Abstract
Purpose
Intermittent hypoxia (IH), a defining feature of obstructive sleep apnea (OSA), is associated with heart damage and linked to transient receptor potential canonical channel 5 (TRPC5). Nonetheless, the function of TRPC5 in OSA-induced cardiac injury remains uncertain. For this research, we aimed to explore the role and potential mechanism of TRPC5 in cardiomyocyte injury induced by intermittent hypoxia.
Methods
30 patients with newly diagnosed OSA and 30 patients with primary snoring(PS) were included in this study. Participants were subjected to polysomnography (PSG) for OSA diagnosis. Echocardiography was used to evaluate the structure and function of the heart, while peripheral blood samples were obtained. Additionally, RT-qPCR was utilized to quantify the relative expression level of TRPC5 mRNA in peripheral blood. H9c2 cells experienced IH or normoxia. TRPC5 levels in H9c2 cells were determined via RT-qPCR and Western blotting (WB) methods. H9c2 cells overexpressing TRPC5 were subjected to either normoxic or intermittent hypoxia conditions. Cell viability was determined by CCK8, the apoptosis rate, reactive oxygen species(ROS) levels, and Ca2+ concentration were assessed by flow cytometry, and the protein levels of TRPC5, Bcl-2, Bax, and Caspase-3 were analyzed by WB. Mitochondrial membrane potential(MMP), mitochondrial membrane permeability transition pore(mPTP), and transmission electron microscopy(TEM) were employed to observe mitochondrial function and structure. After inhibiting ROS with N-acetylcysteine (NAC), apoptosis, mitochondrial function and structure, and the concentration of Ca2+ were further detected.
Results
TRPC5 and left atrial diameter (LAD) were higher in OSA individuals, while the E/A ratio was lower(all P<0.05). IH impaired cell viability, triggered cell apoptosis, and enhanced TRPC5 expression in H9c2 cells(all P<0.05). The effects of IH on apoptosis, cell viability, mitochondrial function and structure damage, and oxidative stress (OxS) in H9c2 cells were accelerated by the overexpression of TRPC5(all P<0.05). Furthermore, cell apoptosis and mitochondrial structural and functional damage caused by overexpression of TRPC5 were attenuated by ROS inhibition.
Conclusion
TRPC5 is associated with structural and functional cardiac damage in patients with OSA, and TRPC5 promotes IH-induced apoptosis and mitochondrial damage in cardiomyocytes through OxS. TRPC5 may be a novel target for the diagnosis and treatment of OSA-induced myocardial injury.
Keywords: TRPC5, oxidative stress, apoptosis, intermittent hypoxia, OSA
Introduction
Obstructive sleep apnea(OSA) involves repetitive upper airway obstruction, leading to interruptions in breathing, Intermittent hypoxia (IH), hypercapnia, or awakenings during sleep.1 It is estimated that there are approximately 936 million individuals aged 30–69 years with OSA worldwide.2 Studies have indicated that OSA is associated with heart-related complications, serving as a predictor for hypertension, coronary artery disease, heart failure, arrhythmia, and stroke.3 Continuous positive airway pressure ventilation (CPAP) therapy is currently the recommended treatment for OSA and is particularly suitable for patients with moderate-to-severe OSA.4 However, in some patients, the risk of cardiovascular events has not been reduced accordingly after CPAP therapy, despite improvement in intermittent hypoxia.5 Therefore, it is important to elucidate the relationship between OSA and myocardial injury and to identify possible molecular mechanisms.
IH is the most fundamental pathogenic factor of OSA.6 The major pathophysiological change in OSA is oxidative stress (OxS), induced by IH. It increases the reactive oxygen species (ROS) production and reduces antioxidant capacities,7,8 and has been reported to promote myocardial injury.9–11 Mitochondria are the main source of ROS12 and intracellular Ca2+ stores, playing an important contribution in regulating intracellular Ca2+ concentration.13 Ca2+ plays a crucial role as a key secondary messenger in cells, participating in apoptosis, autophagy, the cell cycle, and numerous other physiological processes.14 Furthermore, Ca2+ is an important regulator of ROS, generating ROS through its involvement in the tricarboxylic acid cycle and the electron transport chain.15 In turn, ROS affects intracellular Ca2+ signaling.16 Excessive accumulation of ROS, which can stimulate the activation of mitochondrial calcium channels, promotes calcium homeostasis imbalance.17
Calcium channels are important for the maintenance of intracellular calcium homeostasis. TRPC5, part of the TRPC family, is a Transient Receptor Potential (TRP) Canonical Channel. As a nonselective calcium cation channel, it is expressed in the brain, heart, and kidney,18 and is primarily involved in Ca2+ permeation, transporting extracellular free calcium into the cell. TRPC5 has been reported to be associated with myocardial injury and OxS. TRPC5 was elevated in myocardial tissue from patients with end-stage heart failure and positively correlated with myocardial injury.19 Patients with non-valvular atrial fibrillation have significantly increased TRPC5 gene expression.20 TRPC5 expression was upregulated in myocardial tissue from patients with end-stage idiopathic dilated cardiomyopathy.21 Inhibiting TRPC5 may impair metastasis of colon cancer cells by reducing the production of the oxidative stress product −4-Hydroxynonenal(4HNE).22 Inhibition of TRPC5 could attenuate oxidative stress and ameliorate cognitive dysfunction in a rat model of Parkinsonism.23 Our previous study showed that TRPC5 is associated with myocardial injury in a rat model of intermittent hypoxia.24 Still, the effect and the mechanism of TRPC5 in IH-induced myocardial injury remains uncertain.
Therefore, the aim of this study was to evaluate TRPC5 expression in peripheral blood mononuclear cells (PBMCs) from patients with OSA and its correlation with structural and functional cardiac damage. In addition, we investigated the potential mechanisms by which TRPC5 regulates myocardial injury in vitro, which provides new ideas for the diagnosis and treatment of myocardial injury caused by OSA.
Materials and Methods
Participants and Study Design
From February 2023 to April 2024, sixty individuals aged 18–75 years were recruited from the Health Examination Center of the First Affiliated Hospital of Xinjiang Medical University. Polysomnography (PSG) was employed for the diagnosis of OSA25 in accordance with the clinical practice guidelines of the American Sleep Medicine Association. Based on the apnea-hypopnea index (AHI), individuals were categorized into the primary snoring group (PS, AHI<5 events per hour) or the OSA group (AHI≥5 events per hour). Exclusion criteria included: (1) central or mixed sleep apnea; (2) pulmonary fibrosis, chronic obstructive pulmonary disease, bronchial asthma, and other unstable respiratory system diseases; (3) conditions that could impact both the function and structure of the heart, like heart valve disease, cardiomyopathy, congenital heart disease, and severe arrhythmia; (4) diabetes, hyperthyroidism, hypothyroidism; (5) severe liver and kidney dysfunction, chronic inflammation, chronic wasting diseases, and malignant tumors; (6) other factors like cognitive impairment, sequelae of cerebrovascular diseases, and insomnia. Finally, 30 participants were included in each group.
Clinical data collection included age, gender, drinking and smoking history, and body mass index (BMI). Participants were required to fast for a minimum of 8 hours before blood sample collection. The Roche C8000 biochemical analyzer was utilized to analyze blood samples, measuring creatinine, uric acid, triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) levels. Simultaneously, cardiac troponin I (cTN-I), N-terminal pro-B-type (NT-proBNP), and creatine kinase isoenzymes (CK-MB) were assessed.
The research adhered to the Helsinki Declaration guidelines and received ethical approval from the Ethics Committee of the First Affiliated Hospital of Xinjiang Medical University (ethical batch number: IACUC-20210326-08). All enrolled participants provided informed consent before participation.
Polysomnography (PSG)
All participants underwent at least a 4-hour PSG (Australia, Compumedics E-series). Monitoring indicators included blood oxygen saturation, chest and abdominal breathing movements, snoring, and oral and nasal airflow. After the monitoring, professional technicians conducted data analysis and recorded the AHI, oxygen desaturation index (ODI), average blood oxygen saturation (MSaO2), and lowest arterial oxygen saturation (LSaO2).
Collection of Peripheral Blood Specimens
To collect the blood, we used EDTA anticoagulant tubes. PBMCs were isolated from blood components using density gradient centrifugation. Through density gradient centrifugation, the PBMCs were separated from the blood components. Afterward, they were stored at −80 °C until being used.
