Abstract
For the first time the International Symposium on Fungal Stress was joined by the XIII International Fungal Biology Conference. The International Symposium on Fungal Stress (ISFUS), always held in Brazil, is now in its fourth edition, as an event of recognized quality in the international community of mycological research. The event held in São José dos Campos, SP, Brazil, in September 2022, featured 33 renowned speakers from 12 countries, including: Austria, Brazil, France, Germany, Ghana, Hungary, México, Pakistan, Spain, Slovenia, USA, and UK. In addition to the scientific contribution of the event in bringing together national and international researchers and their work in a strategic area, it helps maintain and strengthen international cooperation for scientific development in Brazil.
Keywords: morphogenesis, organellar dynamics, cytoskeleton, cell wall synthesis, fungal interactions, biofuels, agricultural mycology, industrial mycology, medical mycology, control of fungal growth, fungal stress mechanisms and responses
1. Introduction
The Fungal Kingdom contains the largest organisms on the planet. Fungi display exceptional adaptivity and are ubiquitous in the environment. The life span of a fungus can range from several hours to several centuries. They are essential for life on our planet, due to their role in carbon recycling as main decomposers and have symbiotic associations with many plant species. With each passing year, there is a growing recognition of the importance of fungi in human health, food, fermentation, biofuel production, agriculture, and ecological stability. With a warmer planet, fungi are adapting to higher temperatures. This increases the risk of opportunistic infections to endothermic hosts, which are otherwise immune due to their high body temperatures. Human fungal pathogens are a major medical concern, which nonetheless have often been overlooked compared with bacterial pathogens and other diseases. Given their importance, unfortunately, fungi remain under investigation. For example, the languages of fungi used for intra-species and inter-species communication are barely explored, yet many genera produce an abundance of bioactive molecules.
The paragraph above captures what some of the leading experts on fungi spontaneously wrote on napkins at the opening dinner of the XIII International Fungal Biology Conference (IFBC) and the IV International Symposium on Fungal Stress (ISFUS) (Figure 1). They came to this conference to present their research and these mycologists from throughout the world formed a tight-knit group because of this conference. The Symposium took place in São José dos Campos, São Paulo, Brazil on September 25 to 29, 2022 (Figure 2). Although the size of the symposium was relatively small, the depth and breadth of presentations and discussions were impressive.
Figure 1.

Introduction of this manuscript written on a napkin during a pizza and wine night at Armazen da Pizza in São José dos Campos, SP, Brazil
Figure 2:

The official Logo of the IV International Symposium on Fungal Stress (ISFUS) & XIII International Fungal Biology Conference (IFBC).
The IFBC/ISFUS was organized by Drauzio E.N. Rangel and his wife Alene Alder-Rangel, with the help of Jesús Aguirre and Claudia B.L. Campos. The convivial atmosphere among the organizers and all participants permeated the whole meeting. The Rangels encouraged everyone to socialize and make lasting friendships and collaborations (Figure 3). The small size of the symposium also facilitated close interactions between the leading experts in the field as well as students who were attending their first international meeting. Nadine Hochenegger (a PhD student at TU Wien in Vienna, Austria) wrote: “I loved the conference! It was the second biggest conference I attended, and I was overwhelmed by the hospitality of Drauzio and Alene. I learned so much on a scientific level, and also I made friends with such wonderful and interesting people. For me, as a rather young and unexperienced scientist, it was a momentous event that I won’t ever forget!” According to Simon Avery (University of Nottingham, Nottingham, England, UK) “There is a different ‘feel’ compared with other conferences… as it creates a great atmosphere for science as well as for making new friends.” Joan Bennett (Rutgers, State University of New Jersey, New Brunswick, NJ, USA) stated that “Those of us who love fungi, also love to talk with other people who love fungi. The ISFUS venue is the perfect setting to engage in our passion.” Audrey Gasch (University of Wisconsin, Madison, WI, USA) said: “Thank you Drauzio, Alene, and Amanda for such a wonderful time of science, fungi, fairy rings, positive thoughts, and caipirinhas! What a fun time it has been getting to know you better and seeing inspirational science.” (Electronic Supplementary Material 1)
Figure 3.

Speakers and participants holding hands and sharing happy moments at the opening ceremony.
The joint meetings IV ISFUS and XIII IFBC featured 33 speakers from 12 countries (Figure 4, Electronic Supplementary Material 2). The meeting received grant funding from the Brazilian agencies FAPESP and CAPES. It also received support from the Deutsche Forschungsgemeinschaft (DFG) and the British Mycological Society. Elsevier (Fungal Biology) and the Journal of Fungi sponsored the student awards. The hosting institutions were the Universidade Brasil and the Universidade Tecnológica Federal do Paraná.
Figure 4.

Speakers of the IV International Symposium on Fungal Stress (ISFUS) & XIII International Fungal Biology Conference (IFBC) in 2022 held in São José dos Campos, SP, Brazil. Front row from left to right: Alene Alder-Rangel, Amanda E. A. Rangel, Drauzio E. N. Rangel. Second row: Raquel Nascimento (DFG Brazil), Audrey P. Gasch (USA), Nina Gunde-Cimerman (Slovenia), Laila P. Partida-Martínez (Mexico), Xiaorong Lin (USA), Iris Eisermann (UK), Joan W. Bennett (USA), Martine Bassilana (France), Janet Quinn (UK), Fulvia Verde (USA). Third row: Mavis A. Acheampong (Ghana), Meritxell Riquelme (Spain), Robert Arkowitz (USA), Alfredo H. Herrera Estrella (Mexico), Rosa Reyna Mouriño Pérez (Mexico), Nancy P. Keller (USA), Attila Gácser (Hungary). Fourth row: Cristina (DFG), Simon Avery (UK), Geoffrey M. Gadd (UK), Ulrich Terpitz (Germany), Jesús Aguirre (Mexico), Jean-Paul Latgé (France), Joshua D. Nosanchuk (USA), Joseph Heitman (USA). Speakers not in the picture: Alexandre Melo Bailão (Brazil), Claudia B. L. Campos (Brazil), Gerhard Braus (Germany), Irina S. Druzhinina (Austria), Nemat Keyhani (USA), Sehar Afshan Naz (Pakistan), and Thiago Olitta Basso (Brazil).
2. The XIII International Fungal Biology Conference
The International Fungal Biology Conference (IFBC) started as the First International Fungal Spore Symposium, organized by Professor Lilian Hawker https://en.wikipedia.org/wiki/Lilian_Hawker in Bristol, England in 1965. The second symposium of these series was organized by Darrell J. Weber and Wilford M. Hess at Brigham Young University, Provo, Utah, USA, in 1975 (Weber and Hess, 1976), with the aim of bringing together scientists interested in studying the form and function of fungal spores. Salomón Bartnicki-García, at that time working at the University of California, Riverside, USA, attended this symposium and became an active promoter of these series of meetings; later he became the chair of the scientific steering committee. From the beginning, the intention of these conferences was to emphasize cell biology and morphological aspects of fungal growth and development in many cases using electron microscopy techniques.
In 1980, the third symposium of these series was organized by Gilbert Turian (University of Geneva, Switzerland) and Hans R. Hohl (University of Zurich, Switzerland) in Gwat, Switzerland (Turian and Hohl, 1981). In 1987, the name of the conference changed to the IV International Fungal Spore Conference, and it was held at the University of Stirling, in Scotland, UK. In 1991, the V International Fungal Spore Conference was organized by William Timberlake (University of Georgia, Athens, GA, USA), in Helen, Georgia, USA. The sixth conference of this series was organized by Kurt W. Mendgen (University of Konstanz, Germany) in Konstanz, Germany, in 1996.
The name of the conference changed to the VII International Fungal Biology Conference in 1999 and was organized in Groningen, The Netherlands by Johannes Hans Sietsma. In 2002, the VIII IFBC was organized by Jesús Aguirre (Universidad Nacional Autonoma de México, México City, México) and José Ruiz-Herrera (1935–2023) (Cinvestav, Irapuato México) in Guanajuato, México (Gurr, 2003), https://funguscongress.ucr.edu. At that time, Jesús Aguirre became the chair of the IFBC Steering Committee. The next conference of the series was a joint meeting with the XVI New Phytologist Symposium, organized by Francis Martin (INRAE, Nancy, France), Nicholas Talbot (The Sainsbury Laboratory, Norwich, UK), and Holly Slater (Lancaster University, UK) in 2006 in Nancy, France. The conference returned to México in 2009, when a team headed by Salomón Bartnicki-García (University of California, Riverside, USA) organized the X IFBC, which was held jointly with the VIII National Congress of the Cellular and Molecular Biology of Fungi branch of the Mexican Society of Biochemistry in Ensenada, Baja California, México (https://funguscongress.ucr.edu/home.php?lang=eng-US). Reinhard Fischer, Jörg Kämper, and Natalia Requena, all from the Karlsruhe Institute of Technology, Germany, organized the XI IFBC in Karlsruhe, Germany, in 2013 (http://www.iab.kit.edu/conference/index.php). The IFBC was held in Asia in 2017, when Kap-Hoon Han (Wonkwang University, Korea) organized the XII IFBC in Songdo, Incheon, South Korea. Finally, the XIII IFBC was organized in 2022 as a joint meeting along with the IV ISFUS meeting by Drauzio E.N. Rangel, and Alene Alder-Rangel, Claudia B.L. Campos, and Jesús Aguirre, in São José dos Campos, SP, Brazil (Figure 5).
Figure 5.

