Abstract
Purpose
The aim of the research was to observe the variations in brain activity between young cervical spondylosis patients with chronic neck pain (CNP) and healthy volunteers in the resting state and to investigate the central remodeling mechanisms in the patients.
Patients and methods
Our study recruited 31 patients with chronic neck pain from cervical spondylosis and 30 healthy volunteers. Eventually, 29 patients (CNP group) and 29 healthy volunteers (HC group) completed the acquisition of clinical data and resting-state functional magnetic resonance (rs BOLD-fMRI) amplitude of low-frequency fluctuations (ALFF) data; in addition, we assessed the relationship between differentially active brain regions and clinical indicators.
Results
The CNP group found greater ALFF values in the insula, cingulate gyrus, prefrontal lobe, and other brain regions. The occipital, parietal, and other brain regions had lower ALFF values. In addition, there was a negative connection between the duration of the sickness in the CNP group and the ALFF value of the right superior parietal gyrus (SPG.R). The level of tenderness threshold exhibited a negative correlation with the ALFF value of the left insula (INS.L). In addition, the NPQ score showed a negative association with the ALFF value of the ORBinf.R and a positive correlation with the ALFF value of the CC1.L. Finally, the HADS-A score exhibited a positive correlation with the ALFF value of the right anterior cingulate and paracingulate gyrus (ACG.R).
Conclusion
Young patients with chronic neck pain show extensive central remodeling, with altered functional activity in pain-emotion brain areas (such as the cingulate gyrus and insula), pain-cognition brain areas (such as the prefrontal lobe), and other special sensory brain areas (such as the parietal and occipital lobes). These changes are linked to clinical tenderness, functional disability, and negative emotion indicators.
Keywords: cervical spondylosis, chronic neck pain, central remodeling, resting-state functional magnetic resonance, young population
Introduction
Cervical spondylosis is a common chronic degenerative musculoskeletal disease, which is based on cervical intervertebral disc degeneration and secondary pathological changes involving the surrounding soft tissues, nerve roots, vertebral arteries, sympathetic nerves, and spinal cord. It causes a series of clinical symptoms and signs. Pain is one of the most important manifestations, especially when the pain symptoms are prolonged for more than 3 months, which may lead to cervical spondylosis-related chronic neck pain (CNP).1 The cause of this pain may come from muscle injury, cervical spine degeneration, nerve irritation, and so on.2 According to epidemiological surveys, the incidence of neck pain is 30% to 50% among adults worldwide,3,4 especially in China, where up to 65% of the population suffers from neck pain-related disorders.5 Neck pain is the 3rd most prevalent chronic pain disorder in the United States and the 4th most prevalent chronic pain disorder globally.6,7 In terms of prevalence, CNP also has a tendency to become younger. A cross-sectional survey conducted by Osama et al in a group of college students showed a high prevalence of neck discomfort 75.7%.8 Furthermore, considering that neck pain in young patients with CNP is mostly nonspecific, it is a nonspecific neck pain. And existing studies rarely focus on brain function-specific changes in young CNP patients,9 which is an important reason why young CNP patients were selected for observation in this study.
In patients with CNP, pain is primarily mild to moderate, intermittent, and evocative at the beginning of the disease course. It can progress to persistent pain later, significantly affecting functional activities in the neck and adjacent areas. In addition to the discomfort caused by the pain itself, the longer duration and the recurrent nature of the disease will also cause emotional, cognitive, memory, and sleep dysfunction. These impairments significantly impact the patient’s overall physical and mental well-being, resulting in a decreased quality of life and bringing a higher economic burden to the family and society.10
The generation and inhibition mechanism of chronic neck pain in cervical spondylosis is complex, involving multi-level, multi-pathway, and multi-substance regulatory mechanisms at the peripheral-spinal cord-brain level, especially the central regulatory mechanism of the brain, which is still unclear.11 Functional magnetic resonance imaging (fMRI) technology is regarded as a crucial imaging technique for studying diseases’ central damage and compensatory mechanisms. Due to its noninvasive, dynamic, and high-resolution characteristics, it plays an important part in evaluating dysfunction, determining effectiveness, and predicting the prognosis of chronic pain.12 The amplitude of low-frequency fluctuation (ALFF) is a crucial feature of fMRI in the resting state (rs-fMRI), which can effectively observe the signal fluctuation of blood oxygen level in the brain, evaluate the amplitude of each voxel in the brain region from an energy perspective, and better reflect the change in neuronal spontaneous activity.13 The ALFF achieves an optimal balance between reliability and validity and is therefore commonly used in studies of functional brain states.14
Studies in the literature have shown a remodeling of both the structure and function of their brain in individuals with chronic pain due to prolonged and abnormal neural signaling afferents, information integration, and motor signaling efferents to the center.15,16 Regarding structural brain alterations, the structural magnetic resonance of chronic musculoskeletal pain disorders that include CNP has shown that chronic pain is linked to reduced gray matter volume and altered white matter density in the brain.17 In addition, Woodworth et al found that neck pain patients exhibited cortical thinning in the superior frontal gyrus, anterior cingulate gyrus, and precuneus and reduced shell nucleus volume compared with healthy populations and that this alteration was positively correlated with patients’ neurological symptoms and worsening of pain levels.18 Concerning functional changes in the brain, two analyses using resting-state regional homogeneity (ReHo) fMRI studies showed that CNP patients in cervical spondylosis were characterized by altered time-series consistency of spontaneous activity in specific brain regions with neighboring neurons compared with controls. Yu et al showed that compared with healthy people, the patient group had significantly higher ReHo values in the bilateral middle frontal gyrus and significantly lower ReHo values in the left insula, superior frontal gyrus, middle cingulate gyrus, supplementary motor area, right postcentral gyrus, and parietal lobe. These brain regions are implicated in the formation of the brain’s default mode network, the cingulate-insula network, and the sensorimotor network, which is associated with the patient’s increased pain.19 A study by Chen et al discovered that the rs-ReHo of the right temporoparietal junction and the left sensorimotor cortex was reduced in patients with chronic neck pain in cervical spondylosis, and it was positively associated with the scores of the NPQ. In particular, the temporoparietal junction cortex is crucial in the pathophysiology and development of the CNP.20 In addition, some scholars have used the ALFF technique to explore the mechanism of chronic pain, showing that patients have altered spontaneous functional activity in certain regions such as the precuneus, prefrontal, anterior cingulate gyrus, and paracentral lobule. However, most of the diseases studied were chronic lower back pain,21,22 frozen shoulder pain,23 and chronic lumbar disc herniation pain,24 and did not adequately reflect the spontaneous neurological activity in CNP patients.