Echocardiography
Expert ultrasound practitioners operated an American PHILIPS IE33 color Doppler ultrasonic diagnostic instrument while the individuals were positioned in the left supine posture. The echocardiogram was performed with the patient in a relaxed breathing state to observe the structure and morphology of each cardiac slice. Measurements followed the guidelines set by the American Society of Echocardiography,26 including left atrial diameter (LAD), left ventricular end-diastolic diameter (LVEDD), left ventricular end-systolic diameter (LVESD), left ventricular posterior wall thickness (LVPWT), interventricular septal thickness (IVST), right ventricular diameter (RVD), right atrial diameter (RAD), e’ peak, E peak, and A peak. Additionally, the E/A ratio and left ventricular ejection fraction (LVEF) were calculated.
Cell Culture and Treatment
Cells of the H9c2 line were acquired from Procell (CL-0089, Wuhan, China). In a 5% CO2 humidified atmosphere incubator at 37°C, the cells underwent culture in DMEM medium (Procell, PM150210, China), supplemented with 10% fetal bovine serum (Gibco, 10099–141, USA), 100 mg/mL streptomycin, and 100 U/mL penicillin (Gibco, 15140122, USA). Two hours before IH treatment, the cells were exposed to N-acetylcysteine (NAC, MCE, HY-B0215, USA) for the experiments. Stock solutions of NAC at 1M were made in appropriate solvents and refrigerated at 4°C. Subsequent working solutions were prepared through the dissolution of the stock solution in the culture medium, with an equal amount of NAC added to every well. The NAC final concentration was 6 mM.27,28
IH Treatment
Upon reaching 70–80% confluence, H9c2 cells underwent IH stimulation, following a previously described method29 with modifications. The cells were exposed to either IH (35 minutes at 1% O2 followed by 25 minutes at 21% O2, 1 cycle/hour) or Normoxia (21% O2 balanced in 5% CO2) for 24 hours.
RNA Isolation and Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
RNA extraction utilized the Trizol reagent (Invitrogen, 10296028, USA), and subsequent cDNA synthesis employed the SuperScript III RNA PCR kit (ABI-Invitrogen, 11752050, USA). RT-qPCR was conducted with SYBR™ Select mix (ABI-Invitrogen, 4472920, USA) employing the StepOne™ Real-time quantitative PCR system (Applied Biosystems, StepOne Software, USA). Gene expression quantification utilized the 2− ΔΔCT method. For the endogenous control, we used β-actin.
Cell Transfection
H9c2 cells were growing in a 6-well culture plate at a density of 105 cells per well, and transfection took place upon reaching a confluency of 50–60%. H9c2 cells were then introduced into another 6-well plate, 48 hours after the addition of TRPC5 lentivirus or a lentivirus vector-negative control. Afterward, fresh medium was added to replenish the cells. The efficiency of lentivirus transfection was confirmed by assessing green fluorescence, which was found to exceed 80%. qRT-PCR method was employed to validate the TRPC5 overexpression.
Cell Viability Assay
H9c2 cell viability was assessed using the CCK-8 kit (Beyotime, C0037, China). After seeding and culturing for 24 hours, the cells underwent incubation with CCK-8 solution at 37°C for 2 hours. Subsequently, we measured the optical density (OD) for each well at 450 nm.
Flow Cytometry Analysis
The H9c2 cell apoptotic rate was evaluated with the Annexin V-PE apoptosis Kit (Beyotime, C1062L, China). After IH or Normoxia treatment, 195 μL of binding buffer was added, followed by 5 μL of Annexin V tagged with FITC and 10 μL of PI. The mixture was then left to incubate in darkness at room temperature for 15 minutes. The early and late-stage percentages of apoptosis in the samples were determined using flow cytometry (BeamCyte-1026, BEAM DIAG). Within the flow cytometry scatter plot, Q3 indicated early apoptotic cells, whereas Q2 indicated late apoptotic cells.
Detection of Ca2+ and ROS
To measure intracellular Ca2+ concentration, the Fluo4-AM kit (Beyotime, S1061S, China) was employed. Following treatment, H9c2 cells were suspended in Fluo4-AM working solution at 37°C for 30 minutes in darkness. Using flow cytometry with excitation at 485 nm and emission at 530 nm (green), intracellular Ca2+ concentration was determined.
For the determination of ROS, the Reactive Oxygen Species Assay Kit (Beyotime, S003S, China) was utilized. Using flow cytometry, fluorescence intensity was monitored with excitation at 485 nm and emission at 530 nm.
Determination of Mitochondrial Membrane Potential (MMP)
In accordance with the manufacturer’s guidelines, alterations in MMP were assessed using a JC‐1 mitochondrial membrane potential assay kit (Beyotime, C2006, China). At 37°C, H9c2 cells (1×105) were treated with JC-1 staining buffer (10 mg/mL) for 20 minutes. Subsequently, the cells were rinsed with PBS and examined via flow cytometry. The proportion of aggregates, marked by red fluorescence representing the controlled MMP, compared to monomers, marked by green fluorescence indicating MMP loss, served as an indicator for unhealthy mitochondria.
Determination of Mitochondrial Membrane Permeability Transition Pore (mPTP)
Following the manufacturer’s guidelines, alterations in mPTP were assessed utilizing an MPTP kit (Beyotime, C2009S, China). H9c2 cells (1×105) were subjected to Calcein AM (1μL/mL) staining solution at 37°C for 30 minutes. Subsequently, the cells underwent PBS rinsing and underwent flow cytometry analysis.
Transmission Electron Microscopy
H9c2 cells were harvested following treatment with various groups, then rinsed with PBS prior to fixation in 3% glutaraldehyde overnight at 4°C. Following this, cells underwent fixation in 1% osmium tetroxide for a duration of 1 hour. Once embedded in Epon resin, the sections were stained using uranyl acetate and lead citrate. Ultimately, slides were observed using a transmission electron microscope (Hitachi-7800, Japan).
Western Blot (WB)
Proteins underwent extraction and separation via 10% SDS-PAGE electrophoresis, followed by transfer onto PVDF membranes. Subsequently, the membranes were subjected to incubation with respective primary and secondary antibodies. The antibodies utilized were: anti-TRPC5 (1:1000, Abcam, ab240872), anti-Bcl2 (1:1000, Abcam, ab19695), anti-Bax (1:1000, Abcam, ab32503), and anti-Caspase3 (1:1000, Affinity, AF6311). Goat Anti-Rabbit IgG H&L (1:4000, Abcam, ab205718) and Goat Anti-Mouse IgG H&L (1:3000, Abcam, ab205719) were employed. The Enhanced Chemiluminescence System (CLINX, ChemiScope6100) facilitated band visualization, while Image J software was employed to quantify band densities, normalized to β-actin.
Statistical Analysis
Data were analyzed using SPSS 25.0 software. Quantitative data that conformed to normal distribution was expressed as mean ± standard deviation, and an independent samples t-test was used for the comparison of the two groups. One-way ANOVA was used for the comparison of more than two groups, and the Bonferroni method was used for comparison between groups. Quantitative data not conforming to normal distribution was expressed as median (P25, P75), and a non-parametric test was used for comparison between groups. The chi-square test was used for qualitative data. Spearman’s test was used for correlation analysis of non-normally distributed data. A significance threshold of P < 0.05 was applied for statistical interpretation.
Results
Participant Characteristics
Table 1 provided a summary of the clinical and demographic features of the two patient groups. There were no significant differences between the two groups of age, gender, BMI, smoking history, drinking history, MaSO2, uric acid, creatinine, TG, HDL-C, NT-proBNP, CK-MB, cTnI, LVEDD, LVESD, IVST, LVPWT, RVD, E peak, A peak, E/e’ and LVEF. OSA patients had higher AHI, ODI, TC, LDL-C, LAD, and RAD, and lower LSaO2 and E/A ratio. The observed differences were statistically significant (all P<0.05).
Table 1.