The Meet the Speakers Poster for the International Fungal Biology Conference
The current IFBC Steering Committee, composed of Hans A.B. Wosten, Antonio Di Pietro, Kap-Hoon Han, Reinhard Fischer, Natalia Requena, Neil Gow, Meritxell Riquelme, Drauzio Rangel, and Jesús Aguirre, has recently elected Meritxell Riquelme as the new chair, to continue to promote the conference series, characterized by bringing together scientists interested in research at the frontiers of fungal cell biology. The IFBC has evolved as a unique forum that favors close academic and social interactions among scientists that use different fungal models to approach fundamental questions in cell biology. The following section summarizes the presentations at the XIII IFBC.
2.1. IFBC – Session 1: Cell biology of fungal interactions.
Fungi are exposed to many different external stressors affecting their life cycle. As cosmopolitan organisms, fungi share a niche with multiple organisms and must deal with different competitors and predators. The three speakers in this session presented recent results regarding fungi that were exposed to other organisms, symbionts, or physical stressors, respectively. Fungivorous insects injure fungal mycelia during grazing, and the attacked hypha needs to activate defense mechanisms based on a complex molecular interplay including specialized kinases and micro-RNAs. A failing defense may lead to a situation in which organisms successfully exploit the fungus and survive there as bacterial and viral symbionts. In this case, the entire fungal biology of the fungus could be influenced by the symbiont, and both the fungus and the symbiont should be considered as a holobiont. In their natural habitat, fungi distinguish light and dark, as well as different wavelengths of light (e.g., green light detection by rhodopsins), and adapt accordingly to avoid cellular stress induced by irradiation.
Alfredo H. Herrera-Estrella (Centro de Investigación y de Estudios Avanzados del IPN, Irapuato, Guanajuato, México) described how the filamentous fungus Trichoderma atroviride responds to mechanical injury and attack by a fungivorous insect. He pointed out that fungi, like plants and animals, respond to injury by recognizing Damage-Associated Molecular Patterns (DAMPs) that activate Ca2+ and Mitogen-Activated Protein Kinase (MAPK) dependent signaling for the activation of defense mechanisms (Medina-Castellanos et al., 2018). He described how grazing by larvae of the fruit-fly (Drosophila melanogaster) on mycelia inhibited the transcriptional activation of genes required for hyphal regeneration, and triggered the fungal innate immune and chemical defense responses (Atriztan-Hernandez and Herrera-Estrella, 2022; Medina-Castellanos et al., 2014). Herrera-Estrella further discussed the participation of micro-RNA-like molecules in response to injury. His group found that elimination of a single milRNA phenocopied the main defects observed in an RNAi dcr2 mutant (Villalobos-Escobedo et al., 2022). This work demonstrated the essential role of milRNAs in hyphal regeneration and asexual development by post-transcriptionally regulating cellular signaling processes.
Laila P. Partida-Martínez (Centro de Investigación y de Estudios Avanzados del IPN, Irapuato, Guanajuato, México) explained that the biological concept of a holobiont refers to a eukaryotic host plus its microbiota and has been used for plants and animals. However, fungi, as microbial eukaryotes, should also be considered holobionts (Partida-Martinez, 2017). She showed that several species of the genus Rhizopus harbor bacterial (Mycetohabitans spp.) and viral (Narnavirus spp.) symbionts that live in the fungal cytoplasm and are vertically inherited (Espino-Vazquez et al., 2020; Partida-Martinez and Hertweck, 2005; Partida-Martinez et al., 2007). These symbionts affect the fitness of their Rhizopus hosts in several ways, including the production of toxins employed by the fungus to attack plants and animals (Partida-Martinez and Hertweck, 2005), and influence its fungal host’s asexual and sexual reproduction (Espino-Vazquez et al., 2020; Mondo et al., 2017; Partida-Martinez et al., 2007). Altogether, these microbial symbioses are relevant for understanding fungal biology and evolution.
Ulrich Terpitz (Julius-Maximilians-Universität Würzburg, Wuerzburg, Germany) explained that fungal rhodopsins are membrane-embedded, green-light-sensing retinal proteins functioning as either light-driven proton pumps or putative light-sensory proteins (Adam et al., 2018; Panzer et al., 2019). Fungal rhodopsins are located in plasmalemma and/or tonoplast and underlie dynamic relocalization processes with so far unknown function (Adam et al., 2018; Panzer et al., 2019). As green light is highly abundant in the plant environment, a role of rhodopsins in the fungus-plant interaction is suggested. Accordingly, rhodopsin coding genes are predominantly found in genomes of ascomycetes associated with plants (Adam et al., 2018; García-Martínez et al., 2015). Furthermore, light-induced rhodopsin expression in fungi is upregulated in planta compared to axenic conditions, and the auxin indole-acetic acid augments the pump activity of CarO-like rhodopsins (Adam et al., 2018; Lyu et al., 2016; Panzer et al., 2019). Fungal rhodopsins are also involved in many other physiological processes, such as growth regulation, germination, and pathogenesis (Adam et al., 2018; Lyu et al., 2016; Wang et al., 2018).
2.2. IFBC – Session 2: Fungal morphogenesis and polar growth
This session discussed diverse aspects of fungal morphogenesis and polar growth in human and plant pathogens. The talks in this exciting field illustrated new ideas on how pathogens regulate morphology and developmental switches as well as on effective antifungal strategies. Keller described how two endogenous lipids control the balance between hyphal tip growth and hyphal lateral growth (aka branching). Eisermann discussed how important turgor pressure and septins are for appressorium development and the promotion of polarized cell growth. Lin showed briefly that fungal morphogenesis can be explored to develop vaccines. She then detailed how host receptors to detect fungi are employed in developing DectiSomes to increase the efficacy of antifungals. Finally, Verde expanded on the role of the kinase Orb6 in cell morphogenesis and stress response.
Nancy P. Keller (University of Wisconsin, Madison, WI, USA) presented work on endogenous signals that direct Aspergillus fumigatus hyphal branching. Programmed branching is important for colony formation and growth in host substrates where hyphal tip growth is balanced with hyphal lateral growth. Two endogenous lipids direct this balance. The oxylipin 5,8-diHODE induces lateral branching (Niu et al., 2020) and sulfated lipo-chitoologosaccharides (LCOs) inhibit lateral branching (Rush et al., 2020). Keller, in collaboration with Drs. Ane and Roy, developed a computational program called GRAsp (gene regulation of A. fumigatus) to elucidate the signaling networks directing 5,8-diHODE and LCO regulation of branching.
Iris Eisermann (University of East Anglia, Norwich, England, UK) presented new research on the turgor-driven, septin-dependent infection mechanism of the rice blast fungus Magnaporthe oryzae. Rice blast is one of the most important crop diseases globally. Infection proceeds via a specialized cell called an appressorium, and its development is regulated by the Pmk1 MAP kinase signaling pathway. A quantitative phosphoproteomic approach has identified many direct downstream targets of Pmk1, which include proteins related to cytoskeleton remodeling, vesicle trafficking, and cell cycle control. A turgor-sensing histidine-aspartate kinase, Sln1, enables the appressorium to sense when a threshold of turgor has been reached, and facilitates host penetration. A mechanosensory membrane-spanning dye has been used to reveal spatial variations in membrane tension caused by appressorium turgor. Cytoskeletal proteins called septins play a major role during appressorium-mediated plant infection and four M. oryzae septins form a hetero-oligomeric ring structure at the base of the appressorium. A combination of Ultra-High-Throughput Yeast Two Hybrid analysis coupled with an in vivo immunoprecipitation tandem mass spectrometry has identified a wide range of novel interacting partners during appressorium development, including polarity determinants, cytoskeletal components, and a range of regulatory proteins. The role of these proteins in rice blast disease is being investigated (Dagdas et al., 2012; Eseola et al., 2021; Osés-Ruiz et al., 2021; Ryder et al., 2022).
Xiaorong Lin (University of Georgia, Athens, GA, USA) discussed how the treatment of invasive mycoses relies on a few classes of antifungals. Host toxicity and insufficient fungal killing at clinically relevant doses of current antifungals present major barriers for successful treatment. To increase antifungal efficacy, Lin and her collaborators developed DectiSomes: liposomes packaged with antifungals that are coated with host C-type lectin receptors (dectins). Like a guided missile, dectins that are present on DectiSomes deliver drugs specifically to fungi in infected tissues and reduce unspecific drug distribution to host cells, thus achieving higher selectivity and improving drug efficacy. Relative to untargeted liposomal drug, DectiSomes bound stronger to all fungal pathogens tested and achieved better efficacy in mouse models of pulmonary aspergillosis and invasive candidiasis in terms of organ fungal burden and animal survival. Thus, DectiSomes have the potential to usher in a new antifungal drug treatment paradigm (Ambati et al., 2022; Meagher et al., 2021). Lin also discussed exploration about the ability of Cryptococcus neoformans filamentous ZNF2OE strain to elicit protective host responses to identify potential vaccine candidates. Cryptococcal antigens recognized by antibodies of ZNF2OE -vaccinated animals reside within the capsule. Her group is currently focused on GPI-anchored mannoproteins because many are regulated by Znf2 and represent the major immunogens present on cryptococcal cell surface (Lin et al., 2022; Zhai et al., 2015).
Fulvia Verde (University of Miami, Miami, FL, USA) focused on the role of conserved nuclear Dbf2-related (NDR) kinase Orb6 in cell morphogenesis and stress response in the fission yeast Schizosaccharomyces pombe. Orb6 kinase spatially regulates the activity of Cdc42 GTPase, a key morphology control factor, to promote cell shape emergence (Das et al., 2012; Das et al., 2015; Das et al., 2009). Orb6 also inhibits the degradation of specific mRNAs, thereby promoting polarized cell growth (Nunez et al., 2016). Orb6 kinase activity is downregulated by a variety of stimuli, such as nutritional deprivation (Chen et al., 2019). Verde and colleagues have discovered a role for Orb6 kinase in regulating chronological lifespan and identified novel mechanisms of control of Orb6 kinase activity in response to environmental stress. Verde proposed that an important role of NDR kinase in eukaryotic cells is to enable alternative physiological states, from active cell growth to cell quiescence, to promote cell resilience in the face of stress.
2.3. IFBC – Session 3: Cytoskeletal dynamics and growth patterns
This session focused on understanding how fungal cells organize their growth, cellular components, and processes alongside development. Four different presentations explored how the Fungal kingdom may have employed sexual reproduction even before the division of the sexes. Furthermore, phospholipids, especially phosphatidylinositol 4-phosphate, and their transport and regulation are important for invasion and virulence in the pathogenic yeast Candida albicans. Other speakers focused on how the complex fungal COP9 signalosome is assembled in Aspergillus nidulans through the sequential assembly of distinct heterotrimeric subcomplexes, and how microtubule organizing centers become ordered with the aid of septal pores in the filamentous fungus Neurospora crassa.
Joseph Heitman (Duke University, Durham, NC, USA) presented his studies that have revealed novel modes of sexual reproduction involving either one or two parents, including unisexual and pseudosexual reproduction, which results in the production of either clonal or recombinant progeny. He also showed evidence that illustrates the capacity for sexual reproduction to generate diversity de novo. His findings revealed parallels in modes of selfing shared with sexual reproduction in plants and animals. Studies by his group also provided insights into the evolutionary trajectory of sexual reproduction in eukaryotes and may provide insights into how sex first evolved. These findings suggest that there may have been an evolutionary epoch in which there was sexual reproduction before there were mating types or sexes (Heitman, 2015; Lee et al., 2010; Yadav et al., 2021).
Martine Bassilana (University Côte d’Azur, Nice, France) focused on the regulation by phospholipids of Candida albicans hyphal invasive growth in response to external stimuli (Bassilana et al., 2020). Phospholipids, such as phosphatidylserine (PS) and the phosphatidylinositol phosphates PI(4)P and PI(4,5)P2, localize preferentially at the hyphal apex and this distribution is maintained both by enzymatic and transporter activities. In particular, the PI4-kinase Stt4, which generates plasma membrane (PM) PI(4)P, and the flippase Drs2, which transports PS from the external/luminal to the cytoplasmic membrane leaflet, are critical for hyphal growth (Labbaoui et al., 2017; Vernay et al., 2012). Quantitative analyses of mutants in Stt4, as well as in its regulators Efr3 and Ypp1, indicated that PI(4)P levels correlate with virulence in systemic candidiasis (Garcia-Rodas et al., 2022). Deletion of the lipid transfer protein Osh4 specifically restores hyphal invasive growth in a drs2 mutant, indicating that a balance in Drs2 and Osh4 activities regulates invasive growth via PI(4)P (Basante-Bedoya et al., 2022). Together, these results highlight the importance of plasma membrane PI(4)P regulation for invasive growth and virulence.
Gerhard Braus (University of Goettingen, Goettingen, Germany) described how fungal COP9 signalosome (CSN) coordinates protein degradation for stress responses, development, and secondary metabolism (Busch et al., 2007; Nahlik et al., 2010). CSN assembly is initiated through a seven-subunit pre-CSN, which is activated by the integration of the catalytic CsnE/Cs5 deneddylase subunit (Beckmann et al., 2015). This deneddylase activity is required to exchange F-box substrate receptors of SCF ubiquitin ligases, which label proteins for degradation (von Zeska Kress et al., 2012). Numerous F-box receptors are located in the nucleus and interact with regulatory proteins (Sarikaya Bayram et al., 2022). The assembly of the native Aspergillus nidulans pre-CSN was dissected in vivo by combined genetic and biochemical approaches. Two distinct heterotrimeric CSN subcomplexes were identified as pre-CSN assembly intermediates. Surveillance mechanisms control accurate CSN subunit amounts and correct cellular localization for sequential assembly.
Rosa Mouriño-Pérez (Centro de Educación Científica y de Educación Superior de Ensenada, Ensenada, B. C. México) explained how microtubule organizing centers (MTOCs) are organized in the filamentous fungus Neurospora crassa. She talked about the different MTOCs in fungi, such as the spindle pole body (SPB) (Kollman et al., 2010; Moritz et al., 2000) as the functional equivalent of the centrosome and the non-centrosomal MTOCs present in S. pombe (Bartolini and Gundersen, 2006; Horio and Oakley, 2003) and A. nidulans (Zekert et al., 2010; Zhang et al., 2017). Mouriño-Pérez showed that the N. crassa SPB is embedded in the nuclear envelope, with the γ-TuRC targeting proteins PCP-1Pcp1/PcpA located at the inner face and APS-2Mto1/ApsB located at the outer face of the SPB. PCP-1 is a specific component of nuclear MTOCs while APS-2 is also present in the septal pore. Although γ-tubulin was only detected in the nucleus, spontaneous MT nucleation occurred in the apical and subapical cytoplasm during recovery from benomyl-induced MT depolymerization experiments. MT dynamics were monitored with the MT plus end tracking protein MTB-3 and revealed MT polymerization from septa (Lin et al., 2015; Straube et al., 2003). Septal MT polymerization depends on the septal proteins SPA-10Spa10 and SPA-18Mto2/Spa18 (i.e., the APS-2/SPA-18 protein complex) (Ramirez-Cota et al., 2022). The main conclusions presented were that the SPB is the only MT nucleator site, but the septal pore aids MT network arrangement through the anchorage of the MT plus-ends.
2.4. IFBC – Session 4: Subcellular heterogeneity and organelle dynamics and interactions
A characteristic feature of filamentous fungi is their ability to coordinate polar growth for hyphal tip expansion (Riquelme et al., 2018). To achieve this task, environmental, nutritional, and developmental signals must be integrated and converted into cellular processes involving signaling cascades including kinases and GTPases. Ultimately, the underlying signaling network leads to altered expression or localization of key proteins involved in membrane trafficking to support the function of the Spitzenkörper at the growth apex. The precise spatiotemporal expression of these proteins is tightly regulated at both the transcriptional and the posttranscriptional level. Another critical aspect is the coordination of intracellular transport of proteins, mRNAs, and even entire organelles including endosomes. Furthermore, organelles like mitochondria are essential hubs that coordinate signaling and energy supply to support hyphal growth. In this session, Aguirre described how the generation of reactive oxygen species links signaling to mitochondrial functions. Feldbrügge reported how regulation at the level of transport and stability of mRNAs coordinates the polar growth of hyphae. A particular focus is placed on key RNA-binding proteins needed to execute this type of regulation. Riquelme described how the underlying membrane trafficking is needed to orchestrate secretion at the Spitzenkörper. Arkowitz extended this view by reporting how the key machinery of polar growth is used to determine the shape and branching of hyphae. This session demonstrates that a holistic view of cellular processes at various levels is critical to understand the fundamental growth principles of fungi.
Meritxell Riquelme (Centro de Investigación Científica y de Educación Superior de Ensenada, Ensenada, México) studies hyphal morphogenesis in the model filamentous fungus Neurospora crassa. She discussed the role of the exocyst, an octameric tethering complex conserved in all eukaryotes, in the last exocytosis stages of the secretory vesicles in hyphae. Her group found that exocyst subunits SEC-3, SEC-5, SEC-6, SEC-8, and SEC-15 localize at the plasma membrane of hyphal apices, while subunits EXO-70 and EXO-84 occupy the frontal outer layer of the Spitzenkörper (SPK), occupied by macrovesicles (Riquelme et al., 2014; Riquelme and Sánchez-León, 2014). Recently, they investigated exocyst subunit SEC-10, which also localizes at the apical plasma membrane. Hyphae of a strain expressing a GFP C-terminally tagged version of SEC-10 displayed growth and polarity defects, and lacked a SPK, indicative of exocyst dysfunction (Figueroa-Meléndez et al., 2022). Proteomics and transmission electron microscopy analyses confirmed the essential role of SEC-10 in exocyst assembly and/or stability, organization of macrovesicles at the SPK, and tip growth.
Michael Feldbrügge (Heinrich-Heine University Düsseldorf, Düsseldorf, Germany) discussed the critical roles of RNA-binding proteins (RBPs) in Ustilago maydis. The RBP Rrm4 is a key factor for endosomal mRNA transport, an evolutionarily conserved process across fungi, plants, and neurons (Müller et al., 2019; Müntjes et al., 2021). Addressing the crucial question of how mRNAs attach to endosomes, his group showed that Rrm4 carries a C-terminal binding platform containing three MademoiseLLE domains (Devan et al., 2022). Specifically, a novel third MLLE domain is essential for interaction with the PAM2L motifs of the endosomal adapter protein Upa1, thus linking mRNAs to transport endosomes. Shifting gears, they found that the RBP Khd4 (Vollmeister et al., 2009) binds distinct mRNAs encoding regulatory proteins functioning in membrane trafficking. By controlling mRNA stability, Khd4 orchestrates precise gene expression control of the membrane trafficking process during pathogenic development. Essentially, these examples demonstrate the importance of RNA biology in fungi at the level of transport and stability.
Robert Arkowitz (University Côte d’Azur, Nice, France) focused on Candida albicans morphogenesis at different temporal and spatial scales. His group has been investigating the reorganization of different organelles during germ tube formation, subsequent hyphal growth (Silva et al., 2019; Weiner et al., 2019), and more recently hyphal branch formation. In addition, they have been examining the physical characteristics of the cytoplasm in these different growth states, using a micro-rheology probe (Delarue et al., 2018). Using loss of- and gain of- function mutants to probe the link between filament morphology and growth rate, they found a correlation between filament diameter and extension rate, which appears to be mediated by the Spitzenkörper (Puerner et al., 2021). Based upon analyses of growth rate, morphology, and distribution/dynamics of a range of cellular reporters, their results indicate that hyphal branching and growth of the main hyphal filament are distinct developmental states. These studies provide a framework for studying hyphal branching in this human fungal pathogen.
Jesús Aguirre (Universidad Nacional Autonoma de México, México City, México) explained how his research on reactive oxygen species (ROS) signaling mediated by the stress MAPKs SakA and MpkC (Garrido-Bazan et al., 2018a, b; Lara-Rojas et al., 2011) revealed that the same H2O2 treatment that activates this MAPK pathway induces a widespread mitochondrial division (Jaimes-Arroyo et al., 2015). The dynamin-like GTPase DnmA (Drp1 in animals) and its receptor FisA are essential for mitochondrial division. The lack of mitochondrial division in Aspergillus nidulans results in a notable decrease in hyphal growth, asexual and sexual development, as well as an increase in mitochondrial ROS (Garrido-Bazan et al., 2020). Mutants lacking DnmA and FisA were used to demonstrate that H2O2 induces extensive mitochondrial constrictions prior to actual division in a process that depends on mitochondrial depolarization, intracellular calcium, and close contact between mitochondria and the endoplasmic reticulum, mediated by the ERMES complex (Garrido-Bazan and Aguirre, 2022). These results indicate that H2O2 plays a prominent role in the regulation of mitochondrial dynamics.
3. The International Symposium on Fungal Stress
Fungi are exposed to a multifaceted set of abiotic or biotic stresses in the environment, including during fungal pathogenesis when infecting plants or animals, and during industrial processes for the production of biotechnological goods. Understanding how benevolent and dangerous fungi perceive stress is important to augment the benefits and mitigate the harm. Although research on fungal stress is not new, for example, Anita Panek published her first studies around 1960 on the importance of trehalose in yeasts (Panek, 1959, 1962, 1963), the International Symposium on Fungal Stress is the only meeting that specifically brings together renowned scientists exploring this area. (Alder-Rangel et al., 2018; Alder-Rangel et al., 2020; Rangel and Alder-Rangel, 2020; Rangel et al., 2015a; Rangel et al., 2015b; Rangel et al., 2018). The four ISFUS meetings to date have featured a total of 126 speakers from 24 countries and all continents (Figure 6).
Figure 6.
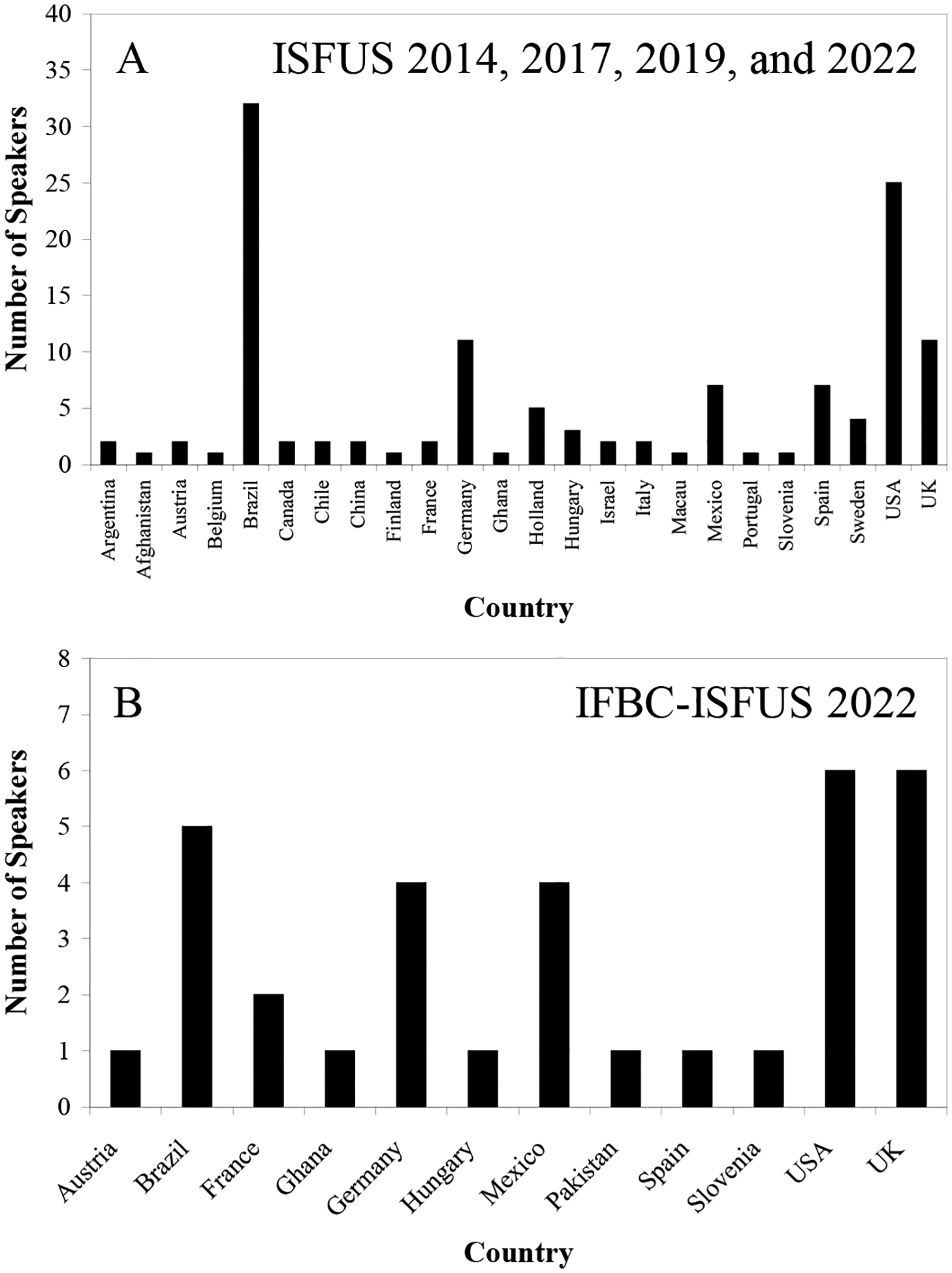
A) The countries of speakers at the ISFUS 2014, 2017, 2019, and IFBC/ISFUS 2022. B) The countries of speakers at the IFBC/ISFUS 2022
The ISFUS is the brainchild of Dr. Drauzio E.N. Rangel. In July 2013, he began to organize the Symposium, invite speakers, and apply for grants. His wife, Alene Alder-Rangel became the co-chairman and brings in her experience as an event planner. ISFUS has always been organized by the Rangels in Brazil and has become a well-recognized international fungal conference (Figure 7).
Figure 7.

