Abstract
Background: Schistosomiasis is considered one of the most devastating parasitic diseases globally, coming second only to malaria in terms of morbidity. The disease-causing parasite can inhabit the body for over a decade, leading to imbalances in the host's metabolic systems. The flukes and their eggs can illicit various immunological and metabolic complications resulting in the generation of reactive oxygen species (ROS). These are known to have several devastating effects on the host through increased oxidative stress, DNA mutation, and gene modifications, which can lead to fibrosis and cancer.
Main Body: Here, we discuss oxidative stress and cancer risk in Schistosoma infection. The concept of ROS generation and the complex antioxidant systems that enable the parasite to evade oxidant insults and prolong its life span in the host are explored. Further, the various roles of ROS during the initiation and progression of schistosomiasis and its influence on the host are discussed. Finally, mechanisms linked to the risk of bladder cancer in Schistosoma haematobium (S. haematobium) infections are elucidated.
Conclusion: Finally, we provide an opinion on how some of these mechanisms could give directions for future studies as well as provide a springboard for diagnostics and drug targeting in schistosomiasis.
Keywords: bladder cancer, oxidative stress, Schistosoma haematobium, Schistosoma mansoni
1. Introduction
Schistosomiasis, also known as bilharzia, is a tropical parasitic disease caused by trematodes of the genus Schistosoma. It is mainly transmitted through a cycle that involves the contamination of surface water with excreta while employing specific freshwater snails as the intermediate host. Schistosomiasis contributes enormously to the global disease burden considering that it is prevalent in some 74 countries and affects over 200 million people worldwide [1–3]. Further, it is estimated that at least 700 million people are at risk of Schistosoma infection, with an estimated worldwide annual mortality rate of around 20,000 [3–5]. A variety of schistosomiasis causative worms exist; however, among humans, Schistosoma mansoni (S. mansoni), Schistosoma haematobium, and Schistosoma japonicum (S. japonicum) are the major schistosomiasis causing species. Schistosoma haematobium, which causes urinary schistosomiasis, is more prevalent in Africa and the Arabian Peninsula and is transmitted by an intermediate host, Bulinus Snail. Schistosoma mansoni, which is more associated with the liver and intestines is transmitted by Biomphalaria snails, and it is commonly found in Africa, the Arabian Peninsula, and South America. The intestinal and hepatosplenic schistosomiasis widely seen in the Philippines, Indonesia, and China could be caused by S. japonicum transmitted by the amphibian snail, Oncomelania [6]. The economic importance of the burden of Schistosoma infections is based on the ability of the disease state to degenerate into serious complications such as colorectal and liver cancers, as has been previously observed in the S. mansoni and S. japonicum species [7, 8]. However, the most crucial association has been the link between S. haematobium and bladder cancer. Consequently, both the WHO and IARC have classified S. haematobium as a Class 1 carcinogen [9]. Schistosomiasis is characterized by over-dispersed population distribution, such that in most endemic areas, children between 5 and 15 years constitute the most significant proportion associated with high infection intensities [10].
The matured schistosome flukes colonize the blood vessels of humans for years with unique abilities to evade the immune system. They rely on blood components such as globulins, plasma proteins, and red blood cells to meet their nutritional needs [6]. During these periods, the worms lay thousands of eggs daily, which could either be trapped in adjacent tissues or be excreted through feces or urine. Several local and systemic pathological conditions are triggered based on the specific immune-mediated granulomatous responses elicited by tissue-trapped eggs. This effect may range from urogenital inflammation (as in the case of haematobium), anemia, stunted growth in children, reduced physical fitness, cognitive impairment, periportal fibrosis (PPF), hepatosplenism, and portal hypertension [11]. In some cases, immune resistance could be slowly acquired through a set of complex immune mechanisms [6]. It has long been argued that the parasite's ability to modulate the host's immune response depends on the deposition of eggs. Severe tissue damage and inflammation can result from strong immunological reactions triggered by the release of eggs into host tissues. However, new data supports the idea that all stages of the infection could contribute to immune modulation [12]. Migrating schistosomula, larval, adult worms, and the skin-penetrating cercariae can release biomolecules that modulate both the innate and adaptive immune responses to facilitate parasitic evasion of the host's immune system. This immune modulation may serve dual purposes. On one hand, the attenuated immune responses enhance parasite survival in the host, while at the same time limiting severe host reactions through the modulation of critical immunopathology [13]. Thus, various mechanisms such as the down or upregulation of inflammation or inhibition of cytokines production, coupled with Type 1 helper (Th1) and Type 2 helper (Th2) immune response switches are employed [14]. During the first phase of the parasitic infection, Type 1 inflammatory immune responses within the plasma and tissues are propagated by interferon-γ, IL-1, IL-12, and TNF- α. However, as the disease progresses to a chronic phase, antigens by deposited eggs trigger the release of CD4+ Th2 responses [15].