Basic Information of Participants
| PS(n=30) | OSA(n=30) | t / χ2/Z | P value | |
|---|---|---|---|---|
| Age(years) | 56.53±10.83 | 58.37±8.79 | −0.720 | 0.474 |
| Male(n,%) | 20(66.67) | 23(76.67) | 0.739 | 0.390 |
| BMI(Kg/m2) | 25.21±4.11 | 26.36±2.66 | −1.278 | 0.206 |
| Smoking history(n,%) | 19(63.33) | 16(55.33) | 0.617 | 0.432 |
| Drinking history(n,%) | 20(66.67) | 19(63.33) | 0.073 | 0.787 |
| AHI(events/hour) | 1.30(0.68,2.73) | 19.99(14.40,36.02) | −6.645 | <0.001 |
| ODI(events/hour) | 3.31(0.81,7.29) | 15.88(8.50,24.37) | −4.828 | <0.001 |
| MSaO2(%) | 93.60(92.30,94.40) | 93.40(91.90,94.00) | −1.213 | 0.225 |
| LSaO2(%) | 84.50(81.80,90.00) | 80.50(74.80,85.00) | −2.873 | 0.004 |
| Uric acid (mmol/L) | 307.93±87.46 | 327.66±81.43 | −0.896 | 0.374 |
| Creatinine (mmol/L) | 63.68±14.69 | 69.07±12.68 | −1.590 | 0.117 |
| TC(mmol/L) | 3.37(2.78,3.79) | 3.86(3.18,4.52) | −2.403 | 0.016 |
| TG(mmol/L) | 1.27(0.92,1.53) | 1.47(1.04,2.07) | −1.804 | 0.071 |
| HDL-C(mmol/L) | 0.95(0.81,1.16) | 0.96(0.85,1.12) | −1.70 | 0.865 |
| LDL-C(mmol/L) | 2.45(1.66,2.68) | 2.66(2.24,3.19) | −2.499 | 0.012 |
| NT-proBNP(ng/L) | 58.70(20.00,151.00) | 81.95(25.70,284.25) | −1.247 | 0.212 |
| CK-MB(ng/mL) | 0.64(0.97,0.92) | 0.62(0.44,0.87) | −0.177 | 0.859 |
| cTnI(μg/L) | 0.009(0.010,0.012) | 0.010(0.009,0.012) | −0.683 | 0.494 |
| LAD(mm) | 33(32,37) | 37(35,39) | −3.197 | 0.001 |
| LVEDD(mm) | 48(46,51) | 49(48,50) | −0.693 | 0.488 |
| LVESD(mm) | 32(31,34) | 32(32,34) | −0.525 | 0.600 |
| IVST(mm) | 9(9,10) | 9(9,10) | −1.129 | 0.259 |
| LVPWT(mm) | 9(9,10) | 9(9,10) | −0.916 | 0.360 |
| RAD(mm) | 33(32,34) | 33(33,34) | −2.177 | 0.030 |
| RVD(mm) | 18(18,20) | 19(18,20) | −0.764 | 0.445 |
| E (m/s) | 0.75(0.61,0.84) | 0.68(0.56,0.83) | −0.771 | 0.441 |
| A (m/s) | 0.68(0.65,0.78) | 0.77(0.68,0.84) | −1.873 | 0.061 |
| E/A | 1.03(0.78,1.31) | 0.78(0.68,1.20) | −2.130 | 0.033 |
| E/e’ | 7.16(6.63,7.99) | 7.03(5.91,7.75) | −1.198 | 0.231 |
| LVEF(%) | 61.79(58.68,62.75) | 61.90(58.71,62.95) | −0.163 | 0.871 |
The Levels of TRPC5 mRNA Expression in the PBMCs of Participants
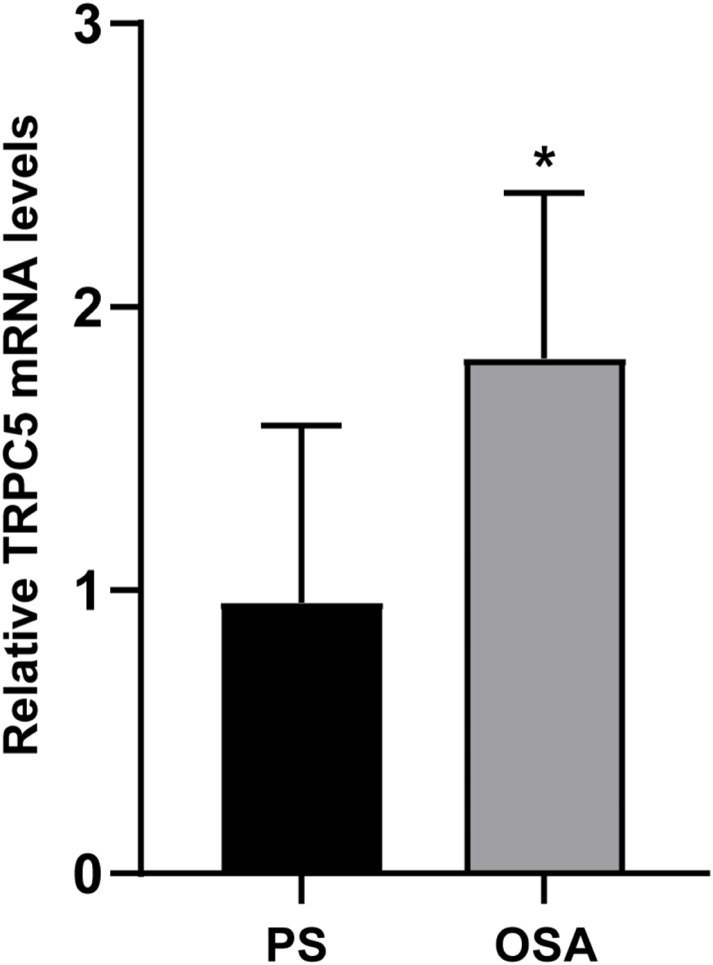
The relative levels of TRPC5 mRNA among participants are depicted in Figure 1. The findings indicated a notable rise in the relative TRPC5 mRNA expression among patients diagnosed with OSA (P<0.05).
Figure 1.
TRPC5 mRNA Levels in PBMCs of Participants. TRPC5 mRNA levels were identified through RT-qPCR analysis. Statistical significance (*P < 0.05) was observed compared to the PS group.
Association of TRPC5 mRNA Expression with Sleep and Echocardiography Parameters
Table 2 presented the relationship between TRPC5 and sleep features, along with echocardiography parameters, in individuals diagnosed with OSA. The findings indicated a positive correlation between the expression levels of TRPC5 mRNA and AHI (ρ = 0.476, P = 0.008), ODI (ρ = 0.415, P = 0.023), as well as LAD (ρ = 0.365, P = 0.045), and a negative correlation with E/A ratio (ρ = 0.0047, P = 0.042).
Table 2.
Association of TRPC5 mRNA Expression with Sleep and Echocardiography Parameters in OSA Individuals
| ρ | P value | |
|---|---|---|
| AHI(events/hour) | 0.476 | 0.008 |
| ODI(events/hour) | 0.415 | 0.023 |
| LSaO2(%) | −0.065 | 0.733 |
| LAD(mm) | 0.365 | 0.047 |
| RAD(mm) | 0.145 | 0.446 |
| E/A | −0.374 | 0.042 |
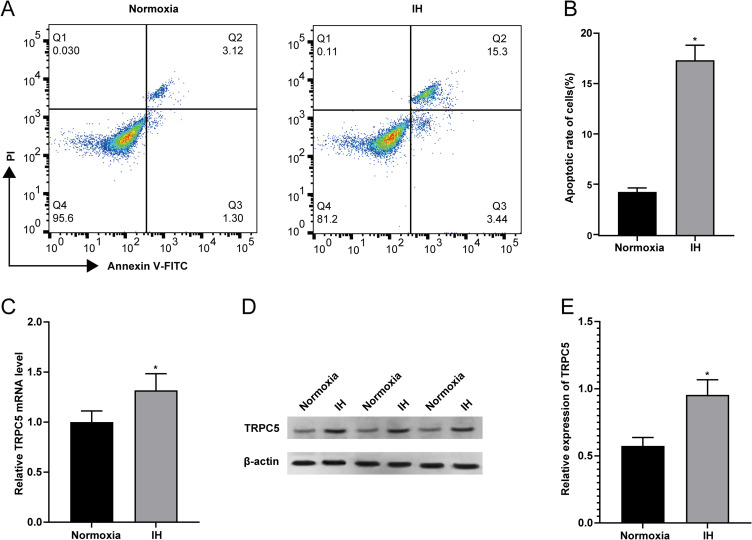
IH Induced Myocardial Injury and Enhanced the Expression of TRPC5 in H9c2 Cells
After exposure to IH, flow cytometry was employed to assess the apoptotic rate of H9c2 cells. The findings revealed a notable rise in the apoptotic rate of H9c2 cells following exposure to IH (Figure 2A–B)(P<0.05). Subsequent RT-qPCR and WB results (Figure 2C–E) showed an elevation in TRPC5 expression after IH treatment (all P<0.05), indicating a positive correlation between TRPC5 and IH-induced apoptosis of H9c2 cells.
Figure 2.
