the extra-cellular space due to their nanosize.63 To overcome these challenges, there is a need to develop cost-effective exosome extraction kits and automate the process to reduce human error and enhance the efficiency and simplicity of exosome isolation. A significant advancement in this field is the ultrafast exosome isolation system invented by Chen et al, which employs negative pressure oscillation and dual-coupled harmonic oscillators to vibrate membranes, achieving automated extraction and efficient purification, thus offering a favorable solution for exosome research.152 Currently, research on ADSC-Exos is largely confined to rodent models, necessitating a transition to larger experimental animals such as pigs or non-human primates to enhance clinical relevance and facilitate the establishment of a database to advance ADSC-Exos research. In the clinic studies, recent concerns of ADSC-Exos are mainly focused on wound healing and skin repair and also referred less to MSDs. It is imperative to enhance foundational research on the impact of ADSC-Exos on cellular bioactivity and tissue repair, based on the anatomy and functionality of the musculoskeletal system, which drives the translation to clinical trials, aimed at validating the therapeutic efficacy and safety of ADSC-Exos in the context of MSDs. In addition, the translation of exosome treatment is related to the optimisation of various parameters, such as the delivery method, injection concentration, and treatment frequency. These experimental conditions also require meticulous refinement and validation to ensure consistent and standardized outcomes across clinical applications. Therefore, for further efforts to be enhanced, the overall process should be standardized so that ADSC-Exo therapy can be applied more extensively to the clinic treatment of MSDs.
Conclusion
Adipose mesenchymal stem cell-derived exosomes have been shown to have powerful therapeutic potential in the field of musculoskeletal disorders. These exosomes can more efficiently reach impaired sites and communicate with surrounding cells via their unique low immunogenicity and high targeting. The various nucleic that is carried by ADSC-Exos as their intercellular transfer signal directly mediates the biological processes of receptor cells, thus promoting injured tissue regeneration and repair. Moreover, due to the accessibility, sufficient reserves, and renewability of adipose tissue, ADSC-Exos have been used for more applications in the treatment of MSDs with their sustainable development than bone marrow and other familiar sources. Presently, there is a lot of animal research devoted to exploring the underlying mechanisms of ADSC-Exos in the MSDs treatment and clinical studies to explore safety and efficacy in the human body are still further refined. ADSC-Exos therapy is thus a promising direction for the treatment of MSDs with potential to be explored in the future.
Acknowledgments
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Funding Statement
This review was supported by the National Natural Science Foundation of China (82174494) and Zhongnan Hospital of Wuhan University Science, Technology and Innovation Seed Fund (CXPY2022041).
Data Sharing Statement
Data supporting the results of this study are available from the corresponding author upon request.
Author Contributions
Ao Tang and Qing Shu have contributed equally to this work and share first authorship. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; Tang. and Lai. took part in drafting, revising or critically reviewing the article; Shu. and Jia gave final approval of the version to be published; Tian. has agreed on the journal to which the article has been submitted, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Reddy MZAP. Stem Cells for Treatment of Musculoskeletal Conditions - Orthopaedic/Sports Medicine Applications. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165624. [DOI] [PubMed] [Google Scholar]
- 2.Safiri S, Kolahi AA, Cross M, et al. Prevalence, Deaths, and Disability‐Adjusted Life Years Due to Musculoskeletal Disorders for 195 Countries and Territories 1990–2017. Arthritis & Rheumatology. 2021;73:702–714. [DOI] [PubMed] [Google Scholar]