The above results in the literature suggest the presence of alterations with CNP in both the structure and function of brain, which may be correlated with sensory, motor, emotional, and cognitive dysfunctions; however, the specific brain networks related to CNP are still unknown, and the spontaneous neuronal activity of brain regions in patients with CNP in the resting state has not yet gained much attention. In addition, to our knowledge, this is the only study to observe brain function-specific changes in young chronic neck pain patients. Our study aimed to (1) compare the differences in spontaneous activity in the brain between the young CNP group and the HC group by using the ALFF analysis technique of fMRI and (2) explore the potential association between ALFF abnormalities and pain characteristics in young CNP patients.
Materials and Methods
Participants
The study included participants from Anhui Provincial Hospital of Chinese Medicine and Anhui University of Chinese Medicine National Medical Hall. We recruited 61 participants, with 31 individuals recruited in the CNP group and 30 in the HC group. Inclusion criteria: (1) Meet the diagnostic criteria for soft tissue cervical spondylosis in China’s “Expert Consensus on Typology, Diagnosis and Non-surgical Treatment of Cervical Spondylosis”: stiffness and pain in the neck, limitation of movement, with corresponding pressure points; a few patients may have reflex shoulder, arm and hand pain, swelling and numbness, and occasional dizziness; cervical spine X-ray shows no abnormality of cervical vertebrae or change in cervical physiological curvature, and mild stenosis of cervical vertebral spine interspace; (2) Neck pain as the main symptom, and the time is more than 12 weeks; (3) Age 18–44 years old; (4) Voluntary participation in the study, and can cooperate to complete all the treatment plan, signed informed consent; (5) No contraindications to MRI, and no serious diseases, such as heart, liver, kidney, and so on. Exclusion criteria: (1) Neck pain caused by other reasons such as fracture, dislocation, infection, trauma, etc; (2) Neck pain accompanied by obvious nerve root, vertebral artery and spinal cord symptoms; (3) Serious cardiovascular and cerebral vascular diseases, cognitive ambiguity; (4) Under treatment at the same period of time; (5) Pregnancy, breastfeeding women, and people with severe osteoporosis; (6) Metal foreign bodies in the body or the combination of other contraindications to MRI, etc. The study underwent a thorough review and received approval from the Medical Ethics Committee of Anhui Provincial Hospital of Chinese Medicine (Ethical Review Consent No. 2023AH-17). Before the trial, precautions were explained in detail to all subjects to ensure that they fully understood the purpose, process, risks, and possible benefits of the trial.
Clinical Data Collection
Tenderness threshold assessment: The same professional used a Wagner Force TenTM-Model FDX digital dynamometer (produced by Wagner Instruments, USA) and placed it on the patient’s most painful part to measure the pressure pain threshold. Repeat the operation three times, and take the average value.
Northwick Park Questionnaire (NPQ): The NPQ is a commonly used scale to assess the pain level of patients with CNP, which is divided into nine items, including the ninth item about driving, which does not need to be answered if the patient does not have driving experience, and even if it is responded to, it is not included in the total score. Each item is scored from 0 to 4 out of 36, and higher scores indicate greater levels of neck dysfunction in the patient.
Hospital Anxiety and Depression Scale (HADS): The HADS scale can simultaneously assess the anxiety and depression status of CNP patients. It consists of a total of 14 items, with seven items to measure anxiety, which is called the anxiety subscale (HADS-A), and another seven items to evaluate depression, which is called the depression subscale (HADS-D). The scoring system assigns a numerical value ranging from 0 to 3 to each item. As the score increases, the severity of the patient’s anxiety or depression symptoms also increases.
Functional Magnetic Resonance Data Acquisition
Subjects who met the enrollment criteria underwent cranial magnetic resonance scanning in the Department of Medical Imaging of the First Affiliated Hospital of Anhui Medical University (Gaoxin Hospital District). A GE Premier 3.0T MRI was used to collect data. Before entering the MRI room, the same specialized imaging staff instructed the subjects to complete the pre-scanning preparations, providing them with necessary instructions and guidance, including reminding them to empty their bladder, wear earplugs, and remove metal objects from their bodies to avoid safety problems during the scanning process. The subject was placed in a supine position with the head resting inside the scanning table coil, and the subject was required to remain stationary during the scanning process. Routine T2 Flair examination was performed first to exclude subjects with apparent intracranial organic lesions, followed by three-dimensional T1 high-resolution structural image data acquisition using the MPRAGE sequence, sagittal scanning, and scanning parameters: echo time of 3.0 ms, repetition time of 7.3 ms, imaging field of view of 256×256 mm, flip angle of 9 °, matrix 256 × 256, the layer thickness of 1 mm, layer spacing of 0 mm, number of layers 208, time 5 min 27s. Finally, resting-state magnetic resonance data acquisition was performed using EPI sequence, transverse bit scanning, and scanning parameters: echo time 30 ms, repetition time 1500 ms, imaging field of view 220×220 mm, flip angle 90 °, matrix 64 × 64, layer thickness 3.4 mm, layer spacing 0 mm, number of layers 48, a total of 200 Time points, and time of 5 min.
Functional Magnetic Resonance Data Processing
Based on the MATLAB R2018a data processing platform, the following preprocessing was performed on the magnetic resonance data using the DPABI 5.1 data processing software. Exclusion of the resting-state functional magnetic resonance data of the first 10 time points of all subjects to ensure data stability; temporal layer correction; head-motion correction (exclusion of the subjects with head-motion displacements >3 mm or rotations >3°); spatial normalization: all the subjects’ corrected cranial MRI images were aligned to the MNI standardized space and resampled according to each voxel of 3 mm × 3 mm × 3 mm; smoothing: through half of the full width by 6 mm; de-linearization drift, removal of covariates (including 24 head movement parameters, cerebrospinal fluid and cerebral white matter signals), and filtering: low-frequency filtering was applied to the smoothed data (filtering range 0.01–0.1 hz). By Fourier transform, the ALFF value of each voxel was obtained, and the ALFF value of each voxel was divided by the mean ALFF value of the whole brain to obtain the standardized ALFF value and the statistical parameter map based on the standardized ALFF value.
