Abstract
Background
Acne is a common inflammatory dermatosis. Although gender-related differences in prevalence and age of onset have been documented. Other gender-related characteristics of acne have not been well elucidated yet.
Objective
We compared characteristics of skin lesions, severity and risk factors of acne between males and females in Chinese.
Methods
Investigator-administered questionnaire was used to collect demographic data, clinical feature and risk factors of acne from May 2020 to August 2021. All participants were from outpatient clinics in seven cities, China.
Results
A total of 13085 participants, including 4746 males (36.27%) and 8339 females (63.73%), aged 8 to 35 years old, completed the questionnaire. The age of onset of acne was significantly younger in males than in females(p<0.001). More females than males had comedones(83.76% vs 75.22%, p<0.001). In contrast, more males than females had pustules, cyst/nodules, scars and severer lesions(p<0.001). Moreover, the major risk factors for acne were constipation, cosmetics, dairy and sweet foods for females, while the major risk factors for males were computer usage/playing electronic games, staying up late at night, intake of hot or spicy foods. More females than males experienced good response to photodynamic treatment(79.52% vs 52.86%, p<0.05).
Conclusion
Acne appears earlier in males than in females. Clinical features and risk factors for acne differ between males and females. More females than males experience good response to photodynamic therapy.
Limitation
This questionnaire survey was carried out in Chinese aged 8 to 35 years old. Gender-related characteristics in other ages and regions remain to be explored.
Keywords: acne, risk factors, gender, severity, treatment
Introduction
Gender differences in biology and pathogenesis of diseases have long been known. In general, females have longer life expectancy than males do.1 Previous studies showed that excessive exposure to UV irradiation induces a higher transepidermal water loss rates in males than in females, while the rate of epidermal permeability barrier recovery is significant higher in females than in males following acute abrogation of barrier function with repeated tap-stripping.2 The slower barrier recovery in males is in part attributable to sex hormone because estrogen (female hormone) accelerates while testosterone delays barrier development in fetal skin.3 Castration of male mice accelerates the recovery of epidermal permeability barrier.4 The thickness of the skin is thicker in males than in females.5 Gender differences in skin disorders have also been demonstrated. For example, the prevalence of psoriasis and erythroderma in males is twice as high as that in females.6 Similarly, the incidence of non-melanoma skin cancers is higher in males than in females.7 In contrast, more females than males suffer from systemic lupus erythematosus.8 Likewise, prevalence of atopic dermatitis is higher in females than in males.9 Moreover, gender differences also reflect in disease severity. For instance, Psoriasis Area Severity Index is lower in females than in males.10,11 But the age of psoriasis onset is younger in females than in males.10 Taken together, this line of evidence indicates variation of cutaneous conditions with gender.
Acne is a common, chronic inflammatory skin disorder. The prevalence of acne varies greatly with age, gender and ethnicity. For example, prevalence of acne is over 40% in children aged 7–9 years old in Lithuania,12 whereas no acne patients were observed in children under 9 years old in China.13 Prevalence of acne is higher in girls than in boys aged 7 to 12 years old, but lower in girls than in boys aged 16 to 18 years old.14 Similarly, prevalence of acne is higher in boys than in girls aged 15 to 29 years old in Chinese.13 However, the prevalence of acne is higher in girls than in boys aged 9 to 16 years old in Ghanaian.15 Moreover, the age of onset of acne is younger in females than in males.16 Furthermore, males with acne have more facial pores than females, while the number of facial pores is correlated positively with the severity of acne.17 Higher portion of males than females have severe acne (15% vs 3.9%).18 Males have higher risk for both severe acne (adjusted odds ratio=1.485, p<0.05) and acne scarring (adjusted odds ratio=2.460, p=0.001).19 Additionally, females with acne have higher Dermatology Life Quality Index score and Cardiff acne disability index than males do.20 However, gender-related differences in acne severity and triggering/aggravating factors in Chinese are still limited. We assessed here the gender-related characteristics of disease severity and risk factors in Chinese with acne.
Participants and Methods
An investigator-administered questionnaire (Supplementary material) was used to collect demographic data, clinical features and risk factors of acne from May 2020 to August 2021. All participants were from outpatient clinics in 7 cities (Suzhou; Huzhou; Nanjing; Baoshan; Luzhou; Zhengzhou; Shanghai). This study was approved by institutional review board of the Fifth Affiliated Hospital of Dali University and carried out in accordance with the Declaration of Helsinki. Informed consent was obtained from all participants and the legal guardian or the parent of individuals under age of 18 years prior to taking the questionnaire survey. Staying up late at night was defined as individuals went to bed after middle night ≥3 times per week. Playing electronics meant watching TV, playing electronic devices (phone, notebook and computer). Changes in living environment meant temporarily changing living place.