Oxidative stress is a major factor in the pathophysiology of schistosomiasis. Hence, the breakdown of the parasite's defensive mechanisms depends heavily on the antioxidant potential of the host. Certain cells are considered significant sources of reactive oxygen species (ROS) and include eosinophils (E), macrophages (M), and neutrophils (N). Inflammations characterized by the infiltration of these cells serve as a hallmark of urogenital schistosomiasis [16]. A clear association between schistosomiasis and endogenous generation of ROS and reactive nitrogen species (RNS)-mediated chronic inflammation has been established [17]. Further, it is known that inducible nitric oxide synthase (iNOS)-mediated oxidative stress is elevated in infectious diseases in response to inflammation [18]. Additionally, oxidative stress associated with lipid peroxidation and DNA damage could aggravate the disease itself. Evidence suggests that schistosomiasis with accompanying oxidative stress is significantly connected with bladder cancer [9, 19]
Therefore, it is vital to understand the state and role of oxidative stress and how it modifies the pathogenesis of urinary schistosomiasis and its consequences, in particularly cancer.
2. Oxidative Stress as an Early Process in Schistosomiasis
The host's immune system produces ROS in the early stages of Schistosoma infection as a means of combating the parasites' invasion [12]. This results in oxidative stress, especially in the host liver and spleen tissues where the parasites migrate and start the maturation process. Oxidative stress in these organs could lead to tissue damage and inflammation as well as potential damage to cellular constituents such as proteins, lipids, and DNA [12, 20]. This is associated with an early release of cytokines including TNF-α, IL-1β, and IFN-γ by the host immune system. ROS plays several physiological roles, as they are usually formed as oxygen metabolic byproducts or due to the presence of environmental stressors and xenobiotics. ROS may also alter genetic and epigenetic information [21–23].
To compensate for the oxidative damage caused by the host's immunological response, Schistosoma parasites employ a range of antioxidant enzymes, such as catalase, glutathione peroxidase (GPx), and superoxide dismutase (SOD), to neutralize the ROS and enable the parasites to survive in the host's hostile environment [12, 20]. These enzymes are essential in the early stages of infection when the parasites are vulnerable to the immune system's oxidative attack. Even though high levels of oxidative stress can cause tissue damage, fibrosis, and persistent inflammation. It is possible for the parasites' antioxidant defenses to take charge and evade immune destruction leading to the development of a chronic infection [20]. As far back as 1987, it was known that many parasites, including schistosomiasis, have increased susceptibility to oxidative insults compared to their host. This was seen in either their susceptibility to ROS in vivo, increased reserve antioxidant capacity of the host, or drugs that induce oxidative stress [24].
2.1. ROS Production by the Host
Generally, M produce ROS to fight invading pathogens, thus, contributing majorly to the net ROS synthesis in hepatic fibrosis [25–27]. In hepatic granuloma cells, M regulate granulomatous inflammation depending on which effector phenotype is activated [28]. In this regard, the differentially activated M M1 and M2 function differently in regulating the inflammation's initiation, progression, and resolution. For instance, in Schistosomiasis, M1 induces a cytotoxic effect on the schistosomula, reducing the risk of hepatic fibrosis in the host [28]. In contrast, M2 macrophage-rich granulomas are generally induced by antigens secreted by schistosome eggs which trigger the release of CD4+ Th2 responses. This prevents acute mortality but enhances the development of liver fibrosis in the chronic stages [15, 28–30]. Interestingly, the differentiation of M2 macrophage depends on ROS but this is not the case for M1 macrophage [31, 32]. In S. japonicum infected mice, ROS generation was extensively observed in the liver and it was further demonstrated that S. japonicum eggs antigen stimulates the increase in ROS levels in M through the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 2 (NOX2) pathway [20].
This is in line with the theory that ROS generation during infection is stimulated by the activation of M and is critical for M2 macrophage differentiation. Again, available evidence indicates that S. japonicum antigen-associated ROS will preferentially propagate the differentiation of M2 M [33]. This is also true for S. mansoni, as the maximum intensity of ROS intermediates was observed in M2 macrophage-rich granulomas near S. mansoni eggs [34]. Eosinophil cells are associated with schistosome-induced granulomas derived from superoxide and hydroxyl radicals [35]. However, the consequences of ROS generation in schistosomiasis are largely still unknown.