Impact of IH on Myocardial Injury and TRPC5 Levels in H9c2 Cells. (A–B) Flow cytometry was utilized to assess the apoptotic rate of H9c2 cells. In RT-qPCR (C) and WB (D–E) results, the difference in TRPC5 expression between IH and the Normoxia cohorts was observed. Statistical significance (*P < 0.05) compared to the Normoxia group.
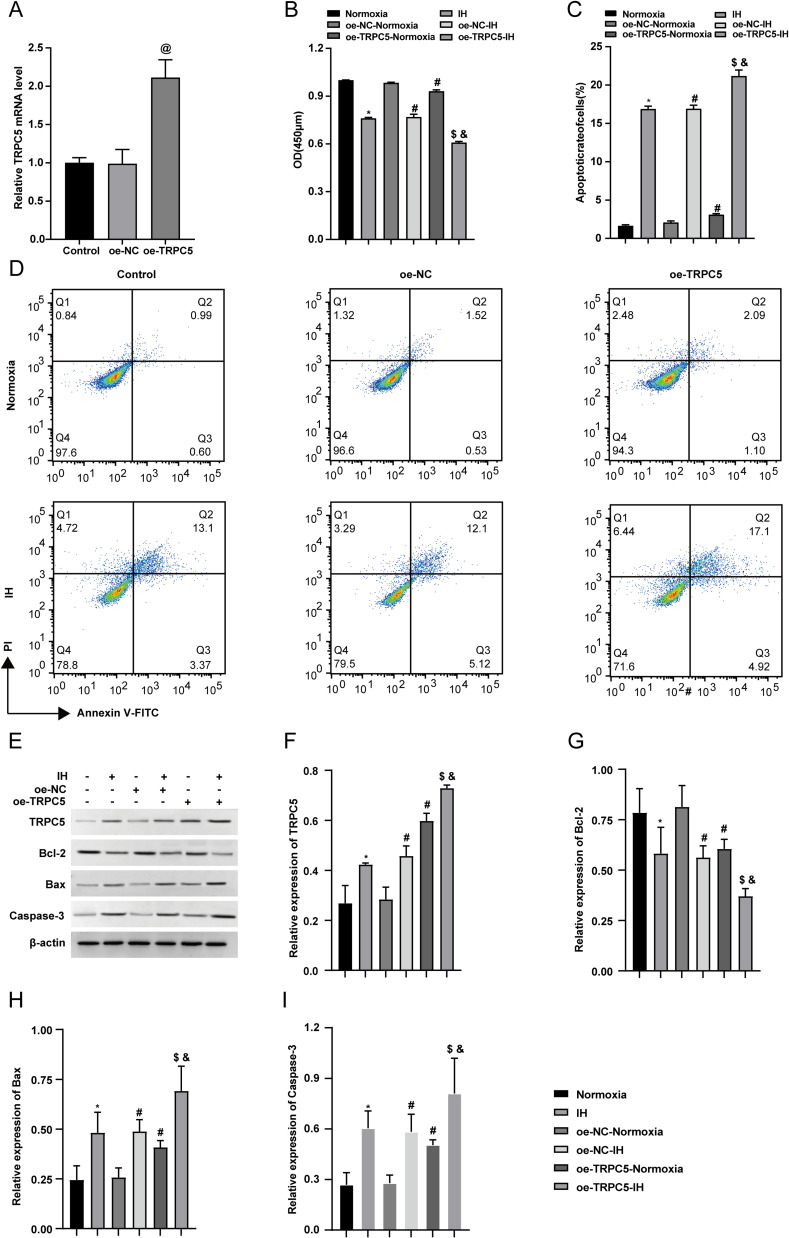
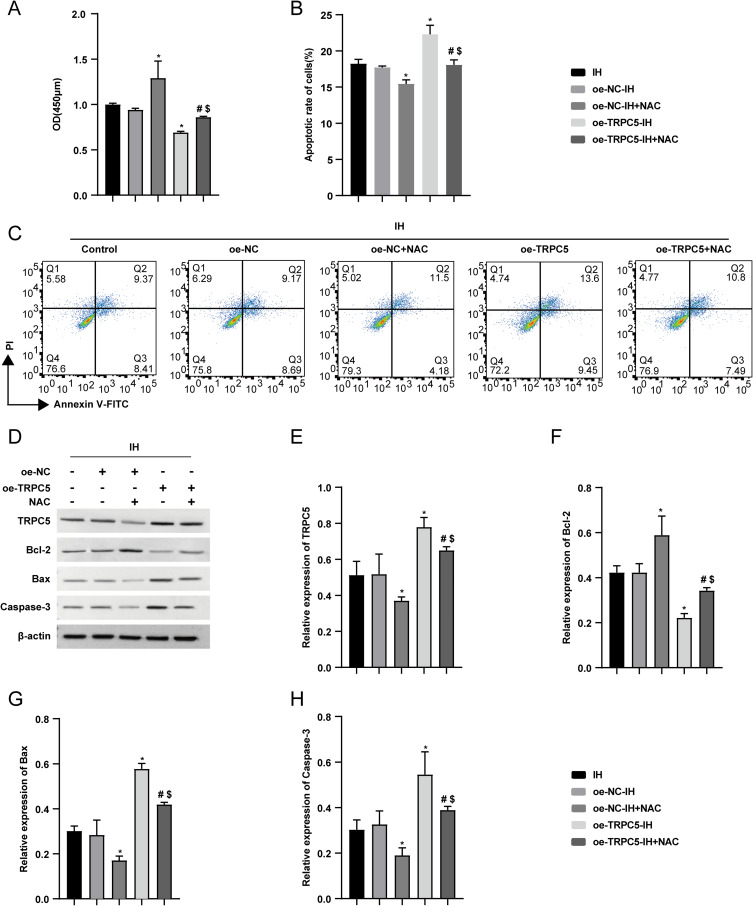
Overexpression of TRPC5 Promoted IH-Induced Apoptosis in H9c2 Cells
Since TRPC5 is lowly expressed in the heart,18 to examine its function in OSA, H9c2 cells were transfected with lentivirus to overexpress TRPC5. Post-transfection, RT-qPCR revealed a significant increase in TRPC5 mRNA expression in the oe-TRPC5 group (P<0.05), with no difference observed between the oe-NC and the Control groups (Figure 3A). Cell viability was assessed using CCK-8, which revealed that IH inhibited cell viability, and the inhibition was further enhanced by TRPC5 overexpression (Figure 3B) (P<0.05). Subsequent flow cytometry results showed that IH promoted apoptosis in H9c2 cells, which was exacerbated by TRPC5 overexpression (Figure 3C–D)(P<0.05). WB results demonstrated IH-induced upregulation of Bax and Caspase-3, and downregulation of Bcl-2, with TRPC5 overexpression exacerbating these changes (Figure 3E–I) (P<0.05). Collectively, these findings suggested that TRPC5 upregulation exacerbates IH-induced apoptosis in H9c2 cells.
Figure 3.
Overexpression of TRPC5 promoted IH-induced apoptosis in H9c2 cells. (A) The transduction efficiency was analyzed using RT-qPCR. (B) H9c2 cells viability were determined using the CCK-8 assay. (C–D) Through flow cytometry analysis, the apoptotic rate of H9c2 cells was measured. (E–I) WB results were employed to analyze the TRPC5, Bcl-2, Bax, and Caspase-3 levels. *P < 0.05 compared to the Normoxia group, #P < 0.05 compared to the the oe-NC-Normoxia group, $P < 0.05 compared to the oe-TRPC5-Normoxia group, &P < 0.05 compared to the the oe-NC-IH group, and @P < 0.05 compared to the control group.
