ABSTRACT
Background:
Globally, breast cancer is the most common malignant tumor in both developed and developing nations, with an incidence of 2 million cases every year and around 6 lakh deaths. Even after the availability of healthcare facilities, delays in the management of breast cancer are seen in both developed and developing countries.
Objectives:
To assess the patient and system-level delays and to determine the factors that cause the identified delays in women with breast cancer from central rural India.
Methodology:
The present Mixed-method study was conducted in a tertiary care hospital in central rural India among 128 female breast cancer patients. Socio-demographic and clinical information of the patients was summarized using frequency and proportions. Delays were reported using a median number of days and interquartile range. To assess the factors associated with the delays, we used Negative log-binomial regression analysis. Qualitative data analysis was done by manual thematic analysis.
Results:
The mean age of the study participants was 50.54, SD was 10.46, Median was 50, and the Interquartile range (IQR) was 43-58 years. A median patient delay of 45 days, and IQR was 15-120. A median system delay of 19 days and IQR was 7-35 days. We identified seven themes addressing the factors influencing delays at various levels by thematic analysis. Negative log-binomial regression models were built for the association of the socio- demographic and clinical variables with patient and system delays.
Conclusion:
Our study concludes that there is a substantial delay at patient level reporting to healthcare care providers, which needs an increase in awareness levels in the community through dedicated Behavior Change Communication strategies along with addressing identified socio-cultural and economic determinants influencing delay at various levels.
Keywords: Breast cancer, mixed method study, patient delays, system delays
Introduction
Breast cancer is the most common malignant tumor globally, affecting 2 million cases and 6 lakh deaths, with 25.8% incidence in India per one lakh women annually.[1] The Indian Council of Medical Research reported a 28.54% relative proportion of breast cancer in our study area from 2012 to 2016.[2] In 2020, 23 lakh new cases of breast cancer were diagnosed in women, surpassing lung cancer, the former most commonly diagnosed cancer.[3] The incidence and mortality rates of diseases in low to middle-income countries (LMIC) are higher than those in high-income countries.[4] Early diagnosis and treatment of breast cancer are crucial to reduce mortality and increase survival rates, especially in developing countries, despite healthcare facilities’ availability, despite delays.[5] Richards et al.’s[6] meta-analysis found that patients with a 6 month delay had a lower 5-year survival rate compared to those with early presentation. Patient delay or provider delay in disease diagnosis and treatment can lead to advanced stages of the disease, making it difficult to treat.[5,7] Early detection offers a promising prognosis for advanced-stage cancers, with high cure rates, while delayed presentations lead to costly treatment options and reduced survival rates.[8]
A 2018 study found an increase in breast cancer incidence in non-Hispanic Asian and Pacific Islander women aged 20-39, while it decreased in non-Hispanic white women over 75 years.[9] Breast cancer deaths increase due to regional, social, and political contexts, affecting economic loss and overall productivity in women, both at work and home.[4] Breast cancer deaths increase due to regional, social, and political contexts, affecting economic loss and overall productivity in women, both at work and home.[10] Breast cancer treatment costs triple that of prostate cancer, and productivity loss in younger women is higher than prostate cancer deaths due to elderly men. Breast cancer leads in detection, treatment, and mortality expenses.[11] Rural India experiences system-related issues leading to delays in early breast cancer diagnosis and treatment initiation due to various factors.
The study investigates healthcare system delays, including late patient presentations, appointments, investigations, diagnosis, and treatment initiation, identifying associated factors. Delays at the patient or care provider level can lead to the progression of cancer stage, resulting in a poor prognosis.[4] The study focuses on recognizing where delays occur in the diagnosis and treatment of breast cancer patients. Family physicians and primary caregivers are often the first points of contact hence they should be senitized to counsel and refer patients suspected of breast cancer to appropriate centers. This study aimed to evaluate patient and system-level delays in women with breast cancer in central rural India and identify the underlying factors.
Material and Methods
Study Settings: Tertiary care medical college hospital located in central rural India.
Study design: Cross-sectional study using a Sequential Explanatory Mixed Method Approach.
Study Participants: All the patients presenting with some breast-related abnormalities confirmed to be breast cancer at the tertiary care hospital during the study duration. The Participants who were eligible and gave consent to participate in study were recruited consecutively from the outpatient and inpatient department of surgery and Oncology till the estimated sample size was achieved.
Inclusion criteria: Women presenting with any breast-related complaint which is confirmed to be breast cancer and willing to consent for participation in the study.
Exclusion Criteria: Terminally ill patients who cannot complete the interview questionnaire were excluded from the study.
Sample size: G* Power 3.1.9.7 version (Franz Faul, Universitat Kiel, Germany) was used to calculate the sample size using a confidence interval of 95%, alpha error probability of 0.05, an effect size of 0.5, and a Power of 0.8. The final sample size was estimated as 128. (n = 128)
Sampling procedure
Quantitative phase: The eligible study participants were enrolled from August 2022 to January 2024 until the desired sample size of 128 was reached.
Data collection tools and procedure -: A structured questionnaire containing the socio-demographic Profile, Questions related to patient-level delay, and system-level delay, details in regards to progression of current ailment from start of first symptoms to first health care consultation sought to treatment and current status along with relevant past, family, and obstetric history was recorded.