Acne was diagnosed by well-trained dermatologists at the time when questionnaire was administered. Only participants who had acne at the time when the questionnaire survey was taken were included in this analysis. Subjects without acne were excluded. According to the severity of disease, acne was classified into 4 categories, ie I, only comedones and a few papules; II, comedones, papules and a few pustules; III, larger inflammatory papules, pustules and a few cysts, involved the face and other body sites; IV, more severe with cysts becoming confluent.21 For risk factors, we did not attempt to quantify the extent of exposure to risk factors. For each item in the questionnaire, only yes-or-no question was provided. Therapeutic outcome of different treatments was based on the surveyees’ response to the questionnaire.
Data Analysis
Data are analyzed with GraphPad Prism software 8.3.0. Descriptive data are expressed percentage while quantitative data are expressed as mean ± sem. Either Chi-square test/Cochran-Mantel-Haenszel test or unpaired student t test was used to determine the significance between males and females.
Results
Demographic Characteristics
A total of 13085 participants, including 4746 males and 8339 females aged 8 to 35 years old, were included in this analysis (Table 1). The average age at onset was younger in males than in females (17.12±0.05 vs 18.56±0.05, p<0.001).
Table 1.
Demographic Characteristics
| Males (N=4746) | Females (N=8339) | P values | ||
|---|---|---|---|---|
| Age (yr) | <20 | 17.47 ± 2.01(47.75%) | 17.81 ± 2.24(29.38%) | <0.001 |
| 20–30 | 24.11 ± 2.54(47.81%) | 24.54 ± 2.63(60.18%) | <0.001 | |
| >30 | 34.55 ± 3.19(4.45%) | 34.48 ± 3.12(10.44%) | 0.782 | |
| Overall | 21.40 ± 0.07(100%) | 23.6 ± 0.06(100%) | <0.001 | |
| Age at onset (yr) | 17.12 ± 0.05 | 18.56 ± 0.05 | <0.001 | |
| Disease duration (yr) | 4.53 ± 0.05 | 5.40 ± 0.05 | <0.001 | |
Note: Data are expressed as Mean ± sem (% of total).
Characteristics of Skin Lesions
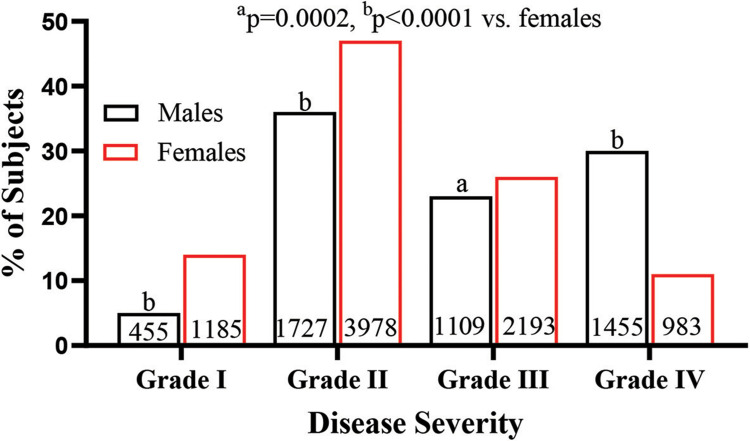
As shown in Table 2, proportion of subjects with comedones was greater in females than in males, while proportion of subjects with papules was comparable between males and females. In contrast, proportion of subjects with pustules, scar and cyst/nodules was higher in males than in females. Similarly, a greater portion of males had severe acne (grade IV) in comparison to that of females (30.66% vs 11.79%, p<0.0001). About 61% of females and 46% of males had grades I and II of acne (Figure 1). These results show that males tend to have severer acne than do females.
Table 2.
Clinical Features
| Age | Males | Females | P | |
|---|---|---|---|---|
| Comedones | <20 | 1819/2266(80.27%) | 2149/2450(87.71%) | <0.001 |
| 20–30 | 1623/2269(71.53%) | 4185/5018(83.4%) | <0.001 | |
| >30 | 128/211(60.66%) | 651/871(74.74%) | <0.001 | |
| Papules | <20 | 1870/2266(82.52%) | 2005/2450(81.84%) | 0.543 |
| 20–30 | 1887/2269(83.16%) | 4097/5018(81.65%) | 0.121 | |
| >30 | 168/211(79.62%) | 665/871(76.35%) | 0.318 | |
| Pustules | <20 | 1003/2266(44.26%) | 791/2450(32.29%) | <0.001 |
| 20–30 | 966/2269(42.57%) | 1674/5018(33.36%) | <0.001 | |
| >30 | 94/211(44.55%) | 295/871(33.87%) | <0.001 | |
| Cyst/nodules | <20 | 634/2266(27.98%) | 243/2450(9.92%) | <0.001 |
| 20–30 | 756/2269(33.32%) | 596/5018(11.88%) | <0.001 | |
| >30 | 65/211(30.81%) | 144/871(16.53%) | <0.001 | |
| Atrophic scar | <20 | 635/2266(28.02%) | 307/2450(12.53%) | <0.001 |
| 20–30 | 849/2269(37.42%) | 889/5018(17.72%) | <0.001 | |
| >30 | 67/211(31.75%) | 129/871(14.81% | <0.001 | |
| Hypertrophic scar | <20 | 182/2266(8.03%) | 40/2450(1.63%) | <0.001 |
| 20–30 | 277/2269(12.21%) | 102/5018(2.03%) | <0.001 | |
| >30 | 31/211(14.69%) | 27/871(3.10%) | <0.001 |
Note: N/total (%).