2.2. Antioxidant Systems of Schistosomes
Adult Schistosoma worms can persist in the human body, an indication that the parasite is efficient in evading or resisting the host's immune response. Parasites in their early developmental stages (including the schistosomula and cercaria phases) are less exposed to redox insults than adult stages [36, 37]. During the schistosomula stage, the parasites have highly developed adequate protective mechanisms for their survival. These include the sequestration of the glycolipids of host erythrocytes at the tegument surface and the lowering of protein antigenic expression [38, 39]. Further, the parasites can enhance complement C3 degradation [40] and initiate a specific mechanism geared towards antioxidant defense buildup in the course of its development [41–43]. Adult Schistosoma worms encounter myriads of redox challenges derived from the digestion of blood, activated immune cells, and self-derived aerobic metabolism [44]. Thus, the parasite can survive over a long period by tolerating and detoxifying ROS in the human host, as seen in S. mansoni [44]. Specifically for S. mansoni, specialized improvement in its antioxidant defense system led to an increase in its capacity to decompose hydrogen peroxide (H2O2) in the vertebrate host [44–57]. In the bloodstream of the vertebrate host, adult S. mansoni can utilize continuous exposure to molecular oxygen (O2) to sustain egg production [48, 49], while maintaining cellular energy through oxidative phosphorylation [50, 51]. In S. mansoni parasites, various enzymes that metabolize ROS including glutathione reductase, SOD, cytochrome c peroxidase, and GPx have been identified [41]. In schistosomes, there is a developmental-based regulation of redox activity. This is expressed in the levels of antioxidant enzymes such as GPx, cytosolic SOD (CT-SOD), glutathione S-transferase (GST), and signal peptide-containing SOD (SP-SOD). These are minimally expressed in the schistosomula with a relatively elevated expression during schistosome development in the mammalian host, thus, enhancing the resistance action of parasites in the presence of ROS [58, 59]. Similarly, Mei et al. [52] showed that GPx activity was developmentally regulated by observing elevated enzyme activity levels in the extract of adult flukes compared to the larval stages. One of the most important antioxidant enzymes that protects the parasites against oxidant insults is thioredoxin reductase which modulates the intracellular redox environment due to its reductive effect on thioredoxin [53]. Other specific antioxidants detected in S. mansoni include 2-cysperoxiredoxins (Prx) 2 and 3 which enable organic peroxides and H2O2 decomposition by employing glutathione and thioredoxin as electron donors [54–56]. It is interesting to note that, apart from the developmental stages, schistosomes' redox regulation may also be enhanced by the sexes of adult worms. In S. mansoni worms, sexual preferences influence nutrient utilization which could regulate ROS generation and endogenous parasite O2 consumption. This is linked to the differential contribution of male and female adult worms to redox biology [45]. The nature of the detoxification between male and female adult S. mansoni flukes follows different mechanistic pathways. While the male redox homeostasis mechanism is geared towards reducing the generation rate, females are more geared towards detoxification than repression of ROS generation [57, 60, 61].
There is also an important selenoenzyme thioredoxin glutathione reductase (TGR) that prolongs the survival of schistosomes during redox insults in the mammalian host [62]. TGR is a chimeric flavo-enzyme that naturally occurs through the fusion of a thioredoxin reductase domain with a glutaredoxin domain [63, 64]. It mainly functions in the parasites' ROS detoxification pathway ensuring its survival and presents a unique drug target against the parasite [65]. However, irrespective of these extensive mechanisms, the antioxidant capacity of schistosomes is limited compared to the human host [66]. Apart from the lack of catalase [67], lower activity of schistosome GPx proteins in the presence of hydrogen peroxide has also been observed. Due to this, schistosome worms are more likely to be affected by oxidative insults than the human redox pathways. Hence the parasite's redox pathways are possible targets for drugs [68]. Recent studies have shown that auranofin, a gold-containing compound can significantly lower the burden of S. mansoni in mice by inhibiting TGR [69].
3. Markers of Oxidative Stress in Schistosoma infection
3.1. Lipid Peroxidation in Schistosome Infection
Lipid peroxidation occurs when the lipid component of the liver cell membrane gets damaged by excess ROS produced from the immunological response to Schistosoma infection. Byproducts of lipid peroxidation can damage the liver cells and adjacent tissues by releasing inflammatory molecules and causing cell death. Hepatic stellate cells (HSCs) and fibroblasts are activated by the continued generation of inflammatory cytokines and ROS, which promotes fibrogenesis [12, 70]. Collagen and extracellular matrix constituents are produced leading to PPF and scarring in the liver tissues. More serious liver diseases including portal hypertension and liver failure may eventually result from lipid peroxidation [12]. Studies have shown that treating S. mansoni not only lowers infection levels but also improves clinical symptoms of hepatosplenomegaly and progressive PPF [68, 71]. A study by Ewuzie et al. [67] suggests that people with current schistosome infection have a 2.5-fold higher chance of developing PFF than people who are not. Ultimately, this can lead to more severe liver conditions like portal hypertension and liver failure [67].