The Meet the Speakers Poster for the International Symposium on Fungal Stress
The first ISFUS occurred on October 25–31, 2014, at the Universidade do Vale do Paraiba (Univap) in São José dos Campos, São Paulo http://web.univap.br/isfus. As a visiting professor in Goiás at the Universidade Federal de Goiás, Rangel organized the second ISFUS held in Goiânia, Goiás, from May 8 to 11, 2017 https://isfus.wordpress.com. The third and fourth ISFUSs were both held at the Hotel Nacional Inn, in São José dos Campos. The third ISFUS occurred May 20–23, 2019 https://isfus2019.wordpress.com. To continue the biennial schedule, this fourth ISFUS was originally scheduled to take place in May 2021, but was delayed until the fall of 2022 https://isfus2022.wordpress.com because of the Covid-19 pandemic (Rangel et al., 2020).
Since the first meeting in 2014, ISFUS has achieved great success, receiving substantial research grants from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), both from Brazil, to bring together the top international scientists (Figure 8). The grant money provided for ISFUS, in Brazilian currency, from both grant agencies has been similar for all the symposia, approximately 100 thousand Brazilian Reais from FAPESP and approximately 45 thousand Brazilian Reais from CAPES (Figure 9). However, the serious devaluation of the Brazilian currency against the US Dollar in recent years limited the ability to pay for international travel to Brazil in 2022, but many of the invited speakers gladly paid their own expenses to participate in this high-level meeting (Figure 9).
Figure 8.

