Abstract
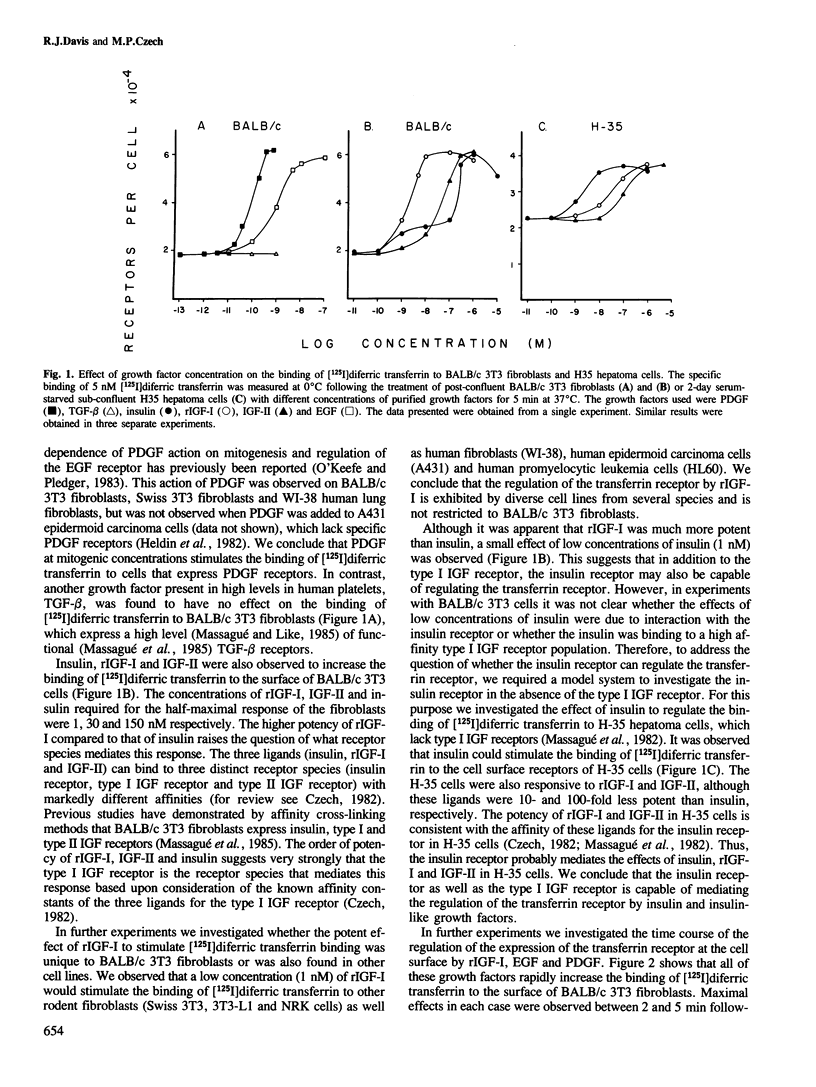
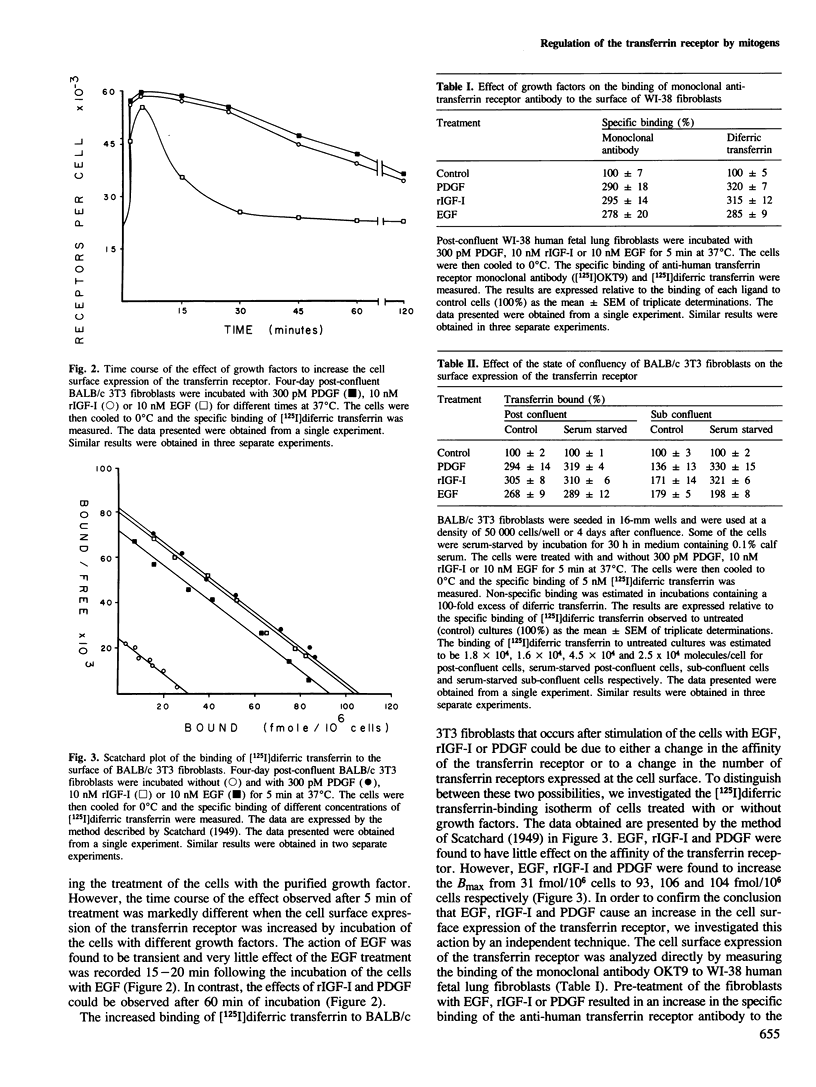
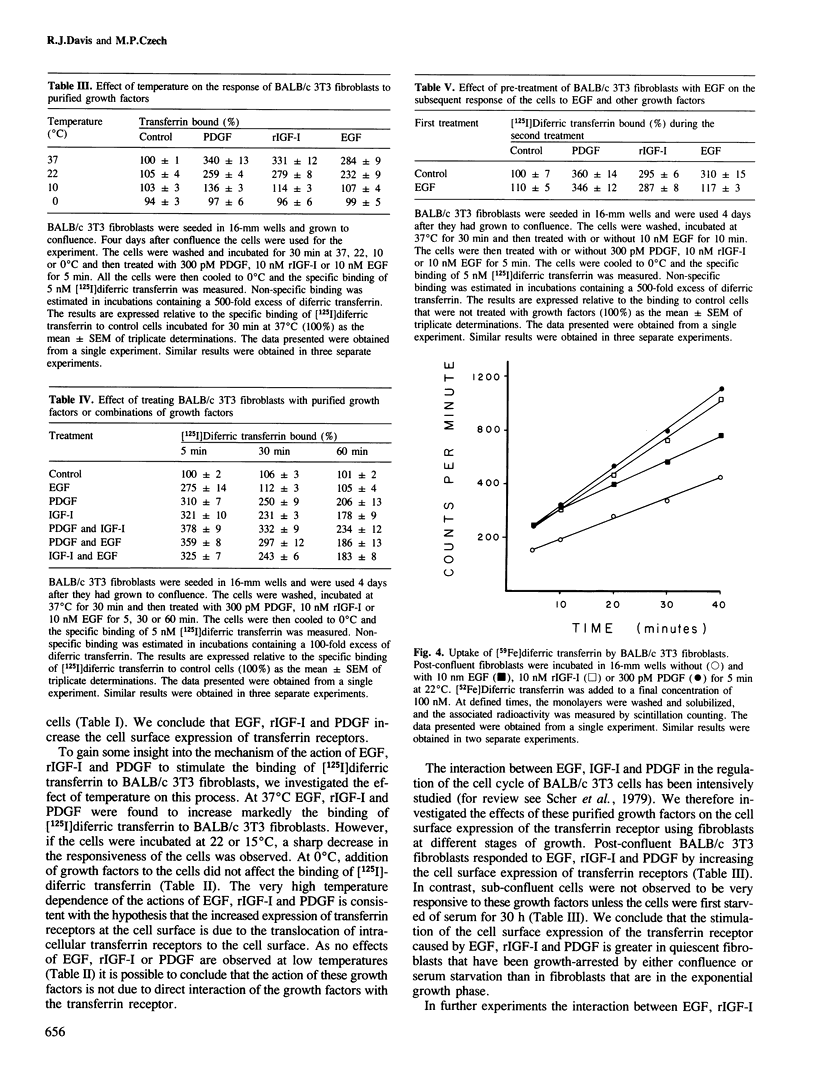
Addition of platelet-derived growth factor (PDGF), recombinant insulin-like growth factor I (rIGF-I) or epidermal growth factor (EGF) to BALB/c 3T3 fibroblasts causes a marked increase in the binding of [125I]diferric transferrin to cell surface receptors. This effect is very rapid and is complete within 5 min. The effect of EGF is transient, with [125I]diferric transferrin binding returning to control values within 25 min. In contrast, PDGF and rIGF-I cause a prolonged stimulation of [125I]diferric transferrin binding that could be observed for up to 2 h. The increase in the binding of [125I]diferric transferrin caused by growth factors was investigated by analysis of the binding isotherm. Epidermal growth factor, PDGF and rIGF-I were found to increase the cell surface expression of transferrin receptors rather than to alter the affinity of the transferrin receptors. This result was confirmed in human fibroblasts by the demonstration that EGF, PDGF and rIGF-I could stimulate the binding of a monoclonal antibody directed against the transferrin receptor (OKT9) to the cell surface. Furthermore, PDGF and rIGF-I stimulated the sustained uptake of [59Fe]diferric transferrin by BALB/c 3T3 fibroblasts, while EGF transiently increased uptake. Thus the effect of these growth factors to increase the cell surface expression of the transferrin receptor appears to have an important physiological consequence.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisen P., Listowsky I. Iron transport and storage proteins. Annu Rev Biochem. 1980;49:357–393. doi: 10.1146/annurev.bi.49.070180.002041. [DOI] [PubMed] [Google Scholar]
- Besancon F., Bourgeade M. F., Testa U. Inhibition of transferrin receptor expression by interferon-alpha in human lymphoblastoid cells and mitogen-induced lymphocytes. J Biol Chem. 1985 Oct 25;260(24):13074–13080. [PubMed] [Google Scholar]
- Booth A. G., Wilson M. J. Human placental coated vesicles contain receptor-bound transferrin. Biochem J. 1981 Apr 15;196(1):355–362. doi: 10.1042/bj1960355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buys S. S., Keogh E. A., Kaplan J. Fusion of intracellular membrane pools with cell surfaces of macrophages stimulated by phorbol esters and calcium ionophores. Cell. 1984 Sep;38(2):569–576. doi: 10.1016/0092-8674(84)90511-7. [DOI] [PubMed] [Google Scholar]