A study that sought to elucidate the possible contribution of products of lipid peroxidation in the hepatic pathophysiology of S. mansoni-infected individuals, revealed a significant elevation of plasma malondialdehyde (MDA) among the patients with schistosomiasis compared to the control group [72]. A positive correlation between plasma MDA levels and two hepatic fibrosis parameters: ultrasonography-graded PPF and serum hyaluronic acid has been reported [72]. An insignificant (10%) elevation in plasma MDA among patients infected with S. mansoni compared to the controls was reported in another study, which however employed a rather reduced sample size of 18 [58]. Contrary to the above findings, Eboumbou et al. [59] reported that hepatitis rather than Schistosoma infection was more likely to induce a higher level of MDA among co-infected individuals. In animal models, S. mansoni-infected mice showed higher levels of MDA, which was reduced after the administration of vitamin E and selenium [73]. Due to the scarcity of data and the inconsistencies in existing data, more studies are required to establish the exact role of MDA in Schistosoma infection.
Similar to the formation of MDA, conjugated dienes and lipid hydroperoxides, among other products, are formed during the oxidation of polyunsaturated fatty acids and other lipids by intermediate ROS [74]. The levels of erythrocyte-conjugated dienes were significantly elevated in S. mansoni individuals compared to the controls [58]. Further, schistosomiasis has been shown to enhance lipid peroxidation by reducing the activity of lecithin–cholesterol acyltransferase, a plasma enzyme essential for cholesterol esterification and the regulation of cell membrane lipid composition in schistosomiasis-infected patients [75]. In mice, excess lipid peroxide generation due to S. mansoni infection caused a reduction in antioxidant capacity leading to liver damage [34].
3.2. Markers of Oxidative DNA and Protein Damage in Schistosomiasis
The alterations to DNA molecules resulting from ROS overproduction could occur in diverse ways. These include mutations, modifications of purines and pyrimidine bases, and changes in the DNA sugar backbone. ROS can also produce breakages in either a single- or double-stranded DNA, leading to mutations such as deletions or translocations. Typically, ROS-associated oxidative DNA damage occurs by oxidizing purines and pyrimidines at apurinic/apyrimidinic (abasic) DNA sites [76]. Major ROS modifications of DNA that occur endogenously include 2,6-diamino-4-hydroxy-5-formamidopyrimidine and 8-oxo-7,8-dihydroguanine (8-oxoGua). An 8-hydroxy-7,8-dihydroguanyl radical is generated when a hydroxyl radical is added to the C8 position of a guanine ring. Subsequent oxidation or reduction of the 8-hydroxy-7,8-dihydroguanyl radical produces either an 8-oxoGua or the ring-opened hydroxy-5-formamidopyrimidine (FapyGua) [77, 78]. The frequency and extent of these DNA alterations correspond to the intensity and quality of oxidative stress and other accompanying factors. DNA glycosylases in humans are responsible for repairing damaged DNA caused by oxidative-stress-induced base changes such as the development of 8-oxo-2′-deoxyguanosine (8-oxo-dG). By cleaving the damaged base from the DNA backbone, this enzyme starts a base excision repair to prevent long-term genetic instability [79, 80].
In schistosomiasis, there is an increased deposition of eggs in the subepithelial tissues of infected subjects which causes chronic inflammation. This enhances ROS release and makes the DNA prone to oxidative stress lesions [22, 81]. Antigens released by S. haematobium eggs deposited in the bladder walls modulate the levels of TNF-α, which mediates inflammation in the mononuclear cells of the peripheral blood. The activation of NF-κB leads to iNOS-mediated overproduction of nitric oxide, an essential precursor to 8-nitroguanine and 8-oxodG production. Ma et al. [82] observed that, in cystitis and bladder cancer patients, S. haematobium-mediated inflammation increases the population of mutant stem cells. Activation of F-κB leads to DNA damage and subsequent tumorigenesis mediated by iNOS release [82]. In terms of the significant oxidative stress which results in DNA lesions, 8-oxo-dG formation is the most prominent mainly due to its pernicious nature as it is known to cause an impairment in the CpG island methylation of the promoter region of genes [22]. Salim et al. [17] observed an increased level of 8-hydroxy-2-deoxyguanosine (8-OHdG) in schistosomiasis-associated squamous cell carcinomas compared to nonschistosomal carcinomas. In line with that, the researchers observed a significant association of the 8-OHdG with an elevated expression of 8-oxoguanine-DNA-glycosylase and apurinic/apyrimidinic endonuclease which are known DNA repair genes [17]. Again, others have demonstrated the formation of 8-oxo-dG and 8-nitroguanine in tissues of S. haematobium-infected bladder cancer patients and further showed a strong correlation between S. haematobium infection and oxidative DNA damage [17, 82]. Thus, it may not be surprising that in 8-nitroguanine-positive schistosomiasis-associated bladder tumor cells, NF-κB was colocalized with an increased expression of iNOS [83].