The data confidentiality was assured to the participants and were explained that the data confidentiality and privacy shall be maintained and data shall be used for academic purpose only.
Qualitative phase: Our study is based on the pragmatism paradigm and is in alignment with our research study question. We conducted face-to-face, in-depth interviews using a semi-structured interview guide which was validated by postgraduate guide. 10 interviews were conducted initially using the purposive sampling method, and then we continued to recruit the participants until no more new themes emerged, i.e., till data saturation was achieved.[12] Finally, we conducted 12 in-depth interviews with 3 oncologists, 3 nursing staff, 3 medical social workers, and 3 patients.
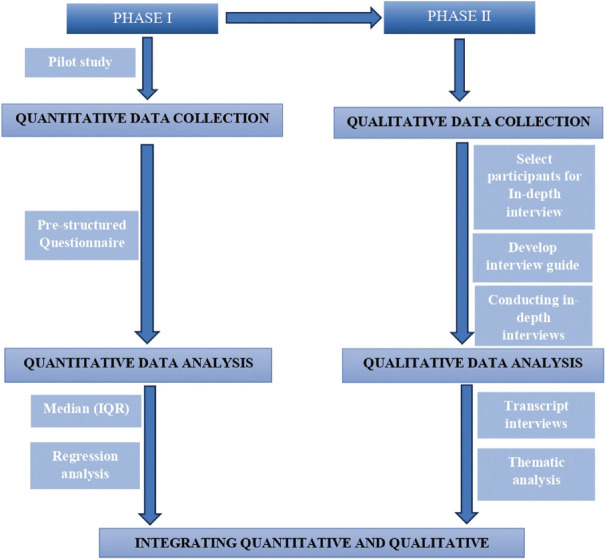
The schematic representation of study procedure is depicted in the flow chart in Figure 1.
Figure 1.
Flow chart of Sequential Explanatory Mixed Method Study
Statistical analysis
Data was entered into Microsoft Excel Worksheet 2019, and the analysis was done using R Studio version 4.1.2. Core Team (2023). _R: A Language and Environment for Statistical Computing_. R Foundation for Statistical Computing, Vienna, Austria.
Socio-demographic and clinical information of the patients was summarized using numbers (N) and proportions (%). Delays were reported using median number of days and IQR (interquartile range). To assess the factors associated with the delays, we used Negative log-binomial regression analysis.
Qualitative data analysis was done by manual thematic analysis. We used a mixed approach, i.e., deductive and inductive approaches, for data analysis to focus on our study objectives and allow new information to emerge during the coding process. Two investigators coded the text transcript independently and verified the coding by discussing it together. Thus, all the transcripts were coded line by line using a descriptive strategy. After careful examination of the codes and their similarity and differences, coherent categories were created. Finally, after creating categories, we identified clusters of similar or related categories and generated themes. All transcripts were used to perform thematic analysis based on the approach described by Braun and Clarke.[13] All the findings of the qualitative phase have been reported according to the ‘Consolidated Criteria for Reporting Qualitative Research’ (COREQ) guidelines.[14]
Operational definitions
Patient delay- Patient delay refers to the delay of over 30 days between symptom onset and healthcare facility consultation. After consultation with stakeholders, a 30-day cut-off was chosen due to availability and accessibility.
Systems delay- System delay refers to the delay between healthcare provider presentation and diagnosis (>7 days) or treatment initiation (>15 days), determined after consultation with stakeholders and postgraduate guide.
Ethical consideration
The study protocol was submitted to the Institutional Ethics Committee of DMIHER (DU) and was approved vide letter number Ref. No- DMIMS (DU)/IEC/2022/22, dated 15th July 2022. The study protocol was also submitted to the Clinical Trial Registry of India and was approved vide reference letter CTRI/2023/06/053915.
Results
As depicted in Table 1, The study participants’ mean age was 50.54 years, with a standard deviation of 10.46 and a median age of 50, with an interquartile range of 43-58 years.
Table 1.
Sociodemographic characteristics of study Participants
| Characteristics | n | (%) | |
|---|---|---|---|
| Age | 26-44 | 40 | 31.25 |
| 45-64 | 73 | 57.03 | |
| >65 | 15 | 11.72 | |
| Total | 128 | 100 | |
| Mean | 50.54 | ||
| Standard Deviation (SD) | 10.46 | ||
| Median | 50 | ||
| Interquartile Range (IQR) | 43-58 | ||
| Religion | Hindu | 113 | 88.28 |
| Muslim | 12 | 9.38 | |
| Other | 3 | 2.34 | |
| Total | 128 | 100 | |
| Education | Primary education | 42 | 32.81 |
| Secondary education | 20 | 15.63 | |
| SSC completed | 22 | 17.19 | |
| HSC completed | 10 | 7.81 | |
| Graduate | 10 | 7.81 | |
| Postgraduate | 1 | 0.78 | |
| No formal education | 23 | 17.97 | |
| Total | 128 | 100 | |
| Socio-economic status | Upper class | 4 | 3.13 |
| Upper middle class | 22 | 17.19 | |
| Middle class | 26 | 20.31 | |
| Lower middle class | 43 | 33.59 | |
| Lower class | 33 | 25.78 | |
| Total | 128 | 100 | |
| Time to travel the nearest health facility (mins) | <15 mins | 50 | 39.06 |
| 16-60 mins | 72 | 56.25 | |
| >60 mins | 6 | 4.69 | |
| Total | 128 | 100 | |
| 1st visit for diagnosis was done in which healthcare facility | Family physician (private) | 10 | 7.81 |
| Government hospital (PHC, civil/district hospital) | 55 | 42.97 | |
| Private hospital (other than a family physician) | 51 | 39.84 | |
| Tertiary care centre (current facility) | 12 | 9.38 | |
| Total | 128 | 100 | |
The study found that 17.97% of participants had no formal education, with the majority having completed primary education and 33.59% of participants (314 out of 128) belonged to the lower middle class, while only 3.12% belonged to the upper-class socio-economic class.