- 3.Kavadar G, EroĞLu DemİR S, Akbal EAY. Use of traditional and complementary medicine for musculoskeletal diseases. Turkish Journal of Medical Scie. 2019;49:809–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malekpour K, Hazrati A, Zahar M, et al. The Potential Use of Mesenchymal Stem Cells and Their Derived Exosomes for Orthopedic Diseases Treatment. Stem Cell Reviews and Reports. 2021;18:933–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee S, Chae D-S, Song B-W, et al. ADSC-Based Cell Therapies for Musculoskeletal Disorders: a Review of Recent Clinical Trials. International Journal of Molecular Sciences. 2021;22:10586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horwitz EM, Le Blanc K, Dominici M, et al. Clarification of the nomenclature for MSC: the International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393–395. [DOI] [PubMed] [Google Scholar]
- 7.Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Experimental & Molecular Medicine. 2013;45:e54–e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. [DOI] [PubMed] [Google Scholar]
- 9.Hwang NS, Zhang C, Hwang Y-S, Varghese S. Mesenchymal stem cell differentiation and roles in regenerative medicine. Wiley Interdiscip Rev Syst Biol Med. 2009;1:97–106. [DOI] [PubMed] [Google Scholar]
- 10.Han Y, Li X, Zhang Y, et al. Mesenchymal Stem Cells for Regenerative Medicine. Cells. 2019;8:886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joo HS, Suh JH, Lee HJ, Bang ES, Lee JM. Current Knowledge and Future Perspectives on Mesenchymal Stem Cell-Derived Exosomes as a New Therapeutic Agent. Int J Mol Sci. 2020;21:727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pashoutan Sarvar D, Shamsasenjan K, Akbarzadehlaleh P. Mesenchymal Stem Cell-Derived Exosomes: new Opportunity in Cell-Free Therapy. Adv Pharm Bull. 2016;6:293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iraci N, Leonardi T, Gessler F, Vega B, Pluchino S. Focus on Extracellular Vesicles: physiological Role and Signalling Properties of Extracellular Membrane Vesicles. Int J Mol Sci. 2016;17:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong P, Yang H, Wu Y, Li K, Tang Z. The functions and clinical application potential of exosomes derived from adipose mesenchymal stem cells: a comprehensive review. Stem Cell Research & Therapy. 2019;10:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J, Li S, Li M, et al. Exosome and exosomal microRNA: trafficking, sorting, and function. Genomics Proteomics Bioinformatics. 2015;13:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen Z, Huang W, Liu J, et al. Effects of Mesenchymal Stem Cell-Derived Exosomes on Autoimmune Diseases. Frontiers in Immunology. 2021;12:749192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li W, Liu Y, Zhang P, et al. Tissue-Engineered Bone Immobilized with Human Adipose Stem Cells-Derived Exosomes Promotes Bone Regeneration. ACS Appl Mater Interfaces. 2018;10:5240–5254. [DOI] [PubMed] [Google Scholar]
- 18.Lyu K, Liu T, Chen Y, et al. A “cell-free treatment” for tendon injuries: adipose stem cell-derived exosomes. Eur J Med Res. 2022;27:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xing H, Zhang Z, Mao Q, et al. Injectable exosome-functionalized extracellular matrix hydrogel for metabolism balance and pyroptosis regulation in intervertebral disc degeneration. J Nanobiotechnology. 2021;19:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marban AIE. Exosomes: fundamental Biology and Roles in Cardiovascular Physiology. Annu Rev Physiol. 2016;78:67–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.HC A. Vesicles associated with calcification in the matrix of epiphyseal cartilage. J Cell Biol. 1969;41:59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). Journal of Biological Chemistry. 1987;262:9412–9420. [PubMed] [Google Scholar]
- 23.Zaborowski MP, Balaj L, Breakefield XO, Lai CP. Extracellular Vesicles: composition, Biological Relevance, and Methods of Study. Bioscience. 2015;65:783–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Behera TN. Exosomes: mediators of bone diseases, protection, and therapeutics potential. Oncoscience. 2018;23:181–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LeBleu RKVS. The biology, function, and biomedical applications of exosomes. Science (New York, N.Y.). 2020;367:6478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gould DMPSJ. Exosomes. Annu Rev Biochem. 2019;88:487–514. [DOI] [PubMed] [Google Scholar]