Funding and sponsors of the IV International Symposium on Fungal Stress (ISFUS) & XIII International Fungal Biology Conference (IFBC)
Figure 9.
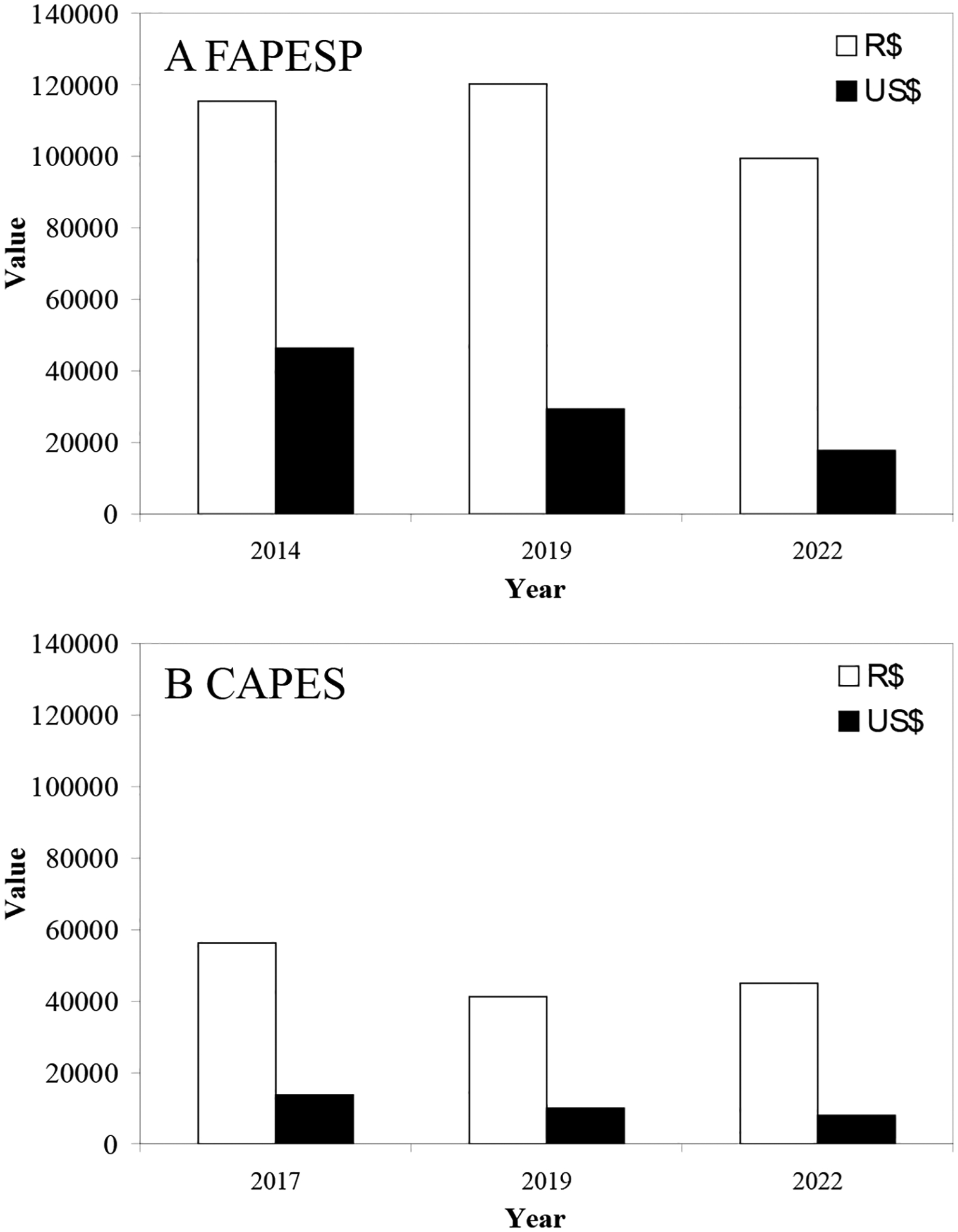
Values of the grants obtained from A) FAPESP and B) CAPES
Most speakers have published an article related to their talks in a special issue in leading journals in the field. In the first meeting, all speakers published in a special issue in Current Genetics. From the second to the fourth meeting, articles from the speakers have been published in three special issues in the top mycology journal, Fungal Biology, published by Elsevier on behalf of the British Mycological Society. The number of articles in the special issues increased from 19 articles in ISFUS 2014, 27 articles in ISFUS 2017, to 32 articles in ISFUS 2019. Approximately 18 articles will be published in this Special Issue for IFBC-ISFUS 2022 (Figure 10).
Figure 10.
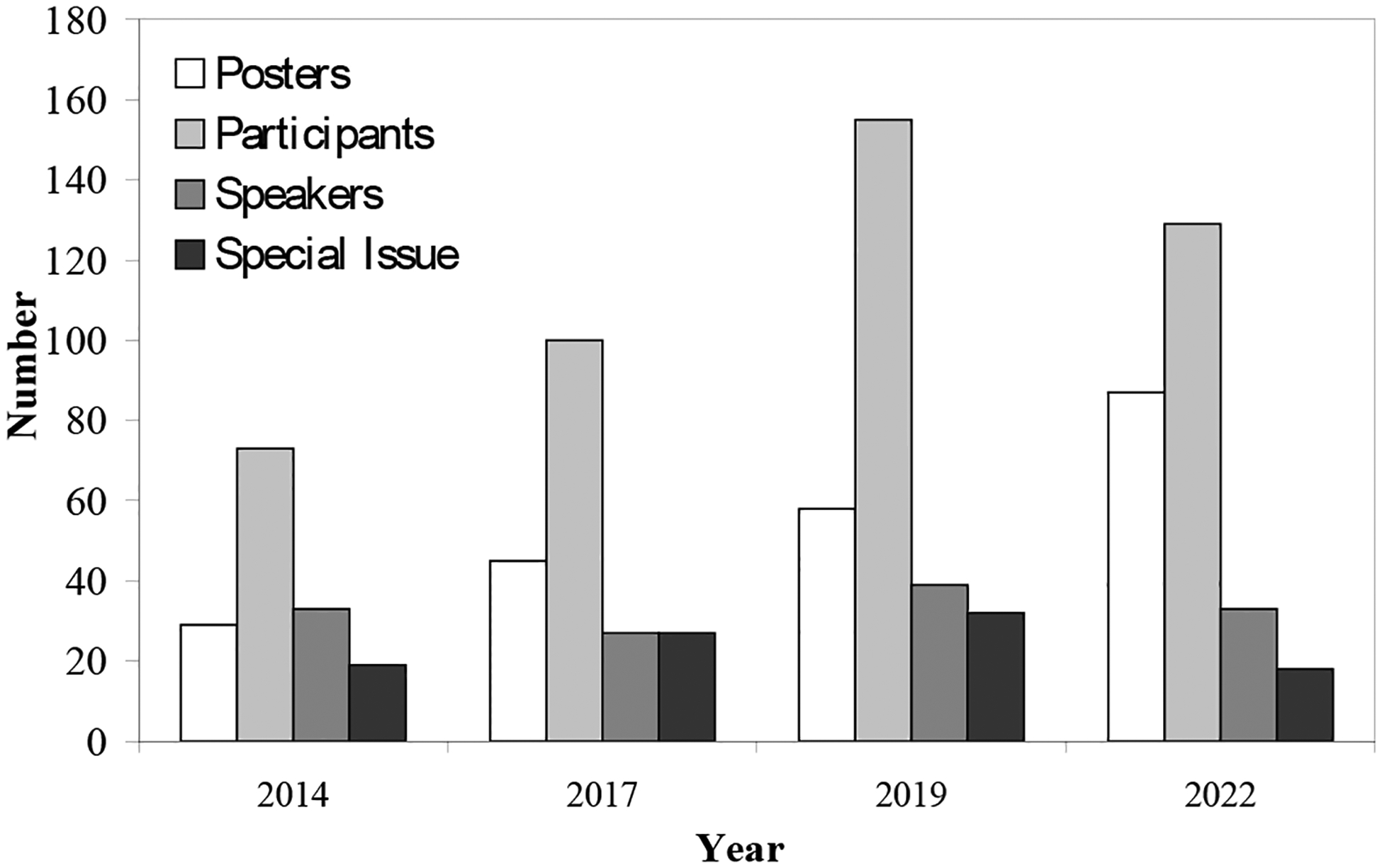
Number of presented posters, number of participants, number of speakers, and number of publications in each special issue.
The number of poster presentations has also increased from 29 poster presentations in ISFUS 2014, 45 posters in ISFUS 2017, 58 posters in ISFUS 2019, to 87 posters in IFBC-ISFUS 2022 (Figure 10).
The number of participants rose from 73 people in ISFUS 2014, 100 people in ISFUS 2017, to 155 people in ISFUS 2019 (Figure 10). The small reduction to 129 participants in the IFBC-ISFUS 2022 was due to uncertainties related to the Covid 19 pandemic (Rangel et al., 2020) as well as increased airfare prices. The following section summarizes the presentations at the VI ISFUS.
3.1. ISFUS – Session 1: Fungal biology in the environment
Extraordinary discoveries are continuously being made about the biology of fungi in all kinds of habitats. The organic and inorganic transformations that fungi carry out for ecosystem function and human health were discussed in this session. The production of volatile organic compounds by fungi is well known, but their significance in inter-organismal communication or in affecting human health is less understood. The latter might be significant in buildings where flooding, water ingress, and condensation can lead to health-threatening fungal proliferation. Black melanized fungi are now known to be ubiquitous in all kinds of environments, including those with to extremes of temperatures, desiccation, and solar irradiation. Certain halotolerant “black yeasts” thrive in marine habitats of high salinity. Studies of these extreme fungi have extended the known physico-chemical parameters for fungal survival and revealed unique cellular mechanisms underlying halotolerance. The formation of minerals is a feature of many fungal metal and mineral transformations, which is important in elemental cycles for many metals and other elements, e.g., phosphorus. The fungal production of nanoscale minerals can have surprising benefits for the organism, e.g., certain metal oxyhydroxide nanoparticles forming a protective sheath around hyphae can catalyze key organic and inorganic environmental transformations, including pollutant degradation.
Joan W. Bennett (Rutgers, State University of New Jersey, New Brunswick, NJ, USA) was planning to continue her research on mycotoxins when her home in New Orleans got flooded by hurricane Katrina and its contents became fodder for fungi (Bennett, 2015). The horrible odor associated with the flooded house redirected her research to fungal volatile organic compounds (VOCs). Subsequently, her laboratory has studied the physiological effects of these low molecular weight molecules in genetic models and found that 1-octen-3-ol can cause Parkinsonian symptoms in a Drosophila melanogaster model (Inamdar et al., 2013) and that decene can enhance growth in Arabidopsis thaliana (Lee et al., 2019). Numerous other fungal and plant VOCs have major – but underappreciated – physiological consequences in inter-organismal communication (Gomes et al., 2020; Hung et al., 2015; Inamdar et al., 2020; Jaddaoui et al., 2023).
Nina Gunde-Cimerman (University of Ljubljana, Ljubljana, Slovenia) explained that Hortaea werneckii is a cosmopolitan black yeast, which was known only as the primary etiological agent of tinea nigra. Later, it was discovered that marine eutrophic solar salterns around the world are its primary ecological niche. H. werneckii can grow across a wide range of NaCl concentrations, from 0% to 32% at saturation, with a broad optimum range of 6% to 14% NaCl. It is an important model organism for the study of halotolerance in Eukarya (Gunde-Cimerman et al., 2018; Gunde-Cimerman et al., 2000). The molecular responses to extremely saline conditions involve rigorous changes in gene expression, influencing synthesis of compatible solutes, regulation of intracellular alkali-metal cations, cell membrane fluidity, and changes in cell wall ultrastructure (Gunde-Cimerman et al., 2018). Genome sequencing of the reference H. werneckii strain (EX – F 2000) revealed an almost 50 Mb genome, initially considered as a result of genome endoreplication. Sequencing of genomes of 50 strains originating from around the world revealed that one-third of the population of H. werneckii is represented by 1n genomes, while two-thirds are 2n, due to recent hybridization events. The resulting hybrids are stable. They do not undergo recombination, as typical for sexual fungi, or loss of chromosomes, characteristic for parasexual processes. This new mode of reproduction, for the first time described in wild populations of extremophilic fungi, was named “stable parasexuality” (Gostincar et al., 2022).
Geoffrey Michael Gadd (University of Dundee, Dundee, Scotland, UK) discussed fungal biomineralization that occurs in natural and human-influenced habitats. Common fungal biominerals include oxides, carbonates, phosphates, and oxalates. Highly insoluble minerals immobilize toxic metals, e.g., lead and uranium phosphates, while biomineralization is also evident in fungal biodeterioration of the built environment and cultural heritage. Some fungal biomineralization processes result in nanoscale mineral and metallic products (Li et al., 2022). Nanoparticles of e.g., Ag, Se, and Te are produced by a range of fungal species. Nanominerals, including oxides, carbonates, and phosphates, can incorporate metals such as Cu, Cd, Zn, Mn, Ni, Fe, Pb, and Sr (Liu et al., 2021). Such nanoscale products have applications as electrochemical materials and catalysts (Chi et al., 2022a; Li et al., 2022; Liu et al., 2021). Some nanoparticles benefit the fungus because of their catalytic properties, behaving as nanozymes that exhibit enzyme-like properties (Chi et al., 2022a; Yu et al., 2020). Fungal formation of reactive metal oxyhydroxide nanoparticles results in a cytoprotective “exoskeleton” around hyphae, which has peroxidase activity, conferring protection from oxidative stress, ensuring iron acquisition, and enabling organic pollutant breakdown through production of oxidant HO• radicals (Chi et al., 2022b). Such findings highlight the significance of biogenic nanomaterials in evolution of the biosphere and the potential of fungal biomineralization in nanobiotechnology.
3.2. ISFUS – Session 2: Stress in fungal pathogenesis
This session on the effects of stress on fungal pathogenesis presented some complex challenges and exciting work in fungal biology. Latgé described how rigorous studies over a half-century have not fully elucidated the mechanisms of cell wall dynamicity. For example, Aspergillus fumigatus has different regulatory processes for the walls of hyphae and conidia. The adaptability of fungi was highlighted by Gácser who detailed how Candida parapsilosis and C. auris readily acquire resistance and cross-resistance after antifungal exposure, and the complexity of the resistance mechanisms. The effect of the host environment was discussed by Nosanchuk, who demonstrated how Histoplasma capsulatum changes its production of extracellular vesicles in response to stressors such as nutritional resources and antibody binding. Further exploring the importance of interactions between fungi and other cells, Naz characterized how bacteria, such as Pseudomonas aeruginosa, produce peptides with antimicrobial activity against diverse Candida species and how these peptides could be harnessed as antifungal compounds. Quinn expanded on the role of bacteria in polymicrobial communities through the lens of Type VI secretion systems producing antifungal effectors that inhibit diverse microbes, including pathogenic fungi.
Sehar Afshan Naz (Federal Urdu University of Arts, Science and Technology, Gulshan Iqbal, Karachi, Pakistan) discussed the emergence of fungal infections at an alarming rate, particularly among the population of immune compromised individuals. Candidiasis is one of the predominant fungal infections with manifestations ranging from mild superficial infections to severe invasive infections. These infections are difficult to treat because of the growing resistance to antifungal drugs. To overcome this issue, antimicrobial peptides produced by bacteria are being investigated for potential antifungal activity. Pseudomonas aeruginosa HS 28, a Gram-negative bacterium, demonstrates appreciable bioactivity against C. albicans and non-albicans Candida species (C. tropicalis, C. glabrata, C. kruzei, C. lipolytica., etc.). This antifungal metabolite was isolated, purified, and characterized. Exposure to low temperature (4 to −20 ℃), high temperature (up to 40 ℃), varying pH (5–8), metallic salts (BaCl2, CaCl2, HgCl2, etc.), and organic solvents (acetone, butanol, ethanol, etc.) has no drastic effects on its bioactivity. Further demonstration of its antifungal activity in vivo is required before presenting it as an addition to the current antifungal chemotherapy (Naz et al., 2015; Simons et al., 2020; Tumbarski et al., 2021).
Janet Quinn (Newcastle University, Newcastle upon Tyne, England, UK) discussed the emerging role of the bacterial Type VI secretion system (T6SS) in surviving polymicrobial environments by producing antifungal effectors against fungal competitors. She explained that although the primary role assigned to the T6SS is in inter-bacterial competition, recently it was shown that this ‘anti-bacterial’ T6SS is also a potent antifungal weapon, able to kill model and pathogenic yeasts by delivering two dedicated antifungal effectors, Tfe1 and Tfe2 (Trunk et al., 2018). Antifungal T6SS is likely to be widespread and possibly shape many diverse polymicrobial communities (Trunk et al., 2019).
Jean-Paul Latgé (University of Crete Heraklion, Greece) explained that the fungal cell is surrounded by a thick cell wall that protects the fungus against external environments. Despite 50 years of molecular and biochemical studies, the cell wall remains poorly understood, especially the role of its modifications during growth in different environments. At the level of the cell wall, A. fumigatus has set up several stratagems to fight stress. First, the distinct cell wall structural organization between the conidium and mycelium facilitates the completion of its life cycle and survival in different habitats. Second, the heterogeneity of the cell wall composition of each cell within a conidial population or hyphal cells within the same colony will help the fungus handle hostile surroundings. Finally, tolerance and resistance to antimicrobials are concepts well studied in the bacterial world but are just beginning to be investigated in mycology.
Joshua D. Nosanchuk (Albert Einstein College of Medicine, The Bronx, NY, USA) discussed the complexity of environment sensing by fungi (Liu et al., 2023). In response to their environment, diverse fungi form and release extracellular vesicles (EVs) carrying a broad array of compounds. An example of this is the modifications in the characteristics of EVs produced by the human pathogenic fungus, Histoplasma capsulatum, in response to changes in nutritional resources (Cleare et al., 2020). The binding of an antibody to the fungal surface is often simply looked at as a docking of a protein to the cell that enables host effector cells to more effectively recognize and phagocytose the invading microbe. However, the binding of a monoclonal antibody to heat shock protein 60 on the surface of H. capsulatum yeasts can induce a cascade of changes, including significantly altering the loading and release of EVs (Matos Baltazar et al., 2016). This antibody-mediated process of EV formation and release is dynamic and highly regulated as changes in antibody concentration markedly shift the compositions of the H. capsulatum-derived EVs (Baltazar et al., 2018). These findings demonstrated a new mechanism for antibody function in microbial pathogenesis. Furthermore, antibody binding to the surface of H. capsulatum results in global shifts in MAPK signaling, sterol metabolism, ubiquitin-mediated proteolysis, and fatty acid metabolism (Burnet et al., 2020). The study of EVs from H. capsulatum has provided new and interesting insights into the biology of fungi (Zamith-Miranda et al., 2021) and opened diverse avenues for further study.
Attila Gácser (University of Szeged, Szeged, Hungary) lamented the limited number of applicable antifungal drugs. Therefore, it is essential to understand the possible mechanisms of antifungal resistance development and their effect on virulence to optimize antifungal treatment strategies in the clinical setting. To address this later question, Gácser and colleagues used directed adaptation-evolution experiments to generate Candida parapsilosis and C. auris strains with acquired resistance to azoles and echinocandins. Their study aimed to examine the effect of acquired antifungal resistance on various physiological features – such as antifungal cross-resistance, stress response, membrane sterol composition, efflux pump activity – as well as virulence characteristics. Gácser’s data indicate that resistance development may occur through different mechanisms that can also alter the virulence of both C. parapsilosis and C. auris. These results highlight the consequences of prolonged drug use and suggest the need to develop alternative antifungal treatment strategies in clinical practice (Bohner et al., 2022; Papp et al., 2020; Papp et al., 2018).
3.3. ISFUS – Session 3: Stress mechanisms and responses in fungi
The stress mechanisms and responses discussed during this session covered a broad range of topics, including single-cell responses to salt stress in yeast, physiological conditions affecting fungal conidiation, control of fungal pathogens and fungal spoilage, and stress responses of fungal-beetle mutualists. In salt stress, yeast continues to provide novel insights on how repression of genetic networks allows for coupling to translational programs that help maximize the ability of cells to rapidly respond and recover from osmotic stress. Conidiation can be considered as the last step in the lifecycle of many fungi, and the results presented reinforced the concept that these cells are dynamic and that their viability and ability to respond to stress are intimately linked to the conditions in which conidiation occurs and conidia mature. Stress can also be exploited to control fungal pathogens, and manipulation of stress conditions in terms of mitigating fungal-caused spoilage can lead to decreased usage of fungicides. Finally, fungi were also recognized as having mutualistic interactions with animals, in this case, ambrosia beetles. The way in which stress (pH) may affect their interaction was investigated.
Audrey Gasch (University of Wisconsin Madison, Madison, WI, USA) presented new results focusing on single-cell responses of Saccharomyces cerevisiae to salt stress. Gasch highlighted the power of genome-scale studies of stress response, including transcriptomic analysis to study how the transcriptome changes in response to diverse stresses (Gasch et al., 2000). A major question in the field has been the purpose of repressing genes involved in growth as part of the common environmental stress response (ESR), a conserved stress response activated in other fungi and organisms. Gasch’s research followed dynamic changes in transcription factor localization using live, single-cell microscopy. Remarkably, cells with stronger activation of the repressor of growth-promoting genes showed faster growth acclimation after stress. Gasch integrated results from several recent studies (Ho et al., 2018; Lee et al., 2011) to present a model in which stress-activated transcription repression in fungi helps to redirect translational capacity of stress-induced genes to promote acclimation.
Drauzio E.N. Rangel (Universidade Tecnológica Federal do Paraná, Dois Vizinhos, PR, Brazil) explained how physiological, physical, and chemical conditions during mycelial growth may induce conidial priming. Conidial priming is defined as an exposure of a fungus to gentle stress during mycelial growth that leads to the development of conidia, which primes the conidia to become more tolerant to the same or other stress conditions. Mycelial growth under nutritive stress (Rangel et al., 2006), heat shock, osmotic and oxidative stresses (Rangel et al., 2008), salicylic acid (Rangel et al., 2012), hypoxia and alkaline stress (Rangel et al., 2015c), white and blue light (Dias et al., 2020; Dias et al., 2022; Dias et al., 2021), hypoxia and anoxia (Silva et al., 2023), or biotic stress caused by a deadlock formed with Metarhizium robertsii and Trichoderma atroviride dual culture (Medina et al., 2020) all induce higher conidial tolerances to UV-B radiation, heat, osmotic, or oxidative stresses, which is caused by higher accumulation of trehalose and mannitol inside the conidia (Rangel et al., 2008; Rangel et al., 2015c). Here, to study the magnetic and electric fields on conidial priming, the insect-pathogenic fungus M. robertsii was cultured in the dark on potato dextrose agar medium (PDA = control), on PDA medium under magnetic field (MF) or electric field (EF), and under nutritive stress minimal medium (MM = positive control). The tolerance of conidia produced in these conditions to oxidative and osmotic stress, heat, and UV-B radiation were evaluated and the fungus showed no difference in vegetative growth. However, the three treatments (PDA, MF, and EF) yielded more conidia than the control on the MM. M. robertsii conidia produced under MF and EF were more tolerant to oxidative and osmotic stress, heat, and UV-B radiation than the conidia produced on PDA medium alone (control). Both treatments (MF and EF) produced conidia with similar tolerance to all stress conditions tested, except for osmotic stress, where conidia from MF were more tolerant than conidia from EF. On the other hand, conidia produced under MF and EF were less tolerant than conidia produced on MM, which causes nutritional stress. In conclusion, both MF and EF induced priming on M. robertsii but at lower level than nutritive stress.
Simon Avery (University of Nottingham, Nottingham, UK) emphasized how traditional approaches to control fungal pathogens and fungal spoilage that rely on the use of chemical actives suffer from problems of resistance and tightening regulations. He described how combinations of agents that synergistically stress fungi can reduce total chemical usage, and this approach may be particularly appealing for synergies between natural products, as these are more accepted (Augostine and Avery, 2022; Davies et al., 2021; Harvey et al., 2023). Avery explained the mechanistic basis for synergy before progressing onto how fungal control measures could avoid chemical actives altogether. Specifically, polymer materials that passively resist fungal attachment were discovered in high-throughput screening programs (Vallieres et al., 2020). This blocks a key first step that precedes many of the problems that fungi cause. He presented data supporting applications of this approach in medical materials and crop protection.
Nemat Keyhani (University of Florida, Gainesville, FL, USA) focused on Harringtonia (prev. Raffaelea) lauricola (Ascomycete: Ophiostomataceae), a filamentous fungus that forms obligate mutualisms with ambrosia beetles in the genus Xyleborus. It is also the causative agent of laurel wilt, a devastating disease that affects members of the Lauraceae family, including avocado. Vectored by its beetle symbiont within specialized fungal transport organs termed mycangia, this fungus can infect the vascular system of healthy trees, leading to wilting and death (Joseph and Keyhani, 2021). Keyhani showed that R. lauricola grows at an optimal pH range from 5–7, displays cold adaptation, and can utilize a wide range of phosphorus and sulfur containing compounds, as well as its fungicide susceptibility (Joseph et al., 2021; Zhou et al., 2018). Aspects of mycangial colonization that have been examined include the dynamics of initial colonization as well as fungal growth and proliferation within the mycangia. These data are the first steps toward identifying determinants of fungal-insect symbionts as a new model system for understanding the diversity of fungal genetics and development (Zhou et al., 2020).
3.4. ISFUS – Session 4: Fungal stress in industry; Fungal stress in agriculture, medicine; ultraviolet radiation, heat, and other stresses in fungal biology
The final section discussed wars fought with opposing objectives, while in industry and agriculture that harness the benefits of fungi or their products, the war is against the fragility of fungi to stress, in medicine, the battle is to fight against fungal resilience to stress. On the one hand, Basso and Acheampong study stress in fungi to look for ways to increase their resistance or mitigate its deleterious effects; on the other hand, Bailão and Campos study stress in fungi to be able to reduce resistance to them. In any case, the physiological and molecular mechanisms behind resistance to stress must be known to achieve the goal of war: be able to manipulate the resistance of fungi to different types of stress. What unites these very different areas is the need to know and understand the mechanisms underlying adaptation and to apply this knowledge for the benefit of society.
Thiago Olitta Basso (Universidade de São Paulo, São Paulo, SP, Brazil) stated that industrial biofuel production is severely affected by bacterial contamination (Walker and Basso, 2020). These bacteria interact with the fermenting yeast and compromise yeast fermentative efficiency, leading to a drop in industrial yield and productivity (Basso et al., 2014). Additional obstacles arise in the second-generation ethanol production process, where lignocellulosic residues are the substrates for fermentation. The pretreatment processes of these substrates generate a variety of molecules (furanic compounds, phenolic compounds, and organic acids) that inhibit microbial metabolism (Cola et al., 2020). Basso discussed the effects of furanic compounds on the physiology of lactic acid bacteria (LAB) strains that are potential contaminants in ethanol production. In general, homo-and heterofermentative LAB are affected differently by furanic compounds (Giacon et al., 2022). Studies involving co-cultivation of S. cerevisiae and species of lactobacilli were used to understand the potential effects of bacterial contamination in second-generation bioprocesses.
Mavis A. Acheampong (University of Ghana, Accra, Ghana) presented on three biological trait studies (temperature tolerance, humidity requirement, and UV sensitivity) of selected entomopathogenic fungal isolates being developed for use in citrus Integrated Pest Management programs in South Africa. Her studies aimed to establish why three highly virulent entomopathogenic fungal isolates yielded good control of subterranean stages of the key phytosanitary pest of citrus, false codling (Thaumatotibia leucotreta), following soil application, while foliar application using the same isolates resulted in insignificant control of citrus mealybug (Planococcus citri) and thrips (Scirtothrips aurantii). The findings showed that neither temperature nor humidity, when considered alone, is likely to significantly influence the efficacy of any of the isolates in the field, given that they are active within the temperature and humidity ranges experienced in South African citrus orchards (Acheampong et al., 2020a). However, all isolates investigated were highly sensitive to UV radiation (Acheampong et al., 2020b). These findings indicated that a suitable UV protectant formulation of these fungi or a different application strategy would be required for success against P. citri and S. aurantii.
Alexandre Melo Bailão (Universidade Federal de Goiás, Goiânia, GO, Brazil) explained that the human pathogenic fungus H. capsulatum is poisoned with copper in the macrophage phagosome. The increase of Cu in phagosomes is mediated by ATP7a since its silencing decreases fungicidal activity of phagocytes. To avoid copper toxicity, H. capsulatum activates the copper efflux pump Crp1. Furthermore, the copper chaperone Ccs1 is induced in high copper conditions, which likely buffer free copper inside the fungal cells. The adaptive mechanism to high Cu is coordinated by the transcription factor Ace1, as it activates Crp1 and Ccs1 in such a condition. The Cu detoxifying machinery is essential to H. capsulatum virulence since depletion of both Crp1 and Ace1 decreases fungal burden in macrophages. Additionally, excess copper promotes changes in cell wall structure/composition, increases ROS content, and activates lipid turnover, as revealed by proteomic analysis.
Claudia B. L. Campos (Universidade Federal de São Paulo, São José dos Campos, SP, Brazil) works with Paracoccidioides spp., the agents of a systemic human mycosis endemic in Brazil and other South American countries. Campos’ group aimed to understand how calcineurin regulates fundamental processes that support cellular adaptation of Paracoccidioides spp. to environmental changes, such as thermo-dimorphism and proliferation, which are two hallmark virulence factors that enable these fungi to succeed within the host and cause disease. Proteome analysis showed that calcineurin inhibition by cyclosporine A in yeast cells of P. brasiliensis led to an overall reprograming of the primary metabolism, including lipid synthesis and degradation. Using anti-PbCNA, Campos demonstrated that calcineurin is found in few yeasts in exponential cell cultures and that most of the calcineurin-labeled yeast cells have a low number or lack lipid droplets (LD). Campos’ group evidenced that calcineurin suits metabolism to the conditions imposed by the environment and that the regulation of the production/consumption of LD is part of the adaptation changes required for P. braziliensis fitness (Campos et al., 2008; Matos et al., 2013; Ribeiro et al., 2018).
4. Student Awards
The presentation of the student awards added to the family atmosphere of the IFBC/ISFUS because Amanda E. Alder Rangel, the 10-year-old daughter of the organizers, announced the awardees and handed out the awards. The international invited speakers judged the students’ work before (Elsevier award) and during the symposium for their poster presentations (Journal of Fungi award).
4.1. Elsevier student awards
Similar to the past three ISFUSs, Elsevier sponsored an award to recognize student research. To apply for the award, students had to submit a manuscript and present their research. Only one student submitted an article. Karla Cecilia Licona Juárez, a PhD student from the Instituto Tecnológico de Celaya in México studying under Humberto Medina, submitted the article: Tolerance to UV-B radiation of the entomopathogenic fungus Metarhizium rileyi to develop a microbial agent for management of the main lepidopteran species in soybean and cotton crops. She received a bronze award and US$200.00 and received a big hug from Amanda (Figure 11).
Figure 11.