Table 2 shows, Out of 128 study participants, 57.81% (74) presented late, with a median delay of 45 days. System delay was found in 89 (69.53%) participants, with a median delay of 19 days, while no system delay was observed in 39 (30.47%).
Table 2.
Duration of Patient and System delay in study participants
| Type of Delay | Presence of Delay | n | (%) |
|---|---|---|---|
| Duration of patient delay in days | Delay (>30 days) | 74 | 57.81 |
| No delay | 54 | 42.19 | |
| Total | 128 | 100 | |
| Median | 45 | ||
| IQR | 15-120 | ||
| Duration of systems delay in days | Delay | 89 | 69.53 |
| No delay | 39 | 30.47 | |
| Total | 128 | 100 | |
| Median | 19 | ||
| IQR | 7-35 |
The study found high patient and system delays prevalence, and the odds ratio overestimated relative risk. Therefore, PR was reported, as OR was not appropriate for larger outcomes over 10%.
As shown in Table 3, Muslim participants have a 1.36 times higher prevalence of system delay compared to Hindu participants, while other religions have a 0.67 times increased prevalence.
Table 3.
Socio-demographic and clinical factors associated with delays among the study participants
| Characteristics | Median Patient delay (IQR)** | PR *(prevalence ratio) | 95% CI | P | Median Systems delay (IQR)** | PR* | 95% CI | P |
|---|---|---|---|---|---|---|---|---|
| Age group (in years) | ||||||||
| Age*** | 45 (15.00-120.00) | 1 | 0.99-1.02 | 0.675 | 19 (7.00-35.00) | 1 | 0.99-1.01 | 0.619 |
| Religion | ||||||||
| Hindu | 90 (60.0-255.0) | Ref | 27.5 (15.75-45.00) | Ref | ||||
| Muslims | 365 (360-365) | 0.7 | 0.35-1.39 | 0.314 | 32 (26.0-95.5) | 1.36 | 1.10-1.68 | 0.005 |
| Other | 37.5 (36.25-38.75) | 1.12 | 0.49-2.53 | 0.778 | 16 (15.5-16.5) | 0.67 | 0.44-2.23 | 0.983 |
| Caste | ||||||||
| Open | 75.5 (60.0-247.5) | Ref | 24 (14.5-33.5) | Ref | ||||
| OBC | 90 (60.0-365.0) | 0.93 | 0.67-1.3 | 0.69 | 31 (20.00-63.50) | 0.91 | 0.71-1.16 | 0.476 |
| SC/ST | 120 (60.0-135.0) | 0.86 | 0.52-1.39 | 0.532 | 35 (17-50) | 0.82 | 0.56-1.21 | 0.328 |
| Other | 60 (NA)# | 1 | - | - | 20 (20-20) | 1 | ||
| Education | ||||||||
| None | 60.5 (60.0-232.5) | 1.28 | 0.73-2.23 | 0.386 | 20 (14.50-31.50) | 0.67 | 0.40-1.11 | 0.12 |
| Primary education | 120 (60.0-315.0) | 1.35 | 0.82-2.23 | 0.241 | 33 (15.5-93.0) | 1.03 | 0.75-1.43 | 0.843 |
| Secondary education | 90 (60.0-272.5) | 1.15 | 0.68-1.95 | 0.603 | 24.5 (18.25-36.25) | 1.06 | 0.77-1.47 | 0.692 |
| HSC and Above | 90 (48.75-333.75) | Ref | 37 (28.5-66.0) | Ref | ||||
| Place of residence district | ||||||||
| <100 Kms distance | 60 (60.0-105.0) | 0.75 | 0.54-1.05 | 0.098 | 22 (13.75-37.25) | 0.93 | 0.73-1.19 | 0.593 |
| >100 Kms distance | 120 (60.0-365.0) | Ref | 32 (20.00-50.00) | Ref | ||||
| Socioeconomic class | ||||||||
| Upper class | 212.5 (136.2-288.8) | Ref | 96 (NA)# | Ref | ||||
| middle class | 95 (60.0-365.0) | 1.14 | 0.42-3.09 | 0.793 | 27 (19.0-39.5) | 2.9 | 0.53-15.90 | 0.22 |
| Lower class | 90 (60-120) | 1.21 | 0.44-3.35 | 0.711 | 29.5 (14.25-112.00) | 2.66 | 0.48-14.80 | 0.262 |
| Marital Status | ||||||||
| Unmarried/widow | 90 (60.0-150.0) | Ref | 24.5 (15.75-53.50) | Ref | ||||
| Married | 90 (60.0-365.0) | 0.79 | 0.53-1.18 | 0.259 | 29 (19.00-45.00) | 1.15 | 0.91-1.46 | 0.23 |
| How did you notice the symptoms | ||||||||
| Accidental findings | 90 (60.0-363.8) | 1.05 | 0.76-1.45 | 0.748 | 27 (19.00-47.50) | 1.22 | 0.93-1.61 | 0.143 |
