ABSTRACT
Background:
About a third of the world’s population is estimated to suffer from anaemia, and iron deficiency is expected to account for about half of all anaemia cases. This study was designed to get an estimate of the proportion of patients with iron deficiency anaemia (IDA) who have a significant gastrointestinal (GI) pathology, in particular a GI malignancy, and to identify any risk factors or predictors for the same.
Methods:
This cross-sectional study was conducted at a hospital in Eastern India. The study population comprised males above the age of 18 and postmenopausal females with IDA, excluding those haemodynamically unstable or with chronic diseases. Data collection included a detailed history, sociodemographic details, dietary habits, GI symptoms, and severity of anaemia. Faecal occult blood tests (OBTs) were conducted, and patients were referred for upper and lower GI endoscopy with biopsies.
Results:
Out of the 257 patients, 50.97% (n = 131) had a significant GI pathology, and 25.68% (n = 66) had a GI malignancy. Male gender (AOR: 5.203, 95% CI: 1.725–15.698) and a positive stool OBT (AOR: 6.516, 95% CI: 2.255–18.828) were found to be independent risk factors for any GI pathology. Age 40 years or above (AOR: 11.376, 95% CI: 1.199–107.946), loss of appetite (AOR: 15.548, 95% CI: 1.416–170.735), pain abdomen (AOR: 5.566, 95% CI: 1.149–26.953), dysphagia (AOR: 7.945, 95% CI: 1.036–60.915), family history of malignancy (AOR: 46.726, 95% CI: 4.076–535.645), and positive OBT (AOR: 22.430, 95% CI: 3.933–127.915) were found to be independent risk factors of GI malignancy.
Conclusions:
This study shows that a large proportion of adult males and postmenopausal females presenting with IDA in India have significant GI pathology. Furthermore, a significant proportion of them have GI malignancies. Thus, bidirectional endoscopy should be considered for these patients. Male patients, age >40, those with history of loss of appetite or weight, pain abdomen or dysphagia, positive family history, and positive OBT should be prioritised for the investigation.
Keywords: Anaemia, endoscopy, gastrointestinal, gastrointestinal diseases, gastrointestinal neoplasms, iron-deficiency, occult blood
Introduction
About a third of the world’s population is estimated to suffer from anaemia.[1] Iron deficiency is expected to account for about half of all anaemia cases.[2] Furthermore, iron deficiency is the largest nutritional deficiency worldwide.[3] In the developed world, the prevalence of iron deficiency anaemia (IDA) among adult men and postmenopausal women is estimated to be 2–5%.[4] In India, more than half of the women in the reproductive age group and a quarter of the men in the age group of 15–49 years suffer from anaemia.[5,6]
IDA may result from various causes like dietary deficiency, menstrual blood loss, hookworm infestation, and GI abnormalities that lead to blood loss or malabsorption. There is significant geographical variation in the proportion of the different aetiologies that contribute to the overall burden of IDA in a population.[1] Significant GI pathologies associated with IDA include duodenal ulcers, gastric ulcers, angiodysplasia, coeliac disease, inflammatory bowel disease, gastric cancer, and colorectal carcinoma.[7] GI malignancy is much less common in iron-deficient premenopausal women compared with adult men and postmenopausal women with iron deficiency.[8] Thus, upper and lower GI endoscopy is advised for all adult men and postmenopausal women with unexplained IDA.[9,10]
In developing countries like India, due to the high prevalence of the condition and limited capacity to offer endoscopies, it is difficult to adhere to these guidelines. Moreover, parasitic infestation and dietary deficiency are more common compared to the West.[11,12] At the same time, large bowel malignancies are less common.[13] This brings into question the applicability of western guidelines in the Indian population. In the absence of local guidelines, clinicians often have to depend on their clinical judgement in deciding who should be investigated with bidirectional endoscopy. Uniform cost-effective guidelines will help in early detection of GI pathologies, especially malignancies. An estimation of the proportion of GI abnormalities in patients with IDA is crucial for framing evidence-based guidelines. Currently, there are limited data from India on this topic.
This study was designed to estimate the proportion of patients with IDA presenting to a tertiary centre in eastern India who have a significant GI pathology, in particular, GI malignancy, and to identify any risk factors or predictors for the same.
Materials and Methods
Study design and duration
This was a hospital-based cross-sectional study conducted in eastern India for a duration of 1 year (November 2020 to October 2021).
Study population
The study population comprised males above the age of 18 and postmenopausal females with IDA who presented during the study period. IDA was defined as a haemoglobin concentration of less than 130 g/L for men and 120 g/L for women with a ferritin value of less than 15 mcg/L or a mean corpuscular volume below the lower limit of the reference range, along with any one of the following: low serum iron, low transferrin saturation, raised total iron binding capacity, and haemoglobin electrophoresis negative for thalassemia. Haemodynamically unstable patients; patients with chronic liver disease, chronic kidney disease, or congestive cardiac failure; and patients unwilling to participate in the study were excluded from the study.
Sample size and sampling technique
The sample size was calculated based on a previous study that reported gastrointestinal pathology in about 60% of patients presenting with IDA.[14] Based on this and taking 10% relative precision and 95% confidence, a sample size of 257 was calculated using an online sample size calculator, Statulator.
A convenience sampling technique was adopted with the aim of including as many eligible patients as possible during the study period. Data collection was done between December 2020 and October 2021.