Amanda E.A. Rangel delivering the Elsevier award to Karla Cecilia Licona Juárez from the Technological Institute of Celaya, Guanajuato, México
4.2. Journal of Fungi student poster award
Of the 87 posters presented at the IFBC/ISFUS, 20 were eligible for the student poster award sponsored by the Journal of Fungi. The student had to be the first author and present their work to the judges during the poster sessions. The posters reflected the wide variety of topics presented at the Symposium. Moreover, the awardees were a good representation of the international participation in the Symposium (Figure 12). Two gold awards won R$ 1000 (US$ 200) each and five silver awards won R$ 600 (US$ 120) each. The silver awards were presented to the following students.
Figure 12.

Delivery of the Journal of Fungi Award for the student posters delivered by Amanda E.A. Rangel (Brazil) and Alene Alder-Rangel (United States). From left to the right: Bhagya C Thimmappa (India), João Neves da Rocha Fonseca (Brazil), Philipp Ernst (Germany), Lennart Greifenhain (France), Natalia Sayuri Wassano (Brazil), Everton Paschoal Antoniel (Brazil), and Nadine Hochenegger (Austria).
Philipp Ernst is a PhD student studying Industrial Biotechnology at the Forschungszentrum Jülich, in Germany, with Nick Wierckx. His poster was about the production and characterization of the itaconic acid-derived compounds 2-hydroxyparaconic and itatartaric acid.
Nadine Hochenegger is a PhD student studying Microbiology at TU Wien in Vienna, Austria, and she is supervised by Dr. Robert Mach. Her poster looked at the transcription factor Xpp1 interacting with SREBP Sah2 to regulate primary and secondary metabolism in Trichoderma reesei.
Lennart Greifenhain was a master’s student in Molecular Biotechnology at Ruprecht-Karls-University Heidelberg, Germany. He presented the study based on bioimaging that he did during his internship with Robert Arkowitz at the Côte d’Azur University in Nice, France. Greifenhain’s poster was about the physical properties of the cytoplasm and cell wall in the human fungal pathogen Candida albicans during morphogenesis and antifungal drug stress.
Natalia Sayuri Wassano is a PhD student in the Program in Functional and Molecular Biology at the Universidade Estadual de Campinas, in Campinas, SP, Brazil. She is studying with André Ricardo de Lima Damasio. Her poster looked at understanding the roles of sirtuins in A. fumigatus.
Everton Paschoal Antoniel is a PhD student in Applied Microbiology at the Universidade Estadual de Campinas, in Campinas, SP, Brazil also studying with André Ricardo de Lima Damasio. His poster was entitled: Identification of transcription factors involved in Aspergillus nidulans adaptation to recombinant protein production.
The gold award went to João Neves da Rocha Fonseca, who is studying for his PhD in Genetics with Nilce Maria Martinez Rossi at the Universidade de São Paulo in Ribeirão Preto, SP, Brazil. His poster discussed a transcriptome meta-analysis about how alternative splicing modulates fungal metabolism by establishing a crosstalk between transcription and post-transcription machineries.
The top poster was presented by Bhagya C. Thimmappa, who is originally from India and currently studying for her PhD at the University of Montreal in Quebec, Canada, with Gertraud Burger. Her poster was titled: Plant pathogen resistance and growth promotion: the role of fungal endosymbionts (Secret life inside plants: the role of fungal endosymbionts). In addition, she was honored to be awarded by Joseph Heitman with a book on Mycota.
5. Research in Germany – Fellowships, Exchange, and Collaboration Programs
On Monday afternoon of IFBC/ISFUS, there was a special presentation by the organization and sponsor DFG in cooperation with DAAD. Deutsche Forschungsgemeinschaft (DFG) is the central self-governing research-funding organization in Germany. The DFG funds research projects and facilitates national and international collaboration among researchers. Scientific and academic excellence, the advancement of early career researchers, interdisciplinary and internationality, and gender equality in research are key elements in the work of the DFG. In cooperation, the German Academic Exchange Service (DAAD) is the largest organization promoting academic and scientific exchange in the world. The DAAD acts as a mediator of German foreign scientific, university, and development policy.
The goal of their presentation at IFBC/ISFUS was to offer comprehensive information on the German research landscape and on funding opportunities for researchers at all career levels. The program included the participation of Michael Feldbrügge of Heinrich-Heine University Düsseldorf, Germany, who presented some of his research history in the field of microbiology, and exemplified his work in international cooperation, citing the project “Determinants of polarized growth in fungi,” conducted in partnership with México and funded by the DFG and CONACYT.
In addition, Katherine Kistner (DFG Department of Life Sciences 1) and Christina Peters (Director of DFG Office Latin America) presented to the audience the main DFG research funding instruments and gave application tips for those who want to develop their research in Germany or in cooperation with Germany. Francine Camelim from DAAD Brazil also presented DAAD’s fellowship modalities for doctorates. It was an excellent opportunity to exchange ideas (Figure 13).
Figure 13.

Poster session at IFBC/ISFUS and the stand of the initiative “Research in Germany” from the DFG Office Latin America and DAAD Brazil
6. Excursion
Several scientific collaborations have begun during the excursions after the ISFUSs, proving that the excursions are an important part of the meeting and a very good way to become better acquainted with colleagues.
The ticket for the excursion included the bus to São Sebastião (Figure 15), schooner expedition (boat trip) (Figure 16), and barbecue onboard the boat. However, the stormy weather prevented the barbecue onboard. Instead, the boat picked up the participants at the pier in São Sebastião and took everyone to the island of Ilhabela (which means Beautiful Island), which is about 50 minutes by boat. On Ilhabela, the weather cooperated, and everyone enjoyed strolling through the old villa and purchasing their souvenirs (Figure 17). Amanda even played in the water.
Figure 15.

Participants and speakers of the IFBC/ISFUS in the excursion bus to the beach in São Sebastião, São Paulo, Brazil.
Figure 16.

Participants and speakers of the IFBC/ISFUS onboard of the boat going from São Sebastião to the island Ilhabela, São Paulo, Brazil.
Figure 17.

Participants and speakers of the IFBC/ISFUS during the excursion to the beach in São Sebastião, São Paulo, Brazil: A) Senior editors of Fungal Biology Geoffrey Michael Gadd and Simon Avery enjoying the Barequeçaba beach. B) The fun-guys Joe Heitman and his wife Mary Tayal, Martine Bassilana, Robert Arkowitz, and Drauzio Rangel having some “caipirinhas” and enjoying a good conversation the Barequeçaba beach. C) Speakers and Participants disembarking the schooner boat to visit the small villa in Ilhabela (Beautiful Island). D) The meeting chairs Jesús Aguirre and Drauzio Rangel visiting a colonial church in Ilhabela.
After spending about two hours on Ilhabela, the boat returned to São Sebastião, and the participants returned to the Hotel Brisa do Mar Barê in Barequeçaba beach where a Brazilian barbecue was waiting for the famished participants. Despite the temperate weather, many enjoyed swimming in the warm Atlantic water.
7. Conclusion
Some doggerel for the Rangel’s to say thank you
How to say it, so that it sounds new?
Our collective gratitude is deep and true.
Who would ever have guessed,
It could be such fun to study fungal stress.
Hard work behind this meeting scene
Was done by Drauzio and Alene
Despite hard pandemic living
Their passion for systemic giving
Brought us together in a Brazilian city
They formed with Amanda -- our very own welcome committee.
We know that fungi are the best
As models much better than the rest.
Among our topics academic
Was lots of membrane traffic
A good break from war in Ukraine
To focus on the fungal membrane.
Cell wall images with GFP stain
Here in the land of sugar cane.
Hyphal branching, yeast cells dancing
Heitman’s data, most complex
Hints that fungi invented sex.
Tales of a germinating spore,
And so very much more
(How I wish something rhymed with Spitzenkörper).
Let me end with a cliché
Warm hospitality is never passé
May God bless the Rangels
To them we’re all thankful.
May you like the contents
Of these mycological presents.
By Joan W. Bennett, member of the US National Academy of Sciences
Joan Bennet read this poem as part of the closing ceremony of the IFBC/ISFUS. After that, everyone (speakers and participants) was invited to the stage for a final picture to celebrate and commemorate our time together (Figure 18). Then Amanda stood at the exit of the auditorium offering hugs to everyone willing to receive them.
Figure 18.