Figure 1.
Comparison of disease Severity between males and females.
Notes: Disease severity was graded as described in the Materials and Methods section. Chi-square test was used to determine the significance between males and females. The number of subjects and significance are indicated in the figure ap=0.002 and bp<0.0001, males vs females.
Risk Factors for Acne Differ Between Males and Females
We next analyzed the risk factors for acne in males and females. The major risk factors for acne were taking spicy foods and staying up late at night in both males and females (Table 3). Significantly higher portion of females aged ≤30 years old proclaimed that dairy diets, seafoods, sweet foods, changes in living environment, genetic factor and psychological stress are the triggering and/or aggravating factors for acne (p<0.001 for males vs females). Similarly, more females than males considered skin care products and constipation as the triggering and/or aggravating factors for acne (p<0.001). In contrast, more males than females aged 21–30 years old, but not in other age groups, experienced triggering and/or aggravating acne by changing season (p<0.001). Moreover, the influences of dairy diets and psychological stress on acne varied with age in both females (p<0.0001 for both) and males (p=0.0021 for dairy diets, p=0.0014 for psychological stress). But the age-dependent effect of sweet foods on acne was only observed in females (p<0.0001), but not in males. These results demonstrate that the risk factors of acne vary with gender.
Table 3.
Triggering/Aggravating Factors
| Age | Males | Females | P | |
|---|---|---|---|---|
| Dairy diets | <20 | 253(11.17%) | 512(20.9%) | <0.001 |
| 20–30 | 318(14.01%) | 1245(24.81%) | <0.001 | |
| >30 | 17(8.06%) | 106(12.17%) | 0.115 | |
| Spicy food | <20 | 1959(86.45%) | 213587.14%) | 0.491 |
| 20–30 | 1880(82.86%) | 4149(82.68%) | 0.867 | |
| >30 | 179(84.83%) | 723(83.01%) | 0.539 | |
| Sweet food | <20 | 670(29.57%) | 1117(45.59%) | <0.001 |
| 20–30 | 744(32.79%) | 2667(53.15%) | <0.001 | |
| >30 | 63(29.86%) | 304(34.9%) | 0.169 | |
| Seafood | <20 | 925(40.82%) | 1108(45.22%) | 0.002 |
| 20–30 | 1007(44.38%) | 2480(49.42%) | <0.001 | |
| >30 | 76(36.02%) | 420(48.22%) | 0.002 | |
| Constipation | <20 | 1019(44.97%) | 1517(61.92%) | <0.001 |
| 20–30 | 1253(55.22%) | 3563(71%) | <0.001 | |
| >30 | 122(57.82%) | 607(69.69%) | 0.001 | |
| Genetics | <20 | 1100(48.54%) | 1413(57.67%) | <0.001 |
| 20–30 | 1206(53.15%) | 2960(58.99%) | <0.001 | |
| >30 | 117(55.45%) | 456(52.35%) | 0.419 | |
| Staying up late | <20 | 1797(79.3%) | 2031(82.9%) | 0.002 |
| 20–30 | 1994(87.88%) | 4371(87.11) | 0.358 | |
| >30 | 174(82.46%) | 702(80.6%) | 0.535 | |
| Overtiredness | <20 | 1501(66.24%) | 1788(72.98%) | <0.001 |
| 20–30 | 1711(75.41%) | 3836(76.44%) | 0.342 | |
| >30 | 160(75.83%) | 630(72.33%) | 0.342 | |
| Cosmetics | <20 | 893(39.41%) | 1537(62.73%) | <0.001 |
| 20–30 | 1042(45.92%) | 3581(71.36%) | <0.001 | |
| >30 | 85(40.28%) | 553(63.49%) | <0.001 | |
| Electronics | <20 | 1695(74.8%) | 1828(74.61%) | 0.893 |
| 20–30 | 1739(76.64%) | 3693(73.6%) | 0.006 | |
| >30 | 137(64.93%) | 622(71.41%) | 0.066 | |
| Changes in living environment | <20 | 1083(47.79%) | 1474(60.16%) | <0.001 |
| 20–30 | 1429(62.98%) | 3514(70.03%) | <0.001 | |
| >30 | 116(54.98%) | 509(58.44%) | 0.393 | |
| Changes in Season | <20 | 1587(70.04%) | 1655(67.55%) | 0.066 |
| 20–30 | 1658(73.07%) | 3367(67.1%) | <0.001 | |
| >30 | 151(71.56%) | 564(64.75%) | 0.061 | |
| Psychological stress | <20 | 1160(51.19%) | 1498(61.14%) | <0.001 |
| 20–30 | 1244(54.83%) | 3130(62.38%) | <0.001 | |
| >30 | 92(43.6%) | 431(49.48%) | 0.125 |
Note: N (%).