Study tool and procedure
Patients who fulfilled the eligibility criteria based on a complete haemogram, ferritin level, and iron studies as described above were enrolled in the study after obtaining their informed consent. A detailed history was taken from each participant. The following information was noted down on the research proforma: socio-demographic details like age, sex, dietary habits (vegetarian or non-vegetarian), and history of any malignancy, as well as GI malignancy in first- and second-degree relatives; presence or absence of GI symptoms, namely, vomiting, dysphagia, dyspepsia, pain abdomen, loss of appetite, weight loss, altered bowel habits, and melaena or bleeding per rectum; and the severity of anaemia (defined by haemoglobin concentration: mild > 90 g/L, moderate 60–90 g/L, and severe < 60 g/L). Faecal occult blood test (OBT) was done using OnSite FOB-Hi Rapid Test, and the patient was referred for upper and lower GI endoscopy with a biopsy of the second part of the duodenum (D2) and any suspicious lesions. A forward-viewing adult video endoscope (Olympus, GIF H-170) was employed for upper GI endoscopy, while a video colonoscope (Olympus, CF H-170) was used for colonoscopy. Conventional endoscopic biopsy forceps were used for obtaining biopsies. The biopsy samples were sent in vials with diluted formalin. A histological examination was performed by an expert pathologist. The findings of these investigations were noted once the reports were available. Hiatus hernia, gastritis, duodenitis, and hyperplastic polyps were not counted as significant pathology on upper GI endoscopy. Melanosis coli was not considered to be a significant lower GI pathology.
Statistical analysis
The collected data were transferred to Microsoft Excel 2019. Data analysis was done using IBM SPSS statistics 22.0 (IBM Corp., Armonk, New York, USA) software. The proportion of patients with upper GI pathology and upper GI malignancy among those who underwent upper GI endoscopy, lower GI pathology, and lower GI malignancy among those who underwent lower GI endoscopy and overall gastrointestinal pathology and malignancy among all patients included in the study, respectively, were calculated along with 95% confidence intervals (CIs). A univariate binomial logistic regression was done for each variable to detect any association with overall pathology or malignancy and the crude odds ratio (COR) with 95% CI was noted. All the variables with a P value <0.20 were loaded together to run a multivariable binary logistic regression model, and an adjusted odds ratio (AQR) with 95% CI was reported in the final model to find out the risk factors associated with abnormal GI pathology and malignant GI pathology. Overall statistical significance was attributed to P < 0.05.
Ethical approval
Ethical approval was obtained from the institutional ethics committee and informed consent was obtained directly from the patients.
Results
Demographic details of the patients
Of the 257 patients, 67.7% (n = 174) were male and 32.3% (n = 83) were female. The mean age of the study subjects was 53.04 ± 14.67 years. The mean haemoglobin concentration was 90.6 ± 24.2 g/L. Distribution according to the severity of anaemia is shown in Table 1.
Table 1.
Distribution of patients according to the severity of anaemia (n=257)
| Severity of anaemia | Frequency | Percentage |
|---|---|---|
| Mild (>90 g/L) | 153 | 59.5% |
| Moderate (60-90 g/L) | 71 | 27.6% |
| Severe (<60 g/L) | 33 | 12.8% |
The proportion of overall GI abnormalities and GI malignancies
A total of 218 out of 257 patients underwent upper GI endoscopy. Among these, 34.4% (n = 75) had a significant upper GI pathology and 19.7% (n = 43) had a malignant pathology. Of the 43 patients with upper GI malignancy, three were in clinical (TNM) stage I, eight in stage II, 14 in stage III, and 13 in stage IV, while information could not be obtained in five patients.
Similarly, 147 out of 257 patients underwent lower GI endoscopy. 44.21% of these (n = 65) were found to have a significant lower GI pathology, with malignancy in 15.65% (n = 23). Of the 23 patients with lower GI malignancy, two were in clinical stage I, three in stage II, ten in stage III, and four in stage IV, while information could not be obtained in the remaining four patients [Figure 1].
Figure 1.
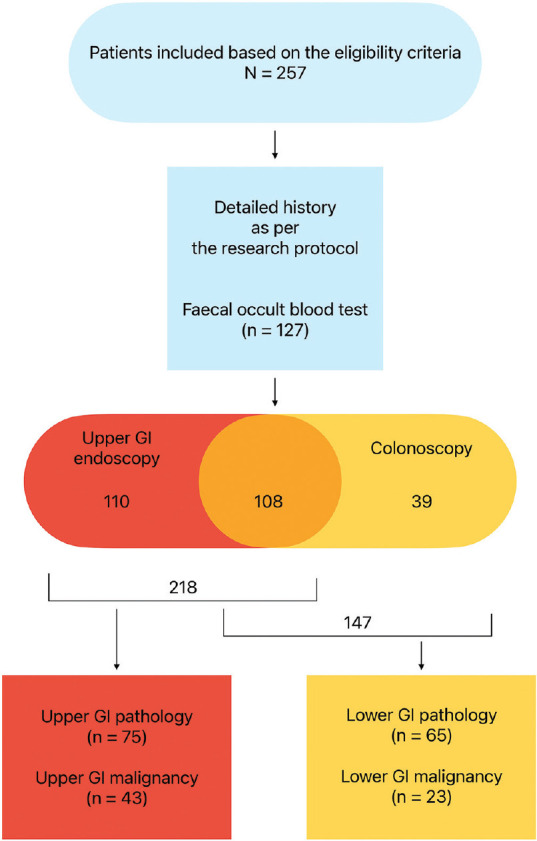
Flow chart of the study
Overall, out of the 257 patients, 50.97% (n = 131, 95% CI: 44.9–57.1%) had a significant GI pathology, and 25.68% (n = 66, 95% CI: 20.7–31.3%) had a GI malignancy.