- 27.Baglio SR, Rooijers K, Koppers-Lalic D, et al. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Research & Therapy. 2015;6:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ren S, Wang C, Guo S. Review of the Role of Mesenchymal Stem Cells and Exosomes Derived from Mesenchymal Stem Cells in the Treatment of Orthopedic Disease. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research. 2022;28:e935937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Narayanan K, Kumar S, Padmanabhan P, et al. Lineage-specific exosomes could override extracellular matrix mediated human mesenchymal stem cell differentiation. Biomaterials. 2018;182:312–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li P, Kaslan M, Lee SH, Yao J, Gao Z. Progress in Exosome Isolation Techniques. Theranostics. 2017;7:789–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thery C, Boussac, P M, Veron P, et al. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol. 2001;166:7309–7318. [DOI] [PubMed] [Google Scholar]
- 32.Wang LMDMZ. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells. 2019;8:727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Yu M, Tian W. Physiological and pathological impact of exosomes of adipose tissue. Cell Proliferation. 2016;49:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J, Liu X, Li H, et al. Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signaling pathway. Stem Cell Research & Therapy. 2016;7:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Narayanan R, Huang CC, Ravindran S. Hijacking the Cellular Mail: exosome Mediated Differentiation of Mesenchymal Stem Cells. Stem Cells International. 2016;2016:3808674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang B, Chen Y, Shi J. Exosome Biochemistry and Advanced Nanotechnology for Next-Generation Theranostic Platforms. Adv Mater. 2019;31:e1802896. [DOI] [PubMed] [Google Scholar]
- 37.He C, Zheng S, Luo Y, Wang B. Exosome Theranostics: biology and Translational Medicine. Theranostics. 2018;8:237–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lorenc T, Klimczyk K, Michalczewska I, et al. Exosomes in Prostate Cancer Diagnosis, Prognosis and Therapy. Int J Mol Sci. 2020;21:2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang X, Liu T, Ran C, et al. Immunoregulatory paracrine effect of mesenchymal stem cells and mechanism in the treatment of osteoarthritis. Frontiers in Cell and Developmental Biology. 2024;12:1411507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Z, Wu Y, Zhao Z, Liu C, Zhang L. Study on Transorgan Regulation of Intervertebral Disc and Extra-Skeletal Organs Through Exosomes Derived From Bone Marrow Mesenchymal Stem Cells. Frontiers in Cell and Developmental Biology. 2021;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Z, Tong K, Zhu J, et al. BMSC-derived exosomes promote tendon-bone healing after anterior cruciate ligament reconstruction by regulating M1/M2 macrophage polarization in rats. Stem Cell Research & Therapy. 2022;13:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xinjia GYW. The Role and Mechanism of Exosomes from Umbilical Cord Mesenchymal Stem Cells in Inducing Osteogenesis and Preventing Osteoporosis. Cell Transplantation. 2021;30:9636897211057465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Di Taranto G, Cicione C, Visconti G, et al. Qualitative and quantitative differences of adipose-derived stromal cells from superficial and deep subcutaneous lipoaspirates: a matter of fat. Cytotherapy. 2015;17:1076–1089. [DOI] [PubMed] [Google Scholar]
- 44.Han YD, Bai Y, Yan XL, et al. Co-transplantation of exosomes derived from hypoxia-preconditioned adipose mesenchymal stem cells promotes neovascularization and graft survival in fat grafting. Biochem Biophys Res Commun. 2018;497:305–312. [DOI] [PubMed] [Google Scholar]
- 45.Wang X, Xia J, Yang L, Dai J, He L. Recent progress in exosome research: isolation, characterization and clinical applications. Cancer Gene Therapy. 2023;30:1051–1065. [DOI] [PubMed] [Google Scholar]
- 46.Cai Y, Li J, Jia C, He Y, Deng C. Therapeutic applications of adipose cell-free derivatives: a review. Stem Cell Research & therapy. 2020;11:312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang ZG, He ZY, Liang S, et al. Comprehensive proteomic analysis of exosomes derived from human bone marrow, adipose tissue, and umbilical cord mesenchymal stem cells. Stem Cell Research & Therapy. 2020;11:511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen TS, Lai RC, Lee MM, et al. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res. 2010;38:215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Toh WS, Lai RC, Zhang B, Lim SK. MSC exosome works through a protein-based mechanism of action. Biochem Soc Trans. 2018;46:843–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma S, Zhang Y, Nie J, et al. BMSC-Derived Exosomal CircHIPK3 Promotes Osteogenic Differentiation of MC3T3-E1 Cells via Mitophagy. Int J Mol Sci. 2023;24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fazaeli H, Kalhor N, Naserpour L, et al. A Comparative Study on the Effect of Exosomes Secreted by Mesenchymal Stem Cells Derived from Adipose and Bone Marrow Tissues in the Treatment of Osteoarthritis-Induced Mouse Model. BioMed Research International. 2021;2021:9688138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yaghoubi Y, Movassaghpour A, Mehdizadeh A, et al. Human umbilical cord mesenchymal stem cells derived-exosomes in diseases treatment. Life Sci. 2019;233:116733. [DOI] [PubMed] [Google Scholar]