| Other reasons | 90 (60.0-187.5) | Ref | 29.5 (14.25-43.75) | Ref | ||||
| First diagnosis at current facility | ||||||||
| Yes | 60 (60.0-120.0) | Ref | 19.5 (13.75-34.75) | Ref | ||||
| No | 120 (60.0-365.0) | 1.15 | 0.82-1.61 | 0.429 | 30 (20.00-63.00) | 1.23 | 0.93-1.63 | 0.148 |
| Number of visits to this health facility | ||||||||
| No of visits *** | 45 (15.00-120) | 0.98 | 0.91-1.06 | 0.699 | 19 (7.00-35.00) | 0.92 | 0.92-0.92 | 0 |
| Time required to travel to nearest health facility | ||||||||
| Time required in mins *** | 45 (15.00-120) | 1.003 | 1.002-1.003 | 0 | 19 (7.00-35.00) | 1.002 | 1.001-1.002 | 0 |
| Heard of breast cancer | ||||||||
| Yes | 120 (60.0-365.0) | Ref | 25 (15.25-40.00) | Ref | ||||
| No | 90 (60-225) | 1.26 | 0.93-1.71 | 0.133 | 32 (20.00-47.50) | 0.85 | 0.68-1.07 | 0.168 |
| Checked breast by SBE | ||||||||
| Yes | 240 (60.0-365.0) | Ref | 35 (28.0-105.0) | Ref | ||||
| No | 90 (60.0-195.0) | 0.73 | 0.54-0.99 | 0.042 | 25 (15.00-45.00) | 0.78 | 0.62-0.98 | 0.036 |
| Comorbidities | ||||||||
| Yes | 75 (60.0-180.0) | Ref | 33.5 (16.25-59.25) | Ref | ||||
| No | 90 (60.0-365.0) | 0.95 | 0.68-1.34 | 0.778 | 27 (17.50-42.50) | 1.21 | 0.88-1.66 | 0.243 |
| Number of children you have | ||||||||
| No. of children *** | 45 (15.00-120) | 1.17 | 1.12-1.21 | 0 | 19 (7.00-35.00) | 1.02 | 0.92-1.12 | 0.701 |
| Family history of breast cancer | ||||||||
| Yes | 90 (90-90) | Ref | 25 (17.0-34.5) | Ref | ||||
| No | 90 (60.0-360.0) | 1.69 | 0.83-3.47 | 0.15 | 29 (17.50-56.50) | 0.97 | 0.68-1.38 | 0.867 |
| Health insurance | ||||||||
| None | 90 (60.0-270.0) | Ref | 29.5 (16.75-46.25) | Ref | ||||
| govt | 180 (82.5-365.0) | 1.23 | 0.89-1.69 | 0.196 | 26.5 (15.00-74.25) | 0.97 | 0.72-1.29 | 0.819 |
| Private | 90 (60.0-105.0) | 1.17 | 0.72-1.92 | 0.51 | 25 (20.00-35.00) | 1.19 | 0.87-1.62 | 0.283 |
*Univariate analysis. **Interquartile range. ***continuous variable. #NA (single value, hence no IQR)
The median patient and system delay in breast check-ups by SBE decreased by 0.73 times and 0.78 times, respectively, compared to those who did not check breasts by SBE, with a median delay of 35 days and 25 days, respectively.
Negative log-binomial (NLB) regression is a novel generalized linear model used in cross-sectional studies to control bias caused by overestimating OR values from unconditional binary logistic regression models.
Tables 4 and 5 show the negative log-binomial regression model’s association with socio-demographic variables and delays, revealing no collinearity as the variance inflation factor is less than 10.
Table 4.
Negative log-binomial regression model for the association of various characteristics and Patient delay among the study participants
| Variables | PR* | 95% CI | P | |
|---|---|---|---|---|
| Checked breast by SBE | Yes | Ref | ||
| No | 0.82 | 0.36-1.84 | 0.633 | |
| Time travelled to the nearest health facility** | 1.00 | 0.99-1.01 | 0.617 | |
| Number of children** | 1.12 | 0.87-1.44 | 0.379 | |
| Heard of breast cancer before | Yes | Ref | ||
| No | 1.14 | 0.622-2.11 | 0.661 | |
| Family history of breast cancer | Yes | Ref | ||
| No | 1.72 | 0.56-5.30 | 0.345 | |
| Health insurance | Govt | 1.14 | 0.56-2.32 | 0.714 |
| Pvt | 1.30 | 0.45-3.72 | 0.625 | |
| None | Ref | |||
| Native place | Within 100 km of study settings | 0.78 | 0.42-1.46 | 0.435 |
| Other | Ref | |||
*Prevalence ratio (negative Log-binomial regression model) **Continuous variable
Table 5.