3.3. Antioxidant Levels in Schistosomiasis
Naturally, during parasitic infections, various bioactive compounds counteract the progress of infection either as an antioxidant or through an oxidative insult which evades the parasite's antioxidant system [84]. Antioxidant enzymes play a significant role in reducing the severity and progression of schistosomiasis. Thus, with the recognition of schistosome infection as a state of oxidative stress, antioxidants that combat the myriads of ROS released into the host system are essential for recovery. Interestingly, in schistosomal infected baboons, the use of SOD (specifically S. mansoni CT-SOD and S. mansoni extracellular SOD) and GPx as vaccine components was able to reduce the number of worms compared to controls that were not vaccinated [85]. Again, the enzymatic antioxidant profile in mice models of schistosomiasis revealed a reduction in antioxidants such as catalase, SOD2, and glutathione levels [86, 87]. Others have shown that lipid peroxidation products and nitric oxide were increased with a concomitant decrease in antioxidants such as vitamin E, glutathione, SOD, and catalase activities in the spleen, kidney, and liver of mice infected with S. mansoni [88, 89].
4. Oxidative Stress and Cancer Risk in Schistosomiasis
Elevated levels of oxidative DNA damage markers such as 8-OHdG and elevated expression of DNA repair genes, 8-oxoguanine-DNA-glycosylase, and apurinic/apyrimidinic endonuclease have been observed in S. haematobium infections [17]. The involvement of ROS in carcinogenesis is mainly explained by two mechanisms. First is the ability of ROS to induce genetic mutations stemming from cellular injury. Second, is the effect of ROS on transcriptional factors and signal transducers. However, various factors such as the stress level and type of ROS involved might direct the specific mechanism it follows [90]. ROS are known to cause mutations in some telomere genes as well as damage to some cell cycle-related and tumor suppressor genes such as p53. Again, ROS is a major contributor to the activation of oncogenes (such as Fos and Jun), transcriptional factor NF-κB, and some protein kinases [91].
Schistosoma haematobium infection may cause chronic granulomatous cystitis, a precursor to the formation of squamous metaplasia of transitional epithelium leading to subsequent squamous cell carcinoma [92]. Indeed, there is a high incidence of squamous cell carcinoma Schistosomiasis endemic areas [93]. Specifically, Rambau, Chalya, and Jackson [92] reported that, among patients living in the western part of Tanzania, Schistosoma eggs were retrieved from about 44.9% of cases diagnosed with urogenital bladder cancer. Again, they reported that schistosomiasis-associated bladder cancer showed more aggressive behavior, as they quickly invaded the muscularis propria of the bladder [92].
Similarly, in the Sokoto region of northern Nigeria, a strong association of chronic schistosomiasis with bladder cancer was observed [94]. Others have shown that, in endemic areas, effective schistosomiasis treatment led to an attenuated occurrence of squamous cell carcinoma [87, 95]. The specific involvement of S. haematobium in squamous cell carcinoma could be traced to a myriad of mechanisms. For instance, the deposition of S. haematobium eggs on the bladder walls induces fibrosis with its attendant proliferation, hyperplasia, and metaplasia [96]. Again chronic urinary infection promotes the synthesis of nitrosamines from their urine precursors [97]. Therefore, it is not surprising that both the WHO and IARC have classified S. haematobium as a Class 1 carcinogen [9].
Interestingly, nitrosamines modulate the production of nitrogen dioxide radicals, hydroxyl radicals, and superoxide anion radicals, thereby, inducing oxidative stress [98]. Further, evidence shows that during S. haematobium infection, both the miracidia and adult schistosomes influence the production of high levels of urinary β-glucuronidase with the subsequent release of carcinogenic amines in urine [99]. Schistosomiasis can also induce the overexpression of cyclooxygenase 2 [100], a known promoter of oncogenesis [101]. Finally, in S. haematobium associated squamous cell carcinoma, multiple oncogenic mutations and abnormal expression of certain vital genes and proteins (e.g., c-erbB-2, p53, and epidermal growth factor receptors) have been observed [102, 103]. An illustration of the interaction between S. haematobium infection, oxidative stress, and the risk of bladder cancer is shown in Figure 1.