- 53.Wu ZB, Han P, Sun X, et al. HucMSC exosome-delivered 14-3-3ζ alleviates ultraviolet radiation-induced photodamage via SIRT1 pathway modulation. Aging. 2021;13:11542–11563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li D, Gupta P. Sgaglione.D.A. Grande. Exosomes Derived from Non-Classic Sources for Treatment of Post-Traumatic Osteoarthritis and Cartilage Injury of the Knee: in Vivo Review. J Clin Med. 2021;10:2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang X, Jiang Y, Huang Q, et al. Exosomes derived from adipose-derived stem cells overexpressing glyoxalase-1 protect endothelial cells and enhance angiogenesis in type 2 diabetic mice with limb ischemia. Stem Cell Research & Therapy. 2021;12:403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shi JY, Hu R, Lian W, et al. Exosomes derived from mmu_circ_0000250-modified adipose-derived mesenchymal stem cells promote wound healing in diabetic mice by inducing miR-128-3p/SIRT1-mediated autophagy. Am J Physiol Cell Physiol. 2020;318:C848–C856. [DOI] [PubMed] [Google Scholar]
- 57.Hu J, Jiang Y, Wu X, et al. Exosomal miR-17-5p from adipose-derived mesenchymal stem cells inhibits abdominal aortic aneurysm by suppressing TXNIP-NLRP3 inflammasome. Stem Cell Research & Therapy. 2022;13:349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Deng S, Zhou X, Ge Z, et al. Exosomes from adipose-derived mesenchymal stem cells ameliorate cardiac damage after myocardial infarction by activating S1P/SK1/S1PR1 signaling and promoting macrophage M2 polarization. Int J Biochem Cell Biol. 2019;114:105564. [DOI] [PubMed] [Google Scholar]
- 59.Wu B, Feng J, Guo J, et al. ADSCs-derived exosomes ameliorate hepatic fibrosis by suppressing stellate cell activation and remodeling hepatocellular glutamine synthetase-mediated glutamine and ammonia homeostasis. Stem Cell Research & Therapy. 2022;13:494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li Q, Wang Z, Xing H, Wang Y, Guo Y. Exosomes derived from miR-188-3p-modified adipose-derived mesenchymal stem cells protect Parkinson’s disease. Mol Ther Nucleic Acids. 2021;23:1334–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu JM, Ji H, Zhang M, Liu W, Wang A. Hypoxic pretreatment of adipose-derived stem cell exosomes improved cognition by delivery of circ-Epc1 and shifting microglial M1/M2 polarization in an Alzheimer’s disease mice model. Aging. 2022;14:3070–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hu RJ, Chen XC, Xu L, et al. MiR-181a-5p Delivered by Adipose-Derived Mesenchymal Stem Cell Exosomes Alleviates Klebsiella pneumonia Infection-Induced Lung Injury by Targeting STAT3 Signaling. Mediators Inflamm. 2022;2022:5188895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yanez-Mo M, Siljander PR, Andreu Z, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li Q, Yu H, Sun M, et al. The tissue origin effect of extracellular vesicles on cartilage and bone regeneration. Acta Biomater. 2021;125:253–266. [DOI] [PubMed] [Google Scholar]
- 65.Kang Y, Xu C, Meng L, et al. Exosome-functionalized magnesium-organic framework-based scaffolds with osteogenic, angiogenic and anti-inflammatory properties for accelerated bone regeneration. Bioactive Materials. 2022;18:26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu NYY. The Role of the Immune Microenvironment in Bone Regeneration. Int J Med Sci. 2021;18:3697–3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang X, Yu F, Ye L. Epigenetic control of mesenchymal stem cells orchestrates bone regeneration. Front Endocrinol (Lausanne). 2023;14:1126787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schindeler A, McDonald MM, Bokko P, Little DG. Bone remodeling during fracture repair: the cellular picture. Semin Cell Dev Biol. 2008;19:459–466. [DOI] [PubMed] [Google Scholar]
