Abstract
Introduction
Transposition of the great arteries (TGA), especially with intact ventricular septum (TGA-IVS), presents unique challenges during fetal-to-neonatal transition, which can contribute to developing persistent pulmonary hypertension of the newborn (PPHN).
Case Presentation
A male newborn with TGA-IVS, delivered via caesarean section, presented with hypoxemia and tachycardia immediately after birth (preductal SpO2: 50–60%, post-ductal SpO2: 70–75%). Echocardiography revealed a floppy interatrial septum and two interatrial connections with bidirectional shunting. Ductal flow showed systolic right-to-left shunting, suggesting high pulmonary vascular resistance. Immediate post-birth management included non-invasive respiratory support with continuous positive airway pressure at 100% oxygen and administration of prostaglandin E2 to maintain ductal patency. Despite initial low oxygen saturation levels, escalation of intensive treatments was deferred based on continuous trend monitoring of vital signs and echocardiographic indicators. Oxygenation and circulation gradually improved within the first 2 h after birth to normal values, obviating escalation of intensive interventions like intubation, nitric oxide and/or balloon atrial septostomy. Arterial switch operation at day 3 post-birth was successful.
Conclusion
This case highlights the possible contribution of fetal-to-neonatal transition in TGA-IVS to developing PPHN, which may subside after transition. Moreover, this case highlights the potential for providing a gentle hemodynamic transition without invariably needing early invasive interventions after birth.
Keywords: Congenital heart disease, Transposition of the great vessels, Persistent pulmonary hypertension of the newborn, Watchful waiting, Case report
Established Facts
Fetal-to-neonatal transition involves significant and abrupt pulmonary and cardiovascular changes required for a fetus to adapt to life outside the uterus.
Infants with transposition of the great arteries and intact ventricular septum (TGA-IVS) have a high risk of developing persistent pulmonary hypertension of the newborn (PPHN) immediately after birth and transition can contribute to this.
Infants with TGA-IVS and postnatal hypoxemia, indicative of suspected PPHN, often require respiratory interventions, as well as balloon atrial septostomy to improve oxygenation and circulation.
Novel Insights
Continuous trend monitoring of vital signs and echocardiographic indicators allows for individualized decisions regarding the need for escalating interventions.
In the presence of adequate interatrial communication, infants with TGA-IVS could benefit from watchful waiting during the fetal-to-neonatal transition after receiving non-invasive respiratory therapy with oxygen supply, potentially averting early invasive intervention.
Delivery room strategies to reduce PVR and stabilization of SVR could offer additional benefit.
Introduction
Fetal-to-neonatal transition refers to the significant and abrupt pulmonary and cardiovascular changes required for a fetus to adapt to life outside the uterus [1]. While most healthy newborns transition independently and uneventfully, the normal physiological changes may be compromised by congenital heart disease (CHD) [2]. Transposition of the great arteries (TGA) is a CHD characterized by discordant ventriculoarterial connection, resulting in a parallel pulmonary and systemic circulation. Postnatally, in infants with TGA, an adequate communication between the two circulations (at atrial and/or ventricular level) is necessary for sufficient arterial oxygen saturation and tissue oxygenation [3]. Newborns with TGA, especially those with an intact ventricular septum (TGA-IVS), may develop severe hypoxemia due to ineffective parallel circulation. Additionally, they are at increased risk of developing persistent pulmonary hypertension of the newborn (PPHN) after birth, characterized by failure of normal pulmonary vascular adaptation at or soon after birth, resulting in persistently elevated pulmonary vascular resistance [4]. Previous studies have reported PPHN in up to 21.5% of infants, with the highest prevalence in TGA-IVS cases [5, 6]. These infants often require non-invasive and invasive respiratory interventions, as well as balloon atrial septostomy to improve SpO2 values and circulation [5, 7]. However, PPHN often proves refractory and therapy resistant to conventional treatment measures [5].
Although there have been advancements in prenatal diagnoses, planned deliveries and better postnatal monitoring, altered fetal-to-neonatal transition can contribute to the development of intrinsic PPHN in TGA-IVS. Determining the appropriate timing for interventions in postnatal hypoxemia, or deciding to refrain from these interventions, is challenging and requires careful consideration. This case highlights the potential for providing a gentle hemodynamic transition for infants with TGA-IVS without invariably needing early invasive interventions after birth.