- Ciechanover A., Schwartz A. L., Lodish H. F. Sorting and recycling of cell surface receptors and endocytosed ligands: the asialoglycoprotein and transferrin receptors. J Cell Biochem. 1983;23(1-4):107–130. doi: 10.1002/jcb.240230111. [DOI] [PubMed] [Google Scholar]
- Cushman S. W., Wardzala L. J. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell. Apparent translocation of intracellular transport systems to the plasma membrane. J Biol Chem. 1980 May 25;255(10):4758–4762. [PubMed] [Google Scholar]
- Czech M. P. Structural and functional homologies in the receptors for insulin and the insulin-like growth factors. Cell. 1982 Nov;31(1):8–10. doi: 10.1016/0092-8674(82)90399-3. [DOI] [PubMed] [Google Scholar]
- Dautry-Varsat A., Ciechanover A., Lodish H. F. pH and the recycling of transferrin during receptor-mediated endocytosis. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2258–2262. doi: 10.1073/pnas.80.8.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith R. M., Galbraith G. M. Expression of transferrin receptors on mitogen-stimulated human peripheral blood lymphocytes: relation to cellular activation and related metabolic events. Immunology. 1981 Dec;44(4):703–710. [PMC free article] [PubMed] [Google Scholar]
- Haigler H. T., Maxfield F. R., Willingham M. C., Pastan I. Dansylcadaverine inhibits internalization of 125I-epidermal growth factor in BALB 3T3 cells. J Biol Chem. 1980 Feb 25;255(4):1239–1241. [PubMed] [Google Scholar]
- Hamilton T. A. Regulation of transferrin receptor expression in concanavalin A stimulated and Gross virus transformed rat lymphoblasts. J Cell Physiol. 1982 Oct;113(1):40–46. doi: 10.1002/jcp.1041130109. [DOI] [PubMed] [Google Scholar]
- Harding C., Heuser J., Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983 Aug;97(2):329–339. doi: 10.1083/jcb.97.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin C. H., Wasteson A., Westermark B. Interaction of platelet-derived growth factor with its fibroblast receptor. Demonstration of ligand degradation and receptor modulation. J Biol Chem. 1982 Apr 25;257(8):4216–4221. [PubMed] [Google Scholar]
- Hopkins C. R. Intracellular routing of transferrin and transferrin receptors in epidermoid carcinoma A431 cells. Cell. 1983 Nov;35(1):321–330. doi: 10.1016/0092-8674(83)90235-0. [DOI] [PubMed] [Google Scholar]