- 69.Partridge JASNC. Physiological Bone Remodeling: systemic Regulation and Growth Factor Involvement. Physiology (Bethesda). 2016;31:233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Majidinia M, Sadeghpour A, Yousefi B. The roles of signaling pathways in bone repair and regeneration. J Cell Physiol. 2018;233:2937–2948. [DOI] [PubMed] [Google Scholar]
- 71.Gruber J, Yee Z, Tolwinski NS. Developmental Drift and the Role of Wnt Signaling in Aging. Cancers (Basel). 2016;8:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Krishnan V, Bryant HU, Macdougald OA. Regulation of bone mass by Wnt signaling. J Clin Invest. 2006;116:1202–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang D, Xiao W, Liu C, et al. Exosomes Derived from Adipose Stem Cells Enhance Bone Fracture Healing via the Activation of the Wnt3a/beta-Catenin Signaling Pathway in Rats with Type 2 Diabetes Mellitus. Int J Mol Sci. 2023;24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lu Z, Chen Y, Dunstan C, Roohani-Esfahani S, Zreiqat H. Priming Adipose Stem Cells with Tumor Necrosis Factor-Alpha Preconditioning Potentiates Their Exosome Efficacy for Bone Regeneration. Tissue Eng Part A. 2017;23:1212–1220. [DOI] [PubMed] [Google Scholar]
- 75.Walmsley GG, Ransom RC, Zielins ER, et al. Stem Cells in Bone Regeneration. Stem Cell Reviews and Reports. 2016;12:524–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Qin Y, Sun R, Wu C, Wang L, Zhang C. Exosome: a Novel Approach to Stimulate Bone Regeneration through Regulation of Osteogenesis and Angiogenesis. Int J Mol Sci. 2016;17:712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dong X, Shen LH, Yi Z, He LH, Yi Z. Exosomes from Adipose-Derived Stem Cells Can Prevent Medication-Related Osteonecrosis of the Jaw. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research. 2021;27:e929684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nan K, Zhang Y, Li D, et al. Exosomes from miRNA-378-modified adipose-derived stem cells prevent glucocorticoid-induced osteonecrosis of the femoral head by enhancing angiogenesis and osteogenesis via targeting miR-378 negatively regulated suppressor of fused (Sufu). Stem Cell Research & Therapy. 2021;12:331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gerber VT, HP RAM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999;5:623–628. [DOI] [PubMed] [Google Scholar]
- 80.Al-Dujaili SA, Lau E, Al-Dujaili H, et al. Apoptotic osteocytes regulate osteoclast precursor recruitment and differentiation in vitro. J Cell Biochem. 2011;112:2412–2423. [DOI] [PubMed] [Google Scholar]
- 81.Kobayashi K, Nojiri H, Saita Y, et al. Mitochondrial superoxide in osteocytes perturbs canalicular networks in the setting of age-related osteoporosis. Scientific Reports. 2015;5:9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ren L, Song ZJ, Cai QW, et al. Adipose mesenchymal stem cell-derived exosomes ameliorate hypoxia/serum deprivation-induced osteocyte apoptosis and osteocyte-mediated osteoclastogenesis in vitro. Biochem Biophys Res Commun. 2019;508:138–144. [DOI] [PubMed] [Google Scholar]
- 83.Zhu LT, Hu CT, Zou YH. Exosomes secreted by mice adipose-derived stem cells after low-level laser irradiation treatment reduce apoptosis of osteocyte induced by hypoxia. European Review for Medical and Pharmacological Sciences. 2017;21:5562–5570. [DOI] [PubMed] [Google Scholar]
- 84.Tang D, Tare RS, Yang LY, et al. Biofabrication of bone tissue: approaches, challenges and translation for bone regeneration. Biomaterials. 2016;83:363–382. [DOI] [PubMed] [Google Scholar]
- 85.Storti SM, G KBS, Orlandi A, Cervelli V. Adipose-Derived Stem Cells in Bone Tissue Engineering: useful Tools with New Applications. Stem Cells International. 2019;2019:3673857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen S, Tang Y, Zhang P, et al. Exosomes derived from miR-375-overexpressing human adipose mesenchymal stem cells promote bone regeneration. Cell Proliferation. 2019;52:e12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li R, Wang H, Chen K, et al. Exosomes from adipose-derived stem cells regulate M1/M2 macrophage phenotypic polarization to promote bone healing via miR-451a/MIF. Stem Cell Research & Therapy. 2022;13:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li G, Zhang Y, Wu J, et al. Adipose stem cells-derived exosomes modified gelatin sponge promotes bone regeneration. Frontiers in Bioengineering and Biotechnology. 2023;11:1096390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jiang Y, Zhao J, Wang M, et al. Mesenchymal stem cell-derived exosomes can alleviate GVHD and preserve the GVL effect in allogeneic stem cell transplantation animal models. Frontiers in Immunology. 2023;14:1284936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zheng Q, Zhang S, Guo W-Z, Li X-K. The Unique Immunomodulatory Properties of MSC-Derived Exosomes in Organ Transplantation. Frontiers in Immunology. 2021;12:659621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lane RL, Khan SN JM. Osteoporosis. Clin Orthop Relat Res. 2000;372:139–150. [DOI] [PubMed] [Google Scholar]