Statistical Analysis
Clinical index data such as pressure pain values, NPQ scale scores, and HADS scale scores, which were quantitative data conforming to the normal distribution, were expressed as “mean ± standard deviation (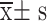 )” and were statistically analyzed using SPSS 23.0. Magnetic resonance data were based on the Matlab R2018a data processing platform, SPM 12 software was used for statistical analysis, and a two-sample t-test was used to compare the differences in standardized ALFF values between the CNP group and the control group. Pearson’s correlation analysis was used to correlate and analyze the neuroimaging data with clinical features.
)” and were statistically analyzed using SPSS 23.0. Magnetic resonance data were based on the Matlab R2018a data processing platform, SPM 12 software was used for statistical analysis, and a two-sample t-test was used to compare the differences in standardized ALFF values between the CNP group and the control group. Pearson’s correlation analysis was used to correlate and analyze the neuroimaging data with clinical features.
Results
Status of Cases
The CNP group recruited 31 cases, of which 2 cases dropped out in the middle, and finally, 29 cases completed the examination in the CNP group. The HC group recruited 30 cases, of which 1 case was dislodged, and finally, 29 cases completed the examination in the HC group.
Demographic Data and Clinical Characteristics
The CNP group consisted of 10 males and 19 females, with ages ranging from 22 to 38 years, with a mean age of 25.62±3.51 years, a mean educational background of 16.59±1.68 years, a disease duration of 12 to 54 months, with a mean duration of 34.97±11.14 months, a mean pressure-pain value of 13.73±2.69, a mean NPQ score of 29.12±7.84, and a mean HADS-A score was 9.07±2.46, and mean HADS-D score was 8.17±2.28. There were 14 males and 15 females in the control group, with ages ranging from 22 to 37 years; the mean age was 25.48±3.59 years, and the mean educational background was 16.03±3.26 years. The two groups of subjects were comparable in gender, age, and educational background (all P > 0.05), as shown in Table 1.
Table 1.
Demographic Data and Clinical Characteristics of Subjects
| Gender (Male /Female) | Age (Years) | Years of Education (Years) | Course of Disease (Months) | Tenderness Value | NPQ Score | HADS-A Score | HADS-D Score | |
|---|---|---|---|---|---|---|---|---|
| CNP | 10/19* | 25.62±3.51* | 16.59± 1.68* | 34.97± 11.14 | 13.73±2.69 | 29.12±7.84 | 9.07±2.46 | 8.17±2.28 |
| HC | 14/15 | 25.48±3.59 | 16.03± 3.26 | / | / | / | / | / |
Note: *Represents p >0.05.
Abbreviations: CNP, chronic neck pain group; HC, health control group; NPQ, Northwick Park Questionnaire; HADS-A, Hospital anxiety and depression scale- anxiety; HADS-D, Hospital anxiety and depression scale- depression.
Comparison of Brain Function Specificity Between the CNP Group and HC Group
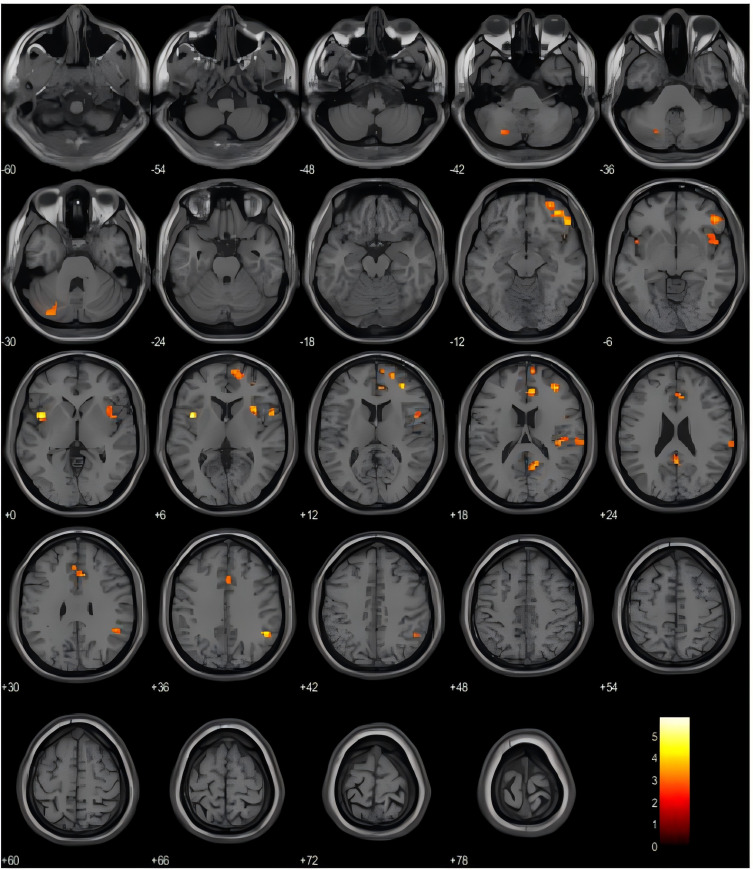
Our study identified a number of brain areas with increased amplitude of low-frequency fluctuations (ALFF) in the CNP group compared to the HC group. These regions include the left insula (INS.L), right inferior frontal gyrus orbital part (ORBinf.R), right angular gyrus (ANG.R), right anterior cingulate and paracingulate gyrus (ACG.R), right middle frontal gyrus (MFG.R), right insula (INS.R), right precuneus (PCUN.R), right rolandic operculum (ROL.R), right superior temporal gyrus (STG.R), right dorsolateral superior frontal gyrus (SFGdor.R), and left cerebellar crus area 2 (CC2.L) (Uncorrected, P<0.005, Cluster block ≥20), Detailed information can be found in Table 2 and Figure 1.