Case Presentation
A male newborn was delivered at 38 weeks and 3 days’ gestation (birthweight 3.6 kg) via planned caesarean section due to a maternal history of a fourth-degree perineal tear. A prenatal diagnosis of TGA-IVS was made after a second trimester anomaly scan indicated a potential CHD. At 36+5 weeks’ gestation, no signs of a restrictive foramen ovale (FO) were present (online suppl. Video 1; for all online suppl. material, see https://doi.org/10.1159/000542723). The FO flap was mobile, opening up to two-thirds into the left atrium and allowing bidirectional flow. Maximum velocity in the pulmonary veins was 22 cm/s. The patient’s pathway from fetal diagnosis to surgery is shown in Table 1.
Table 1.
Patients pathway from fetal diagnosis to surgery
| Age | Event |
|---|---|
| 20 w + 5 d | Prenatal diagnosis of TGA-IVS, no signs of a restrictive foramen ovale (FO) |
| 36 w + 5 d | Prenatal ultrasound showing a mobile FO flap, opening up to two-thirds into the left atrium and allowing bidirectional flow. Does not meet criteria for an emergency BAS. Planned caesarean at 38 weeks, followed by admission to the neonatology intensive care unit with the possibility of urgent BAS |
| 0 d 38 w + 3 d | Delivered by planned caesarean section due to maternal indication. Apgar score at 1, 5, and 10 min were 6, 7 and 8, respectively. First saturation measurement at 5 min was 25% (preductal). Heart rate was >100 bpm, and the infant was crying loudly |
| CPAP with PEEP 8 cmH2O was started, and FiO2 was incrementally increased to 1.0, with pre-and post-ductal saturation measurements of 50% and 70%, respectively | |
| 0–1 h | CPAP with PEEP 7 (FiO2 1.0) was continued at the Neonatal ICU, with pre-and post-ductal saturations of 45–55% and 70–75%, respectively |
| 0–6 h | Gradual increase of oxygen saturation to 85–90% (preductal) and 90–93% (post-ductal) with CPAP PEEP 6 and FiO2 0.8 |
| Blood gas analysis | |
| |
| Confirmation of diagnosis: TGA-IVS, aorta in right anterior position to the pulmonary artery, tricuspid semilunar valves, coronary anatomy 1LCx-2R (according to the Leiden Convention coronary coding system)1 | |
| 3 d | Successful arterial switch operation with Lecompte maneuver and closure of the FO |
| 32 m | Patient is doing well, no developmental delays or disorders. Good biventricular systolic function, mild neo-aortic root dilatation, trivial neo-aortic valve regurgitation, no pulmonary branch stenosis |
BAS, balloon atrial septostomy; CPAP, continuous positive airway pressure; FiO2, fraction of inspired oxygen; FO, oval fossa; IVS, intact ventricular septum; h, hours; m, months; PEEP, positive end-expiratory pressure; TGA, transposition of the great arteries; w, weeks.
1Gittenberger-de Groot et al. J Thorac Cardiovasc Surg 2018;156:2260-9.
After birth and upon initial assessment, the neonate showed signs of severe hypoxia (preductal SpO2 of 25% at 5 min) and tachycardia (170 bpm), and the umbilical cord was clamped at 1 min. Non-invasive respiratory support was started via continuous positive airway pressure (CPAP) with positive end-expiratory pressure 8 and fraction of inspired oxygen of 1.0 (FiO2 1.0), aiming to enhance pulmonary blood flow (PBF). This would facilitate pulmonary venous return, leading to an increase in left atrial pressure to promote interatrial mixing of oxygenated and deoxygenated blood. Initially, this did not improve preductal oxygen saturations levels, which remained at approximately 50%. Prostaglandin E2 was administered to maintain ductal patency (12.5 μg/kg/min). The neonate was then transferred to the neonatal intensive care unit, continuing respiratory support (CPAP PEEP 8, FiO2 1.0).