Males and Females Differentially Respond to the Treatments
Next, we compared the response to treatments between males and females. As shown in Table 4, males and females did not respond differently to most of the topical treatments except to topical benzoyl peroxide and clindamycin, which females displayed poor therapeutical response (for benzoyl peroxide, p=0.005 for grade I acne; for clindamycin, p=0.023 for grade IV acne). Likewise, the responses to either oral isotretinoin or antibiotics were comparable between males and females. Moreover, males and females displayed similar responses to the treatment with either laser or red blue light. However, more females with grades II and III acne exhibited better therapeutical response to photodynamic therapy (p=0.031 for grade II and p=0.003 for grade III). These results indicate gender-related differences in response to the treatments of acne. Notably, about 70% of subjects experienced good response to the treatment with tretinoin, while less than 40% of subjects declared good therapeutic response to erythromycin and clindamycin.
Table 4.
Response to Topical Treatments
| Treatments | Severity | Males | Females | P |
|---|---|---|---|---|
| Tretinoin | I | 71/97(73.2) | 234/319(73.35) | 0.975 |
| II | 266/366(72.68) | 893/1263(70.7) | 0.463 | |
| III | 201/284(70.77) | 554/757(73.18) | 0.438 | |
| IV | 301/446(67.49) | 300/408(73.53) | 0.053 | |
| Benzoyl peroxide | I | 20/22(90.91) | 30/52(57.69) | 0.005 |
| II | 68/91(74.73) | 185/273(67.77) | 0.212 | |
| III | 42/65(64.62) | 108/164(65.85) | 0.859 | |
| IV | 45/105(42.86) | 41/115(35.65) | 0.274 | |
| Erythromycin | I | 28/82(34.15) | 66/206(32.04) | 0.731 |
| II | 105/296(35.47) | 224/705(31.77) | 0.255 | |
| III | 78/206(37.86) | 146/421(34.68) | 0.434 | |
| IV | 104/323(32.2) | 81/242(33.47) | 0.75 | |
| Clindamycin | I | 15/46(32.61) | 52/128(40.63) | 0.338 |
| II | 83/174(47.7) | 178/426(41.78) | 0.185 | |
| III | 48/134(35.82) | 103/284(36.27) | 0.929 | |
| IV | 66/209(31.58) | 35/166(21.08) | 0.023 | |
| Fusidic acid | I | 28/38(73.68) | 93/139(66.91) | 0.426 |
| II | 145/213(68.08) | 517/720(71.81) | 0.292 | |
| III | 73/146(50) | 207/420(49.29) | 0.882 | |
| IV | 93/283(32.86) | 78/227(34.36) | 0.722 | |
| Sulfur lotion | I | 17/30(56.67) | 12/34(35.29) | 0.087 |
| II | 77/105(73.33) | 109/173(63.01) | 0.076 | |
| III | 34/73(46.58) | 41/105(39.05) | 0.317 | |
| IV | 45/135(33.33) | 30/70(42.86) | 0.179 | |
| Glucocorticoids | I | 3/11(27.27) | 6/18(33.33) | 0.732 |
| II | 15/26(57.69) | 26/42(61.9) | 0.73 | |
| III | 10/16(62.5) | 27/41(65.85) | 0.812 | |
| IV | 18/34(52.94) | 9/13(69.23) | 0.312 | |
| Chemical peeling | I | 40/53(75.47) | 113/165(68.48) | 0.333 |
| II | 113/158(71.52) | 364/533(68.29) | 0.441 | |
| III | 64/100(64) | 182/287(63.41) | 0.917 | |
| IV | 61/145(42.07) | 48/132(36.36) | 0.332 |
Notes: Data are expressed as percentage of subjects who positively responded to topical treatments [N/total (%)]. Chi-square test is used to determine the significance between males and females. Italic bold indicates significant difference.
Discussion
Previous studies showed gender differences in prevalence and age of onset of acne.13–16 Correspondingly, we showed here that the age of onset was younger in boys than in girls. The earlier onset of acne in boys can be attributable to the higher testosterone levels. Previous studies demonstrated a link between high testosterone levels to the development of acne.22,23 First, serum testosterone levels are higher in individuals with acne than those without acne, and are passively correlated with the severity of acne.24 Second, anti-androgenic therapy is effective for acne.25,26 Because testosterone levels are higher in boys than in girls at puberty age,27,28 the age of onset is younger in boys than in girls. The other gender-related difference is acne severity. Our results were consistent with prior study,20 showing that males have more severe acne than females. This is not surprising. As mentioned above, boys have higher levels of serum testosterone, which is linked to acne. Also, females usually have greater cosmetic concern compared to males. Hence, females seek medical care in a timely manner when they find lesions on the skin. Appropriate early treatment can mitigate the progression of acne. Thus, proportion of severe acne are greater in males than in females.