- Hopkins C. R., Trowbridge I. S. Internalization and processing of transferrin and the transferrin receptor in human carcinoma A431 cells. J Cell Biol. 1983 Aug;97(2):508–521. doi: 10.1083/jcb.97.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchings S. E., Sato G. H. Growth and maintenance of HeLa cells in serum-free medium supplemented with hormones. Proc Natl Acad Sci U S A. 1978 Feb;75(2):901–904. doi: 10.1073/pnas.75.2.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsson A., Heldin C. H., Westermark B., Wasteson A. Platelet-derived growth factor: identification of constituent polypeptide chains. Biochem Biophys Res Commun. 1982 Jan 15;104(1):66–74. doi: 10.1016/0006-291x(82)91941-6. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Ashwell G., van Renswoude J., Harford J. B., Bridges K. R. Binding of apotransferrin to K562 cells: explanation of the transferrin cycle. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2263–2266. doi: 10.1073/pnas.80.8.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner R. D., Harford J., van Renswoude J. Rapid internalization of the transferrin receptor in K562 cells is triggered by ligand binding or treatment with a phorbol ester. Proc Natl Acad Sci U S A. 1984 May;81(10):3005–3009. doi: 10.1073/pnas.81.10.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J. E., Ray F., Ward J. H., Kushner J. P., Kaplan J. Internalization and subcellular localization of transferrin and transferrin receptors in HeLa cells. J Biol Chem. 1983 Jul 25;258(14):8751–8758. [PubMed] [Google Scholar]
- Massague J., Guillette B. J., Czech M. P. Affinity labeling of multiplication stimulating activity receptors in membranes from rat and human tissues. J Biol Chem. 1981 Mar 10;256(5):2122–2125. [PubMed] [Google Scholar]
- Massagué J., Blinderman L. A., Czech M. P. The high affinity insulin receptor mediates growth stimulation in rat hepatoma cells. J Biol Chem. 1982 Dec 10;257(23):13958–13963. [PubMed] [Google Scholar]
- Massagué J., Kelly B., Mottola C. Stimulation by insulin-like growth factors is required for cellular transformation by type beta transforming growth factor. J Biol Chem. 1985 Apr 25;260(8):4551–4554. [PubMed] [Google Scholar]
- Massagué J., Like B. Cellular receptors for type beta transforming growth factor. Ligand binding and affinity labeling in human and rodent cell lines. J Biol Chem. 1985 Mar 10;260(5):2636–2645. [PubMed] [Google Scholar]