Ending of the IFBC/ISFUS meeting with most of the participants and speakers on stage
The IV International Symposium on Fungal Stress (ISFUS) and the XIII International Fungal Biology Conference (IFBC) brought together scientists from around the world to present their work, learn from each other, and make friends or strengthen existing bonds. As with previous versions of these meetings, the focus was on fungal stress and fungal biology, and convened ~120 colleagues over four days of outstanding scientific presentations. An important contribution to the meeting was vibrant poster sessions featuring students and fellows, and their recognition through the awards program. As is a tradition with this meeting, the invited participants had a post-meeting shared adventure at the Brisa do Mar Barê Praia Hotel located on the seafront, right on the sand on Barequeçaba beach, in São Sebastião, including a boat trip to visit the nearby island. As a community, we are indebted to Drauzio Rangel for his vision and his effort organizing this highly successful international meeting over the years.
Given the success of this meeting, plans are already well underway for the V International Symposium on Fungal Stress (ISFUS) to be held September 22 – 26, 2024 https://isfus2024.wordpress.com. The meeting will be sponsored by the Universidade Tecnológica Federal do Paraná (UTFPR) – Dois Vizinhos, PR, Brazil. It will be held in Iguazu Falls, Brazil, which will facilitate the post-meeting adventure to visit the famous and stunningly gorgeous Iguaçu Falls from both the Brazilian and the Argentinian vistas. We welcome you to join us for a continued celebration and exploration of the luster of the Fungal Kingdom and the beauty of the natural world.
Supplementary Material
Figure 14.