Negative log-binomial regression model for the association of various characteristics and Systems delay among the participants
| Variables | PR* | 95% CI | P | |
|---|---|---|---|---|
| Religion | Hindu | Ref | ||
| Muslim | 1.30 | 0.52-3.25 | 0.574 | |
| Others | 1.11 | 0.17-7.24 | 0.912 | |
| Time travelled to nearest health facility** | 1.00 | 0.99-1.01 | 0.767 | |
| Checked breast by SBE | Yes | Ref | ||
| No | 0.71 | 0.33-1.55 | 0.394 | |
| No. of visits to this health facility** | 1.05 | 0.92-1.19 | 0.472 | |
| First diagnosis at current facility- | Yes | Ref | ||
| No | 1.11 | 0.58-2.11 | 0.75 | |
| How did you notice your symptoms | Accidental | 1.14 | 0.62-2.09 | 0.669 |
| Other | Ref | |||
| Heard of breast cancer before | Yes | Ref | ||
| No | 0.82 | 0.45-1.45 | 0.495 | |
| Education | None | 0.71 | 0.26-1.95 | 0.505 |
| Primary | 0.99 | 0.43-2.32 | 0.999 | |
| Secondary | 1.05 | 0.45-2.43 | 0.907 | |
| HSC *** and above | Ref | |||
*Prevalence ratio (negative Log-binomial regression model) **Continuous variable. ***Higher secondary education
Participants without a family history of breast cancer have higher patient delay prevalences, while those from less than 100 km away have decreased delays, but not statistically significant.
The study found no significant association between variables and system delay among participants referred after diagnosis at the current facility, despite the expected 1.11 higher prevalence.
The participants who were not diagnosed in the current facility and were referred after diagnosis at the current health facility are expected to have a 1.11 [95% CI: 0.58-2.11] greater prevalence of having system delay than those who were diagnosed in the current facility but it was not found to be statistically significant. After adjusting all the variables included, we found that no variable was found to be significantly associated with system delay.
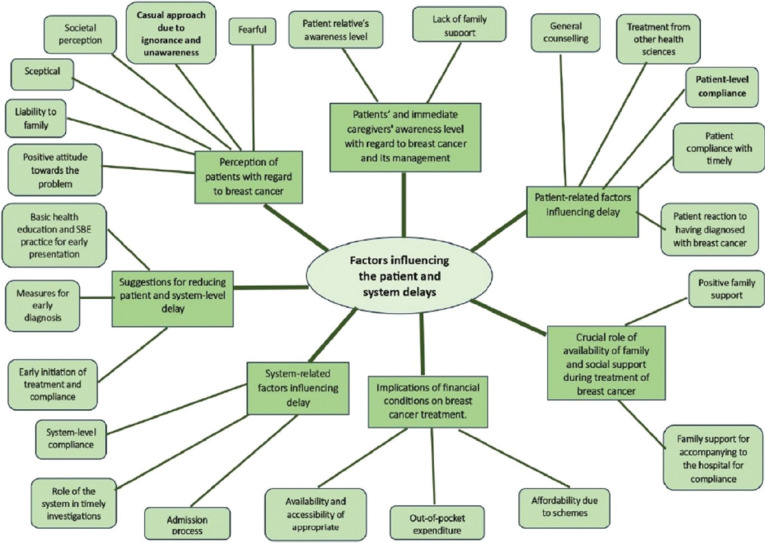
From the qualitative phase of our study, we obtained the following themes by thematic analysis, as depicted in Figure 2.
Figure 2.
Schematic depiction of identified codes, categories and themes of the factors influencing the delays
Implications of financial conditions on breast cancer treatment: Availability and accessibility of appropriate healthcare services was one of the major concerns of the participants; they also agreed that due to the schemes for the treatment of breast cancer it was Affordable yet Out-of-pocket expenditure was another inevitable concern for the poorest of the participants.
Patients’ and immediate caregivers’ awareness level with regard to breast cancer and its management and the Crucial role of availability of family and social support during treatment of breast cancer were the two themes identified with respect to the support the participants expect in a situation like this, but not all receive it. One of the relatives said, “she is already old, what good will come out in such age after treating her with surgery and some many medicines?” recalled one of the Auxiliary nurse midwife (ANM).
Another theme that emerged was the Perception of patients with regard to breast cancer. Some patients have a positive attitude towards the problem, but some think of themself as a Liability to family; others might be Sceptical and fearful. Many are afraid of Societal perception, and some have a casual approach due to ignorance and unawareness.
Patient-related factors influencing delay included choosing alternative options of treatment and Patient compliance with the timely investigation. Many people in rural setups are still skeptical of allopathic treatment; more often, they fear surgical, chemo, and radiotherapy and hence opt for less invasive treatment. Some patients get afraid of the tests. They don’t go for further tests because of their past experiences. “The needle test caused the lesion to burst, and that is why it spread; now it became so painful, I didn’t go when they called me for another big needle test”, said the participant who had undergone fine needle aspiration cytology (FNAC) for her abscess on the left breast.