Previous studies demonstrated that females have more comedones than males.29 We show here that the prevalence of comedones was higher in females than in males (83.76% vs 75.22%, p<0.001). The underlying mechanisms accounting for such gender difference are unknown. Another remarkable gender-related difference was a higher prevalence of acne scars in males than in females (23.13% vs 12.69%, p<0.0001), consistent with previous observation.30 Such gender-related difference is likely due to a higher sebum production in males than in females.17,31 Sebum can induce inflammation,32,33 resulting in severe acne, which is known to be linked to the development of acne scars.34 Therefore, high prevalence of severe acne and acne scars in males is attributed to higher testosterone levels, resulting in increased sebum production and inflammation.
Although acne can occur as early as at age 6 in girls in the United States,35 the present study showed the youngest Chinese with acne were 8 years old in girls. In comparison to Americans, Chinese children, especially those live in suburban areas, eat less protein and dairy products, while nutrition determines the onset of puberty.36 The excessive nutrition is linked to the development of acne.37 Thus, the age of onset is older in Chinese than in Americans. Previous study showed the age of onset of acne was ≥10 years in Chinese reported in 2011,13 a two-year older than that shown in the present study. Because the trend of pubertal age is decreasing,38,39 the difference in the age of onset between the present and previous studies could reflect the trend of decrease in pubertal age.
The risk factors for acne include diet, life style, psychological stress, etc. We show here that over 80% of the subjects claimed spicy food is triggering/aggravating factor, which is in contrast to previous study.40 Thus, the pathogenic role of spicy food in acne remains to be explored. The present study also demonstrate that over 80% of subjects declared staying up late at night as a risk factor for acne, which can be attributed to the increased sebum production.41 Since acne is an inflammatory dermatosis, late bedtime-induced inflammation can also provoke and/or exacerbate acne.42 A striking difference between males and females is the risk factors, such as skin care products (relative risk=2.71) and psychological stress (relative risk=1.154), both of which prevalence were higher in females than in males. Females tend to frequently use more skin care products. Skin care products can worsen acne because some ingredients in skin care products can irritate the skin or cause acne.43–45 Thus, skin care products become a major risk factor for acne in females. In agreement with previous study,46 psychological stress is one of the risk factors in females. Gender differences in response to psychological stress are due to psychological and biological differences as well as sex hormone.47 Females usually experience a higher level of stress than males.48,49 Psychological stress increases the secretion of adrenal androgens, resulting in sebaceous hyperplasia,46 consequently leading to the development and exacerbation of acne. Regarding the sweet foods, females tend to eat more sweet foods than males.50 Intake of excessive sugar can increase serum insulin, which lowers sex hormone-binding globulin concentration while elevating androgen concentration, resulting in the development and exacerbation of acne.51 Taken together, gender-related difference in risk factors for acne can be attributable to the differences in psychology, biology and life habits between males and females. Regarding the underlying mechanisms accounting for the gender differences in response to the treatments, it is unclear. Nevertheless, the results suggest that gender should be considered in the management of acne. Avoidance of sweet foods and proper management of constipation can possibly mitigate acne, especially in females. Benzoyl peroxide is more effective for mild acne in males than in females.
In conclusions, intake of spicy food, sweet food, playing electronics, constipation and late bedtime are the major risk factors for acne. Males and females with acne differ in age of onset, disease severity, some of risk factors and response to some treatments. In the management of acne, these gender differences should be considered.
Limitation
This questionnaire survey was carried out in Chinese aged 8 to 35 years old in southern China. Because sun-exposure can affect the severity of acne,52,53 gender-related characteristics of acne in other ages and regions such as northern China remain to be explored. Moreover, the disease duration and the number of subjects were not the same between males and females. And larger sample size can increase analysis power. It would be ideally to elucidate the gender-related differences between males and females with similar disease duration, age and sample size.
Funding Statement
This work was supported in part by National Natural Science Foundation of China(NSFC82103756, TZ), and Joint Medical Research Program, Baoshan City, Yunnan Province, China (2023bskjylzd001, SY).
Data Sharing Statement
All data supporting the findings of this study are available within the paper and its Supplementary Information.
Disclosure
Authors declare no conflict of interests for this article.