The distribution of various abnormalities identified on endoscopy and biopsy is shown in Figure 2. Carcinoma of the stomach (12.8%, n = 33) was the most common pathology identified, followed by carcinoma of the colon (5.4%, n = 14).
Figure 2.
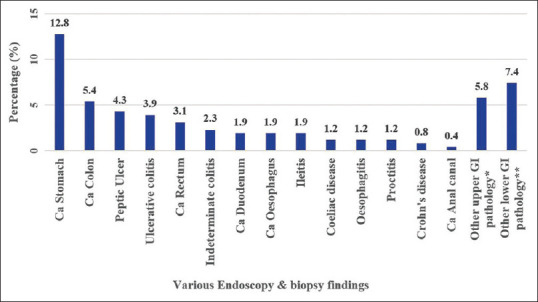
Distribution of abnormal findings on endoscopy and biopsy (N = 257). *Oesophageal aspergillosis 1, oesophageal candidiasis 2, benign oesophageal stricture 1, gastric mucormycosis 1, gastric submucosal bulging lesion 1, inflammatory thickening of the proximal stomach 1, vascular malformation in the stomach 1, gastric outlet obstruction 5, duodenal diverticulum 1, and duodenal lymphangiectasia 1.**Haemorrhoids 6, rectal polyp 1, colonic polyp 1, colonic lipoma 2, colonic ulcer 1, colonic diverticulosis 2, extrinsic compression of the sigmoid colon 1, sigmoid stricture 1, terminal ileal ulcer 3, and post-infectious widely open ileocecal valve 1
Factors associated with abnormal GI pathology
On univariate analysis, male gender, a non-vegetarian diet, weight loss, a history of vomiting, loss of appetite, melaena, pain in the abdomen, and a positive test for stool for occult blood were found to be significantly associated with a significant GI pathology.
These potential risk factors were further subjected to multivariate analysis. In addition to the above-mentioned factors, adjustments were also made for the severity of anaemia (COR: 1.566, 95%CI: 0.743–3.302), altered bowel habits (COR: 1.630, 95%CI: 0.921–2.887), dysphagia (COR: 1.659, 95%CI: 0.792–3.477), and age above 40 years (COR: 0.841, 95%CI: 0.439–1.614). Male gender (AOR: 5.203, 95% CI: 1.725–15.698) and a positive stool test for occult blood (AOR: 6.516, 95% CI: 2.255–18.828) were found to be independent risk factors for abnormal gastrointestinal pathology [Table 2].
Table 2.
Various risk factors associated with abnormal gastrointestinal lesions (n=257)
| Variables | Categories | Abnormal gastrointestinal lesions | Crude OR (95% CI) | Adjusted OR (95% CI) | |
|---|---|---|---|---|---|
|
| |||||
| Absent n=131 | Present n=126 | ||||
| Age 40 or more$ | No (n=44) | 20 (45.5%) | 24 (54.5%) | 1 | - |
| Yes (n=213) | 106 (49.8%) | 107 (50.2%) | 0.0841 (0.439-1.614) | ||
| Severity of anaemia$ | Mild-moderate (n=224) | 113 (50.4%) | 111 (49.6%) | 1 | - |
| Severe (n=33) | 13 (39.4%) | 20 (60.6%) | 1.566 (0.743-3.302) | ||
| Loss of weight$ | No (n=65) | 48 (73.8%) | 17 (26.2%) | 1 | - |
| Yes (n=192) | 78 (40.6%) | 114 (59.4%) | 4.127 (2.212-7.699) | ||
| Vomiting$ | No (n=145) | 81 (55.9%) | 64 (44.1%) | 1 | - |
| Yes (n=112) | 45 (40.2%) | 67 (59.8%) | 1.884 (1.143-3.107) | ||
| Melaena$ | No (n=165) | 90 (54.5%) | 75 (45.5%) | 1 | - |
| Yes (n=92) | 36 (39.1%) | 56 (60.9%) | 1.867 (1.111-3.136) | ||
| Loss of appetite$ | No (n=76) | 48 (63.2%) | 28 (36.8%) | 1 | - |
| Yes (n=181) | 78 (43.1%) | 103 (56.9%) | 2.264 (1.305--3.928) | ||
| Pain abdomen$ | No (n=92) | 57 (62.0%) | 35 (38.0%) | 1 | - |
| Yes (n=165) | 69 (41.8%) | 96 (58.2%) | 2.266 (1.344-3.820) | ||
| Dysphagia$ | No (n=223) | 113 (50.7%) | 110 (49.3%) | 1 | - |
| Yes (n=34) | 13 (38.2%) | 21 (61.8%) | 1.659 (0.792-3.477) | ||
| Gender$ | Female (n=83) | 50 (60.2%) | 33 (39.8%) | 1 | 5.203 (1.725-15.698)* |
| Male (n=174) | 76 (43.7%) | 98 (56.3%) | 1.954 (1.148-3.326) | ||
| Diet$ | Vegetarian (n=75) | 44 (58.7%) | 31 (41.3%) | 1 | - |
| Non-vegetarian (n=182) | 82 (45.1%) | 100 (54.9%) | 1.731 (1.004-2.984) | ||
| Occult blood test$ | Negative (n=92) | 64 (69.6%) | 28 (30.4%) | 1 | 6.516 (2.255-18.828)* |
| Positive (n=35) | 10 (28.6%) | 25 (71.4%) | 5.714 (2.425-13.468) | ||
| Altered bowel habit$ | Negative (n=192) | 100 (52.1%) | 92 (47.9%) | 1 | - |
| Positive (n=65) | 26 (40.0%) | 39 (60.0%) | 1.630 (0.921-2.887) | ||
Nagelkerke’s R2=0.361. Chi-square value (df)=7.200 (8), P=0.515. $Risk factors taken for adjusted odds ratio calculation, *Significant risk factors for AOR
Factors associated with GI malignancy
For GI malignancy, age above 40 years, weight loss, melaena, loss of appetite, pain abdomen, dysphagia, history of malignancy in a first- or second-degree relative, and a positive test for stool for occult blood were found to be significant associations on univariate analysis.