Participants at the IFBC/ISFUS in the auditorium and at the coffee break
Acknowledgments
This article is part of the “Fungal growth, development, and stress responses” special issue for the XIII International Fungal Biology Conference (IFBC) & IV International Symposium on Fungal Stress (ISFUS), which is supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) grant 2021/13614-3 and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) grant 88887.680665/2022-00.
References
- Acheampong MA, Coombes CA, Moore SD, Hill MP, 2020a. Temperature tolerance and humidity requirements of select entomopathogenic fungal isolates for future use in citrus IPM programmes. J Invertebr Pathol 174, 107436. 10.1016/j.jip.2020.107436. [DOI] [PubMed] [Google Scholar]
- Acheampong MA, Hill MP, Moore SD, Coombes CA, 2020b. UV sensitivity of Beauveria bassiana and Metarhizium anisopliae isolates under investigation as potential biological control agents in South African citrus orchards. Fungal Biol 124, 304–310. 10.1016/j.funbio.2019.08.009. [DOI] [PubMed] [Google Scholar]
- Adam A, Deimel S, Pardo-Medina J, García-Martínez J, Konte T, Limón MC, Avalos J, Terpitz U, 2018. Protein activity of the Fusarium fujikuroi rhodopsins CarO and OpsA and their telation to fungus–plant interaction. Int J Mol Sci 19, 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alder-Rangel A, Bailão AM, da Cunha AF, Soares CMA, Wang C, Bonatto D, Dadachova E, Hakalehto E, Eleutherio ECA, Fernandes EKK, Braus GH, Braga GUL, Goldman GH, Malavazi I, Hallsworth JE, Takemoto JY, Fuller K, Selbmann L, Corrochano LM, Bertolini MC, Schmoll M, Pedrini N, Loera O, Finlay RD, Peralta RM, Rangel DEN, 2018. The Second International Symposium on Fungal Stress: ISFUS. Fungal Biol 122, 386–399. 10.1016/j.funbio.2017.10.011. [DOI] [PubMed] [Google Scholar]
- Alder-Rangel A, Idnurm A, Brand AC, Brown AJP, Gorbushina A, Kelliher CM, Campos CB, Levin DE, Bell-Pedersen D, Dadachova E, Bauer FF, Gadd GM, Braus GH, Braga GUL, Brancini GTP, Walker GM, Druzhinina I, Pócsi I, Dijksterhuis J, Aguirre J, Hallsworth JE, Schumacher J, Wong KH, Selbmann L, Corrochano LM, Kupiec M, Momany M, Molin M, Requena N, Yarden O, Cordero RJB, Fischer R, Pascon RC, Mancinelli RL, Emri T, Basso TO, Rangel DEN, 2020. The Third International Symposium on Fungal Stress – ISFUS. Fungal Biol 124, 235–252. 10.1016/j.funbio.2020.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambati S, Pham T, Lewis ZA, Lin X, Meagher RB, 2022. DectiSomes: Glycan targeting of liposomal drugs improves the treatment of disseminated candidiasis. Antimicrob Agents Ch 66, e01467–01421. doi: 10.1128/AAC.01467-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atriztan-Hernandez K, Herrera-Estrella A, 2022. Drosophila attack inhibits hyphal regeneration and defense mechanisms activation for the fungus Trichoderma atroviride. ISME J 16, 149–158. 10.1038/s41396-021-01068-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augostine CR, Avery SV, 2022. Discovery of natural products with antifungal potential through combinatorial synergy. Frontiers in microbiology 13, 866840. 10.3389/Fmicb.2022.866840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltazar LM, Zamith-Miranda D, Burnet MC, Choi H, Nimrichter L, Nakayasu ES, Nosanchuk JD, 2018. Concentration-dependent protein loading of extracellular vesicles released by Histoplasma capsulatum after antibody treatment and its modulatory action upon macrophages. Scientific Reports 8. 10.1038/S41598-018-25665-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolini F, Gundersen GG, 2006. Generation of noncentrosomal microtubule arrays. J Cell Sci 119, 4155–4163. 10.1242/jcs.03227. [DOI] [PubMed] [Google Scholar]
- Basante-Bedoya MA, Bogliolo S, Garcia-Rodas R, Zaragoza O, Arkowitz RA, Bassilana M, 2022. Two distinct lipid transporters together regulate invasive filamentous growth in the human fungal pathogen Candida albicans. PLoS Genet 18, e1010549. PGENETICS-D-22–01026 [pii] 10.1371/journal.pgen.1010549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassilana M, Puerner C, Arkowitz RA, 2020. External signal-mediated polarized growth in fungi. Curr Opin Cell Biol 62, 150–158. 10.1016/j.ceb.2019.11.001. [DOI] [PubMed] [Google Scholar]
- Basso TO, Gomes FS, Lopes ML, de Amorim HV, Eggleston G, Basso LC, 2014. Homo- and heterofermentative lactobacilli differently affect sugarcane-based fuel ethanol fermentation. Anton Leeuw Int J G 105, 169–177. 10.1007/s10482-013-0063-6. [DOI] [PubMed] [Google Scholar]
- Beckmann EA, Köhler AM, Meister C, Christmann M, Draht OW, Rakebrandt N, Valerius O, Braus GH, 2015. Integration of the catalytic subunit activates deneddylase activity in vivo as final step in fungal COP9 signalosome assembly. Molecular Microbiology 97, 110–124. 10.1111/mmi.13017. [DOI] [PubMed] [Google Scholar]
- Bennett JW, 2015. The fungi that ate my house. Science 349, 1018–1018. 10.1126/science.349.6251.1018. [DOI] [PubMed] [Google Scholar]
- Bohner F, Papp C, Gacser A, 2022. The effect of antifungal resistance development on the virulence of Candida species. FEMS Yeast Res 22. 10.1093/femsyr/foac019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnet MC, Zamith-Miranda D, Heyman HM, Weitz KK, Bredeweg EL, Nosanchuk JD, Nakayasu ES, 2020. Remodeling of the Histoplasma Capsulatum membrane induced by monoclonal antibodies. Vaccines-Basel 8. 10.3390/Vaccines8020269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch S, Schwier EU, Nahlik K, Bayram Ö, Helmstaedt K, Draht OW, Krappmann S, Valerius O, Lipscomb WN, Braus GH, 2007. An eight-subunit COP9 signalosome with an intact JAMM motif is required for fungal fruit body formation. Proceedings of the National Academy of Sciences 104, 8089–8094. doi: 10.1073/pnas.0702108104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos CB, Di Benedette JP, Morais FV, Ovalle R, Nobrega MP, 2008. Evidence for the role of calcineurin in morphogenesis and calcium homeostasis during mycelium-to-yeast dimorphism of Paracoccidioides brasiliensis. Eukaryot Cell 7, 1856–1864. 10.1128/EC.00110-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Rodriguez Pino M, Haller PR, Verde F, 2019. Conserved NDR/LATS kinase controls RAS GTPase activity to regulate cell growth and chronological lifespan. Mol Biol Cell 30, 2598–2616. 10.1091/mbc.E19-03-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi ZL, Yu GH, Kappler A, Liu CQ, Gadd GM, 2022a. Fungal-mineral interactions modulating intrinsic peroxidase-like activity of iron nanoparticles: Implications for the biogeochemical cycles of nutrient elements and attenuation of contaminants. Environ Sci Technol 56, 672–680. 10.1021/acs.est.1c06596. [DOI] [PubMed] [Google Scholar]
- Chi ZL, Yu GH, Teng HH, Liu HG, Wang J, Liu CQ, Shen QR, Gadd GM, 2022b. Molecular trade-offs between lattice oxygen and oxygen vacancy drive organic pollutant degradation in fungal biomineralized exoskeletons. Environmental Science & Technology 56, 8132–8141. 10.1021/acs.est.2c01388. [DOI] [PubMed] [Google Scholar]
- Cleare LG, Zamith D, Heyman HM, Couvillion SP, Nimrichter L, Rodrigues ML, Nakayasu ES, Nosanchuk JD, 2020. Media matters! Alterations in the loading and release of Histoplasma capsulatum extracellular vesicles in response to different nutritional milieus. Cell Microbiol 22, e13217. 10.1111/cmi.13217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cola P, Procopio DP, Alves ATD, Carnevalli LR, Sampaio IV, da Costa BLV, Basso TO, 2020. Differential effects of major inhibitory compounds from sugarcane-based lignocellulosic hydrolysates on the physiology of yeast strains and lactic acid bacteria. Biotechnology Letters 42, 571–582. 10.1007/s10529-020-02803-6. [DOI] [PubMed] [Google Scholar]
- Dagdas YF, Yoshino K, Dagdas G, Ryder LS, Bielska E, Steinberg G, Talbot NJ, 2012. Septin-mediated plant cell invasion by the rice blast fungus, Magnaporthe oryzae. Science 336, 1590–1595. 10.1126/science.1222934. [DOI] [PubMed] [Google Scholar]
- Das M, Drake T, Wiley DJ, Buchwald P, Vavylonis D, Verde F, 2012. Oscillatory dynamics of Cdc42 GTPase in the control of polarized growth. Science 337, 239–243. doi: 10.1126/science.1218377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M, Nunez I, Rodriguez M, Wiley DJ, Rodriguez J, Sarkeshik A, Yates JR 3rd, Buchwald P, Verde F, 2015. Phosphorylation-dependent inhibition of Cdc42 GEF Gef1 by 14-3-3 protein Rad24 spatially regulates Cdc42 GTPase activity and oscillatory dynamics during cell morphogenesis. Mol Biol Cell 26, 3520–3534. 10.1091/mbc.E15-02-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M, Wiley DJ, Chen X, Shah K, Verde F, 2009. The conserved NDR kinase Orb6 controls polarized cell growth by spatial regulation of the small GTPase Cdc42. Curr Biol 19, 1314–1319. 10.1016/j.cub.2009.06.057. [DOI] [PubMed] [Google Scholar]
- Davies CR, Wohlgemuth F, Young T, Violet J, Dickinson M, Sanders JW, Vallieres C, Avery SV, 2021. Evolving challenges and strategies for fungal control in the food supply chain. Fungal Biol Rev 36, 15–26. 10.1016/j.fbr.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delarue M, Brittingham GP, Pfeffer S, Surovtsev IV, Pinglay S, Kennedy KJ, Schaffer M, Gutierrez JI, Sang D, Poterewicz G, Chung JK, Plitzko JM, Groves JT, Jacobs-Wagner C, Engel BD, Holt LJ, 2018. mTORC1 controls phase separation and the biophysical properties of the cytoplasm by tuning crowding. Cell 174, 338–349 e320. 10.1016/j.cell.2018.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devan SK, Schott-Verdugo S, Muentjes K, Bismar L, Reiners J, Hachani EP, Schmitt LP, Hoeppner AP, Smits SHP, Gohlke HP, Feldbruegge MP, 2022. A MademoiseLLE domain binding platform links the key RNA transporter to endosomes. Plos Genetics 18. 10.1371/journal.pgen.1010269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias LP, Pedrini N, Braga GUL, Ferreira PC, Pupin B, Araújo CAS, Corrochano LM, Rangel DEN, 2020. Outcome of blue, green, red, and white light on Metarhizium robertsii during mycelial growth on conidial stress tolerance and gene expression. Fungal Biol 124, 263–272. 10.1016/j.funbio.2019.04.007. [DOI] [PubMed] [Google Scholar]
- Dias LP, Pupin B, Roberts DW, Rangel DEN, 2022. Low- or high-white light irradiance induces similar conidial stress tolerance in Metarhizium robertsii. Arch Microbiol 204, 83. 10.1007/s00203-021-02730-8. [DOI] [PubMed] [Google Scholar]
- Dias LP, Souza RKF, Pupin B, Rangel DEN, 2021. Conidiation under illumination enhances conidial tolerance of insect-pathogenic fungi to environmental stresses. Fungal Biol 125, 891–904. 10.1016/j.funbio.2021.06.003. [DOI] [PubMed] [Google Scholar]
- Eseola AB, Ryder LS, Oses-Ruiz M, Findlay K, Yan X, Cruz-Mireles N, Molinari C, Garduno-Rosales M, Talbot NJ, 2021. Investigating the cell and developmental biology of plant infection by the rice blast fungus Magnaporthe oryzae. Fungal Genet Biol 154, 103562. 10.1016/j.fgb.2021.103562. [DOI] [PubMed] [Google Scholar]
- Espino-Vazquez AN, Bermudez-Barrientos JR, Cabrera-Rangel JF, Cordova-Lopez G, Cardoso-Martinez F, Martinez-Vazquez A, Camarena-Pozos DA, Mondo SJ, Pawlowska TE, Abreu-Goodger C, Partida-Martinez LP, 2020. Narnaviruses: novel players in fungal-bacterial symbioses. ISME J 14, 1743–1754. 10.1038/s41396-020-0638-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa-Meléndez A, Martínez-Núñez L, Rico-Ramírez AM, Martínez-Andrade JM, Munson M, Riquelme M, 2022. The C-terminal domain of SEC-10 is fundamental for exocyst function, Spitzenkörper organization and cell morphogenesis in Neurospora crassa. bioRxiv, 2022.2001.2014.475644. 10.1101/2022.01.14.475644. [DOI] [Google Scholar]
- García-Martínez J, Brunk M, Avalos J, Terpitz U, 2015. The CarO rhodopsin of the fungus Fusarium fujikuroi is a light-driven proton pump that retards spore germination. Scientific Reports 5, 7798. 10.1038/srep07798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rodas R, Labbaoui H, Orange F, Solis N, Zaragoza O, Filler SG, Bassilana M, Arkowitz RA, 2022. Plasma membrane phosphatidylinositol-4-phosphate Is not necessary for Candida albicans viability yet Is key for cell wall integrity and systemic infection. MBio 13, e03873–03821. doi: 10.1128/mbio.03873-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido-Bazan V, Aguirre J, 2022. H2O2 induces calcium and ERMES complex-dependent mitochondrial constriction and division as well as mitochondrial outer membrane remodeling in Aspergillus nidulans. Journal of Fungi 8. 10.3390/Jof8080829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido-Bazan V, Jaimes-Arroyo R, Sanchez O, Lara-Rojas F, Aguirre J, 2018a. SakA and MpkC stress MAPKs show opposite and common functions during stress responses and development in Aspergillus nidulans. Frontiers in microbiology 9. 10.3389/Fmicb.2018.02518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido-Bazan V, Jaimes-Arroyo R, Sanchez O, Lara-Rojas F, Aguirre J, 2018b. SakA and MpkC Stress MAPKs Show Opposite and Common Functions During Stress Responses and Development in Aspergillus nidulans. Frontiers in Microbiology 9. ARTN 2518 10.3389/fmicb.2018.02518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido-Bazan V, Pardo JP, Aguirre J, 2020. DnmA and FisA mediate mitochondria and peroxisome fission, and regulate mitochondrial function, ROS production and development in Aspergillus nidulans. Frontiers in microbiology 11. 10.3389/Fmicb.2020.00837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasch AP, Spellman PT, Kao CM, Carmel-Harel O, Eisen MB, Storz G, Botstein D, Brown PO, 2000. Genomic expression programs in the response of yeast cells to environmental changes. Molecular Biology of the Cell 11, 4241–4257. 10.1091/mbc.11.12.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacon TG, Cunha GCDE, Eliodorio KP, Oliveira RPD, Basso TO, 2022. Homo- and heterofermentative lactobacilli are distinctly affected by furanic compounds. Biotechnology Letters 44, 1431–1445. 10.1007/s10529-022-03310-6. [DOI] [PubMed] [Google Scholar]
- Gomes EN, Elsherbiny A, Elsherbiny BA, Bennett JW, 2020. Beyond classical biocontrol: new perspectives on Trichoderma, in: Hasham AE-L et al. , e. (Ed.), Fungal Biotechnology and Bioengineering. Springer Nature, Switzerland, pp. 437–455. [Google Scholar]
- Gostincar C, Sun X, Cernosa A, Fang C, Gunde-Cimerman N, Song Z, 2022. Clonality, inbreeding, and hybridization in two extremotolerant black yeasts. Gigascience 11. 6749380 [pii] giac095 [pii] 10.1093/gigascience/giac095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunde-Cimerman N, Plemenitas A, Oren A, 2018. Strategies of adaptation of microorganisms of the three domains of life to high salt concentrations. FEMS Microbiol Rev 42, 353–375. 10.1093/femsre/fuy009. [DOI] [PubMed] [Google Scholar]
- Gunde-Cimerman N, Zalar P, de Hoog S, Plemenitas A, 2000. Hypersaline waters in salterns - natural ecological niches for halophilic black yeasts. FEMS Microbiology Ecology 32, 235–240. [DOI] [PubMed] [Google Scholar]
- Gurr S, 2003. TRAVEL FUND REPORT: VIII International Fungal Biology Conference, December 2002. https://www.bspp.org.uk/travel-fund-report-viii-international-fungal-biology-conference-guanajuato-mexico-december-2002/. British Society for Plant Pathology, Guanajuato, Mexico. [Google Scholar]
- Harvey HJ, Hendry AC, Archer DB, Avery SV, 2023. Evaluating the potential of natural product combinations with sorbic acid for improving preservative action against food-spoilage yeasts. Fungal Biol. 10.1016/j.funbio.2023.01.004. [DOI] [PubMed] [Google Scholar]
- Heitman J, 2015. Evolution of sexual reproduction: A view from the fungal kingdom supports an evolutionary epoch with sex before sexes. Fungal Biology Reviews 29, 108–117. 10.1016/j.fbr.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y-H, Shishkova E, Hose J, Coon JJ, Gasch AP, 2018. Decoupling yeast cell division and stress defense implicates mRNA repression in translational reallocation during stress. Curr. Biol 28, 2673–2680.e2674. 10.1016/j.cub.2018.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horio T, Oakley BR, 2003. Expression of Arabidopsis gamma-tubulin in fission yeast reveals conserved and novel functions of gamma-tubulin. Plant Physiol 133, 1926–1934. 10.1104/pp.103.027367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung R, Lee S, Bennett JW, 2015. Fungal volatile organic compounds and their role in ecosystems. Applied Microbiology and Biotechnology 99, 3395–3405. 10.1007/s00253-015-6494-4. [DOI] [PubMed] [Google Scholar]
- Inamdar AA, Hossain MM, Bernstein AI, Miller GW, Richardson JR, Bennett JW, 2013. Fungal-derived semiochemical 1-octen-3-ol disrupts dopamine packaging and causes neurodegeneration. Proc Natl Acad Sci U S A 110, 19561–19566. 10.1073/pnas.1318830110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamdar AA, Morath S, Bennett JW, 2020. Fungal volatile organic compounds: More than just a funky smell? Annual Review of Microbiology 74, 101–116. 10.1146/annurev-micro-012420-080428. [DOI] [PubMed] [Google Scholar]
- Jaddaoui IE, Rangel DEN, Bennett JW, 2023. Fungal volatiles have physiological properties. Fungal Biol. 10.1016/j.funbio.2023.03.005. [DOI] [PubMed] [Google Scholar]
- Jaimes-Arroyo R, Lara-Rojas F, Bayram O, Valerius O, Braus GH, Aguirre J, 2015. The SrkA kinase Is part of the SakA mitogen-activated protein kinase interactome and regulates stress responses and development in Aspergillus nidulans. Eukaryotic Cell 14, 495–510. 10.1128/Ec.00277-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph R, Keyhani NO, 2021. Fungal mutualisms and pathosystems: life and death in the ambrosia beetle mycangia. Applied Microbiology and Biotechnology 105, 3393–3410. 10.1007/s00253-021-11268-0. [DOI] [PubMed] [Google Scholar]
- Joseph R, Lasa M, Zhou YH, Keyhani NO, 2021. Unique attributes of the laurel wilt pathogen, Raffaelea lauricola, as revealed by metabolic profiling. Pathogens 10. 10.3390/Pathogens10050528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollman JM, Polka JK, Zelter A, Davis TN, Agard DA, 2010. Microtubule nucleating gamma-TuSC assembles structures with 13-fold microtubule-like symmetry. Nature 466, 879–882. 10.1038/nature09207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbaoui H, Bogliolo S, Ghugtyal V, Solis NV, Filler SG, Arkowitz RA, Bassilana M, 2017. Role of Arf GTPases in fungal morphogenesis and virulence. Plos Pathog 13, e1006205. 10.1371/journal.ppat.1006205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara-Rojas F, Sanchez O, Kawasaki L, Aguirre J, 2011. Aspergillus nidulans transcription factor AtfA interacts with the MAPK SakA to regulate general stress responses, development and spore functions. Molecular microbiology 80, 436–454. 10.1111/j.1365-2958.2011.07581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MV, Topper SE, Hubler SL, Hose J, Wenger CD, Coon JJ, Gasch AP, 2011. A dynamic model of proteome changes reveals new roles for transcript alteration in yeast. Mol Syst Biol 7, 514. 10.1038/msb.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Behringer G, Hung R, Bennett J, 2019. Effects of fungal volatile organic compounds on Arabidopsis thaliana growth and gene expression. Fungal Ecology 37, 1–9. 10.1016/j.funeco.2018.08.004. [DOI] [Google Scholar]
- Lee SC, Ni M, Li W, Shertz C, Heitman J, 2010. The evolution of sex: a perspective from the fungal kingdom. Microbiol Mol Biol Rev 74, 298–340. 10.1128/MMBR.00005-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li QW, Liu FX, Li M, Chen CM, Gadd GM, 2022. Nanoparticle and nanomineral production by fungi. Fungal Biology Reviews 41, 31–44. 10.1016/j.fbr.2021.07.003. [DOI] [Google Scholar]