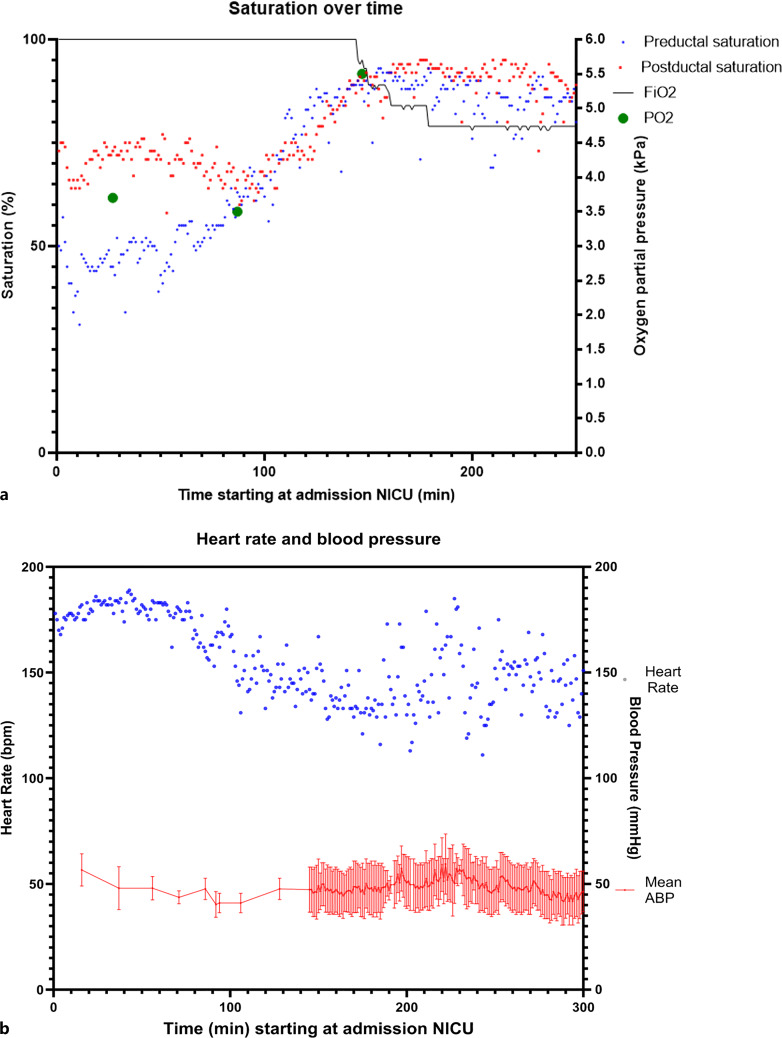
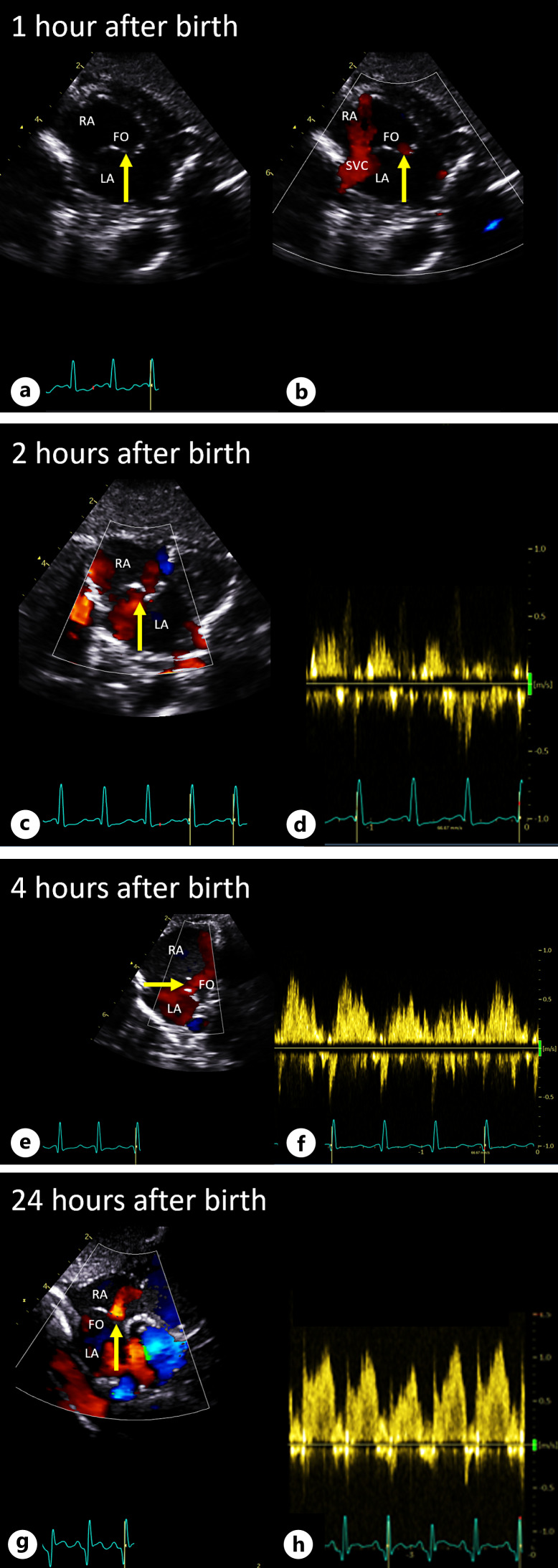
In the first hour after birth, SpO2 levels remained low with preductal SpO2 at 50% and post-ductal SpO2 at 70% (Fig. 1a). This indicated predominant right-to-left shunting at ductal level, suggesting high pulmonary vascular resistance (PVR) and/or a potentially restrictive FO. Clinically, the neonate showed a sufficient breathing pattern along with tachycardia (175–185 bpm, Fig. 1b). First postnatal echocardiography confirmed TGA-IVS, revealing a floppy interatrial septum bulging more toward the left atrium. Two connections were observed in the atrial septum, suggestive of appropriateness in size but demonstrating bidirectional shunting (Fig. 2; online suppl. Videos 2–5). The arterial duct (DA) was widely open, also demonstrating bidirectional shunting (systolic right-to-left and diastolic left-to-right; online suppl. Video 6). This confirmed the hypothesis of high PVR.
Fig. 1.
Trend of hemodynamic parameters and pulse oxygen saturation over time. Changes of saturation (a), heart rate (b) and arterial blood pressure (b) over time (in minutes), starting at NICU admission (±30 min post birth). a Blue dots indicate the preductal saturation and the red dots the post-ductal saturation measurements. b Blue dots indicate heart rate trend and the red line the mean arterial blood pressure (ABP), including systolic and diastolic blood pressure (interval). BP, blood pressure; FiO2, fraction of inspired oxygen; min, minutes; NICU, neonatal intensive care unit; PO2, partial pressure of oxygen.
Fig. 2.
Echocardiographic images of the FO after birth. Postnatal echocardiographic images of the FO at 60 min (a, b), 2 h (c, d), 4 h (e, f), and 24 h (g, h) post-birth. Yellow arrow indicates the interatrial septum with adequate communication (left-to-right shunting through FO) between LA and RA depicted by laminar color Doppler (a–c, e, g) and pulsed wave Doppler (d, f, h). LA, left atrium; RA; right atrium; FO, patent foramen ovale; SVC, superior vena cava.