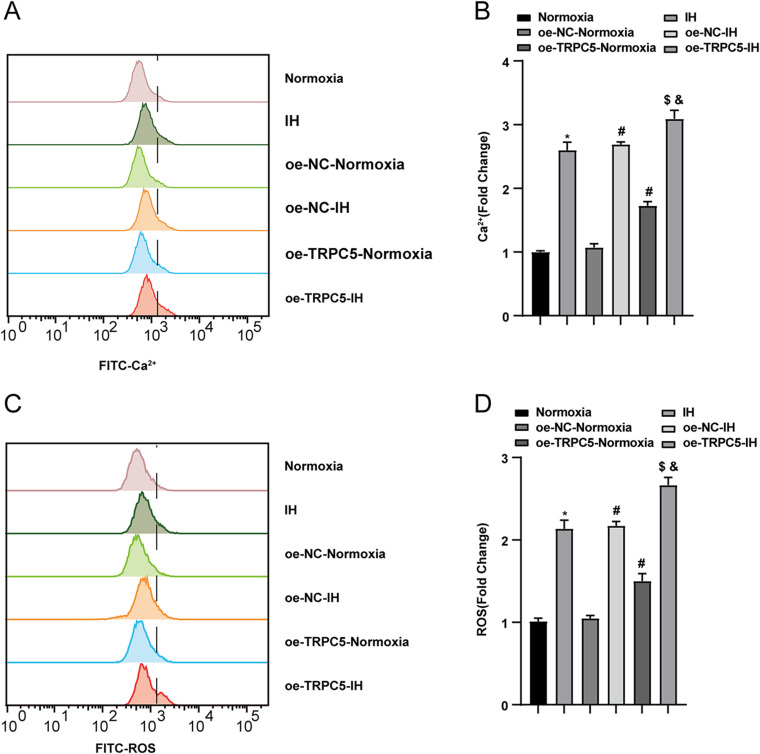
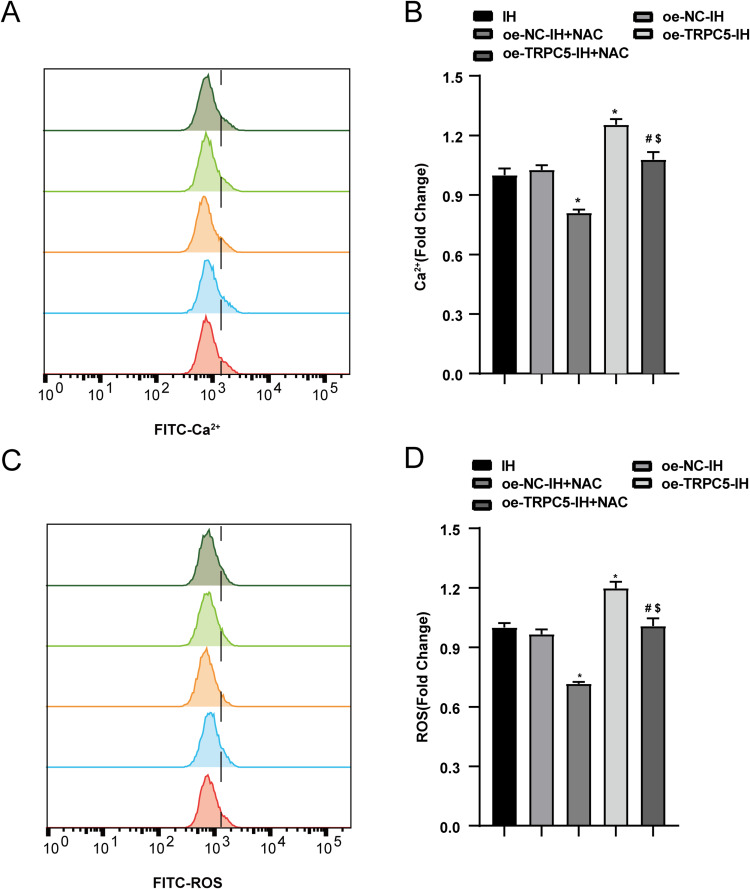
The Overexpression of TRPC5 Promoted IH-Induced Ca2+ Influx and the Oxidative Stress in H9c2 Cells
To explore the impact of TRPC5 on OxS, we assessed Ca2+ and ROS levels in H9C2 cells. Flow cytometry was used to measure intracellular Ca2+ and ROS levels in cells exposed to normoxia or IH conditions. The findings revealed that IH significantly elevated Ca2+ and ROS accumulation in H9c2 cells compared to the Normoxia group(P<0.05). Furthermore, TRPC5 overexpression further amplified Ca2+ levels (Figure 4A–B) and ROS levels (Figure 4C–D) in H9c2 cells (all P<0.05). These results collectively indicated that TRPC5 overexpression exacerbates cardiomyocyte injury by inducing intracellular Ca2+ overload and increasing ROS levels.
Figure 4.
Overexpression of TRPC5 promoted IH-induced Ca2+ influx and the OxS in H9c2 cells. (A–B) Flow cytometry was employed to test the concentration of Ca2+ and the production of ROS (C–D). *P < 0.05 compared to the Normoxia group, #P < 0.05 compared to the oe-NC-Normoxia group, $P < 0.05 compared to the oe-TRPC5-Normoxia group, &P < 0.05 compared to the the oe-NC-IH group.
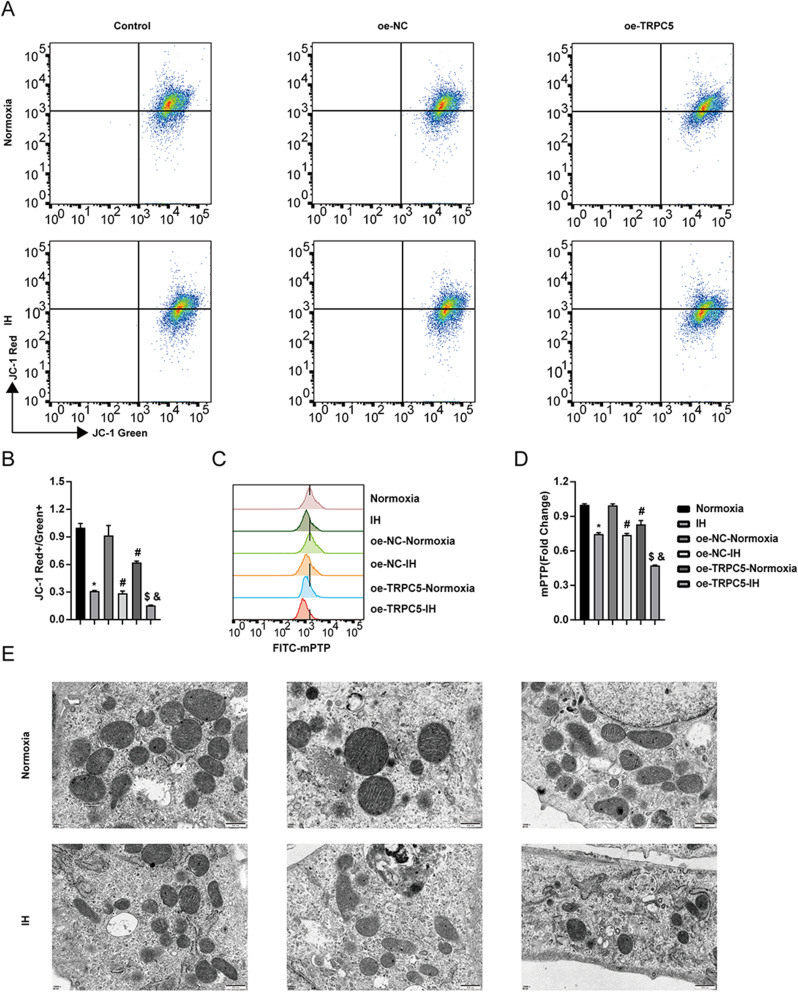
Overexpression of TRPC5 Promoted Mitochondrial Damage Induced by IH in H9c2 Cells
To investigate the impact of TRPC5 on mitochondrial structure and function, we examined the MMP, mPTP, and ultrastructural alterations of mitochondria. The findings indicated that IH induced a reduction in MMP and the opening of mPTP, with TRPC5 overexpression exacerbating these effects (Figure 5A–D) (all P<0.05). TEM results revealed that IH led to the loss of mitochondrial cristae, which was further intensified by TRPC5 overexpression (Figure 5E), suggesting that TRPC5 overexpression contributes to mitochondrial damage in H9c2 cells following IH.
Figure 5.
Overexpression of TRPC5 promoted mitochondrial damage induced by IH in H9c2 cells. Flow cytometry was used to measure the MMP (A–B) and mPTP (C–D) levels. (E) TEM images of mitochondria. *P < 0.05 compared to the Normoxia group, #P < 0.05 compared to the oe-NC-Normoxia group, $P < 0.05 compared to the oe-TRPC5-Normoxia group, &P < 0.05 compared to the the oe-NC-IH group.
NAC Alleviated the Damage to H9c2 Cells Caused by TRPC5 Overexpression Under IH
Since OxS is related to myocardial injury induced by IH, and TRPC5 is associated with OxS, we treated the H9c2 cells with NAC, a ROS inhibitor, to explore the mechanism of TRPC5 on cardiomyocyte injury during IH. The CCK8 assay revealed that NAC mitigated the decline in cell viability prompted by TRPC5 overexpression during IH (Figure 6A)(P<0.05). Flow cytometry assessed the apoptotic rate in each group, revealing NAC alleviating the apoptosis induced by TRPC5 overexpression under IH (Figure 6B–C)(P<0.05). Additionally, WB results revealed the expression of apoptosis-related proteins. The findings indicated NAC’s inhibition of the upregulation of Bax and Caspase-3, as well as the downregulation of Bcl-2 induced by TRPC5 overexpression following IH (Figure 6D–H)(all P<0.05). These results indicated the potential of NAC to alleviate H9c2 cell apoptosis resulting from TRPC5 overexpression during IH.