All the above variables were included in the multivariate analysis. Age above 40 years (AOR: 11.376, 95% CI: 1.199-107.946), loss of appetite (AOR: 15.548, 95% CI: 1.416-170.735), pain abdomen (AOR: 5.566, 95% CI: 1.149-26.953), dysphagia (AOR: 7.945, 95% CI: 1.036-60.915), family history of malignancy (AOR: 46.726, 95% CI: 4.076-535.645), and positive stool test for occult blood (AOR: 22.430, 95% CI: 3.933-) were found to be independent risk factors of gastrointestinal malignancy [Table 3].
Table 3.
Various risk factors associated with malignant gastrointestinal lesions (n=257)
| Variables | Categories | Gastrointestinal Malignancy | Crude OR (95% CI) | Adjusted OR (95% CI) | |
|---|---|---|---|---|---|
|
| |||||
| Absent n=191 | Present n=66 | ||||
| Age 40 or more$ | No (n=44) | 40 (90.9%) | 4 (9.1%) | 1 | 11.376 (1.199-107.946)* |
| Yes (n=213) | 151 (70.9%) | 62 (29.1%) | 4.106 (1.409-11.964) | ||
| Loss of weight$ | No (n=65) | 60 (92.3%) | 5 (7.7%) | 1 | - |
| Yes (n=192) | 131 (68.2%) | 61 (31.8%) | 5.588 (2.136-14.617) | ||
| Melaena$ | No (n=165) | 135 (81.8%) | 30 (18.2%) | 1 | - |
| Yes (n=92) | 56 (60.9%) | 36 (39.1%) | 2.893 (1.626-5.146) | ||
| Loss of appetite$ | No (n=76) | 66 (86.8%) | 10 (13.2%) | 1 | 15.548 (1.416-170.735)* |
| Yes (n=181) | 125 (69.1%) | 56 (30.9%) | 2.957 (1.416-6.172) | ||
| Pain abdomen$ | No (n=92) | 81 (88.0%) | 11 (12.0%) | 1 | 5.566 (1.149-26.953)* |
| Yes (n=165) | 110 (66.7%) | 55 (33.3%) | 3.682 (1.814-7.475) | ||
| Dysphagia$ | No (n=223) | 171 (76.7%) | 52 (23.3%) | 1 | 7.945 (1.036-60.915)* |
| Yes (n=34) | 20 (58.8%) | 14 (41.2%) | 2.302 (1.087-4.874) | ||
| Family history of any malignancy$ | No (n=232) | 178 (76.7%) | 54 (23.3%) | 1 | 46.726 (4.076-535.845)* |
| Yes (n=25) | 13 (52%) | 12 (48%) | 3.043 (1.311-7.060) | ||
| Occult blood test$ | Negative (n=92) | 86 (93.5%) | 6 (6.5%) | 1 | 22.430 (3.933-127.915)* |
| Positive (n=35) | 20 (57.1%) | 15 (42.9%) | 10.750 (3.708-31.167) | ||
Nagelkerke’s R2=0.584. Chi-square value (df)=1.154 (8), P=0.997. $Variables taken for adjusted odds ratio calculation. *Significant risk factors for AOR
Discussion
Bidirectional endoscopy is the standard of care for adult men and postmenopausal women presenting with IDA in the developed world.[9,10] In India and other developing countries, this is not a uniform practice and the decision to do an endoscopy is usually left to the judgement of the clinician. There is a paucity of data on the occurrence of GI pathology in general and GI malignancy in particular in patients presenting with IDA in India. This study attempts to fill this gap. This is the largest study from South Asia so far reporting the occurrence of GI pathology in IDA.
In this study, 51% of the patients were found to have a significant GI pathology and 26% had a GI malignancy. Minor abnormalities like gastritis, duodenitis, hyperplastic polyps of the upper GI tract, hiatus hernia, and melanosis coli were disregarded, thereby reducing the proportion of patients with significant GI pathology. A previous American study of 100 patients reported abnormal endoscopic findings in 62 patients with malignancy in 12.[14] Another study, also from America, of 100 patients with IDA and/or stool positive for occult blood noted a gastrointestinal lesion in 53 patients and malignancy in seven.[15] A smaller study of 70 patients with IDA found GI pathology in 71% (n = 50) and malignancy in 13% (n = 9).[16] An older Australian study of 100 carefully selected patients noted a potential GI cause of iron deficiency in 86 patients with a GI malignancy in 20.[17] A British study of 89 patients found GI pathology in 84% (n = 75) of the patients, with malignancy in 51% (n = 45).[18] A retrospective analysis of 2808 patients referred to the IDA clinic of a district hospital in the UK between 2004 and 2018 found that 86% of the patients underwent endoscopic investigations. Among these, 27% had a GI abnormality with malignancy in 8.3%.[19] It is clear from the above that most studies have reported a high rate of GI pathology in patients with IDA with a significant proportion of these being malignant lesions. Thus, the findings of the current study are consistent with those cited above. It is noteworthy that each of these studies has used a different definition of IDA. This may partly account for the variation in outcomes.