- Matrisian L. M., Larsen B. R., Finch J. S., Magun B. E. Further purification of epidermal growth factor by high-performance liquid chromatography. Anal Biochem. 1982 Sep 15;125(2):339–351. doi: 10.1016/0003-2697(82)90015-x. [DOI] [PubMed] [Google Scholar]
- May W. S., Jacobs S., Cuatrecasas P. Association of phorbol ester-induced hyperphosphorylation and reversible regulation of transferrin membrane receptors in HL60 cells. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2016–2020. doi: 10.1073/pnas.81.7.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckers L. M., Cossman J. Transferrin receptor induction in mitogen-stimulated human T lymphocytes is required for DNA synthesis and cell division and is regulated by interleukin 2. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3494–3498. doi: 10.1073/pnas.80.11.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe E. J., Pledger W. J. A model of cell cycle control: sequential events regulated by growth factors. Mol Cell Endocrinol. 1983 Aug;31(2-3):167–186. doi: 10.1016/0303-7207(83)90147-8. [DOI] [PubMed] [Google Scholar]
- Oka Y., Mottola C., Oppenheimer C. L., Czech M. P. Insulin activates the appearance of insulin-like growth factor II receptors on the adipocyte cell surface. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4028–4032. doi: 10.1073/pnas.81.13.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer C. L., Pessin J. E., Massague J., Gitomer W., Czech M. P. Insulin action rapidly modulates the apparent affinity of the insulin-like growth factor II receptor. J Biol Chem. 1983 Apr 25;258(8):4824–4830. [PubMed] [Google Scholar]
- Pearse B. M. Coated vesicles from human placenta carry ferritin, transferrin, and immunoglobulin G. Proc Natl Acad Sci U S A. 1982 Jan;79(2):451–455. doi: 10.1073/pnas.79.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters M. A., Lau E. P., Snitman D. L., Van Wyk J. J., Underwood L. E., Russell W. E., Svoboda M. E. Expression of a biologically active analogue of somatomedin-C/insulin-like growth factor I. Gene. 1985;35(1-2):83–89. doi: 10.1016/0378-1119(85)90160-x. [DOI] [PubMed] [Google Scholar]
- Rovera G., Ferrero D., Pagliardi G. L., Vartikar J., Pessano S., Bottero L., Abraham S., Lebman D. Induction of differentiation of human myeloid leukemias by phorbol diesters: phenotypic changes and mode of action. Ann N Y Acad Sci. 1982 Dec 10;397:211–220. doi: 10.1111/j.1749-6632.1982.tb43428.x. [DOI] [PubMed] [Google Scholar]