Although escalation of intensive treatment (such as mechanical ventilation, inhaled nitric oxide administration, fluid boluses, and vasopressor therapy) is usually indicated, we opted for an alternative approach and decided to postpone this escalation for the following reasons.
-
1.
The neonate was delivered via caesarean section and the presence of extra lung liquid caused transient tachypnea of the neonate, which could contribute to developing PPHN.
-
2.
Echocardiography indicated two interatrial connections, likely of appropriate size but with bidirectional shunting, leading to the decision that balloon atrial septostomy was not warranted at this time.
-
3.
Pulmonary venous return was present and from the point of admission preductal SpO2 levels were gradually increasing over time to 65% within the first 1.5 h after birth (Fig. 1a).
In the following hours, the clinical condition of the infant improved markedly with significant decrease in heart rate and increase in SpO2 levels, reaching preductal 85% and post-ductal 93% (Fig. 1). Follow-up echocardiography demonstrated rightward shift of the interatrial septum, and primarily left-to-right shunting over the interatrial communication, accompanied by an increased pulmonary venous flow on color Doppler assessment (Fig. 2; online suppl. Videos 2–5). The DA flow pattern shifted toward a predominant left-to-right shunt with a DA of equal size, indicating a decrease in PVR. Additionally, arterial blood gas showed normocapnia, low arterial lactate levels, and normal pH approximately 3 h after birth (Table 1). After 3 days post-birth, the patient underwent an uneventful arterial switch operation. At the age of 3 years, the patient exhibited normal neurodevelopment and showed no signs of significant residual cardiac abnormalities.
Discussion
In this case, we successfully supported the fetal-to-neonatal transition of an infant with TGA-IVS using non-invasive respiratory support, 100% oxygen supply, prostaglandin therapy, continuous monitoring, and watchful waiting. This report highlights the potential contribution of this transition to the development of PPHN and the importance of an individualized approach, avoiding invasive interventions after birth in some cases.