Figure 1.
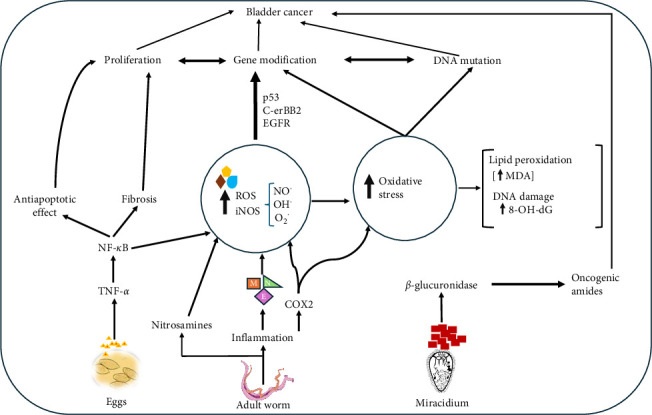
Proposed interplay between Schistosoma haematobium infection, oxidative stress, and the risk of bladder cancer.
Schistosoma haematobium eggs deposited in the bladder walls release antigens which promote TNF-α and NF-κB release. Activated NF-κB leads to iNOS-mediated increased NO, OH. and O2. radicals leading to oxidative stress. Adult worms cause inflammation and release nitrosamines, leading to further elevating ROS. Again, inflammation causes the accumulation of E, M, and N, which also contribute to iNOS and ROS production. Excessive ROS production leads to oxidative stress marked by increased lipid peroxidation (MDA, DNA, and protein oxidation (8-oxo-dG). This eventually influences gene mutation, DNA damage, proliferation, and cancer. Urinary glucuronidase produced by the miracidium and cyclooxygenase-2 (COX 2) generated through inflammation by adult worms could lead to the synthesis of oncogenic amides which could lead to cancer.
The data on S. haematobium's impact on carcinogenesis are the strongest, but it remains unclear if S. mansoni and other S. japonicum species possess a direct carcinogenic potential [104]. Available evidence shows that S. mansoni could generally act as a cofactor for a hepatic lesion in Hepatitis B and C virus infections, potentiating liver injury [105]. In S. mansoni infections, antigens released from schistosome eggs trapped in tissues influence the release of proto-oncogenes associated with hepatocellular carcinoma [106]. Among these are the transcriptional factors STAT3 and c-jun, both of which are crucial in the molecular pathway leading to inflammation and the development of cancer. Therefore, targeting these underlying pathways could be useful in providing therapeutics for schistosomiasis-related carcinogenesis [106].
5. Future Perspective and Conclusion
Schistosomiasis remains a public health burden, especially in less developed countries and the need to find a lasting solution is apparent. More worrying is the fact that some species such as S. haematobium is known to carry some risk for cancer development.
Various studies have established the importance of oxidative stress in schistosome infection. This is enabled through oxidative damage to DNA and lipid peroxidation and direct immunologic complications leading to fibrosis. There is a huge interplay between S. haematobium infection and bladder cancer mediated by oxidative stress. Thus, it is our opinion that treatment of these infections should be considered alongside the remediation and if possible, the reversal of the damages caused by the oxidative insults. In this review, we have elucidated that redox activity in the schistosomes is regulated based on its developmental stage and thus, the expression of antioxidant enzymes by the flukes is minimal in the schistosomula. Therefore, diagnosis and treatment of schistosomiasis in the early stages are crucial to avoid further complications. Therefore, drugs targeting the schistosomula may have maximum effect during the treatment of the infection in the early stages. Consequently, future studies should be directed at elucidating the mechanism needed to overcome the antioxidant systems of the parasite.
Comparatively, there is less data on the role ROS plays in modulating the molecular and metabolic activities involved in S. haematobium and S. japonicum infections compared to S. mansoni. Most attention has been given to oxidative DNA damage caused by S. mansoni species when compared to other human parasitic flukes such as S. haematobium and S. japonicum. Interestingly, it is S. haematobium that has been classified as a class I carcinogen by the WHO. It is, therefore, suggested that more studies focusing on the mechanisms connecting ROS with cancer development and progression should be carried out.
Acknowledgments
The authors have nothing to report.
Contributor Information
Justice Afrifa, Email: jafrifa@ucc.edu.gh.
Eric Gyamerah Ofori, Email: eric.gyamerah@ucc.edu.gh.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Ethics Statement
The authors have nothing to report.