- Lin J, Pham T, Hipsher K, Glueck N, Fan Y, Lin X, 2022. Immunoprotection against Cryptococcosis offered by Znf2 depends on capsule and the hyphal morphology. MBio 13, e02785–02721. doi: 10.1128/mbio.02785-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T. c., Neuner A, Schiebel E, 2015. Targeting of γ-tubulin complexes to microtubule organizing centers: conservation and divergence. Trends in Cell Biology 25, 296–307. 10.1016/j.tcb.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Liu F, Shah DS, Gadd GM, 2021. Role of protein in fungal biomineralization of copper carbonate nanoparticles. Curr Biol 31, 358–368 e353. 10.1016/j.cub.2020.10.044. [DOI] [PubMed] [Google Scholar]
- Liu S, Zamith-Miranda D, Almeida-Paes R, da Silva LBR, Nacharaju P, Nosanchuk JD, 2023. Nitric oxide-loaded nano- and microparticle platforms serving as potential new antifungal therapeutics. Fungal Biol. 10.1016/j.funbio.2023.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu X, Shen C, Fu Y, Xie J, Jiang D, Li G, Cheng J, 2016. The microbial opsin homolog Sop1 is involved in Sclerotinia sclerotiorum development and environmental stress response. Frontiers in microbiology 6. 10.3389/fmicb.2015.01504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos Baltazar L, Nakayasu ES, Sobreira TJ, Choi H, Casadevall A, Nimrichter L, Nosanchuk JD, 2016. Antibody binding alters the characteristics and contents of extracellular vesicles released by Histoplasma capsulatum. mSphere 1. 10.1128/mSphere.00085-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos TG, Morais FV, Campos CB, 2013. Hsp90 regulates Paracoccidioides brasiliensis proliferation and ROS levels under thermal stress and cooperates with calcineurin to control yeast to mycelium dimorphism. Med. Mycol 51, 413–421. 10.3109/13693786.2012.725481. [DOI] [PubMed] [Google Scholar]
- Meagher RB, Lewis ZA, Ambati S, Lin X, 2021. Aiming for a bull’s-eye: Targeting antifungals to fungi with dectin-decorated liposomes. Plos Pathog 17, e1009699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Castellanos E, Esquivel-Naranjo EU, Heil M, Herrera-Estrella A, 2014. Extracellular ATP activates MAPK and ROS signaling during injury response in the fungus Trichoderma atroviride. Front Plant Sci 5, 659. 10.3389/fpls.2014.00659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Castellanos E, Villalobos-Escobedo JM, Riquelme M, Read ND, Abreu-Goodger C, Herrera-Estrella A, 2018. Danger signals activate a putative innate immune system during regeneration in a filamentous fungus. PLoS Genet 14, e1007390. 10.1371/journal.pgen.1007390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina EQA, Oliveira AS, Medina HR, Rangel DEN, 2020. Serendipity in the wrestle between Trichoderma and Metarhizium. Fungal Biol 124, 418–426. 10.1016/j.funbio.2020.01.002. [DOI] [PubMed] [Google Scholar]
- Mondo SJ, Lastovetsky OA, Gaspar ML, Schwardt NH, Barber CC, Riley R, Sun H, Grigoriev IV, Pawlowska TE, 2017. Bacterial endosymbionts influence host sexuality and reveal reproductive genes of early divergent fungi. Nat Commun 8, 1843. 10.1038/s41467-017-02052-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz M, Braunfeld MB, Guenebaut V, Heuser J, Agard DA, 2000. Structure of the gamma-tubulin ring complex: a template for microtubule nucleation. Nat Cell Biol 2, 365–370. 10.1038/35014058. [DOI] [PubMed] [Google Scholar]
- Müller J, Pohlmann T, Feldbrugge M, 2019. Core components of endosomal mRNA transport are evolutionarily conserved in fungi. Fungal Genet Biol 126, 12–16. 10.1016/j.fgb.2019.01.013. [DOI] [PubMed] [Google Scholar]
- Müntjes K, Devan SK, Reichert AS, Feldbrugge M, 2021. Linking transport and translation of mRNAs with endosomes and mitochondria. Embo Rep 22. 10.15252/embr.202152445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahlik K, Dumkow M, Bayram O, Helmstaedt K, Busch S, Valerius O, Gerke J, Hoppert M, Schwier E, Opitz L, Westermann M, Grond S, Feussner K, Goebel C, Kaever A, Meinicke P, Feussner I, Braus GH, 2010. The COP9 signalosome mediates transcriptional and metabolic response to hormones, oxidative stress protection and cell wall rearrangement during fungal development. Mol Microbiol 78, 964–979. 10.1111/j.1365-2958.2010.07384.x. [DOI] [PubMed] [Google Scholar]
- Naz SA, Jabeen N, Sohail M, Rasool SA, 2015. Biophysicochemical characterization of Pyocin SA189 produced by Pseudomonas aeruginosa SA189. Brazilian Journal of Microbiology 46, 1147–1154. 10.1590/S1517-838246420140737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu M, Steffan BN, Fischer GJ, Venkatesh N, Raffa NL, Wettstein MA, Bok JW, Greco C, Zhao C, Berthier E, Oliw E, Beebe D, Bromley M, Keller NP, 2020. Fungal oxylipins direct programmed developmental switches in filamentous fungi. Nat Commun 11, 5158. 10.1038/s41467-020-18999-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez I, Rodriguez Pino M, Wiley DJ, Das ME, Chen C, Goshima T, Kume K, Hirata D, Toda T, Verde F, 2016. Spatial control of translation repression and polarized growth by conserved NDR kinase Orb6 and RNA-binding protein Sts5. eLife 5. 10.7554/eLife.14216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osés-Ruiz M, Cruz-Mireles N, Martin-Urdiroz M, Soanes DM, Eseola AB, Tang B, Derbyshire P, Nielsen M, Cheema J, Were V, Eisermann I, Kershaw MJ, Yan X, Valdovinos-Ponce G, Molinari C, Littlejohn GR, Valent B, Menke FLH, Talbot NJ, 2021. Appressorium-mediated plant infection by Magnaporthe oryzae is regulated by a Pmk1-dependent hierarchical transcriptional network. Nature Microbiology 6, 1383–1397. 10.1038/s41564-021-00978-w. [DOI] [PubMed] [Google Scholar]
- Panek A, 1959. Kinetic study of the formation and the utilization of trehalose by baker’s yeast. C. R. Hebd. Seances. Acad. Sci 249, 333–335. [PubMed] [Google Scholar]
- Panek A, 1962. Synthesis of trehalose by baker’s yeast (Saccharomyces cerevisiae). Arch. Biochem. Biophys 98, 349–355. [DOI] [PubMed] [Google Scholar]
- Panek A, 1963. Function of trehalose in baker’s yeast (Saccharomyces cerevisiae). Arch Biochem Biophys 100, 422–425. [DOI] [PubMed] [Google Scholar]
- Panzer S, Brych A, Batschauer A, Terpitz U, 2019. Opsin 1 and Opsin 2 of the corn smut fungus Ustilago maydis are green light-driven proton pumps. Front. Microbiol 10.3389/fmicb.2019.00735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp C, Bohner F, Kocsis K, Varga M, Szekeres A, Bodai L, Willis JR, Gabaldon T, Toth R, Nosanchuk JD, Vagvolgyi C, Gacser A, 2020. Triazole evolution of Candida parapsilosis results in cross-resistance to other antifungal drugs, influences stress responses, and alters virulence in an antifungal drug-dependent manner. mSphere 5. 10.1128/mSphere.00821-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp C, Kocsis K, Toth R, Bodai L, Willis JR, Ksiezopolska E, Lozoya-Perez NE, Vagvolgyi C, Mora Montes H, Gabaldon T, Nosanchuk JD, Gacser A, 2018. Echinocandin-induced microevolution of Candida parapsilosis influences virulence and abiotic stress tolerance. mSphere 3. 10.1128/mSphere.00547-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partida-Martinez LP, 2017. The fungal holobiont: Evidence from early diverging fungi. Environ Microbiol 19, 2919–2923. 10.1111/1462-2920.13731. [DOI] [PubMed] [Google Scholar]
- Partida-Martinez LP, Hertweck C, 2005. Pathogenic fungus harbours endosymbiotic bacteria for toxin production. Nature 437, 884–888. 10.1038/nature03997. [DOI] [PubMed] [Google Scholar]
- Partida-Martinez LP, Monajembashi S, Greulich KO, Hertweck C, 2007. Endosymbiont-dependent host reproduction maintains bacterial-fungal mutualism. Curr Biol 17, 773–777. 10.1016/j.cub.2007.03.039. [DOI] [PubMed] [Google Scholar]
- Puerner C, Serrano A, Wakade RS, Bassilana M, Arkowitz RA, 2021. A myosin light chain is critical for fungal growth robustness in Candida albicans. MBio 12. 10.1128/mBio.02528-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Cota R, Espino-Vazquez AN, Rodriguez-Vega TC, Macias-Diaz RE, Callejas-Negrete OA, Freitag M, Fischer R, Roberson RW, Mourino-Perez RR, 2022. The cytoplasmic microtubule array in Neurospora crassa depends on microtubule-organizing centers at spindle pole bodies and microtubule plus end-depending pseudo-MTOCs at septa. Fungal Genet Biol 162. 10.1016/J.Fgb.2022.103729. [DOI] [PubMed] [Google Scholar]
- Rangel DEN, Aguirre J, Alder-Rangel A, 2020. Postponing the IV International Symposium on Fungal Stress (ISFUS) and the XIII International Fungal Biology Conference (IFBC) due to COVID-19. Fungal Biol 124, 536. 10.1016/j.funbio.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel DEN, Alder-Rangel A, 2020. History of the International Symposium on Fungal Stress – ISFUS, a dream come true! Fungal Biol-Uk 124, 525–535. 10.1016/j.funbio.2020.02.004. [DOI] [PubMed] [Google Scholar]
- Rangel DEN, Alder-Rangel A, Dadachova E, Finlay RD, Dijksterhuis J, Braga GUL, Corrochano LM, Hallsworth JE, 2015a. The International Symposium on Fungal Stress: ISFUS. Curr. Gen 61, 479–487. 10.1007/s00294-015-0501-2. [DOI] [PubMed] [Google Scholar]
- Rangel DEN, Alder-Rangel A, Dadachova E, Finlay RD, Kupiec M, Dijksterhuis J, Braga GUL, Corrochano LM, Hallsworth JE, 2015b. Fungal stress biology: a preface to the Fungal Stress Responses special edition. Curr. Gen 61, 231–238. 10.1007/s00294-015-0500-3. [DOI] [PubMed] [Google Scholar]
- Rangel DEN, Anderson AJ, Roberts DW, 2006. Growth of Metarhizium anisopliae on non-preferred carbon sources yields conidia with increased UV-B tolerance. J. Invertebr. Pathol 93, 127–134. 10.1016/j.jip.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Rangel DEN, Anderson AJ, Roberts DW, 2008. Evaluating physical and nutritional stress during mycelial growth as inducers of tolerance to heat and UV-B radiation in Metarhizium anisopliae conidia. Mycol. Res 112, 1362–1372. 10.1016/j.mycres.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Rangel DEN, Braga GUL, Fernandes ÉKK, Keyser CA, Hallsworth JE, Roberts DW, 2015c. Stress tolerance and virulence of insect-pathogenic fungi are determined by environmental conditions during conidial formation. Curr. Gen 61, 383–404. 10.1007/s00294-015-0477-y. [DOI] [PubMed] [Google Scholar]
- Rangel DEN, Fernandes EKK, Anderson AJ, Roberts DW, 2012. Culture of Metarhizium robertsii on salicylic-acid supplemented medium induces increased conidial thermotolerance. Fungal Biol 116, 438–442. [DOI] [PubMed] [Google Scholar]
- Rangel DEN, Finlay RD, Hallsworth JE, Dadachova E, Gadd GM, 2018. Fungal strategies for dealing with environmental and agricultural stress. Fungal Biol. 122, 602–612. 10.1016/j.funbio.2018.02.002. [DOI] [PubMed] [Google Scholar]
- Ribeiro GF, Goes CG, Onorio DS, Campos CBL, Morais FV, 2018. Autophagy in Paracoccidioides brasiliensis under normal mycelia to yeast transition and under selective nutrient deprivation. PLoS One 13, e0202529. 10.1371/journal.pone.0202529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riquelme M, Aguirre J, Bartnicki-García S, Braus GH, Feldbrügge M, Fleig U, Hansberg W, Herrera-Estrella A, Kämper J, Kück U, Mouriño-Pérez RR, Takeshita N, Fischer R, 2018. Fungal morphogenesis, from the polarized growth of hyphae to complex reproduction and infection structures. Microbiology and Molecular Biology Reviews 82, e00068–00017. doi: 10.1128/MMBR.00068-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riquelme M, Bredeweg EL, Callejas-Negrete O, Roberson RW, Ludwig S, Beltrán-Aguilar A, Seiler S, Novick P, Freitag M, 2014. The Neurospora crassa exocyst complex tethers Spitzenkörper vesicles to the apical plasma membrane during polarized growth. Molecular Biology of the Cell 25, 1312–1326. 10.1091/mbc.e13-06-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riquelme M, Sánchez-León E, 2014. The Spitzenkörper: a choreographer of fungal growth and morphogenesis. Current Opinion in Microbiology 20, 27–33. 10.1016/j.mib.2014.04.003. [DOI] [PubMed] [Google Scholar]
- Rush TA, Puech-Pagès V, Bascaules A, Jargeat P, Maillet F, Haouy A, Maës AQ, Carriel CC, Khokhani D, Keller-Pearson M, Tannous J, Cope KR, Garcia K, Maeda J, Johnson C, Kleven B, Choudhury QJ, Labbé J, Swift C, O’Malley MA, Bok JW, Cottaz S, Fort S, Poinsot V, Sussman MR, Lefort C, Nett J, Keller NP, Bécard G, .Ané JM, 2020. Lipo-chitooligosaccharides as regulatory signals of fungal growth and development. Nat. Commun 11, 3897. 10.1038/s41467-020-17615-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder LS, Cruz-Mireles N, Molinari C, Eisermann I, Eseola AB, Talbot NJ, 2022. The appressorium at a glance. Journal of Cell Science 135. 10.1242/jcs.259857. [DOI] [PubMed] [Google Scholar]
- Sarikaya Bayram Ö, Bayram Ö, Karahoda B, Meister C, Köhler AM, Thieme S, Elramli N, Frawley D, McGowan J, Fitzpatrick DA, Schmitt K, de Assis LJ, Valerius O, Goldman GH, Braus GH, 2022. F-box receptor mediated control of substrate stability and subcellular location organizes cellular development of Aspergillus nidulans. Plos Genetics 18, e1010502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AM, Pedrini N, Pupin B, Roberts DW, Rangel DEN, 2023. Asphyxiation of Metarhizium robertsii during mycelial growth produces conidia with increased stress tolerance via increased expression of stress-related genes. Fungal Biol-Uk. 10.1016/j.funbio.2023.01.005. [DOI] [PubMed] [Google Scholar]
- Silva PM, Puerner C, Seminara A, Bassilana M, Arkowitz RA, 2019. Secretory vesicle clustering in fungal filamentous cells does not require directional growth. Cell Reports 28, 2231–2245.e2235. 10.1016/j.celrep.2019.07.062. [DOI] [PubMed] [Google Scholar]
- Simons A, Alhanout K, Duval RE, 2020. Bacteriocins, antimicrobial peptides from bacterial origin: Overview of their biology and their impact against multidrug-resistant bacteria. Microorganisms 8. 10.3390/microorganisms8050639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube A, Brill M, Oakley BR, Horio T, Steinberg G, 2003. Microtubule organization requires cell cycle-dependent nucleation at dispersed cytoplasmic sites: Polar and perinuclear microtubule organizing centers in the plant pathogen Ustilago maydis. Molecular Biology of the Cell 14, 642–657. 10.1091/mbc.e02-08-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trunk K, Coulthurst SJ, Quinn J, 2019. A new front in microbial warfare—delivery of antifungal effectors by the type VI secretion system. Journal of Fungi 5, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trunk K, Peltier J, Liu Y-C, Dill BD, Walker L, Gow NAR, Stark MJR, Quinn J, Strahl H, Trost M, Coulthurst SJ, 2018. The type VI secretion system deploys antifungal effectors against microbial competitors. Nature Microbiology 3, 920–931. 10.1038/s41564-018-0191-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumbarski Y, Yanakieva V, Nikolova R, Mineva G, Deseva I, Mihaylova D, 2021. Antifungal effect of a bacteriocin of Bacillus methylotrophicus bm47 and its potential application as a bio preservative in traditional Bulgarian yogurt. . Journal of Microbiology, Biotechnology and Food Sciences 8 659–662. 10.15414/jmbfs.2018.8.1.659-662. [DOI] [Google Scholar]
- Turian G, Hohl HR, 1981. The fungal spore, morphogenetic controls : proceedings of the Third International Fungal Spore Symposium (3IFSS) held 18–22 August 1980 at Gwatt near Thun, Switzerland. Academic Press, .London; New York. [Google Scholar]
- Vallieres C, Hook AL, He YF, Crucitti VC, Figueredo G, Davies CR, Burroughs L, Winkler DA, Wildman RD, Irvine DJ, Alexander MR, Avery SV, 2020. Discovery of (meth)acrylate polymers that resist colonization by fungi associated with pathogenesis and biodeterioration. Sci Adv 6, eaba6574. 10.1126/sciadv.aba6574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernay A, Schaub S, Guillas I, Bassilana M, Arkowitz RA, 2012. A steep phosphoinositide bis-phosphate gradient forms during fungal filamentous growth. J Cell Biol 198, 711–730. 10.1083/jcb.201203099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalobos-Escobedo JM, Martinez-Hernandez JP, Pelagio-Flores R, Gonzalez-De la Rosa PM, Carreras-Villasenor N, Abreu-Goodger C, Herrera-Estrella AH, 2022. Trichoderma atroviride hyphal regeneration and conidiation depend on cell-signaling processes regulated by a microRNA-like RNA. Microb Genom 8. 10.1099/mgen.0.000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmeister E, Haag C, Zarnack K, Baumann S, Konig J, Mannhaupt G, Feldbrugge M, 2009. Tandem KH domains of Khd4 recognize AUACCC and are essential for regulation of morphology as well as pathogenicity in Ustilago maydis. RNA 15, 2206–2218. 10.1261/rna.1817609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Zeska Kress MR, Harting R, Bayram O, Christmann M, Irmer H, Valerius O, Schinke J, Goldman GH, Braus GH, 2012. The COP9 signalosome counteracts the accumulation of cullin SCF ubiquitin E3 RING ligases during fungal development. Mol Microbiol 83, 1162–1177. 10.1111/j.1365-2958.2012.07999.x. [DOI] [PubMed] [Google Scholar]
- Walker GM, Basso TO, 2020. Mitigating stress in industrial yeasts. Fungal Biol-Uk 124, 387–397. 10.1016/j.funbio.2019.10.010. [DOI] [PubMed] [Google Scholar]
- Wang Z, Wang J, Li N, Li J, Trail F, Dunlap JC, Townsend JP, 2018. Light sensing by opsins and fungal ecology: NOP-1 modulates entry into sexual reproduction in response to environmental cues. Molecular Ecology 27, 216–232. 10.1111/mec.14425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber DJ, Hess WM, 1976. The fungal spore : form and function : [proceedings]. Wiley, New York. [Google Scholar]
- Weiner A, Orange F, Lacas-Gervais S, Rechav K, Ghugtyal V, Bassilana M, Arkowitz RA, 2019. On-site secretory vesicle delivery drives filamentous growth in the fungal pathogen Candida albicans. Cellular Microbiology 21. 10.1111/cmi.12963. [DOI] [PubMed] [Google Scholar]
- Yadav V, Sun S, Heitman J, 2021. Uniparental nuclear inheritance following bisexual mating in fungi. eLife 10. 10.7554/eLife.66234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu GH, Chi ZL, Kappler A, Sun FS, Liu CQ, Teng HH, Gadd GM, 2020. Fungal nanophase particles catalyze iron transformation for oxidative stress removal and iron acquisition. Curr. Biol 30, 2943.-+. 10.1016/j.cub.2020.05.058. [DOI] [PubMed] [Google Scholar]
- Zamith-Miranda D, Alves LR, Nakayasu ES, Nosanchuk JD, 2021. Lessons learned from studying Histoplasma capsulatum extracellular vesicles. Curr Top Microbiol 432, 13–18. 10.1007/978-3-030-83391-6_2. [DOI] [PubMed] [Google Scholar]
- Zekert N, Veith D, Fischer R, 2010. Interaction of the Aspergillus nidulans microtubule-organizing center (MTOC) component ApsB with gamma-tubulin and evidence for a role of a subclass of peroxisomes in the formation of septal MTOCs. Eukaryot Cell 9, 795–805. 10.1128/EC.00058-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai B, Wozniak KL, Masso-Silva J, Upadhyay S, Hole C, Rivera A, Wormley FL, Lin X, 2015. Development of protective inflammation and cell-mediated immunity against Cryptococcus neoformans after exposure to hyphal mutants. MBio 6, e01433–01415. doi: 10.1128/mBio.01433-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Gao X, Manck R, Schmid M, Osmani AH, Osmani SA, Takeshita N, Fischer R, 2017. Microtubule-organizing centers of Aspergillus nidulans are anchored at septa by a disordered protein. Molecular Microbiology 106, 285–303. 10.1111/mmi.13763. [DOI] [PubMed] [Google Scholar]
- Zhou YH, Avery PB, Carrillo D, Duncan RH, Lukowsky A, Cave RD, Keyhani NO, 2018. Identification of the Achilles heels of the laurel wilt pathogen and its beetle vector. Applied Microbiology and Biotechnology 102, 5673–5684. 10.1007/s00253-018-9037-y. [DOI] [PubMed] [Google Scholar]
- Zhou YH, Lu DD, Joseph R, Li T, Keyhani NO, 2020. High efficiency transformation and mutant screening of the laurel wilt pathogen, Raffaelea lauricola. Applied Microbiology and Biotechnology 104, 7331–7343. 10.1007/s00253-020-10762-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