- Savage C. R., Jr, Cohen S. Epidermal growth factor and a new derivative. Rapid isolation procedures and biological and chemical characterization. J Biol Chem. 1972 Dec 10;247(23):7609–7611. [PubMed] [Google Scholar]
- Scher C. D., Shepard R. C., Antoniades H. N., Stiles C. D. Platelet-derived growth factor and the regulation of the mammalian fibroblast cell cycle. Biochim Biophys Acta. 1979 Aug 10;560(2):217–241. doi: 10.1016/0304-419x(79)90020-9. [DOI] [PubMed] [Google Scholar]
- Sutherland R., Delia D., Schneider C., Newman R., Kemshead J., Greaves M. Ubiquitous cell-surface glycoprotein on tumor cells is proliferation-associated receptor for transferrin. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4515–4519. doi: 10.1073/pnas.78.7.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Kono T. Evidence that insulin causes translocation of glucose transport activity to the plasma membrane from an intracellular storage site. Proc Natl Acad Sci U S A. 1980 May;77(5):2542–2545. doi: 10.1073/pnas.77.5.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardzala L. J., Simpson I. A., Rechler M. M., Cushman S. W. Potential mechanism of the stimulatory action of insulin on insulin-like growth factor II binding to the isolated rat adipose cell. Apparent redistribution of receptors cycling between a large intracellular pool and the plasma membrane. J Biol Chem. 1984 Jul 10;259(13):8378–8383. [PubMed] [Google Scholar]
- Watts C. Rapid endocytosis of the transferrin receptor in the absence of bound transferrin. J Cell Biol. 1985 Feb;100(2):633–637. doi: 10.1083/jcb.100.2.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley H. S., Kaplan J. Epidermal growth factor rapidly induces a redistribution of transferrin receptor pools in human fibroblasts. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7456–7460. doi: 10.1073/pnas.81.23.7456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham M. C., Hanover J. A., Dickson R. B., Pastan I. Morphologic characterization of the pathway of transferrin endocytosis and recycling in human KB cells. Proc Natl Acad Sci U S A. 1984 Jan;81(1):175–179. doi: 10.1073/pnas.81.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Renswoude J., Bridges K. R., Harford J. B., Klausner R. D. Receptor-mediated endocytosis of transferrin and the uptake of fe in K562 cells: identification of a nonlysosomal acidic compartment. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6186–6190. doi: 10.1073/pnas.79.20.6186. [DOI] [PMC free article] [PubMed] [Google Scholar]