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
J.A. and E.G.O developed the idea. J.A., E.G.O, Y.K.O, K.K.A., R.D.S., I.W.N-F., and R.A. wrote and reviewed the manuscript. J.A. and E.G.O designed the figure. All authors have read and approved the final manuscript.
Funding
No funding was received by the authors for this study.
References
- 1.GROUP R. U. S. World Health Organization; 1995. Identification of High-Risk Communities for Schistosomiasis in Africa: A Multicountry Study. [Google Scholar]
- 2.Hotez P. J., Alvarado M., Basáñez M.-G., et al. The Global Burden of Disease Study 2010: Interpretation and Implications for the Neglected Tropical Diseases. PLoS Neglected Tropical Diseases . 2014;8 doi: 10.1371/journal.pntd.0002865.e2865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mcmanus J. F., Costa K., Ng H. C., et al. Time-Series Transects of Deglacial Circulation Changes in the Deep North Atlantic Ocean. AGU Fall Meeting Abstracts . 2018. pp. PP13A–08.
- 4.Sturrock R. F. Transactions of The Royal Society of Tropical Medicine and Hygiene . Vol. 92. World Health Organization; 1998. Guidelines for the Evaluation of Soil-Transmitted Helminthiasis and Schistosomiasis at Community Level: A Guide for Managers of Control Programmes; pp. 470–471. [Google Scholar]
- 5.Steinmann P., Keiser J., Bos R., Tanner M., Utzinger J. Schistosomiasis and Water Resources Development: Systematic Review, Meta-Analysis, and Estimates of People at Risk. The Lancet Infectious Diseases . 2006;6(7):411–425. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- 6.Gryseels B., Polman K., Clerinx J., Kestens L. Human Schistosomiasis. The Lancet . 2006;368(9541):1106–1118. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- 7.Zhang R., Takahashi S., Orita S.-I., et al. p53 Gene Mutations in Rectal Cancer Associated With Schistosomiasis Japonica in Chinese Patients. Cancer Letters . 1998;131(2):215–221. doi: 10.1016/S0304-3835(98)00154-2. [DOI] [PubMed] [Google Scholar]
- 8.Madbouly K. M., Senagore A. J., Mukerjee A., et al. Colorectal Cancer in a Population With Endemic Schistosoma mansoni: Is This an at-Risk Population? International Journal of Colorectal Disease . 2007;22(2):175–181. doi: 10.1007/s00384-006-0144-3. [DOI] [PubMed] [Google Scholar]
- 9.WHO/IARC. Summary of Data Reported and Evaluation . Lyon, Franc: 1994. IARC Monographs on the Evaluation of Carcinogenic Risk to Humans. Schistosomas, Liver Flukes and Helicobacter Pylori. [PMC free article] [PubMed] [Google Scholar]
- 10.Mnkugwe R. H., Minzi O. S., Kinung’hi S. M., Kamuhabwa A. A., Aklillu E., Diemert D. J. Prevalence and Correlates of Intestinal Schistosomiasis Infection Among School-Aged Children in North-Western Tanzania. PLOS ONE . 2020;15(2) doi: 10.1371/journal.pone.0228770.e0228770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colley D. G., Bustinduy A. L., Secor W. E., King C. H. Human Schistosomiasis. The Lancet . 2014;383(9936):2253–2264. doi: 10.1016/S0140-6736(13)61949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masamba P., Kappo A. P. Immunological and Biochemical Interplay between Cytokines, Oxidative Stress and Schistosomiasis. International Journal of Molecular Sciences . 2021;22(13) doi: 10.3390/ijms22137216.7216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jenkins S. J., Hewitson J. P., Jenkins G. R., Mountford A. P. Modulation of the Host’s Immune Response by Schistosome Larvae. Parasite Immunology . 2005;27(10-11):385–393. doi: 10.1111/j.1365-3024.2005.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janssen L., Silva Santos G. L., Muller H. S., Vieira A. R. A., De Campos T. A., De Paulo Martins V. Schistosome-Derived Molecules as Modulating Actors of the Immune System and Promising Candidates to Treat Autoimmune and Inflammatory Diseases. Journal of Immunology Research . 2016;2016 doi: 10.1155/2016/5267485.5267485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar R., Mickael C., Chabon J., et al. The Causal Role of IL-4 and IL-13 in Schistosoma mansoni Pulmonary Hypertension. American Journal of Respiratory and Critical Care Medicine . 2015;192(8):998–1008. doi: 10.1164/rccm.201410-1820OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosin M. P., Anwar W. A., Ward A. J. Inflammation, Chromosomal Instability, and Cancer: The Schistosomiasis Model. Cancer Research . 1994;54:1929s–1933s. [PubMed] [Google Scholar]