References
- 1.Zarulli V, Kashnitsky I, Vaupel JW. Death rates at specific life stages mold the sex gap in life expectancy. Proc Natl Acad Sci U S A. 2021;118(20):e2010588118.10. doi: 10.1073/pnas.2010588118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Z, Fluhr JW, Song SP, et al. Sun-induced changes in stratum corneum function are gender and dose dependent in a Chinese population. Skin Pharmacol Physiol. 2010;23(6):313–319. doi: 10.1159/000314138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanley K, Rassner U, Jiang Y, et al. Hormonal basis for the gender difference in epidermal barrier formation in the fetal rat. acceleration by estrogen and delay by testosterone. J Clin Invest. 1996;97(11):2576–2584. doi: 10.1172/JCI118706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kao JS, Garg A, Mao-Qiang M, et al. Testosterone perturbs epidermal permeability barrier homeostasis. J Invest Dermatol. 2001;116(3):443–451. doi: 10.1046/j.1523-1747.2001.01281.x [DOI] [PubMed] [Google Scholar]
- 5.Giacomoni PU, Mammone T, Teri M. Gender-linked differences in human skin. J Dermatol Sci. 2009;55(3):144–149. doi: 10.1016/j.jdermsci.2009.06.001 [DOI] [PubMed] [Google Scholar]
- 6.Furue M, Yamazaki S, Jimbow K, et al. Prevalence of dermatological disorders in Japan: a nationwide, cross-sectional, seasonal, multicenter, hospital-based study. J Dermatol. 2011;38(4):310–320. doi: 10.1111/j.1346-8138.2011.01209.x [DOI] [PubMed] [Google Scholar]
- 7.Huang J, Chan SC, Ko S, et al. Global incidence, mortality, risk factors and trends of melanoma: a systematic analysis of registries. Am J Clin Dermatol. 2023;24(6):965–975. doi: 10.1007/s40257-023-00795-3 [DOI] [PubMed] [Google Scholar]
- 8.Izmirly PM, Ferucci ED, Somers EC, et al. Incidence rates of systemic lupus erythematosus in the USA: estimates from a meta-analysis of the centers for disease control and prevention national lupus registries. Lupus Sci Med. 2021;8(1):e000614. doi: 10.1136/lupus-2021-000614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johansson EK, Bergström A, Kull I, et al. Prevalence and characteristics of atopic dermatitis among young adult females and males-report from the Swedish population-based study BAMSE. J Eur Acad Dermatol Venereol. 2022;36(5):698–704. doi: 10.1111/jdv.17929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guillet C, Seeli C, Nina M, Maul LV, Maul JT. The impact of gender and sex in psoriasis: what to be aware of when treating women with psoriasis. Int J Womens Dermatol. 2022;8(2):e010. doi: 10.1097/JW9.0000000000000010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Napolitano M, Mastroeni S, Fania L, et al. Sex- and gender-associated clinical and psychosocial characteristics of patients with psoriasis. Clin Exp Dermatol. 2020;45(6):705–711. doi: 10.1111/ced.14218 [DOI] [PubMed] [Google Scholar]
- 12.Karciauskiene J, Valiukeviciene S, Gollnick H, Stang A. The prevalence and risk factors of adolescent acne among schoolchildren in Lithuania: a cross-sectional study. J Eur Acad Dermatol Venereol. 2014;28(6):733–740. doi: 10.1111/jdv.12160 [DOI] [PubMed] [Google Scholar]
- 13.Shen Y, Wang T, Zhou C, et al. Prevalence of acne vulgaris in Chinese adolescents and adults: a community-based study of 17,345 subjects in six cities. Acta Derm Venereol. 2012;92(1):40–44. doi: 10.2340/00015555-1164 [DOI] [PubMed] [Google Scholar]
- 14.Kilkenny M, Merlin K, Plunkett A, Marks R. The prevalence of common skin conditions in Australian school students: 3. acne vulgaris. Br J Dermatol. 1998;139(5):840–845. doi: 10.1046/j.1365-2133.1998.02510.x [DOI] [PubMed] [Google Scholar]
- 15.Hogewoning AA, Koelemij I, Amoah AS, et al. Prevalence and risk factors of inflammatory acne vulgaris in rural and urban Ghanaian schoolchildren. Br J Dermatol. 2009;161(2):475–477. doi: 10.1111/j.1365-2133.2009.09259.x [DOI] [PubMed] [Google Scholar]
- 16.Schäfer T, Nienhaus A, Vieluf D, Berger J, Ring J. Epidemiology of acne in the general population: the risk of smoking. Br J Dermatol. 2001;145(1):100–104. doi: 10.1046/j.1365-2133.2001.04290.x [DOI] [PubMed] [Google Scholar]
- 17.Kim BY, Choi JW, Park KC, Youn SW. Sebum, acne, skin elasticity, and gender difference - which is the major influencing factor for facial pores? Skin Res Technol. 2013;19(1):e45–53. doi: 10.1111/j.1600-0846.2011.00605.x [DOI] [PubMed] [Google Scholar]