The fetal-to-neonatal transition period presents unique challenges, characterized by significant hemodynamic changes. During this transition, lung aeration is causing an immediate decrease in PVR with an increase in PFB, while the SVR increases with cord clamping and removal of the low-resistance placenta from circulation [8]. Most healthy newborns will transition independently and uneventfully, but there is very limited data on infants with CHD, such as TGA. In TGA-IVS, for instance, when transition is uneventful, PVR decreases, and PBF increases with support from left-to-right shunting through the DA. This leads to adequate pulmonary venous return and increased preload of the left atrium. An appropriate interatrial connection is required and the increasing left atrial pressure facilitates mixing of oxygenated and deoxygenated blood between the pulmonary and systemic venous circulation [8]. However, when neonatal adaptation is poor with subsequent hypoxemia, the fall in PVR is delayed, PBF is reduced and interatrial mixing is compromised [9].
Newborns with TGA-IVS have a high risk of PPHN at or soon after birth. However, there is little data of the exact incidence and causes for severe PPHN in TGA. Literature suggests a complex multifactorial etiology, with factors including intrauterine or early postnatal hypoxemia and acidosis, restrictive FO or fetal ductal constriction due to increased oxygen content in the pulmonary artery in fetuses with TGA, increased pulmonary artery wall thickness with muscularity and intimal proliferation, involvement of mediating pathways such as endothelin-1, prostacyclin-cGMP, nitric oxide-cAMP, and vascular endothelial growth factor; and possibly clinically unrecognized pulmonary microthrombi. Nonetheless, PPHN has been associated with significant mortality in TGA, reported up to 29% [5, 10–13].
These factors contributing to PPHN add complexity to the circulatory challenges during transition, potentially necessitating early invasive interventions. As highlighted in a similar case and literature review by Karimi et al. [14], TGA-IVS with PPHN poses significant challenges and is often resistant to conventional therapies. In managing TGA-IVS after birth, accurate prenatal diagnosis via fetal echocardiography is crucial, as it delineates anatomical features and shunt characteristics. This allows for optimized daytime delivery planning and ensures that the necessary medical team and resources are in place for potential postnatal interventions.
However, the exact timing for interventions in postnatal hypoxemia, or deciding to refrain from immediate interventions, remains challenging. Management strategies based on achieving a more gentle and stable transition to extra-uterine life, such as delayed or physiological-based cord clamping, could be the key to improving fetal-to-neonatal transition, reducing the incidence of PPHN. This approach might help prevent subsequent complications and decrease the need for early invasive interventions after birth [15]. Additionally, this could potentially allow for earlier surgical correction, further optimizing outcomes [16]. In the management strategy for TGA-IVS, considering the presence of transitional physiology could aid treatment decisions.
This case highlights the contribution of transitional issues to the development of PPHN and a tailored management approach in a newborn with TGA-IVS, where factors such as mode of delivery, trend monitoring of vital signs and echocardiographic findings played an important role in discriminating fetal-to-neonatal transition from intrinsic PPHN. Delivery room strategies to reduce PVR and stabilization of SVR could offer additional benefit.
Conclusion
This case report presents the successful postnatal management of a neonate with TGA-IVS and highlights the complexity and importance of neonatal transition in CHD. In the presence of adequate interatrial communication, infants with TGA-IVS could benefit from watchful waiting for fetal-to-neonatal transition, supported by non-invasive respiratory therapy with oxygen supply, potentially preventing early invasive interventions. However, it is important to acknowledge that this is a single case, and that each TGA newborn presents unique challenges. Therefore, treatment strategies should be individualized and tailored to each patient’s specific needs.
Statement of Ethics
Ethical approval was not required for this study in accordance with local and national guidelines.
Written informed consent was obtained from the infant’s parents for publication of the case report and accompanying images.
Conflict of Interest Statement
The authors declare that there is no conflict of interest.
Funding Sources
This research is funded by the Hartekind Foundation (Stichting Hartekind).
Author Contributions
Conception and final approval: Roel L.F. van der Palen. Investigation: Jesse A. Weeda, Roel L.F. van der Palen, and Monique C. Haak. Supervision: Roel L.F. van der Palen, A.B. te Pas, Nico A. Blom, and Monique C. Haak. Writing original draft: Jesse A. Weeda. Writing – review and editing: Roel L.F. van der Palen, Arjan B. te Pas, Nico A. Blom, and Monique C. Haak.