Table 2.
Brain Regions with Enhanced ALFF Values in the CNP Group Compared to the HC Group
| Brain Region | BA | Cluster Size | MNI Coordination | T-Value | P-value | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Left insula | 29 | 30 | −39 | +9 | +3 | 5.86 | 0.000 |
| Right inferior frontal gyrus orbital part | 16 | 88 | +48 | +39 | −9 | 4.73 | 0.000 |
| Right angular gyrus | 66 | 31 | +48 | −48 | 36 | 4.52 | 0.000 |
| Right anterior cingulate and paracingulate gyrus | 32 | 63 | +9 | +36 | +18 | 4.32 | 0.000 |
| Right middle frontal gyrus | 8 | 27 | +36 | +42 | +18 | 4.19 | 0.000 |
| Right insula | 30 | 61 | +36 | +15 | +6 | 3.84 | 0.000 |
| Right precuneus | 68 | 31 | +12 | −54 | +18 | 3.79 | 0.000 |
| Right rolandic operculum | 18 | 23 | +48 | +6 | +15 | 3.79 | 0.000 |
| Right superior temporal gyrus | 82 | 50 | +42 | −24 | +15 | 3.76 | 0.000 |
| Right dorsolateral superior frontal gyrus | 4 | 41 | +21 | +57 | +9 | 3.64 | 0.000 |
| Left cerebellar crus area 2 | 93 | 49 | −30 | −81 | −33 | 3.38 | 0.001 |
Figure 1.
Orange and yellow areas represent brain regions with enhanced ALFF values in CNP group compared to HC group.
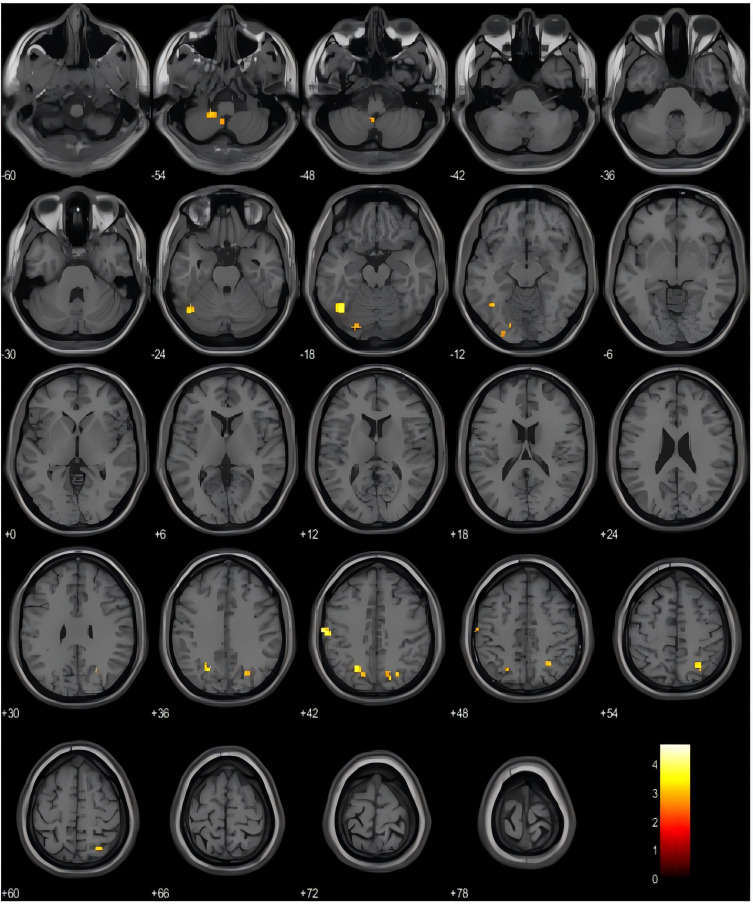
The brain areas exhibiting attenuated ALFF values include the left cerebellar crus area 1 (CC1.L), left superior marginal gyrus (SMG.L), left middle occipital gyrus (MOG.L), right superior parietal gyrus (SPG.R), left cerebellar region 9 (cere9.L), right superior occipital gyrus (SOG.R), and left fusiform gyrus (FFG.L) (Uncorrected, P<0.005, cluster blocks ≥20), Detailed information can be found in See Table 3 and Figure 2.
Table 3.
Brain Regions with Attenuated ALFF Values in the CNP Group Compared to the HC Group
| Brain Region | BA | Cluster Size | MNI Coordination | T-value | P-value | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Left cerebellar crus area 1 | 91 | 48 | −42 | −63 | −21 | 4.71 | 0.000 |
| Left superior marginal gyrus | 63 | 30 | −60 | −18 | +42 | 4.44 | 0.000 |
| Left middle occipital gyrus | 51 | 35 | −24 | −60 | +39 | 4.16 | 0.000 |
| Right superior parietal gyrus | 60 | 28 | +27 | −57 | +51 | 4.03 | 0.000 |
| Left cerebellar region 9 | 105 | 41 | −6 | −54 | −57 | 3.66 | 0.000 |
| Right superior occipital gyrus | 50 | 26 | +27 | −69 | +39 | 3.59 | 0.000 |
| Left fusiform gyrus | 55 | 24 | −18 | −84 | −15 | 3.23 | 0.001 |
Figure 2.
Orange-yellow areas represent brain regions with attenuated ALFF values in CNP group compared to HC group.