- 18.Kaminsky A, Florez-White M, Bagatin E, Arias MI, Iberian Latin American Acne Studies Group (GILEA - Grupo Ibero-Latinoamericano de Estudio del Acne). Large prospective study on adult acne in Latin America and the Iberian Peninsula: risk factors, demographics, and clinical characteristics. Int J Dermatol. 2019;58(11):1277–1282. doi: 10.1111/ijd.14441 [DOI] [PubMed] [Google Scholar]
- 19.Heng AHS, Say YH, Sio YY, Ng YT, Chew FT. Epidemiological risk factors associated with acne vulgaris presentation, severity, and scarring in a Singapore Chinese population: a cross-sectional study. Dermatology. 2022;238(2):226–235. doi: 10.1159/000516232 [DOI] [PubMed] [Google Scholar]
- 20.Singh IP, Poonia K, Bajaj K. Quality of life in young adults with acne: results of a cross-sectional study. J Cosmet Dermatol. 2021;20(12):4017–4023. doi: 10.1111/jocd.14540 [DOI] [PubMed] [Google Scholar]
- 21.Adityan B, Kumari R, Thappa DM. Scoring systems in acne vulgaris. Indian J Dermatol Venereol Leprol. 2009;75(3):323–326. doi: 10.4103/0378-6323.51258 [DOI] [PubMed] [Google Scholar]
- 22.Behayaa HR, Juda TM, Mohammed SB. The effect of androgen hormones in acne pathogenesis: a review. Med J Babylon. 2022;19(3):345–349. doi: 10.4103/MJBL.MJBL_88_22 [DOI] [Google Scholar]
- 23.Schmidt JB, Spona J. The levels of androgen in serum in female acne patients. Endocrinol Exp. 1985;19(1):17–23. [PubMed] [Google Scholar]
- 24.Iftikhar U, Choudhry N. Serum levels of androgens in acne & their role in acne severity. Pak J Med Sci. 2019;35(1):146–150. doi: 10.12669/pjms.35.1.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemay A, Poulin Y. Oral contraceptives as anti-androgenic treatment of acne. J Obstet Gynaecol Can. 2002;24(7):559–567. doi: 10.1016/S1701-2163(16)31058-1 [DOI] [PubMed] [Google Scholar]
- 26.Tan JK, Degreef H. Oral contraceptives in the treatment of acne. Skin Therapy Lett. 2001;6(5):1–3. [PubMed] [Google Scholar]
- 27.Shirtcliff EA, Dahl RE, Pollak SD. Pubertal development: correspondence between hormonal and physical development. Child Dev. 2009;80(2):327–337. doi: 10.1111/j.1467-8624.2009.01263.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sizonenko PC, Paunier L. Hormonal changes in puberty III: correlation of plasma dehydroepiandrosterone, testosterone, FSH, and LH with stages of puberty and bone age in normal boys and girls and in patients with Addison’s disease or hypogonadism or with premature or late adrenarche. J Clin Endocrinol Metab. 1975;41(5):894–904. doi: 10.1210/jcem-41-5-894 [DOI] [PubMed] [Google Scholar]
- 29.Lucky AW, Biro FM, Simbartl LA, Morrison JA, Sorg NW. Predictors of severity of acne vulgaris in young adolescent girls: results of a five-year longitudinal study. J Pediatr. 1997;130(1):30–39. doi: 10.1016/S0022-3476(97)70307-X [DOI] [PubMed] [Google Scholar]
- 30.Agrawal DA, Khunger N. A morphological study of acne scarring and its relationship between severity and treatment of active acne. J Cutan Aesthet Surg. 2020;13(3):210–216. doi: 10.4103/JCAS.JCAS_177_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Man MQ, Xin SJ, Song SP, et al. Variation of skin surface pH, sebum content and stratum corneum hydration with age and gender in a large Chinese population. Skin Pharmacol Physiol. 2009;22(4):190–199. doi: 10.1159/000231524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fabbrocini G, Annunziata MC, D’Arco V, et al. Acne scars: pathogenesis, classification and treatment. Dermatol Res Pract. 2010;2010:893080. doi: 10.1155/2010/893080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo JW, Lin TK, Wu CH, et al. Human sebum extract induces barrier disruption and cytokine expression in murine epidermis. J Dermatol Sci. 2015;78(1):34–43. doi: 10.1016/j.jdermsci.2015.01.010 [DOI] [PubMed] [Google Scholar]
- 34.Layton AM, Henderson CA, Cunliffe WJ. A clinical evaluation of acne scarring and its incidence. Clin Exp Dermatol. 1994;19(4):303–308. doi: 10.1111/j.1365-2230.1994.tb01200.x [DOI] [PubMed] [Google Scholar]
- 35.Pochi PE, Strauss JS, Downing DT. Skin surface lipid composition, acne, pubertal development, and urinary excretion of testosterone and 17-ketosteroids in children. J Invest Dermatol. 1977;69(5):485–489. doi: 10.1111/1523-1747.ep12511753 [DOI] [PubMed] [Google Scholar]