Figure 6.
NAC mitigated H9c2 cell apoptosis induced by TRPC5 overexpression during IH. (A) H9c2 cells viability were determined using the CCK-8 assay. Flow cytometry was used to assess the apoptotic rates of H9c2 cells, including representative figures (C) and analysis (B). (D–H) WB results analyzed relative expression levels of TRPC5, Bcl-2, Bax, and Caspase-3. *P < 0.05 compared to the oe-NC-IH group, #P < 0.05 compared to the oe-TRPC5-IH group, $P < 0.05 compared to the oe-NC-IH+NAC group.
Flow cytometry was utilized to measure intracellular Ca2+ and ROS levels. The results revealed that NAC inhibited the increase in Ca2+ (Figure 7A–B) and ROS levels (Figure 7C–D) induced by TRPC5 overexpression after IH(all P<0.05).
Figure 7.
NAC alleviated the Ca2+ influx in H9c2 cells caused by TRPC5 overexpression under IH. Flow cytometry was used to measure Ca2+ concentration(A–B) and ROS production (C–D). *P < 0.05 compared to the oe-NC-IH group, #P < 0.05 compared to versus the oe-TRPC5-IH group, $P < 0.05 compared to the oe-NC-IH+NAC group.
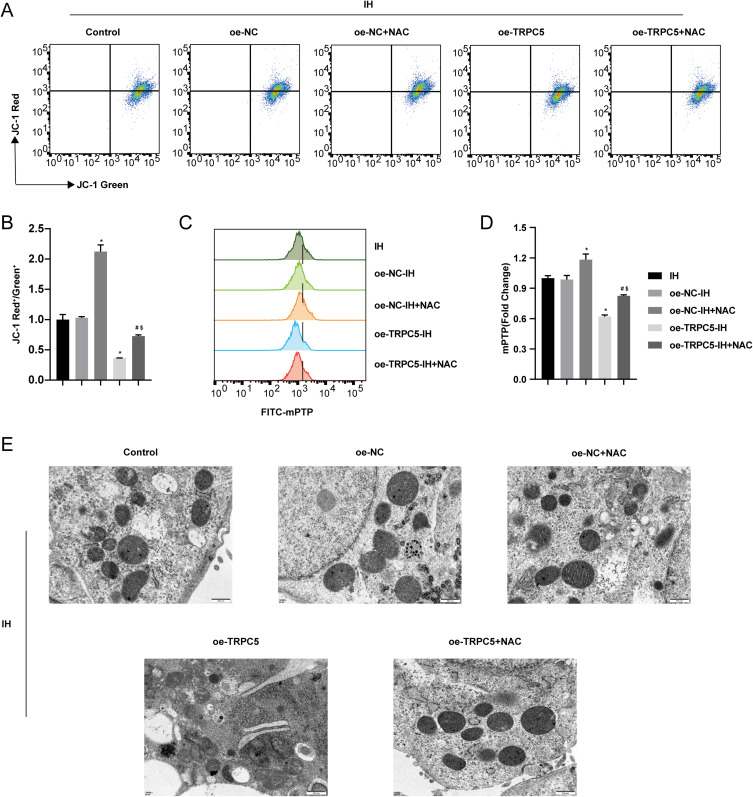
We also assessed the MMP, mPTP, and ultrastructural changes of mitochondria. The findings revealed that NAC mitigated the decrease in MMP (Figure 8A–B) and the opening of mPTP (Figure 8C–D) in H9c2 cells induced by TRPC5 overexpression after IH(all P<0.05). The TEM results showed that NAC could alleviate the disappearance of mitochondrial cristae caused by TRPC5 overexpression under IH (Figure 8E).
Figure 8.
NAC alleviated the mitochondrial damage in H9c2 cells caused by TRPC5 overexpression under IH. Flow cytometry assessed the MMP (A–B) and mPTP opening (C–D). (E) TEM images of mitochondria. *P < 0.05 compared to the oe-NC-IH group, #P < 0.05 compared to the oe-TRPC5-IH group, $P < 0.05 compared to the oe-NC-IH+NAC group.
All of the results indicated that inhibiting oxidative stress could alleviate the cardiomyocyte injury caused by TRPC5 overexpression under IH.
Discussion
Our study demonstrated that TRPC5 was upregulated and was related to structural and functional damage of the heart in OSA patients. It also showed that IH promoted cardiomyocyte apoptosis and upregulated TRPC5 expression. Overexpression of TRPC5 further promoted IH-induced cardiomyocyte apoptosis, disturbed Ca2+ metabolism, oxidative stress, and mitochondrial damage, whereas inhibition of oxidative stress partially reversed these changes.
Myocardial injury is a common complication of OSA, potentially leading to heart failure.30 OSA has been linked to myocardial injury and diastolic dysfunction, with its severity positively correlated with diastolic dysfunction.31 Additionally, left atrial volume is positively associated with OSA severity.32 Our study revealed a larger LAD and lower E/A ratio in OSA patients, indicating myocardial injury, consistent with previous research. However, the mechanism of myocardial injury in OSA remains unclear.
TRPC5, as a non-selective cation channel, primarily promotes Ca2+ influx through protein tyrosine kinases or G protein-coupled receptors and is important in the calcium signaling pathway.22 Studies have shown that TRPC5 is involved in myocardial injury. TRPC5 was found to be increased in the cardiac tissue of individuals suffering from end-stage heart failure.19 TRPC5 knockout attenuated cholinergic-induced tachycardia, myocardial fibrosis, and cardiac hypertrophy.33 Nevertheless, there has been no investigation into the correlation between TRPC5 and myocardial injury in OSA. Our research marks the inaugural effort to assess the association between OSA, myocardial injury, and TRPC5 levels in peripheral blood. The results showed that TRPC5 levels in PBMCs of OSA patients are associated with myocardial injury, suggesting that TRPC5 may be involved in myocardial injury in OSA.
Apoptosis is a natural biological process in which cells undergo programmed cell death following self-injury. Increased apoptosis is a manifestation of cellular injury, which is closely related to Ca2+ homeostasis.34 As a second messenger, Ca2+ establishes a sophisticated and comprehensive network for information exchange between the mitochondria and endoplasmic reticulum, participating in various processes such as cell apoptosis, proliferation, and energy metabolism.35 TRPC5 has been implicated in apoptosis. Knockout of TRPC5 alleviated neuronal apoptosis in mice induced by cerebral ischemia-reperfusion.36 TRPC5 was involved in the injury and apoptosis of SH-SY5Y cells induced by CaCl2.37 HC070, an inhibitor of TRPC5, could suppress apoptosis in SH-SY5Y cells.23 TRPC5 participated in the apoptosis of thyroid cancer via the Wnt/β-catenin pathway.38 Our study demonstrated that IH promoted apoptosis and upregulated TRPC5 expression, indicating the potential role of TRPC5 in regulating apoptosis in H9c2 cells. Overexpression of TRPC5 promoted apoptosis, mitochondrial damage, and elevated levels of Ca2+ and ROS. These findings align with previous studies.
Mitochondria are essential sources of cellular energy, and their abundance is high in myocardial tissue,39 and closely linked to cardiovascular diseases.40 Primarily, mitochondria generate energy through oxidative phosphorylation, playing a crucial role in maintaining cellular metabolic balance and vitality, with Ca2+ serving as a significant regulatory factor in this process.39 When the concentration of Ca2+ remains within the normal range, mitochondria activate normally, sustaining stable respiratory chain function. However, excessive Ca2+ concentration can induce mitochondrial damage, resulting in decreased MMP and mPTP opening, ultimately leading to cell death.41 TRPC5, a non-selective cation channel, is associated with mitochondrial damage. Blocking TRPC5 expression partially reversed the decrease in mPTP caused by MPP+ in SH-SY5Y cells.42 Clemizole, a TRPC5 inhibitor, could ameliorate the decline in MMP and mitochondrial biosynthesis in Parkinson’s rat models.43 In our study, TRPC5 overexpression exacerbated MMP decline, mPTP opening, and mitochondrial cristae disappearance, suggesting that mitochondrial damage occurred, which is related to OxS.44
OxS arises from the harmful impacts of an imbalance between oxidative and antioxidant mechanisms45 and is considered a key factor contributing to heart-related issues in individuals with OSA.46 IH upregulated ROS levels in the heart of rats and promoted cardiomyocyte apoptosis, resulting in cardiac structural and functional damage.24,47 This indicates that ROS levels may be the cause of IH-induced myocardial injury. It has been demonstrated that ROS levels are regulated by Ca2+, and an imbalance in Ca2+ homeostasis results in OxS,48 which eventually leads to injury. Disturbed Ca2+ metabolism promoted ROS production by affecting the electron transport chain,49 which opened the mPTP, decreased the MMP, and ultimately led to apoptosis.50 Our research illustrated that IH increased Ca2+ concentrations and ROS, hindered cell viability, and induced apoptosis in H9c2 cells, suggesting a relationship between OxS and myocardial injury.