Correlation Between Brain Function and Clinical Indicators in the CNP Group
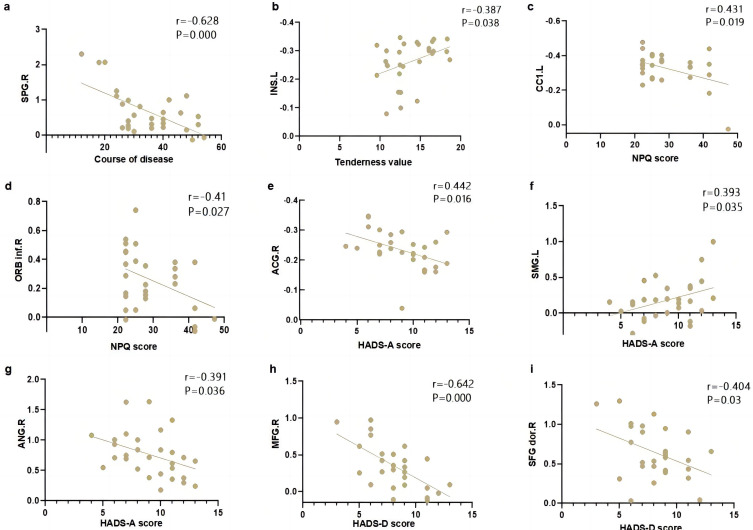
The study found that there is a negative correlation between the course of patients with chronic neuropathic pain (CNP) and the amplitude of low-frequency fluctuations (ALFF) in the right superior parietal gyrus. Additionally, the tenderness threshold exhibited a positive correlation with the ALFF value in the left insula. Furthermore, the NPQ score showed a positive correlation with the ALFF value in the left cerebellar crus area 1, while it displayed a negative correlation with the ALFF value in the right inferior frontal gyrus orbital part. Moreover, the HADS-A score demonstrated a positive correlation with the ALFF value in the right anterior cingulate and paracingulate gyri, as well as the left supramarginal gyrus. Conversely, it exhibited a negative correlation with the ALFF value in the right angular gyrus. Lastly, the HADS-D score displayed a negative correlation with the ALFF value in the right middle frontal gyrus and the right dorsolateral superior frontal gyrus. Refer to Figure 3a–i.
Figure 3.
The correlation analysis between clinical indicators and brain function. (a) Course of disease vs ALFF in SPG.R; (b) Tenderness value vs ALFF in INS.L; (c) NPQ score vs ALFF in CC1.L; (d) NPQ score vs ALFF in ORB inf.R; (e) Hads-A score vs ALFF in ACG.R; (f) HADS-A score vs ALFF in SMG.L; (g) HADS-A score vs ALFF in ANG.R; (h) HADS-D score vs ALFF in MFG.R; (i) HADS-D score vs ALFF in SFG dor.R.
Abbreviations: SPG.R, right superior parietal gyrus; INS.L, left insula; CC1.L, left cerebellar crus area 1; ORB inf.R, right inferior frontal gyrus orbital part; ACG.R, right anterior cingulate and paracingulate gyrus; SMG.L, left superior marginal gyrus; ANG.R, right angular gyrus; MFG.R, right middle frontal gyrus; SFG dor.R, right dorsolateral superior frontal gyrus.
Discussion
The fMRI technique, also known as blood oxygen level-dependent functional magnetic resonance imaging (BOLD-fMRI), operates by detecting spontaneous neuronal activity through hemodynamic changes in the brain. When neurons in a brain region are active, the blood flow to some of the brain regions is increased, which causes an increase in the concentration of venous oxygen and deoxygenation of the Hemoglobin content, which is shown as a signal enhancement on fMRI images, representing activation of the corresponding brain regions, and vice versa, signal weakening, representing inhibition of the corresponding brain regions.11 ALFF is one of the central processing and analyzing methods of rs-fMRI, which requires the participants to detect spontaneous neuronal activity in the brain. At the same time, their eyes are closed, stationary, and relaxed, and they are awake without active thinking. It has high sensitivity in measuring cerebral abnormalities among patients with neuropsychiatric and some functional brain disorders.25 Because it does not require the use of radioactive substances or injections, does not cause any harm to the patient, and can clinically and directly suggest the presence of anomalies in the cerebral areas of patients experiencing pain, it has become an important means of investigating the central mechanisms of pain.26 The previous studies have primarily focused on the impact of different interventions on the functional activity of specific brain areas in patients with CNP, and there is a noticeable dearth of studies concerning the central pathogenic mechanism of CNP, especially the lack of research on the alterations in the spontaneous central activity in young individuals suffering from CNP. In this study, we conducted a comparison between the brain regions of young patients with CNP and those of healthy populations by using the fMRI ALFF technique to make up for the corresponding deficiencies. This study showed that, compared with the healthy population, the increased ALFF values of young cervical spondylosis CNP patients were concentrated in the insula, cingulate gyrus, and prefrontal lobes. The decreased ALFF values were concentrated in the occipital and parietal lobes. Among these, the cingulate gyrus and insula belong to the pain-emotional areas of the brain, which are associated with the generation and transmission of adverse emotions and feelings of pain, and the pain-cognitive brain regions, including the prefrontal lobes, which are involved in the formation of cognition about pain. It suggests young CNP patients have altered functional activity in multiple cortical brain regions involved in chronic pain-related sensations, emotions, and cognition.
One of them, the brain’s insula, is part of the salience network (SN). As one of the essential functional networks in the brain, the SN is involved in coordinating attentional control, emotion regulation, behavioral control, and self-awareness and is especially critical in processing internal and external stimuli and regulating attention and emotional responses.27 The study results showed that the insula’s ALFF function in the patient group was enhanced bilaterally, reflecting the negative aversive emotions and associated perceptual abilities. In addition, correlation studies showed that pressure pain thresholds were negatively correlated with left insula ALFF values, the lower the patient’s pain threshold, the greater the spontaneous activity of the insula and the more pronounced the aversion to pain. Traditionally, nociception’s sensory and emotional components are believed to be conducted by their independent conduction pathways and processed in different brain compartments. With the progress of research, it has been found that the relevant brain regions do not process information about a single dimension of pain in isolation but are capable of processing information about multiple dimensions of pain. It has also been previously documented that brain insulae can be involved in both emotional responses to pain and encode information about the intensity and location of injurious and non-injurious temperature stimuli, which could also be used to explain the results of the present study.28–30
The cingulate gyrus plays a crucial role within the SN network and is divided into the anterior cingulate gyrus (ACC) and posterior cingulate gyrus (PCC). The anterior cingulate gyrus is a significant medial pain transduction system component. Also, it plays an essential role within the limbic system, which is mainly responsible for pain processing. It involves the processes of pain perception, encoding, and the subsequent avoidance responses to painful stimuli.31 When chronic pain patients were compared with healthy populations, it was shown that the ACC exhibited a significant abundance of opioid receptor-binding sites, which demonstrates the pivotal involvement of the ACC in both the development and control of pain.32 When injury stimulates the ACC, it produces inattention, apathy, emotional abnormalities, and autonomic dysfunction. It has been shown that individuals suffering from chronic headaches due to whiplash injuries have reduced gray matter volume in the ACC and dorsolateral prefrontal lobe three months post-injury.33 Thus, it is clear that increased ALFF in the ACC may be one of the critical features of CNP, suggesting that, compared with the healthy population, the ACC of patients with CNP receives more negative pain emotions, and the more they develop adverse emotions and negative interpretations of pain. The HADS-A scale exhibited a positive correlation with the ALFF value of the ACC section, suggesting that as the patient’s anxiety accumulates, there is more spontaneous brain activation in the ACC section.