There are very few studies from India estimating the occurrence of GI pathology in IDA. In a single-centre study of 152 adult males, upper and lower GI endoscopies were done only when “indicated”. The overall prevalence of GI lesions was found to be 65.1%. Only two patients (1.3%) had malignancies.[20] In another study of 102 adult patients with IDA, 56 underwent upper GI endoscopy.[21] Abnormalities were noted in 29. Out of the 30 patients who underwent lower GI endoscopy, an abnormality was noted in 11. Altogether, three cases of GI malignancy were detected. Notably, this study also included menstruating women. Another study found a GI malignancy in 29 out of the 100 patients who were investigated for IDA.[22]
The current study is the largest study from India that attempts to assess the proportion of patients with IDA who have GI pathology and GI malignancy, respectively. Moreover, it was designed to assess all adult males and postmenopausal females detected to have IDA with upper and lower GI endoscopies in a non-selective manner. However, many patients underwent only one of the two procedures because either a pathology that could explain IDA was detected with the first procedure and the endoscopist deemed it unnecessary to carry out the second procedure or the patient chose not to have the second procedure.
It may be noted that a large proportion of the patients in the current study had one or more GI symptoms, namely, change in bowel habits, weight loss, vomiting, melaena, loss of appetite, pain abdomen, dysphagia, or dyspepsia. This may be due to the fact that the study sample was chosen from patients presenting to a tertiary care hospital and may be partly responsible for the high prevalence of GI malignancy observed.
One of the objectives of this study was also to identify risk factors, if any, for GI pathology in general and GI malignancy in particular in patients with IDA. As the sample size was calculated to estimate the proportion of patients with GI pathology in IDA, it was underpowered to detect risk factors. Yet a few important associations emerged. Male gender and a positive result for stool for occult blood were associated with a significantly higher risk of GI pathology. Furthermore, age 40 or more, a history of loss of appetite, pain in the abdomen, dysphagia, a history of malignancy in a first- or second-degree relative, and a positive result for stool for occult blood were associated with a higher occurrence of GI malignancy.
This study found an association between GI pathology and male gender. Previous studies have noted male gender as a risk factor for certain GI pathologies.[23,24,25,26,27] Age 40 or older showed a positive association with GI malignancy. The risk of oesophageal cancer is known to increase with age, with a median age of 67 years at diagnosis.[28,29] Similarly, the peak incidence of gastric cancer is seen between 50 and 70 years of age.[30] Data from the United States show an increase in the incidence of colorectal cancer with age with the highest incidence in those over 75 years.[31] A previous study had noted male gender, age over 50 years, and haemoglobin at presentation less than 9.0 g/dL to be significant risk factors for GI malignancy in iron deficiency anaemia.[32] The current study did not find the severity of anaemia to be a significant risk factor.
On multivariate analysis, loss of appetite, pain in the abdomen, and dysphagia were identified as symptoms associated with a risk of GI malignancy. Loss of appetite has been reported in patients with GI malignancies.[33,34] Loss of weight is closely associated with loss of appetite and is known to occur in patients with GI malignancies.[33,34,35] In the current study, although it was identified as a potential risk factor on univariate analysis, the association was not found to be significant on multivariate analysis after adjusting for other factors. A large cohort study of patients above the age of 40 presenting to primary care with new-onset abdominal pain reported an increased risk of intra-abdominal malignancy with abdominal pain in the elderly.[36] Dysphagia is known to be associated with upper GI malignancies.[37] Upper GI endoscopy is indicated in the evaluation of oesophageal dysphagia, irrespective of IDA.[38,39]
A history of malignancy in a first- or second-degree relative was found to be a significant predictor of GI malignancy. Six patients with carcinoma of the stomach had a family history of malignancy. Two each had a family history of GI malignancy and uterine malignancy, respectively. One each had a family history of carcinoma of the larynx and neck malignancy respectively. One patient with carcinoma of the duodenum had a family history of liver cancer. Five patients with colorectal cancer had a positive family history. Three patients had a family history of GI malignancy. One patient had a family history of malignant brain tumours, while another was not able to recall correctly. Family history was based on patients’ recall, and hence, it is not possible to establish its accuracy. For the same reason, upper and lower GI malignancies could not be distinguished and had to be clubbed together as GI malignancies. While most gastric cancers are sporadic, a small minority may be associated with known genetic mutations. Mutations in the cadherin-1 (CDH1) gene can be associated with gastric cancer and other cancers.[40] Similarly, most colorectal cancers are sporadic. Familial adenomatous polyposis (FAP) and hereditary nonpolyposis colorectal cancer (HPNCC) or Lynch syndrome are the most common of the familial colorectal cancer syndromes. Up to two percent of patients with FAP can develop brain tumours.[41] Individuals with Lynch syndrome are also at increased risk of ovarian, upper GI, pancreatic, biliary, genitourinary, and brain malignancies.[42]
Positive faecal occult blood testing (OBT) was found to be strongly associated with both GI pathology in general and GI malignancy in particular. OBT has been advocated for population screening for colorectal cancer.[43,44,45] A higher incidence of gastric cancer was reported in patients who had a positive faecal OBT but normal colonoscopy, thereby suggesting a potential role for this test in screening for gastric cancer as well.[46] However, its sensitivity for detecting a source of GI blood loss is unsatisfactory.[47] Hence, the existing guidelines do not recommend its use in the work-up of IDA.[9,10] Faecal immunochemical testing has higher sensitivity in detecting colorectal malignancy and is replacing the conventional methods of occult blood testing in colorectal cancer screening programmes.[48,49] Its role in the investigation of IDA is evolving.[50,51]
Strengths and weaknesses
This is the largest study from South Asia reporting the proportion of patients with IDA who have GI pathology and GI malignancy, respectively. Data collection was done prospectively. Endoscopy was offered to all adult males and post-menopausal females in a non-selective manner.