Studies have shown that TRPC5 is associated with OxS. TRPC5 knockout alleviated neuronal death due to the influx of Ca2+ and OxS.51 Through depalmitoylation, TRPC5 shielded striatal neurons from OxS in Huntington’s disease.52 NU6027, an inhibitor of TRPC5, could alleviate the influx of Ca2+, oxidative stress, and the loss of hippocampal dendrites induced by traumatic brain injury.53 The present study revealed that TRPC5 promoted the influx of Ca2+ and the generation of ROS, suggesting that TRPC5 promotes OxS. To further elucidate the mechanism of TRPC5 and OxS, the ROS inhibitor NAC was used to inhibit OxS. After treatment with NAC, the apoptosis rate and cell viability significantly improved, the levels of Ca2+ decreased, and mitochondrial damage was alleviated.
However, our study has limitations: (1)The sample size of clinical data was small, which only demonstrated the correlation between TRPC5 and altered cardiac structural function in patients with OSA. (2)We did not conduct experiments with TRPC5 inhibitors to validate their impact on IH-induced myocardial injury. For future research, we need to expand the sample size and conduct a multicentre study to further validate our findings. Meanwhile, we need to use TRPC5 inhibitors for in vivo and in vitro experiments to validate our findings and enhance their credibility.
Conclusions
Our study is the first to investigate the relationship between TRPC5 and OSA-induced myocardial injury. The study revealed that TRPC5 is associated with myocardial injury in OSA patients. IH promoted apoptosis, and TRPC5 may exacerbate the injury by increasing ROS generation, worsening mitochondrial damage, and disrupting calcium homeostasis. This further elucidates the mechanism of TRPC5 in myocardial injury, suggesting that TRPC5 may be a promising target for the diagnosis and treatment of cardiovascular disease caused by OSA.
Acknowledgments
Xuan Qiu and Yanli Yao are Co-first authors and contributed equally to this work.
Funding Statement
This work was supported by National Natural Science Foundation of China (grant number: 82060058).
Data Sharing Statement
The data that support the findings of this study are available.
Disclosure
The authors declare no conflicts of interest.
References
- 1.Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90(1):47–112. doi: 10.1152/physrev.00043.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjafield AV, Ayas NT, Eastwood PR, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7(8):687–698. doi: 10.1016/S2213-2600(19)30198-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Javaheri S, Barbe F, Campos-Rodriguez F, et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. Journal of the American College of Cardiology. 2017;69(7):841–858. doi: 10.1016/j.jacc.2016.11.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel SR. Obstructive sleep apnea. Ann Intern Med. 2019;171(11):Itc81–itc96. doi: 10.7326/AITC201912030 [DOI] [PubMed] [Google Scholar]
- 5.Peker Y, Glantz H, Eulenburg C, Wegscheider K, Herlitz J, Thunström E. Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive sleep apnea. The RICCADSA randomized controlled trial. Am J Respir Crit Care Med. 2016;194(5):613–620. doi: 10.1164/rccm.201601-0088OC [DOI] [PubMed] [Google Scholar]
- 6.Ji Y, Liang Y, Mak JCW, Ip MSM. Obstructive sleep apnea, intermittent hypoxia and non-alcoholic fatty liver disease. Sleep Med. 2022;95:16–28. doi: 10.1016/j.sleep.2022.04.006 [DOI] [PubMed] [Google Scholar]
- 7.Meliante PG, Zoccali F, Cascone F, et al. Molecular pathology, oxidative stress, and biomarkers in obstructive sleep apnea. Int J Mol Sci. 2023;24(6):5478. doi: 10.3390/ijms24065478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lavalle S, Masiello E, Iannella G, et al. Unraveling the complexities of oxidative stress and inflammation biomarkers in obstructive sleep apnea syndrome: a comprehensive review. Life. 2024;14(4): 425. doi: 10.3390/life14040425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu K, Yang Y, Lan M, et al. Apigenin alleviates oxidative stress-induced myocardial injury by regulating SIRT1 signaling pathway. Eur J Pharmacol. 2023;944:175584. doi: 10.1016/j.ejphar.2023.175584 [DOI] [PubMed] [Google Scholar]
- 10.Al-Salam S, Kandhan K, Sudhadevi M, Yasin J, Tariq S. Early doxorubicin myocardial injury: inflammatory, oxidative stress, and apoptotic role of galectin-3. Int J Mol Sci. 2022;23(20):12479. doi: 10.3390/ijms232012479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsieh PL, Chu PM, Cheng HC, et al. Dapagliflozin Mitigates Doxorubicin-Caused Myocardium Damage by Regulating AKT-Mediated Oxidative Stress, Cardiac Remodeling, and Inflammation. Int J Mol Sci. 2022;23(17):10146. doi: 10.3390/ijms231710146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dan Dunn J, Alvarez LA, Zhang X, Soldati T. Reactive oxygen species and mitochondria: a nexus of cellular homeostasis. Redox Biol. 2015;6:472–485. doi: 10.1016/j.redox.2015.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alevriadou BR, Patel A, Noble M, et al. Molecular nature and physiological role of the mitochondrial calcium uniporter channel. Am J Physiol Cell Physiol. 2021;320(4):C465–c482. doi: 10.1152/ajpcell.00502.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patergnani S, Danese A, Bouhamida E, et al. Various aspects of calcium signaling in the regulation of apoptosis, autophagy, cell proliferation, and cancer. Int J Mol Sci. 2020;21(21):8323. doi: 10.3390/ijms21218323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552(Pt 2):335–344. doi: 10.1113/jphysiol.2003.049478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287(4):C817–833. doi: 10.1152/ajpcell.00139.2004 [DOI] [PubMed] [Google Scholar]
- 17.Dong Z, Shanmughapriya S, Tomar D, et al. Mitochondrial Ca(2+) uniporter is a mitochondrial luminal redox sensor that augments MCU channel activity. Mol Cell. 2017;65(6):1014–1028.e1017. doi: 10.1016/j.molcel.2017.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zholos AV. TRPC5. Handb Exp Pharmacol. 2014;222:129–156. doi: 10.1007/978-3-642-54215-2_6. [DOI] [PubMed] [Google Scholar]
- 19.Dragún M, Gažová A, Kyselovič J, Hulman M, Máťuš M. TRP channels expression profile in human end-stage heart failure. Medicina (Kaunas). 2019;55(7):380. doi: 10.3390/medicina55070380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Düzen IV, Yavuz F, Vuruskan E, et al. Leukocyte TRP channel gene expressions in patients with non-valvular atrial fibrillation. Sci Rep. 2017;7(1):9272. doi: 10.1038/s41598-017-10039-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bush EW, Hood DB, Papst PJ, et al. Canonical transient receptor potential channels promote cardiomyocyte hypertrophy through activation of calcineurin signaling. J Biol Chem. 2006;281(44):33487–33496. doi: 10.1074/jbc.M605536200 [DOI] [PubMed] [Google Scholar]
- 22.Piciu F, Balas M, Badea MA, Cucu D. TRP channels in tumoral processes mediated by oxidative stress and inflammation. Antioxidants. 2023;12(7):1327. doi: 10.3390/antiox12071327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaidya B, Roy I, Sharma SS. Neuroprotective potential of HC070, a potent TRPC5 channel inhibitor in parkinson’s disease models: a behavioral and mechanistic study. ACS Chem Neurosci. 2022;13(18):2728–2742. doi: 10.1021/acschemneuro.2c00403 [DOI] [PubMed] [Google Scholar]