The woman who has suspicion that she has it might go through all forms of emotional disturbance due to confusion and fear. On top of it, some people considered it to be shameful and socially inappropriate to discuss or talk about it.
System-related factors influencing delay included proper counseling of the admission process, system-level compliance, and the role of the system in timely investigations. Many times, the patients or their relatives are not willing to investigate as those are not free of cost. “they are disappointed and as they can’t afford it and hence are not willing to proceed”, said the medical social worker (MSW). One of the participants revealed that she got tested in one hospital a year ago and was confirmed to have malignant cells in fine needle aspiration cytology (FNAC), yet they were told to get higher investigations done from other facilities from Nagpur as it was not available in their setup, but since they (patients) couldn’t afford to go travel that far they didn’t do it.
We also identified a theme proposing suggestions for reducing patient and system-level delay which included organizing awareness camps in the community, availability of comprehensive cancer centres that facilitate all-in-one service, starting from health education for awareness in the community, screening programs for early diagnosis, direct consultation with an onco-specialist, and diagnosis and treatment provision with government schemes assistance. Everyone should be aware and positively practice self-breast examination (SBE)/breast-self examination (BSE). “even the women in my own family who are educated do not practice SBE…they should not only be aware of it but also know the importance of practicing it and should develop it as a habit,” said an onco-specialist.
Discussion
According to our study, the majority of the study participants belonged to the age group 45-64 years, with mean age 50.54 years, and SD 10.46. A similar study done in North-east India by Kumar A et al.[15] in 2019 showed 60.2% of participants in their study were 45-64 years old; another study from Nagpur, Central India, in 2011 conducted by Thakur NA et al.[16] showed that the maximum number of participants belonged to the 41-50 years age group, followed by 31-40 years, and the mean age was 45.99 years SD = 9.61 years, another study conducted by Chintamani et al.[17] in 2011 showed the majority, 39%, belonged to the 40-55 age group.
The socioeconomic status of our study participants showed that the majority belonged to a lower middle class, followed by lower class and middle class. A similar result was found in the study conducted in a geographically adjacent area from Nagpur done by Thakur NA et al.,[16] where the majority of participants belonged to the lower class. Another study from Northeast India by Kumar A et al.[15] showed the majority of participants belonging to the upper class (47.6%) followed by the lower class (27.5%); this difference in results might be due to overall regional and economic differences in the two study settings.
In our study, we observed that the majority of the study participants had primary education, followed by those having no formal education. Another study from Rwanda by Pace LE et al.[4] found that most participants either had no education or studied only up to primary school (75%). A study from China by Zhang H. et al.[5] showed that the majority had at least studied till junior or middle school, and another study from Indonesia done by Hutajulu SH et al.[7] showed that the majority of participants (63.6%) had studied more than junior high school.
A study done on Malaysian women by Norsa’adah B et al.[18] found that the majority (39%) of the participants studied up to high school, a study from Hong Kong by Yau TK et al.[19] reported 61% of participants had at least secondary-level education. This difference in the educational status of participants from studies outside India may be due to the low literacy rate in India and the rural study setting of our research study.
In our study, the majority of the participants belonged to low socioeconomic status; hence, most participants (42.97%) would have preferred going to a government hospital (PHCs, Civil or District hospitals) first in contrast to the study conducted in northeast India by Kumar A et al.,[15] where most participants (70.6%) presented to private hospitals first as majority of them belonged to upper socioeconomic class.
Ten percent of participants in our study have a family history of breast cancer; similar studies showed 3.42%, 8%, and 8.2% in China by Li YL et al., Rwanda by Pace LE et al., and Malaysia by Norsa’adah B et al., respectively had a family history of breast cancer.[4,18,20] Whereas another study from Indonesia by Hutajulu SH et al.[7] and Hong Kong by Yau TK et al.[19] reported 38%, 51% of participants having a family history of breast cancer, respectively.
We found in the qualitative phase of the study that the awareness levels of our participants were low. These results are in alignment with the quantitative results, which showed that out of 128 participants, 47.66% have heard of breast cancer, and only 15.63% used to practice SBE. In the study done in Indonesia by Hutajulu SH et al.[7] they reported that 28% of participants practised SBE at least once a week, 12% would practice it at least once a month, and most of the participants (60%) would rarely practice SBE. The Rwanda study conducted by Pace LE et al.[4] reported that 78% of the study participants had heard of breast cancer before, and 35% checked their own breasts for lumps before developing the current problem.
We found that 57.81% of participants had a patient delay. The median patient delay was 45 days (15-120 IQR). 69.53% of participants had system delays, with a median system delay being 19 days (7-35 IQR). The median presentation delay in the northeast Indian study by Kumar A et al.[15] was 35 days (10-112 IQR), and the median treatment delay was 130 days (75-258 IQR).
In the study by Thakur NA et al.,[16] 50% of participants took more than 6 months to consult for their symptoms. Similar to our study, the median patient delay in the study done in China by Zhang H. et al.[5] was 50 days; 45.9% of participants sought consultation after 29 days of first noticing their symptoms. 35.8% of participants sought delayed treatment by more than 90 days, and 11.8% sought treatment after 366 days.