The prefrontal lobe is a default mode network (DMN) component relevant to cognition, emotion, pain, and behavior management. Among them, the orbitofrontal cortex is closely related to emotional cognition. When pain is increased and recurrent, the frontal orbitofrontal cortex’s cognition and memory of pain are also intensified, so it causes enhanced spontaneous neuronal activity here. The precuneus is also an integral part of the DMN and critical for attention, memory, and self-reflection.34,35 It plays a primary role in the processing of adverse feelings resulting from pain or other forms of discomfort, and it also has a significant impact on cognitive function networks. In our study, increased spontaneous neurological activity in the precuneus among patients with cervical CNP may be related to increased adverse emotions due to chronic pain. Johansson proposes that increased spontaneous neurological activity in the prefrontal and precuneus lobes may reflect the tendency of patients to overthink pain, which can raise the personal threat in a particular situation and enhance the sensitivity and perception of the pain experience.36 The superior temporal gyrus is an auditory system’s information center, and pain, as a complex physiological and psychological phenomenon, has also been shown to affect auditory and visual networks.37 Duke et al carried out an investigation on patients with chronic musculoskeletal pain and showed increased functional connectivity between the patient group’s supratemporal gyrus and the left cerebellum.38 Chen conducted a study on the brain default mode network in individuals suffering from chronic neck pain. Their findings revealed that these patients had enhanced functional connectivity in the right supratemporal gyrus. The outcomes of our research are consistent with the fact that increased ALFF values in the superior temporal gyrus may be associated with excessive preoccupation with pain in patients with CNP, which affects other somatosensory perceptual systems.39
The occipital cortex is responsible for the integration of the hearing, vision, and somatosensory systems. In particular, the middle occipital gyrus is a significant center for visual processing, which could be modulated by attention.40 The most crucial feature of the occipital cortex’s processing of visual information is that the occipital cortex will extract the input from different levels, with upward circuits extracting information from the visual stimulus in terms of “position” and “motion”, while downward circuitry extracts information about the “what” of the object. The occipital lobe is susceptible to injury, which can result in various visual impairments, deficits in memory function, and compromised perception of motion. Studies have shown that the presence of chronic pain is associated with a decrease in the volume of gray matter in the occipital cortex, suggesting the importance of the occipital lobe to pain production and helping to explain the alterations in occipital spontaneous neuronal activity in patients with CNP.39 In addition to the fact that most of the occipital cortex shows reduced spontaneous activity in patients with CNP, parts of the parietal lobe, particularly the back part of the parietal cortex, which is known for its function in spatial perception and body position perception, work in conjunction with the somatosensory cortex to help the brain integrate, localize, and interpret pain signals, reflecting dysfunction in body perception and pain localization in patients with chronic neck pain in comparison to a group of healthy subjects.41
In this study, after analyzing the correlation between neurological function-specific changes and pain characteristics in CNP patients, we found a negative correlation between the ALFF values of the right superior parietal gyrus and the duration of the CNP. It means that if CNP patients fail to receive timely and effective treatment, the disease duration is prolonged, the spontaneous activity of neurons in the right superior parietal gyrus region decreases, and the ALFF value decreases. In addition, The ALFF values in area 1 of the left cerebellar crus exhibited a positive correlation with the scores obtained from the NPQ. The cerebellum can integrate the information received from nociception, proprioception, and kinesthesia; therefore, when more dysfunction exists in a patient with neck pain, it suggests that the patient’s cerebellum is abnormally activated to a higher degree. The ALFF values in the right inferior frontal gyrus orbital exhibited a negative correlation with NPQ scores. Additionally, the ALFF values in the right anterior cingulate/paracentral cingulate gyrus and left supramarginal gyrus displayed a positive correlation with HADS-A scores. Furthermore, the ALFF values in the right angular gyrus demonstrated a negative correlation with HADS-A scores. These findings align with the results of NI et al, who observed significant correlations between the frontal lobe, anterior cingulate/paracentral cingulate gyrus, as well as inferior temporal gyrus and the pain Characteristics in patients with neck pain.42 The ALFF values in the right middle frontal gyrus and right dorsolateral superior frontal gyrus exhibited a negative correlation with HADS-D scores. This finding aligns with the results reported by IHARA, who observed a positive correlation between patients’ dorsolateral prefrontal cortex and scores on the Agoraphobia Scale.43 The above results further suggest the wide range of abnormalities in young CNP patients’ spontaneous functional activity in brain regions. Nevertheless, the neurological mechanisms of impairment of brain network function involving pain sensation, pain emotion, and pain cognition can still be learned.
Limitations
Due to the limitations of various aspects, this trial still has some things that could be improved. Firstly, this trial only focused on the young CNP population, while patients of different age groups and different genders may have differences in clinical manifestations and changes in brain function, so future studies can be conducted to categorize CNP patients of different age groups or different genders. Second, due to the limited number of human patients, the final number of cases included in each group was only 29, which is not a sufficient sample size, and additional cases are imperative to enhance the credibility of the outcomes of trials in future studies. Third, in this study, only the observation of low-frequency oscillation amplitude results of the cerebral cortex in its resting state was compared, so multimodal neuroimaging markers need to be used in future studies to better understand the central remodeling mechanisms in patients with cervical spondylosis CNP.