- 36.González-Mondragón EA, Ganoza-Granados LDC, Toledo-Bahena ME, et al. Acne and diet: a review of pathogenic mechanisms. Bol Med Hosp Infant Mex. 2022;79(2):83–90. doi: 10.24875/BMHIM.21000088 [DOI] [PubMed] [Google Scholar]
- 37.Lucky AW, Biro FM, Huster GA, Morrison JA, Elder N. Acne vulgaris in early adolescent boys. Correlations with pubertal maturation and age. Arch Dermatol. 1991;127(2):210–216. doi: 10.1001/archderm.1991.01680020078009 [DOI] [PubMed] [Google Scholar]
- 38.Goldberg JL, Dabade TS, Davis SA, Feldman SR, Krowchuk DP, Fleischer AB. Changing age of acne vulgaris visits: another sign of earlier puberty? Pediatr Dermatol. 2011;28(6):645–648. doi: 10.1111/j.1525-1470.2011.01643.x [DOI] [PubMed] [Google Scholar]
- 39.Zhou X, Zhang L. The trends of puberty onset among chinese children. Iran J Public Health. 2015;44(1):134–135. [PMC free article] [PubMed] [Google Scholar]
- 40.El Darouti MA, Zeid OA, Abdel Halim DM, et al. Salty and spicy food; are they involved in the pathogenesis of acne vulgaris? A case controlled study. J Cosmet Dermatol. 2016;15(2):145–149. doi: 10.1111/jocd.12200 [DOI] [PubMed] [Google Scholar]
- 41.Shao L, Jiang S, Li Y, et al. Regular late bedtime significantly affects the skin physiological characteristics and skin bacterial microbiome. Clin Cosmet Invest Dermatol. 2022;15:1051–1063. doi: 10.2147/CCID.S364542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alqaderi H, Abdullah A, Finkelman M, et al. The relationship between sleep and salivary and serum inflammatory biomarkers in adolescents. Front Med. 2023;10:1175483. doi: 10.3389/fmed.2023.1175483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Available from: https://www.aad.org/public/diseases/acne/skin-care/habits-stop. Accessed May 2, 2023. ADD. 10 skin care habits that can worsen acne
- 44.Available from: https://lamav.com/blogs/articles/10-common-ingredients-in-cosmetics-that-actually-cause-acne. Accessed May 2, 2023. LAMAV Organic Skin Scienc. 10 common ingredients in comsmentics that may cause acne
- 45.Huang LN, Zhong YP, Liu D, et al. Adverse cutaneous reactions to skin care products on the face vary with age, but not with sex. Contact Dermatitis. 2018;79(6):365–369. doi: 10.1111/cod.13102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zari S, Alrahmani D. The association between stress and acne among female medical students in Jeddah, Saudi Arabia. Clin Cosmet Invest Dermatol. 2017;10:503–506. doi: 10.2147/CCID.S148499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Verma R, Balhara YP, Gupta CS. Gender differences in stress response: role of developmental and biological determinants. Ind Psychiatry J. 2011;20(1):4–10. doi: 10.4103/0972-6748.98407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Graves BS, Hall ME, Dias-Karch C, Haischer MH, Apter C. Gender differences in perceived stress and coping among college students. PLoS One. 2021;16(8):e0255634. doi: 10.1371/journal.pone.0255634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dreno B, Bagatin E, Blume-Peytavi U, Rocha M, Gollnick H. Female type of adult acne: physiological and psychological considerations and management. J Dtsch Dermatol Ges. 2018;16(10):1185–1194. [DOI] [PubMed] [Google Scholar]
- 50.Masella R, Malorni W. Gender-related differences in dietary habits. Clin Manag Issues. 2017;11(2):59–62. [Google Scholar]
- 51.Romańska-Gocka K, Woźniak M, Kaczmarek-Skamira E, Zegarska B. The possible role of diet in the pathogenesis of adult female acne. Postepy Dermatol Alergol. 2016;33(6):416–420. doi: 10.5114/ada.2016.63880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen P, Li S, Xiao Y, et al. Long-term exposure to low levels of ambient UVB are associated with a decreased risk of moderate-to-severe acne: a retrospective cohort study in college students. Photodermatol Photoimmunol Photomed. 2023;39(2):132–139. doi: 10.1111/phpp.12852 [DOI] [PubMed] [Google Scholar]
- 53.Piquero-Casals J, Morgado-Carrasco D, Rozas-Muñoz E, et al. Sun exposure, a relevant exposome factor in acne patients and how photoprotection can improve outcomes. J Cosmet Dermatol. 2023;22(6):1919–1928. doi: 10.1111/jocd.15726 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data supporting the findings of this study are available within the paper and its Supplementary Information.