- 24.Wang M, Wen W, Chen Y, et al. TRPC5 channel participates in myocardial injury in chronic intermittent hypoxia. Clinics. 2024;79:100368. doi: 10.1016/j.clinsp.2024.100368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(3):479–504. doi: 10.5664/jcsm.6506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1–39.e14. doi: 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 27.Ben Othmène Y, Monceaux K, Karoui A, et al. Tebuconazole induces ROS-dependent cardiac cell toxicity by activating DNA damage and mitochondrial apoptotic pathway. Ecotoxicol Environ Saf. 2020;204:111040. doi: 10.1016/j.ecoenv.2020.111040 [DOI] [PubMed] [Google Scholar]
- 28.Bao M, Hua X, Mo H, et al. N-Acetylcysteine, an ROS inhibitor, alleviates the pathophysiology of hyperthyroidism-induced cardiomyopathy via the ROS/Ca(2+) pathway. Biomolecules. 2022;12(9):1195. doi: 10.3390/biom12091195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Q, Lin G, Lin L, et al. LncRNA XR_596701 protects H9c2 cells against intermittent hypoxia-induced injury through regulation of the miR-344b-5p/FAIM3 axis. Cell Death Discov. 2022;8(1):42. doi: 10.1038/s41420-022-00834-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Miao Y, Zhang Q. Causal associations of obstructive sleep apnea with cardiovascular disease: a Mendelian randomization study. Sleep. 2023;46(3). doi: 10.1093/sleep/zsac298 [DOI] [PubMed] [Google Scholar]
- 31.Raut S, Gupta G, Narang R, et al. The impact of obstructive sleep apnoea severity on cardiac structure and injury. Sleep Med. 2021;77:58–65. doi: 10.1016/j.sleep.2020.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korcarz CE, Peppard PE, Young TB, et al. Effects of obstructive sleep apnea and obesity on cardiac remodeling: the Wisconsin sleep cohort study. Sleep. 2016;39(6):1187–1195. doi: 10.5665/sleep.5828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thakore P, Clark JE, Aubdool AA, et al. Transient Receptor Potential Canonical 5 (TRPC5): regulation of heart rate and protection against pathological cardiac hypertrophy. Biomolecules. 2024;14(4):442. doi: 10.3390/biom14040442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu J, Xie Y, Ma J, Chu H. New Ca(2+) based anticancer nanomaterials trigger multiple cell death targeting Ca(2+) homeostasis for cancer therapy. Chem Biol Interact. 2024;393:110948. doi: 10.1016/j.cbi.2024.110948 [DOI] [PubMed] [Google Scholar]
- 35.Ge WD, Du TT, Wang CY, Sun LN, Wang YQ. Calcium signaling crosstalk between the endoplasmic reticulum and mitochondria, a new drug development strategies of kidney diseases. Biochem Pharmacol. 2024;225:116278. doi: 10.1016/j.bcp.2024.116278 [DOI] [PubMed] [Google Scholar]
- 36.Guo J, Li J, Xia L, et al. Transient receptor potential canonical 5-scramblase signaling complex mediates neuronal phosphatidylserine externalization and apoptosis. Cells. 2020;9(3):547. doi: 10.3390/cells9030547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li K, Li W, Yin H, Cheong YK, Ren G, Yang Z. Pretreatment-Etidronate Alleviates CoCl(2) Induced-SH-SY5Y Cell Apoptosis via Decreased HIF-1α and TRPC5 Channel Proteins. Neurochem Res. 2019;44(2):428–440. doi: 10.1007/s11064-018-2696-3 [DOI] [PubMed] [Google Scholar]
- 38.Huang B, Zhang Y, Sun P, Yuan Y, Wang C. MiR-138-5p inhibits thyroid cancer cell growth and stemness by targeting TRPC5/Wnt/β-catenin pathway. Mol Biotechnol. 2024;66(3):544–553. doi: 10.1007/s12033-023-00782-3 [DOI] [PubMed] [Google Scholar]
- 39.Zhao M, Lian A, Zhong L, Guo R. The regulatory mechanism between lysosomes and mitochondria in the aetiology of cardiovascular diseases. Acta Physiol (Oxf). 2022;234(2):e13757. doi: 10.1111/apha.13757 [DOI] [PubMed] [Google Scholar]
- 40.Divya KP, Kanwar N, Anuranjana PV, et al. SIRT6 in regulation of mitochondrial damage and associated cardiac dysfunctions: a possible therapeutic target for CVDs. Cardiovasc Toxicol. 2024;24(6):598–621. doi: 10.1007/s12012-024-09858-1 [DOI] [PubMed] [Google Scholar]
- 41.Carraro M, Bernardi P. The mitochondrial permeability transition pore in Ca(2+) homeostasis. Cell Calcium. 2023;111:102719. doi: 10.1016/j.ceca.2023.102719 [DOI] [PubMed] [Google Scholar]
- 42.Vaidya B, Gupta P, Laha JK, Roy I, Sharma SS. Amelioration of Parkinson’s disease by pharmacological inhibition and knockdown of redox sensitive TRPC5 channels: focus on mitochondrial health. Life Sci. 2023;328:121871. doi: 10.1016/j.lfs.2023.121871 [DOI] [PubMed] [Google Scholar]
- 43.Vaidya B, Gupta P, Biswas S, Laha JK, Roy I, Sharma SS. Effect of clemizole on alpha-synuclein-preformed fibrils-Induced Parkinson’s disease pathology: a pharmacological investigation. Neuromolecular Med. 2024;26(1):19. doi: 10.1007/s12017-024-08785-2 [DOI] [PubMed] [Google Scholar]
- 44.Alqahtani T, Deore SL, Kide AA, et al. Mitochondrial dysfunction and oxidative stress in Alzheimer’s disease, and Parkinson’s disease, Huntington’s disease and amyotrophic lateral sclerosis -an updated review. Mitochondrion. 2023;71:83–92. doi: 10.1016/j.mito.2023.05.007 [DOI] [PubMed] [Google Scholar]
- 45.Lin J, Zheng X, Xiong Z, et al. DJ-1-mediated p62 degradation delays intervertebral disc degeneration by inhibiting apoptosis of nucleus pulposus cells. Apoptosis. 2023;28(9–10):1357–1371. doi: 10.1007/s10495-023-01862-0 [DOI] [PubMed] [Google Scholar]
- 46.Zong D, Liu X, Shen C, Liu T, Ouyang R. Involvement of Galectin-3 in neurocognitive impairment in obstructive sleep apnea via regulating inflammation and oxidative stress through NLRP3. Sleep Med. 2023;101:1–10. doi: 10.1016/j.sleep.2022.09.018 [DOI] [PubMed] [Google Scholar]
- 47.Chi R, Chai C, Liu G, Cao H, Yang B. Chronic intermittent hypoxia-induced BNIP3 expression mitigates contractile dysfunction and myocardial injury in animal and cell model via modulating autophagy. Hum Cell. 2023;36(2):631–642. doi: 10.1007/s13577-022-00851-w [DOI] [PubMed] [Google Scholar]
- 48.Milbourn HR, Toomey LM, Gavriel N, et al. Limiting oxidative stress following neurotrauma with a combination of ion channel inhibitors. Discov Med. 2017;23(129):361–369. [PubMed] [Google Scholar]
- 49.Vercesi AE, Kowaltowski AJ, Grijalba MT, Meinicke AR, Castilho RF. The role of reactive oxygen species in mitochondrial permeability transition. Biosci Rep. 1997;17(1):43–52. doi: 10.1023/A:1027335217774 [DOI] [PubMed] [Google Scholar]
- 50.Hansson MJ, Månsson R, Morota S, et al. Calcium-induced generation of reactive oxygen species in brain mitochondria is mediated by permeability transition. Free Radic Biol Med. 2008;45(3):284–294. doi: 10.1016/j.freeradbiomed.2008.04.021 [DOI] [PubMed] [Google Scholar]
- 51.Park SE, Song JH, Hong C, et al. Contribution of zinc-dependent delayed calcium influx via TRPC5 in oxidative neuronal death and its prevention by novel TRPC antagonist. Mol Neurobiol. 2019;56(4):2822–2835. doi: 10.1007/s12035-018-1258-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hong C, Choi SH, Kwak M, et al. TRPC5 channel instability induced by depalmitoylation protects striatal neurons against oxidative stress in Huntington’s disease. Biochim Biophys Acta Mol Cell Res. 2020;1867(2):118620. doi: 10.1016/j.bbamcr.2019.118620 [DOI] [PubMed] [Google Scholar]
- 53.Park MK, Choi BY, Kho AR, et al. Effects of Transient Receptor Potential Cation 5 (TRPC5) inhibitor, NU6027, on hippocampal neuronal death after traumatic brain injury. Int J Mol Sci. 2020;21(21):8256. doi: 10.3390/ijms21218256 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available.