Conclusion
In summary, chronic pain in young patients induces central remodeling, with changed functional activity in pain-emotion brain areas, which include the cingulate insula and gyrus, and pain-cognition brain areas, which include the prefrontal lobe. Additionally, other special sensory brain regions, which include the occipital lobes and parietal lobes. These alterations correlate with clinical pressure pain, functional impairment, and negative mood indicators of anxiety and depression, and the ALFF alterations in these brain regions can, to some extent, be a neuroimaging marker to reflect pain in young CNP patients objectively.
Acknowledgments
We thank the co-investigators who were not identified as co-authors of this study and all individuals who participated in the scanning process.
Funding Statement
This study was supported by the Key Research Project of Natural Science in Universities of Anhui Province (2023AH050725), the Major Research Project of Natural Science in Universities of Anhui Province (KJ2021ZD0062), and the Special Research Project of “Healthcare Service and Protection Capacity Enhancement Subsidy” of Bozhou Vocational and Technical College (YLFW2308).
Data Sharing Statement
The datasets generated during this study will not be made available to the public due to the subjects’ right to informed consent. However, they may be made available to the corresponding authors upon their reasonable request.
Ethical Statement
This study complied with the Declaration of Helsinki. The study protocol involving human subjects was reviewed and approved by the Medical Ethics Committee of Anhui Provincial Hospital of Chinese Medicine, Ethical Review Consent No. 2023AH-17. All patients and participants who agreed to participate in this study were provided with written informed consent.
Disclosure
No financial or business relationships could have caused conflicts of interest during this study.
References
- 1.Theodore N. Degenerative cervical spondylosis. New Engl J Med. 2020;383(2):159–168. doi: 10.1056/NEJMra2003558 [DOI] [PubMed] [Google Scholar]
- 2.Popescu A, Lee H. Neck pain and lower back pain. Med Clin North Am. 2020;104(2):279–292. doi: 10.1016/j.mcna.2019.11.003 [DOI] [PubMed] [Google Scholar]
- 3.Barreto TW, Svec JH Chronic neck pain: nonpharmacologic treatment. Am Family Phys. 2019100:(3):180–182. [PubMed] [Google Scholar]
- 4.Safiri S, Kolahi AA, Hoy D, et al. Global, regional, and national burden of neck pain in the general population, 1990–2017: systematic analysis of the global burden of disease study 2017. BMJ. 2020;368:m791. doi: 10.1136/bmj.m791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marchesini M, Ippolito C, Ambrosini L, Bignami EG, Fasani M, Abbenante D. Prevalence of low back and cervical back pain in military helicopter crews: an underestimated Italian problem. J Spec Oper Med. 2021;21(2):67–71. doi: 10.55460/MQZT-YXMK [DOI] [PubMed] [Google Scholar]
- 6.Murray CJL, Atkinson C, Bhalla K, et al. The state of US health, 1990–2010. JAMA. 2013;310(6):591–608. doi: 10.1001/jama.2013.13805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoy D, March L, Brooks P, et al. The global burden of low back pain: estimates from the global burden of disease 2010 study. Ann Rheumatic Dis. 2014;73(6):968–974. doi: 10.1136/annrheumdis-2013-204428 [DOI] [PubMed] [Google Scholar]
- 8.Osama M, Ali S, Malik RJ. Posture related musculoskeletal discomfort and its association with computer use among university students. J Pak Med Assoc. 2018;68(4):639–641. [PubMed] [Google Scholar]
- 9.Wang R. Observation on the Efficacy of Extracorporeal Shock Wave Combined with Neck Resistance Training on Chronic Nonspecific Neck Pain in Young People. Chengde Medical College; 2021; doi: 10.27691/d.cnki.gcdyx.2021.000239 [DOI] [Google Scholar]
- 10.Tang B, Meng W, Hägg S, Burgess S, Jiang X. Reciprocal interaction between depression and pain: results from a comprehensive bidirectional Mendelian randomization study and functional annotation analysis. Pain. 2022;163(1):e40–e48. doi: 10.1097/j.pain.0000000000002305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weng CJ, Xie DH. Progress of multimodal MRI study of chronic neck and shoulder pain in cervical spondylosis. Magn Reson Imaging. 2020;11(3):3. [Google Scholar]
- 12.Williams RJ, Specht JL, Mazerolle EL, Lebel RM, MacDonald ME, Pike GB. Correspondence between BOLD fMRI task response and cerebrovascular reactivity across the cerebral cortex. Front Physiol. 2023;14:1167148. doi: 10.3389/fphys.2023.1167148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang B, Jung M, Tu Y, et al. Identifying brain regions associated with the neuropathology of chronic low back pain: a resting-state amplitude of low-frequency fluctuation study. Br J Anaesth. 2019;123(2):e303–e311. doi: 10.1016/j.bja.2019.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen H, Qi G, Zhang Y, et al. Altered dynamic amplitude of low-frequency fluctuations in patients with migraine without aura. Front Hum Neurosci. 2021;15:636472. doi: 10.3389/fnhum.2021.636472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tu Y, Cao J, Bi Y, Hu L. Magnetic resonance imaging for chronic pain: diagnosis, manipulation, and biomarkers. Sci China Life Sci. 2021;64(6):879–896. doi: 10.1007/s11427-020-1822-4 [DOI] [PubMed] [Google Scholar]
- 16.Wager TD, Atlas LY, Lindquist MA, Roy M, Woo CW, Kross E. An fMRI-based neurologic signature of physical pain. New Engl J Med. 2013;368(15):1388–1397. doi: 10.1056/NEJMoa1204471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bishop JH, Shpaner M, Kubicki A, Clements S, Watts R, Naylor MR. Structural network differences in chronic musculoskeletal pain: beyond fractional anisotropy. Elsevier BV. 2018;182:441–455. doi: 10.1016/j.neuroimage.2017.12.021 [DOI] [PubMed] [Google Scholar]