- 92.Genant CC, Poor HK, Reid G, Ehrlich I, G EA. Interim report and recommendations of the World Health Organization Task-Force for Osteoporosis. Osteoporos Int. 1999;10:259–264. [DOI] [PubMed] [Google Scholar]
- 93.Szulc REP. Use of bone turnover markers in postmenopausal osteoporosis. Lancet Diabetes Endocrinol. 2017;5:908–923. [DOI] [PubMed] [Google Scholar]
- 94.Wang SZ, Jia J. lncRNA-KCNQ1OT1: a Potential Target in Exosomes Derived from Adipose-Derived Stem Cells for the Treatment of Osteoporosis. Stem Cells International. 2021;2021:7690006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Du W, Su L, Zhang N, Wang H. Exosomes derived from preadipocytes improve osteogenic differentiation, potentially via reduced miR‑223 expression. Molecular Medicine Reports. 2019;19:951–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yu B, Huo L, Liu Y, et al. PGC-1alpha Controls Skeletal Stem Cell Fate and Bone-Fat Balance in Osteoporosis and Skeletal Aging by Inducing TAZ. Cell Stem Cell. 2018;23:193–209e195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yang S, Guo S, Tong S, Sun X. Exosomal miR-130a-3p regulates osteogenic differentiation of Human Adipose-Derived stem cells through mediating SIRT7/Wnt/beta-catenin axis. Cell Proliferation. 2020;53:e12890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang L, Pan Y, Liu M, et al. Wen-Shen-Tong-Luo-Zhi-Tong Decoction regulates bone-fat balance in osteoporosis by adipocyte-derived exosomes. Pharm Biol. 2023;61:568–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Napoli N, Chandran M, Pierroz DD, et al. Mechanisms of diabetes mellitus-induced bone fragility. Nat Rev Endocrinol. 2017;13:208–219. [DOI] [PubMed] [Google Scholar]
- 100.Zhang L, Wang Q, Su H. Exosomes from adipose derived mesenchymal stem cells alleviate diabetic osteoporosis in rats through suppressing NLRP3 inflammasome activation in osteoclasts. Journal of bioscience and Bioengineering. 2021;131:671–678. [DOI] [PubMed] [Google Scholar]
- 101.Zhang L, Wang Q, Su H, et al. Exosomes from Adipose Tissues Derived Mesenchymal Stem Cells Overexpressing MicroRNA-146a Alleviate Diabetic Osteoporosis in Rats. Cellular and Molecular Bioengineering. 2022;15:87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Greene GW, Banquy X, Lee DW, et al. Adaptive mechanically controlled lubrication mechanism found in articular joints. Proceedings of the National Academy of Sciences. 2011; 108: 5255–5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Armiento AR, Alini M, Stoddart MJ. Articular fibrocartilage - Why does hyaline cartilage fail to repair? Adv Drug Deliv Rev. 2019;146:289–305. [DOI] [PubMed] [Google Scholar]
- 104.Richardson AMBJB. Articular cartilage: structure, injuries and review of management. Br Med Bull. 2008;87:77–95. [DOI] [PubMed] [Google Scholar]
- 105.Archer CW FWP. The chondrocyte. Int J Biochem Cell Biol. 2003;35:401–404. [DOI] [PubMed] [Google Scholar]
- 106.Zhang MJ, Han R, Zhang J. Mesenchymal stem cell related therapies for cartilage lesions and osteoarthritis. American Journal of Translational Research. 2019;11:6275–6289. [PMC free article] [PubMed] [Google Scholar]
- 107.Wang FA, Lu Y, Pan L, et al. Exosome modification to better alleviates endoplasmic reticulum stress induced chondrocyte apoptosis and osteoarthritis. Biochem Pharmacol. 2022;206:115343. [DOI] [PubMed] [Google Scholar]
- 108.Xie A, Xue J, Wang Y, et al. Kartogenin Induced Adipose-Derived Stem Cell Exosomes Enhance the Chondrogenic Differentiation Ability of Adipose-Derived Stem Cells. Dis Markers. 2022;2022:6943630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen J, Huang T, Liu R, et al. Congenital microtia patients: the genetically engineered exosomes released from porous gelatin methacryloyl hydrogel for downstream small RNA profiling, functional modulation of microtia chondrocytes and tissue-engineered ear cartilage regeneration. J Nanobiotechnology. 2022;20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang Z, Huang G, Mao G, Hu S. Characterization of exosomal long non-coding RNAs in chondrogenic differentiation of human adipose-derived stem cells. Mol Cell Biochem. 2021;476:1411–1420. [DOI] [PubMed] [Google Scholar]