Funding Statement
This research is funded by the Hartekind Foundation (Stichting Hartekind).
Data Availability Statement
All relevant data are within the paper, further inquiries can be directed to the corresponding authors.
Supplementary Material.
Supplementary Material.
Supplementary Material.
Supplementary Material.
Supplementary Material.
Supplementary Material.
Supplementary Material.
References
- 1. Chakkarapani AA, Roehr CC, Hooper SB, Te Pas AB, Gupta S; ESPR Neonatal Resuscitation section writing group . Transitional circulation and hemodynamic monitoring in newborn infants. Pediatr Res. 2024;96(3):595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Peyvandi S, Donofrio MT. Circulatory changes and cerebral blood flow and oxygenation during transition in newborns with congenital heart disease. Semin Pediatr Neurol. 2018;28:38–47. [DOI] [PubMed] [Google Scholar]
- 3. Szymanski MW, Moore SM, Kritzmire SM, Goyal A. Transposition of the great arteries. StatPearls StatPearls Publishing; 2023. [PubMed] [Google Scholar]
- 4. Mathew B, Lakshminrusimha S. Persistent pulmonary hypertension in the newborn. Children. 2017;4(8):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Roofthooft MT, Bergman KA, Waterbolk TW, Ebels T, Bartelds B, Berger RM. Persistent pulmonary hypertension of the newborn with transposition of the great arteries. Ann Thorac Surg. 2007;83(4):1446–50. [DOI] [PubMed] [Google Scholar]
- 6. Sallaam S, Natarajan G, Aggarwal S. Persistent pulmonary hypertension of the newborn with D-transposition of the great arteries: management and prognosis. Congenit Heart Dis. 2016;11(3):239–44. [DOI] [PubMed] [Google Scholar]
- 7. Thomas AR, Ma AL, Weinberg DD, Huber M, Ades A, Rychik J, et al. Delivery room oxygen physiology and respiratory interventions for newborns with cyanotic congenital heart disease. J Perinatol. 2021;41(9):2309–16. [DOI] [PubMed] [Google Scholar]
- 8. Hooper SB, Te Pas AB, Lang J, van Vonderen JJ, Roehr CC, Kluckow M, et al. Cardiovascular transition at birth: a physiological sequence. Pediatr Res. 2015;77(5):608–14. [DOI] [PubMed] [Google Scholar]
- 9. Singh Y, Tissot C. Echocardiographic evaluation of transitional circulation for the neonatologists. Front Pediatr. 2018;6:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kumar A, Taylor GP, Sandor GG, Patterson MW. Pulmonary vascular disease in neonates with transposition of the great arteries and intact ventricular septum. Br Heart J. 1993;69(5):442–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Newfeld EA, Paul MM, Muster AJ, Idriss FS. Pulmonary vascular disease in complete transposition of the great arteries: a study of 200 patients. Am J Cardiol. 1974;34(1):75–82. [DOI] [PubMed] [Google Scholar]
- 12. Geiger R, Berger RM, Hess J, Bogers AJ, Sharma HS, Mooi WJ. Enhanced expression of vascular endothelial growth factor in pulmonary plexogenic arteriopathy due to congenital heart disease. J Pathol. 2000;191(2):202–7. [DOI] [PubMed] [Google Scholar]
- 13. Hoshino S, Somura J, Furukawa O, Yanagi T, Maruo Y. The histological findings in transposition of the great artery with severe persistent pulmonary hypertension of the newborn. J Cardiol Cases. 2018;17(5):159–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Karimi M, Kirshbom PM, Kopf GS, Steele MM, Sullivan JM. Persistent pulmonary hypertension in a neonate with transposition of great arteries and intact ventricular septum: a case report and review of the literature. World J Pediatr Congenit Heart Surg. 2015;6(3):462–5. [DOI] [PubMed] [Google Scholar]
- 15. Marzec L, Zettler E, Cua CL, Rivera BK, Pasquali S, Katheria A, et al. Timing of umbilical cord clamping among infants with congenital heart disease. Prog Pediatr Cardiol. 2020;59:101318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Anderson BR, Ciarleglio AJ, Hayes DA, Quaegebeur JM, Vincent JA, Bacha EA. Earlier arterial switch operation improves outcomes and reduces costs for neonates with transposition of the great arteries. J Am Coll Cardiol. 2014;63(5):481–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are within the paper, further inquiries can be directed to the corresponding authors.