- 18.Woodworth DC, Holly LT, Mayer EA, Salamon N, Ellingson BM. Alterations in cortical thickness and subcortical volume are associated with neurological symptoms and neck pain in patients with cervical spondylosis. Ovid Technologies. 2018;84(3):588–598. doi: 10.1093/neuros/nyy066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.xin YC, ting JT, Song H, et al. Abnormality of spontaneous brain activities in patients with chronic neck and shoulder pain: a resting-state fMRI study. SAGE Publications. 2017;45(1):182–192. doi: 10.1177/0300060516679345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J, Wang Z, Tu Y, et al. Regional homogeneity and multivariate pattern analysis of cervical spondylosis neck pain and the modulation effect of treatment. Frontiers Media S A. 2018;12:900. doi: 10.3389/fnins.2018.00900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fan N, Chen J, Zhao B, et al. Neural correlates of central pain sensitization in chronic low back pain: a resting-state fMRI study. Springer Sci Bus Media LLC. 2023;65(12):1767–1776. doi: 10.1007/s00234-023-03237-3 [DOI] [PubMed] [Google Scholar]
- 22.Hu Y, Ma J, Chen B, Pang J, Liang W, Wu W. The duration of chronic pain can affect brain functional changes of the pain matrix in patients with chronic back pain: a resting-state fMRI study. Informa UK Limit. 2024;17:1941–1951. doi: 10.2147/jpr.s457575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li T, Li J, Zhao R, Zhou J, Chu X. Deficits in the thalamocortical pathway associated with hypersensitivity to pain in patients with frozen shoulder. Frontiers Media S A. 2023;14. doi: 10.3389/fneur.2023.1180873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang C, Guo G, Fang S, et al. Abnormal brain activity in lumbar disc herniation patients with chronic pain is associated with their clinical symptoms. Frontiers Media S A. 2023;17:1206604. doi: 10.3389/fnins.2023.1206604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de VF, Koini M, Schouten TM, et al. A comprehensive analysis of resting state fMRI measures to classify individual patients with Alzheimer’s disease. Elsevier BV. 2018;167:62–72. doi: 10.1016/j.neuroimage.2017.11.025 [DOI] [PubMed] [Google Scholar]
- 26.Wang HB, Li LP, Gu WD. Progress of functional magnetic resonance imaging in the study of neuropathic pain. Chin J Pain Med. 2017;23(6):6. [Google Scholar]
- 27.Xu H, Chen Y, Tao Y, et al. Modulation effect of acupuncture treatment on chronic neck and shoulder pain in female patients: evidence from periaqueductal gray‐based functional connectivity. Wiley. 2022;28(5):714–723. doi: 10.1111/cns.13803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Craig AD, Chen K, Bandy D, Reiman EM. Thermosensory activation of insular cortex. Springer Sci Bus Media LLC. 2000;3(2):184–190. doi: 10.1038/72131 [DOI] [PubMed] [Google Scholar]
- 29.Bernhardt BC, Singer T. The neural basis of empathy. Ann Rev. 2012;35(1):1–23. doi: 10.1146/annurev-neuro-062111-150536 [DOI] [PubMed] [Google Scholar]
- 30.Craig A. A new view of pain as a homeostatic emotion. Elsevier BV. 2003;26(6):303–307. doi: 10.1016/s0166-2236(03)00123-1 [DOI] [PubMed] [Google Scholar]
- 31.Budell L, Kunz M, Jackson PL, Rainville P. Mirroring pain in the brain: emotional expression versus motor Imitation. Pub Lib Sci. 2015;10(2):e0107526. doi: 10.1371/journal.pone.0107526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Apkarian AV, Sosa Y, Sonty S, et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. Soc Neurosci. 2004;24(46):10410–10415. doi: 10.1523/jneurosci.2541-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Obermann M, Nebel K, Schumann C, et al. Gray matter changes related to chronic posttraumatic headache. Ovid Technologies. 2009;73(12):978–983. doi: 10.1212/wnl.0b013e3181b8791a [DOI] [PubMed] [Google Scholar]
- 34.Alshelh Z, Marciszewski KK, Akhter R, et al. Disruption of default mode network dynamics in acute and chronic pain states. Elsevier BV. 2018;17:222–231. doi: 10.1016/j.nicl.2017.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Irie K, Matsumoto A, Zhao S, Kato T, Liang N. Neural basis and motor imagery intervention methodology based on neuroimaging studies in children with developmental coordination disorders: a review. Frontiers Media S A. 2021;15. doi: 10.3389/fnhum.2021.620599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johansson E, Xiong HY, Polli A, Coppieters I, Nijs J. Towards a real-life understanding of the altered functional behaviour of the default mode and salience network in chronic pain: are people with chronic pain overthinking the meaning of their pain? MDPI AG. 2024;13(6):1645. doi: 10.3390/jcm13061645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chehadi O, Rusu AC, Konietzny K, et al. Brain structural alterations associated with dysfunctional cognitive control of pain in patients with low back pain. Wiley. 2017;22(4):745–755. doi: 10.1002/ejp.1159 [DOI] [PubMed] [Google Scholar]
- 38.Han SD, Buchman AS, Arfanakis K, Fleischman DA, Bennett DA. Functional connectivity networks associated with chronic musculoskeletal pain in old age. Wiley. 2012;28(8):858–867. doi: 10.1002/gps.3898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Y, Yu CX, Li B, Ji TT. A study of brain default network in chronic neck and shoulder pain patients with neurogenic cervical spondylosis. Magn Reson Imaging. 2020;11(3):5. [Google Scholar]
- 40.Ni X, Zhang J, Sun M, et al. Abnormal dynamics of functional connectivity density associated with chronic neck pain. Frontiers Media S A. 2022;15:880228. doi: 10.3389/fnmol.2022.880228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y, Wang Z, Du J, et al. Regulatory effects of acupuncture on emotional disorders in patients with menstrual migraine without aura: a Resting-state fMRI study. Frontiers Media S A. 2021;15:726505. doi: 10.3389/fnins.2021.726505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gallace A, Bellan V. The parietal cortex and pain perception: a body protection system. Elsevier. 2018;151:103–117. doi: 10.1016/b978-0-444-63622-5.00005-x [DOI] [PubMed] [Google Scholar]
- 43.Ihara N, Wakaizumi K, Nishimura D, et al. Aberrant resting-state functional connectivity of the dorsolateral prefrontal cortex to the anterior insula and its association with fear avoidance belief in chronic neck pain patients. Pub Lib Sci. 2019;14(8):e0221023. doi: 10.1371/journal.pone.0221023 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during this study will not be made available to the public due to the subjects’ right to informed consent. However, they may be made available to the corresponding authors upon their reasonable request.