Another study from China by Li YL et al.[20] reported that 40% of participants had more than 3 months of patient delay, and the median patient delay was 2 months. 15.5% of participants had a health care delay of more than 1 month, and the median care delay was 0.37 months.
The study done in Indonesia by Hutajulu SH et al.,[7] states that the median delay of presentation was 61 days. 43.3% had delayed the consultation by more than 3 months. The median delay for diagnosis confirmation was 1 month, 64.7% of participants had confirmation of diagnosis after 1 month. The median patient delay among the participants of the study in Rwanda by Pace LE et al.[4] was 5 months, and the median system delay was also 5 months. Overall, all similar studies concluded that there were significant delays in reporting the first symptoms to healthcare providers.
A higher prevalence of patient delay with decreasing education was estimated from univariate analysis in our study, which was not statistically significant. A study in northeast India by Kumar A et al.[15] also had similar findings. At a minimum secondary education, the study conducted by Ozmen V et al.[21] in Turkey reported P = 0.028 tended toward shorter Patient Delay.
In our study, lower-class and middle-class participants were found to have a higher prevalence of patient delay and system delay, respectively. However, there was no statistical significance between the associations. Similar results were reported by Kumar A et al.[15] in a study conducted in Northeast India. In the study done in Nagpur by Thakur NA et al.,[16] the delay in the presentation was found to have a significant association with socioeconomic status with χ2 = 5.12, df = 1 P = 0.02.
In our study, it was estimated that the increase in time required to reach the nearest health facility slightly increased the prevalence of the patient as well as system delay. A significant association was found between the distance between the nearest primary health facility and delay in Northeast India by Kumar A. et al.[15] An association between time taken to travel to a healthcare facility and delay was not statistically significant, as reported by a study from Rwanda by Pace LE et al.[4]
A lesser prevalence of delay was found in participants who did not practice SBE. This association with patient delay was statistically significant with PR = 0.73, 95%CI: 0.54-0.99 P = 0.042. This might be due to checking their breast by SBE might or might not be reported by the participants due to generalized unawareness in our study participants, causing more patient delay. Moreover, many of our participants were diagnosed in screening camps and hence were referred and reported at early stages. Hence, less delay might have been seen in participants not practising SBE.
SBE was found to be a Protective factor for patient delay significantly associated with standardized β =0.436, OR = 0.065, 95% CI: 0.007-0.590, P = 0.015 multiple logistic regression in a study done by Zhang H. et al. in China.[5] Patients with stronger self-examination habits at P < 0.001 had shorter delay time, which tended to report shorter Patient Delay Time as per the study done by Ozmen V et al. in Turkey.[21] No significance was found in studies from Rwanda by Pace LE et al.[4] and Indonesia by Hutajulu SH et al.[7]
In our study, after doing regression analysis, we did not find any significant association between education level and patient or system delay.
From the qualitative phase of our study we obtained the following themes by thematic analysis: Revolved around the lack of awareness and knowledge of breast cancer, social and financial constraints, the inadequacy of healthcare personnel in appropriate diagnosis of breast cancer.[22]
The themes highlighted by another mixed method study done by Kumar A. et al.[15] from Northeast India reported on similar lines of reasons for delays like Myths and beliefs, stigma related to the disease, prioritizing the family over health and social responsibilities, attitude of neglect, embarrassment, fear and denial, alternate treatment methods, affordable and accessible healthcare, fear of treatment, fear of side effects, dissatisfied with healthcare delivery system attribution to either presentation delay or treatment delay.
Similar to our study, this also reported suggestions for reducing the delay. It included decentralization as well as free screening facilities, breast cancer awareness, promoting breast self-examination and fear allaying through community health workers, gender-sensitive screening with patient-friendly staff, opportunistic screening at primary healthcare facilities.
The identified themes in the existing published literature were in congruence with the themes we identified in our study, like lack of awareness, financial issues, trust in alternative medicine, family support, sociocultural determinants, etc.
Conclusion
Our study concludes that there is a substantial delay at patient level reporting to healthcare care providers which needs an Increase in awareness levels in the Community in regard to early reporting of symptoms as well as timely initiation of Treatment and compliance to treatment completion. In the qualitative phase of our study, we could explore various social, cultural, and economic factors influencing the presentation and initiation of treatment of breast cancer patients. These socio-cultural and economic factors identified should be addressed to decrease delays in breast cancer management.
Financial support and sponsorship
The study is funded by ICMR, New Delhi, under the ICMR PG thesis grant Vide reference no. 3/2/dec-2022/PG-thesis-HRD (24) dated 1st dec 2023.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We acknowledge the Indian Council of Medical Research (I.C.M.R.), New Delhi, for funding this research study under the I.C.M.R. financial support for the postgraduate thesis grant.
References
- 1.Barwal VK, Sharma GA. IAPSM's textbook of community medicine. Int J Community Med Public Health. 2019;6:4131–1. [Google Scholar]
- 2.Report of National Cancer Registry Programme (ICMR-NCDIR), Bengaluru, India 2020. Available from: https://www.ncdirindia.org/All_Reports/Report_2020/default.aspx .