- 111.Glyn-Jones S, Palmer AJ, Agricola R, et al. Osteoarthritis. Lancet. 2015;386:376–387. [DOI] [PubMed] [Google Scholar]
- 112.Woolf AD PB. Burden of major musculoskeletal conditions. Bull World Health Organ. 2003;81:646–656. [PMC free article] [PubMed] [Google Scholar]
- 113.Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64:1697–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vargas Negrin F, Medina Abellan MD, Hermosa Hernan JC, de Felipe Medina R. Treatment of patients with osteoarthritis. Aten Primaria. 2014;46(1):39–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kwon DG, Kim MK, Jeon YS, et al. State of the Art: the Immunomodulatory Role of MSCs for Osteoarthritis. Int J Mol Sci. 2022;23:1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Molnar V, Pavelic E, Vrdoljak K, et al. Mesenchymal Stem Cell Mechanisms of Action and Clinical Effects in Osteoarthritis: a Narrative Review. Genes (Basel). 2022;13:949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li CY, Wu XY, Tong JB, et al. Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. Stem Cell Research & Therapy. 2015;6:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li Y, Duan J, Lin W, Liu J. Exosomal miR-93-5p regulated the progression of osteoarthritis by targeting ADAMTS9. Open Med (Wars). 2023;18:20230668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li C, Li W, Pu G, Wu J, Qin F. Exosomes derived from miR-338-3p-modified adipose stem cells inhibited inflammation injury of chondrocytes via targeting RUNX2 in osteoarthritis. Journal of Orthopaedic Surgery and Research. 2022;17:567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li F, Xu Z, Sun X, et al. Adipose mesenchymal stem cells-derived exosomes alleviate osteoarthritis by transporting microRNA −376c-3p and targeting the WNT-beta-catenin signaling axis. Apoptosis. 2023;28:362–378. [DOI] [PubMed] [Google Scholar]
- 121.Meng S, Tang C, Deng M, et al. Tropoelastin-Pretreated Exosomes from Adipose-Derived Stem Cells Improve the Synthesis of Cartilage Matrix and Alleviate Osteoarthritis. J Funct Biomater. 2023;14:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Xu Y, Wang Q, Xiang X-N, et al. The Effect of Different Frequencies of Pulsed Electromagnetic Fields on Cartilage Repair of Adipose Mesenchymal Stem Cell–Derived Exosomes in Osteoarthritis. Cartilage. 2022;13:200–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wu J, Kuang L, Chen C, et al. miR-100-5p-abundant exosomes derived from infrapatellar fat pad MSCs protect articular cartilage and ameliorate gait abnormalities via inhibition of mTOR in osteoarthritis. Biomaterials. 2019;206:87–100. [DOI] [PubMed] [Google Scholar]
- 124.Kos N, Gradisnik L, Velnar T. A Brief Review of the Degenerative Intervertebral Disc Disease. Med Arch. 2019;73:421–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vergroesen PP, Kingma I, Emanuel KS, et al. Mechanics and biology in intervertebral disc degeneration: a vicious circle. Osteoarthritis Cartilage. 2015;23:1057–1070. [DOI] [PubMed] [Google Scholar]
- 126.Shapiro MVRIM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol. 2014;10:44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lu K, Li HY, Yang K, et al. Exosomes as potential alternatives to stem cell therapy for intervertebral disc degeneration: in-vitro study on exosomes in interaction of nucleus pulposus cells and bone marrow mesenchymal stem cells. Stem Cell Research & Therapy. 2017;8:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xia Y, Yang R, Hou Y, et al. Application of mesenchymal stem cell-derived exosomes from different sources in intervertebral disc degeneration. Frontiers in Bioengineering and Biotechnology. 2022;10:1019437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang Z, Yang J, Huang J, et al. Influence of extracellular nanovesicles derived from adipose-derived stem cells on nucleus pulposus cell from patients with intervertebral disc degeneration. Exp Ther Med. 2021;22:1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang J, Zhang Y, Liu W, et al. Mesenchymal stem cells-derived exosomes ameliorate intervertebral disc degeneration through inhibiting pyroptosis. J Cell Mol Med. 2020;24:11742–11754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen D, Jiang X, Zou H. hASCs-derived exosomal miR-155-5p targeting TGFbetaR2 promotes autophagy and reduces pyroptosis to alleviate intervertebral disc degeneration. J Orthop Translat. 2023;39:163–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lin TW, Cardenas L, Soslowsky LJ. Biomechanics of tendon injury and repair. J Biomech. 2004;37:865–877. [DOI] [PubMed] [Google Scholar]