However, the study is not without its limitations. As it is a hospital-based study, its findings may not truly reflect the prevalence of GI pathologies, including GI malignancies, in adult males and post-menopausal females in the community. Furthermore, as it is a single-centre study, the results may not necessarily be generalisable to the whole of India. The study was underpowered to achieve its secondary objective of detecting risk factors for GI pathology and GI malignancy, respectively, in patients with IDA. The power was further compromised as out of 257, only 218 underwent upper GI endoscopy and 147 underwent lower GI endoscopy. Family history was recorded on the basis of the participants’ recall rather than any documentary evidence.
Conclusion and Recommendations
This study shows that a large proportion of adult males and postmenopausal females presenting with IDA in India have significant GI pathology. Furthermore, a significant proportion of these have GI malignancies. Thus, bidirectional endoscopy should be considered for these patients. If it is not possible to offer endoscopy universally, the findings of this study suggest that male patients, those above the age of 40, those having a history of loss of appetite or weight, pain in the abdomen or dysphagia, patients with a family history of malignancy, and those testing positive for occult blood should be prioritised for the investigation. Stool OBT has emerged as a strong predictor of both GI pathology and GI malignancy and can be used to identify patients at greater risk. This study highlights the need for larger studies to identify the subset of patients who are at a higher risk of GI pathology, including malignancies, so that informed national guidelines can be framed on who should be offered bidirectional endoscopy in the investigation of IDA.
Ethical approval
Ethical approval was obtained from the institutional ethics committee of AIIMS Patna (Ref. No. AIIMS/Pat/IEC/PGTh/July19/30).
Data availability statement
The datasets are available from the corresponding author upon reasonable request.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We would like to express our sincere gratitude to Dr. Alok Ranjan from the Department of Community and Family Medicine, AIIMS Patna for his help in the statistical analysis. Additionally, we extend our thanks to Mr. Navneet Bhatt for his assistance in the statistical analysis.
References
- 1.Chaparro CM, Suchdev PS. Anemia epidemiology, pathophysiology, and etiology in low- and middle-income countries. Ann N Y Acad Sci. 2019;1450:15–31. doi: 10.1111/nyas.14092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu K, Kaffes AJ. Iron deficiency anaemia: A review of diagnosis, investigation and management. Eur J Gastroenterol Hepatol. 2012;24:109–16. doi: 10.1097/MEG.0b013e32834f3140. [DOI] [PubMed] [Google Scholar]
- 3.Vos T, Allen C, Arora M, Barber RM, Bhutta ZA, Brown A, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1545–602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patterson RN, Johnston SD. Iron deficiency anaemia: Are the British Society of Gastroenterology guidelines being adhered to? Postgrad Med J. 2003;79:226–8. doi: 10.1136/pmj.79.930.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Family Health Survey (NFHS-5) 2019-2021. Ministry of Health and Family Welfare, Government of India. [[Last accessed on 2023 Jul 13]]. Available from: https://dhsprogram.com/pubs/pdf/FR375/FR375.pdf .
- 6.Didzun O, De Neve JW, Awasthi A, Dubey M, Theilmann M, Bärnighausen T, et al. Anaemia among men in India: A nationally representative cross-sectional study. Lancet Glob Health. 2019;7:e1685–94. doi: 10.1016/S2214-109X(19)30440-1. [DOI] [PubMed] [Google Scholar]
- 7.Bosch X, Montori E, Guerra-García M, Costa-Rodríguez J, Quintanilla MH, Tolosa-Chapasian PE, et al. A comprehensive evaluation of the gastrointestinal tract in iron-deficiency anemia with predefined hemoglobin below 9mg/dL: A prospective cohort study. Dig Liver Dis Off J Ital Soc Gastroenterol Ital Assoc Study Liver. 2017;49:417–26. doi: 10.1016/j.dld.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 8.Ioannou GN, Rockey DC, Bryson CL, Weiss NS. Iron deficiency and gastrointestinal malignancy: A population-based cohort study. Am J Med. 2002;113:276–80. doi: 10.1016/s0002-9343(02)01214-7. [DOI] [PubMed] [Google Scholar]
- 9.Snook J, Bhala N, Beales ILP, Cannings D, Kightley C, Logan RP, et al. British Society of Gastroenterology guidelines for the management of iron deficiency anaemia in adults. Gut. 2021;70:2030–51. doi: 10.1136/gutjnl-2021-325210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pasricha SR, Tye-Din J, Muckenthaler MU, Swinkels DW. Iron deficiency. Lancet Lond Engl. 2021;397:233–48. doi: 10.1016/S0140-6736(20)32594-0. [DOI] [PubMed] [Google Scholar]
- 11.Khare A, Samudre S, Arora A. Sneak-peek into iron deficiency anemia in India: The need for food-based interventions and enhancing iron bioavailability. Food Res Int. 2022;162:111927. doi: 10.1016/j.foodres.2022.111927. [DOI] [PubMed] [Google Scholar]