- 3.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 4.Pace LE, Mpunga T, Hategekimana V, Dusengimana JMV, Habineza H, Bigirimana JB, et al. Delays in breast cancer presentation and diagnosis at two rural cancer referral centers in Rwanda. Oncologist. 2015;20:780–8. doi: 10.1634/theoncologist.2014-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang H, Wang G, Zhang J, Lu Y, Jiang X. Patient delay and associated factors among Chinese women with breast cancer. Medicine (Baltimore) 2019;98:e17454. doi: 10.1097/MD.0000000000017454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richards MA, Westcombe AM, Love SB, Littlejohns P, Ramirez AJ. Influence of delay on survival in patients with breast cancer: A systematic review. Lancet. 1999;353:1119–26. doi: 10.1016/s0140-6736(99)02143-1. [DOI] [PubMed] [Google Scholar]
- 7.Hutajulu SH, Prabandari YS, Bintoro BS, Wiranata JA, Widiastuti M, Suryani ND, et al. Delays in the presentation and diagnosis of women with breast cancer in Yogyakarta, Indonesia: A retrospective observational study. PLoS One. 2022;17:e0262468. doi: 10.1371/journal.pone.0262468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Oliveira NPD, Cancela M de C, Martins LFL, de Souza DLB. Spatial distribution of advanced stage diagnosis and mortality of breast cancer: Socioeconomic and health service offer inequalities in Brazil. PLoS One. 2021;16:e0246333. doi: 10.1371/journal.pone.0246333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellington TD, Miller JW, Henley SJ, Wilson RJ, Wu M, Richardson LC. Trends in breast cancer incidence, by race, ethnicity, and age among women aged≥20 years —United States, 1999–2018. MMWR Morb Mortal Wkly Rep. 2022;71:43–7. doi: 10.15585/mmwr.mm7102a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ekwueme DU, Trogdon JG, Khavjou OA, Guy GP. Productivity costs associated with breast cancer among survivors aged 18–44 years. Am J Prev Med. 2016;50:286–94. doi: 10.1016/j.amepre.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Max W, Sung HY, Stark B. The economic burden of breast cancer in California. Breast Cancer Res Treat. 2009;116:201–7. doi: 10.1007/s10549-008-0149-4. [DOI] [PubMed] [Google Scholar]
- 12.Guest G, Bunce A, Johnson L. How many interviews are enough?: An experiment with data saturation and variability. Field Methods, Sage Journals. 2006:18. doi:10.1177/1525822x05279903. [Google Scholar]
- 13.Braun V, Clarke V. Using thematic analysis in psychology. Qualitative Res Psychol. 2006;3:77–101. [Google Scholar]
- 14.Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): A 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19:349–57. doi: 10.1093/intqhc/mzm042. [DOI] [PubMed] [Google Scholar]
- 15.Kumar A, Bhagabaty SM, Tripathy JP, Selvaraj K, Purkayastha J, Singh R. Delays in diagnosis and treatment of breast cancer and the pathways of care: A mixed methods study from a tertiary cancer centre in North East India. Asian Pac J Cancer Prev. 2019;20:3711–21. doi: 10.31557/APJCP.2019.20.12.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thakur NA, Humne AY, Godale LB. Delay in presentation to the hospital and factors affecting it in breast cancer patients attending tertiary care center in Central India. Indian J Cancer. 2015;52:102. doi: 10.4103/0019-509X.175602. [DOI] [PubMed] [Google Scholar]
- 17.Chintamani, Tuteja A, Khandelwal R, Megha T, Bamal R, Jain S, et al. Patient and provider delays in breast cancer patients attending a tertiary care centre: A prospective study. JRSM Short Reports. 2011;2 doi: 10.1258/shorts.2011.011006. doi:10.1258/shorts.2011.011006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Norsa'adah B, Rampal KG, Rahmah MA, Naing NN, Biswal BM. Diagnosis delay of breast cancer and its associated factors in Malaysian women. BMC Cancer. 2011;11:141. doi: 10.1186/1471-2407-11-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yau TK, Choi CW, Ng E, Yeung R, Soong IS, Lee AWM. Delayed presentation of symptomatic breast cancers in Hong Kong: Experience in a public cancer centre. Hong Kong Med J. 2010;16:373–7. [PubMed] [Google Scholar]
- 20.Li YL, Qin YC, Tang LY, Liao YH, Zhang W, Xie XM, et al. Patient and care delays of breast cancer in China. Cancer Res Treat. 2019;51:1098–106. doi: 10.4143/crt.2018.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ozmen V, Boylu S, Ok E, Canturk NZ, Celik V, Kapkac M, et al. Factors affecting breast cancer treatment delay in Turkey: A study from Turkish Federation of Breast Diseases Societies. Eur J Public Health. 2015;25:9–14. doi: 10.1093/eurpub/cku086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim JNW, Potrata B, Simonella L, Ng CWQ, Aw TC, Dahlui M, et al. Barriers to early presentation of self-discovered breast cancer in Singapore and Malaysia: A qualitative multicentre study. BMJ Open. 2015;5:e009863. doi: 10.1136/bmjopen-2015-009863. [DOI] [PMC free article] [PubMed] [Google Scholar]