- 133.Millar NL, Silbernagel KG, Thorborg K, et al. Tendinopathy. Nat Rev Dis Primers. 2021;7:1. [DOI] [PubMed] [Google Scholar]
- 134.Sharma P MN. Tendon injury and tendinopathy: healing and repair. J Bone Joint Surg Am. 2005;87:187–202. [DOI] [PubMed] [Google Scholar]
- 135.Liang X, Zhang L, Wang S, Han Q, Zhao RC. Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. J Cell Sci. 2016;129:2182–2189. [DOI] [PubMed] [Google Scholar]
- 136.Wang C, Zhang Y, Zhang G, Yu W, He Y. Adipose Stem Cell-Derived Exosomes Ameliorate Chronic Rotator Cuff Tendinopathy by Regulating Macrophage Polarization: from a Mouse Model to a Study in Human Tissue. Am J Sports Med. 2021;49:2321–2331. [DOI] [PubMed] [Google Scholar]
- 137.Mikolyzk DK, Wei AS, Tonino P, et al. Effect of corticosteroids on the biomechanical strength of rat rotator cuff tendon. J Bone Joint Surg Am. 2009;91:1172–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li A, Han K, Zhang X, et al. Anti-inflammatory and Tendon-Protective Effects of Adipose Stem Cell-Derived Exosomes with Concomitant Use of Glucocorticoids. Stem Cells International. 2022;2022:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu H, Zhang M, Zhang T, et al. Adipose-derived mesenchymal stromal cell-derived exosomes promote tendon healing by activating both SMAD1/5/9 and SMAD2/3. Stem Cell Research & Therapy. 2021;12:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fu LL, Pan G, Fan Z, Yin F A. Adipose-derived stem cell exosomes facilitate rotator cuff repair by mediating tendon-derived stem cells. Regen Med. 2021;16:359–372. [DOI] [PubMed] [Google Scholar]
- 141.Nichols AEC, Best KT, Loiselle AE. The cellular basis of fibrotic tendon healing: challenges and opportunities. Transl Res. 2019;209:156–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhang X, Cai Z, Wu M, et al. Adipose Stem Cell-Derived Exosomes Recover Impaired Matrix Metabolism of Torn Human Rotator Cuff Tendons by Maintaining Tissue Homeostasis. Am J Sports Med. 2021;49:899–908. [DOI] [PubMed] [Google Scholar]
- 143.Thankam FG, Chandra I, Diaz C, et al. Matrix regeneration proteins in the hypoxia-triggered exosomes of shoulder tenocytes and adipose-derived mesenchymal stem cells. Mol Cell Biochem. 2020;465:75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kuzel BR, Grindel S, Papandrea R, Ziegler D. Fatty infiltration and rotator cuff atrophy. J Am Acad Orthop Surg. 2013;21:613–623. [DOI] [PubMed] [Google Scholar]
- 145.Zhu A, Liu N, Shang Y, Zhen Y, An Y. Signaling pathways of adipose stem cell-derived exosomes promoting muscle regeneration. Chin Med J (Engl). 2022;135:2525–2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang C, Song W, Chen B, Liu X, He Y. Exosomes Isolated From Adipose-Derived Stem Cells: a New Cell-Free Approach to Prevent the Muscle Degeneration Associated With Torn Rotator Cuffs. Am J Sports Med. 2019;47:3247–3255. [DOI] [PubMed] [Google Scholar]
- 147.Wang C, Hu Q, Song W, Yu W, He Y. Adipose Stem Cell-Derived Exosomes Decrease Fatty Infiltration and Enhance Rotator Cuff Healing in a Rabbit Model of Chronic Tears. Am J Sports Med. 2020;48:1456–1464. [DOI] [PubMed] [Google Scholar]
- 148.Jafari D, Shajari S, Jafari R, et al. Designer Exosomes: a New Platform for Biotechnology Therapeutics. BioDrugs. 2020;34:567–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Xu J, Ye Z, Han K, et al. Infrapatellar Fat Pad Mesenchymal Stromal Cell-Derived Exosomes Accelerate Tendon-Bone Healing and Intra-articular Graft Remodeling After Anterior Cruciate Ligament Reconstruction. Am J Sports Med. 2022;50:662–673. [DOI] [PubMed] [Google Scholar]
- 150.Seo Y, Shin TH, Kim HS. Current Strategies to Enhance Adipose Stem Cell Function: an Update. Int J Mol Sci. 2019;20:3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rosochowicz MA, Lach MS, Richter M, Suchorska WM. Trzeciak. Conditioned Medium - Is it an Undervalued Lab Waste with the Potential for Osteoarthritis Management? Stem Cell Reviews and Reports. 2023;19:1185–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chen Y, Zhu Q, Cheng L, et al. Exosome detection via the ultrafast-isolation system: EXODUS. Nature Methods. 2021;18:212–218. [DOI] [PubMed] [Google Scholar]