- 12.Shobha M, Bithika D, Bhavesh S. The prevalence of intestinal parasitic infections in the urban slums of a city in Western India. J Infect Public Health. 2013;6:142–9. doi: 10.1016/j.jiph.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Pathy S, Lambert R, Sauvaget C, Sankaranarayanan R. The incidence and survival rates of colorectal cancer in India remain low compared with rising rates in East Asia. Dis Colon Rectum. 2012;55:900–6. doi: 10.1097/DCR.0b013e31825afc4e. [DOI] [PubMed] [Google Scholar]
- 14.Rockey DC, Cello JP. Evaluation of the gastrointestinal tract in patients with iron-deficiency anemia. N Engl J Med. 1993;329:1691–5. doi: 10.1056/NEJM199312023292303. [DOI] [PubMed] [Google Scholar]
- 15.Zuckerman G, Benitez J. A prospective study of bidirectional endoscopy (colonoscopy and upper endoscopy) in the evaluation of patients with occult gastrointestinal bleeding. Am J Gastroenterol. 1992;87:62–6. [PubMed] [Google Scholar]
- 16.Kepczyk T, Kadakia SC. Prospective evaluation of gastrointestinal tract in patients with iron-deficiency anemia. Dig Dis Sci. 1995;40:1283–9. doi: 10.1007/BF02065539. [DOI] [PubMed] [Google Scholar]
- 17.Cook IJ, Pavli P, Riley JW, Goulston KJ, Dent OF. Gastrointestinal investigation of iron deficiency anaemia. Br Med J Clin Res Ed. 1986;292:1380–2. doi: 10.1136/bmj.292.6532.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hardwick RH, Armstrong CP. Synchronous upper and lower gastrointestinal endoscopy is an effective method of investigating iron-deficiency anaemia. Br J Surg. 1997;84:1725–8. [PubMed] [Google Scholar]
- 19.Stone H, Almilaji O, John C, Smith C, Surgenor SL, Ayres L, Williams EJ, Snook J. The dedicated iron deficiency anaemia clinic: A 15-year experience. Frontline Gastroenterol. 2022;13:20–4. doi: 10.1136/flgastro-2020-101470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh A, Mishra R, Ranjan R. Gastrointestinal lesions and its associated factors in adult males with iron deficiency anaemia: A cross-sectional study from tertiary care centre of North India. Cureus. 14:e26905. doi: 10.7759/cureus.26905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar A, Gupta S, Meena L, Meher M, Rai M, Kumar S, Bharti A. Study to evaluate the etiology of iron deficiency anemia at a teaching hospital in northeastern part of India. J Fam Med Prim Care. 2020;9:3076. doi: 10.4103/jfmpc.jfmpc_3_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kadla SA, Shah NA, Bindroo MA, Khan BA, Farooq A, Yousf W, Wani BA. Evaluation of iron deficiency anaemia for gastrointestinal causes in patients without GI symptoms in high prevalent GI malignancy zones. Arab J Gastroenterol Off Publ Pan-Arab Assoc Gastroenterol. 2016;17:67–72. doi: 10.1016/j.ajg.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Varadarajulu S, Eloubeidi MA, Patel RS, Mulcahy HE, Barkun A, Jowell P, et al. The yield and the predictors of esophageal pathology when upper endoscopy is used for the initial evaluation of dysphagia. Gastrointest Endosc. 2005;61:804–8. doi: 10.1016/s0016-5107(05)00297-x. [DOI] [PubMed] [Google Scholar]
- 24.Lee SW, Chang CS, Yeh HJ, Lien HC, Lee TY, Peng YC. The diagnostic value of alarm features for identifying types and stages of upper gastrointestinal malignancies. Gastroenterol Res. 2017;10:120–5. doi: 10.14740/gr826w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thrumurthy SG, Chaudry MA, Thrumurthy SSD, Mughal M. Oesophageal cancer: Risks, prevention, and diagnosis. BMJ. 2019;366:l4373. doi: 10.1136/bmj.l4373. [DOI] [PubMed] [Google Scholar]
- 26.Qureshi NA, Hallissey MT, Fielding JW. Outcome of index upper gastrointestinal endoscopy in patients presenting with dysphagia in a tertiary care hospital-A 10 years review. BMC Gastroenterol. 2007;7:43. doi: 10.1186/1471-230X-7-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greuter T, Manser C, Pittet V, Vavricka SR, Biedermann L on behalf of Swiss IBDnet, an official working group of the Swiss Society of Gastroenterology. Gender differences in inflammatory bowel disease. Digestion. 2020;101(Suppl 1):98–104. doi: 10.1159/000504701. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y. Epidemiology of esophageal cancer. World J Gastroenterol. 2013;19:5598. doi: 10.3748/wjg.v19.i34.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu C, Ma Y, Qin Q, Wang P, Luo Y, Xu P, et al. Epidemiology of esophageal cancer in 2020 and projections to 2030 and 2040. Thorac Cancer. 2023;14:3–11. doi: 10.1111/1759-7714.14745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354. doi: 10.3748/wjg.v12.i3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Gastroenterol Rev. 2019;14:89–103. doi: 10.5114/pg.2018.81072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.James MW, Chen CM, Goddard WP, Scott BB, Goddard AF. Risk factors for gastrointestinal malignancy in patients with iron-deficiency anaemia. Eur J Gastroenterol Hepatol. 2005;17:1197–203. doi: 10.1097/00042737-200511000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Khalid U, Spiro A, Baldwin C, Sharma B, McGough C, Norman AR, et al. Symptoms and weight loss in patients with gastrointestinal and lung cancer at presentation. Support Care Cancer. 2007;15:39–46. doi: 10.1007/s00520-006-0091-0. [DOI] [PubMed] [Google Scholar]
- 34.Ockenga J, Valentini L. Review article: Anorexia and cachexia in gastrointestinal cancer. Aliment Pharmacol Ther. 2005;22:583–94. doi: 10.1111/j.1365-2036.2005.02628.x. [DOI] [PubMed] [Google Scholar]
- 35.Palesty JA, Dudrick SJ. What we have learned about cachexia in gastrointestinal cancer. Dig Dis. 2003;21:198–213. doi: 10.1159/000073337. [DOI] [PubMed] [Google Scholar]
- 36.Price SJ, Gibson N, Hamilton WT, King A, Shephard EA. Intra-abdominal cancer risk with abdominal pain: A prospective cohort primary care study. Br J Gen Pract. 2022;72:e361–8. doi: 10.3399/BJGP.2021.0552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bai Y, Li ZS, Zou DW, Wu RP, Yao YZ, Jin ZD, et al. Alarm features and age for predicting upper gastrointestinal malignancy in Chinese patients with dyspepsia with high background prevalence of Helicobacter pylori infection and upper gastrointestinal malignancy: An endoscopic database review of 102,665 patients from 1996 to 2006. Gut. 2010;59:722–8. doi: 10.1136/gut.2009.192401. [DOI] [PubMed] [Google Scholar]
- 38.Talley NJ. American gastroenterological association medical position statement: Evaluation of dyspepsia. Gastroenterology. 2005;129:1753–5. doi: 10.1053/j.gastro.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 39.Liu LWC, Andrews CN, Armstrong D, Diamant N, Jaffer N, Lazarescu A, et al. Clinical practice guidelines for the assessment of uninvestigated esophageal dysphagia. J Can Assoc Gastroenterol. 2018;1:5–19. doi: 10.1093/jcag/gwx008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petrovchich I, Ford JM. Genetic predisposition to gastric cancer. Semin Oncol. 2016;43:554–9. doi: 10.1053/j.seminoncol.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 41.Groen EJ, Roos A, Muntinghe FL, Enting RH, de Vries J, Kleibeuker JH, et al. Extra-intestinal manifestations of familial adenomatous polyposis. Ann Surg Oncol. 2008;15:2439–50. doi: 10.1245/s10434-008-9981-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watson P, Vasen HFA, Mecklin JP, Bernstein I, Aarnio M, Järvinen HJ, et al. The risk of extra-colonic, extra-endometrial cancer in the Lynch syndrome. Int J Cancer J Int Cancer. 2008;123:444–9. doi: 10.1002/ijc.23508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strul H, Arber N. Fecal occult blood test for colorectal cancer screening. Ann Oncol Off J Eur Soc Med Oncol. 2002;13:51–6. doi: 10.1093/annonc/mdf076. [DOI] [PubMed] [Google Scholar]
- 44.Hewitson P, Glasziou P, Watson E, Towler B, Irwig L. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): An update. Am J Gastroenterol. 2008;103:1541–9. doi: 10.1111/j.1572-0241.2008.01875.x. [DOI] [PubMed] [Google Scholar]
- 45.Navarro M, Nicolas A, Ferrandez A, Lanas A. Colorectal cancer population screening programs worldwide in 2016: An update. World J Gastroenterol. 2017;23:3632. doi: 10.3748/wjg.v23.i20.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zappa M, Visioli CB, Ciatto S, Grazzini G, Rubeca T, Bonanomi AG, et al. Gastric cancer after positive screening faecal occult blood testing and negative assessment. Dig Liver Dis Off J Ital Soc Gastroenterol Ital Assoc Study Liver. 2007;39:321–6. doi: 10.1016/j.dld.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 47.Lee MW, Pourmorady JS, Laine L. Use of fecal occult blood testing as a diagnostic tool for clinical indications: A systematic review and meta-analysis. Am J Gastroenterol. 2020;115:662–70. doi: 10.14309/ajg.0000000000000495. [DOI] [PubMed] [Google Scholar]
- 48.Borges LV, Mattar R, Silva JMKD, Silva ALWD, Carrilho FJ, Hashimoto CL. Fecal occult blood: A comparison of chemical and immunochemical tests. Arq Gastroenterol. 2018;55:128–32. doi: 10.1590/S0004-2803.201800000-22. [DOI] [PubMed] [Google Scholar]
- 49.Overview |Quantitative Faecal Immunochemical Tests to Guide Referral for Colorectal Cancer in Primary Care |Guidance |NICE. NICE. 2017. [[Last accessed on 2023 Jul 12]]. Available from: https://www.nice.org.uk/guidance/dg30 .
- 50.Monahan KJ, Davies MM, Abulafi M, Banerjea A, Nicholson BD, Arasaradnam R, et al. Faecal immunochemical testing (FIT) in patients with signs or symptoms of suspected colorectal cancer (CRC): A joint guideline from the Association of Coloproctology of Great Britain and Ireland (ACPGBI) and the British Society of Gastroenterology (BSG) Gut. 2022;71:1939–62. doi: 10.1136/gutjnl-2022-327985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clackett W, Barclay ST, Stanley AJ, Cahill A. The value of quantitative faecal immunochemical testing as a prioritisation tool for the endoscopic investigation of patients with iron deficiency. Front Med. 2021;8:700753. doi: 10.3389/fmed.2021.700753. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets are available from the corresponding author upon reasonable request.