- 17.Salim E. I., Morimura K., Menesi A., El-Lity M., Fukushima S., Wanibuchi H. Elevated Oxidative Stress and DNA Damage and Repair Levels in Urinary Bladder Carcinomas Associated With Schistosomiasis. International Journal of Cancer . 2008;123(3):601–608. doi: 10.1002/ijc.23547. [DOI] [PubMed] [Google Scholar]
- 18.Ohshima H., Sawa T., Akaike T. 8-Nitroguanine, a Product of Nitrative DNA Damage Caused by Reactive Nitrogen Species: Formation, Occurrence, and Implications in Inflammation and Carcinogenesis. Antioxidants & Redox Signaling . 2006;8(5-6):1033–1045. doi: 10.1089/ars.2006.8.1033. [DOI] [PubMed] [Google Scholar]
- 19.Mostafa M. H., Sheweita S. A., O’Connor P. J. Relationship Between Schistosomiasis and Bladder Cancer. Clinical Microbiology Reviews . 1999;12(1):97–111. doi: 10.1128/CMR.12.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu Y., Wang J., Wang X., et al. Schistosome Eggs Stimulate Reactive Oxygen Species Production to Enhance M2 Macrophage Differentiation and Promote Hepatic Pathology in Schistosomiasis. PLOS Neglected Tropical Diseases . 2021;15(8) doi: 10.1371/journal.pntd.0009696.e0009696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frei B. Reactive Oxygen Species and Antioxidant Vitamins: Mechanisms of Action. The American Journal of Medicine . 1994;97(3):S5–S13. doi: 10.1016/0002-9343(94)90292-5. [DOI] [PubMed] [Google Scholar]
- 22.Nishida N., Arizumi T., Takita M., et al. Reactive Oxygen Species Induce Epigenetic Instability Through the Formation of 8-Hydroxydeoxyguanosine in Human Hepatocarcinogenesis. Digestive Diseases . 2013;31(5-6):459–466. doi: 10.1159/000355245. [DOI] [PubMed] [Google Scholar]
- 23.Pizzino G., Irrera N., Cucinotta M., et al. Oxidative Stress: Harms and Benefits For Human Health. Oxidative Medicine and Cellular Longevity . 2017;2017(1):13. doi: 10.1155/2017/8416763.8416763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schirmer R., Schöllhammer T., Eisenbrand G., Krauth-Siegel R. Oxidative Stress as a Defense Mechanism against Parasitic Infections. Free Radical Research Communications . 1987;3:3–12. doi: 10.3109/10715768709069763. [DOI] [PubMed] [Google Scholar]
- 25.West A. P., Brodsky I. E., Rahner C., et al. TLR Signalling Augments Macrophage Bactericidal Activity Through Mitochondrial ROS. Nature . 2011;472(7344):476–480. doi: 10.1038/nature09973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nieto N., Friedman S. L., Cederbaum A. I. Cytochrome P450 2E1-Derived Reactive Oxygen Species Mediate Paracrine Stimulation of Collagen I Protein Synthesis by Hepatic Stellate Cells. Journal of Biological Chemistry(2002 . 2002;277(12):9853–9864. doi: 10.1074/jbc.M110506200. [DOI] [PubMed] [Google Scholar]
- 27.De Minicis S., Bataller R., Brenner D. A. NADPH Oxidase in the Liver: Defensive, Offensive, or Fibrogenic? Gastroenterology,(2006) . 2006;131(1):272–275. doi: 10.1053/j.gastro.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 28.Barron L., Wynn T. A. Macrophage Activation Governs Schistosomiasis-Induced Inflammation and Fibrosis. European Journal of Immunology . 2011;41(9):2509–2514. doi: 10.1002/eji.201141869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmed S. F., Oswald I. P., Caspar P., et al. Developmental Differences Determine Larval Susceptibility to Nitric Oxide-Mediated Killing in a Murine Model of Vaccination Against Schistosoma mansoni. Infection and Immunity . 1997;65(1):219–226. doi: 10.1128/iai.65.1.219-226.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noël W., Raes G., Ghassabeh G. H., De Baetselier P., Beschin A. Alternatively Activated Macrophages During Parasite Infections. Trends in Parasitology . 2004;20(3):126–133. doi: 10.1016/j.pt.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Covarrubias A., Byles V., Horng T. ROS Sets the Stage For Macrophage Differentiation. Cell Research . 2013;23(8):984–985. doi: 10.1038/cr.2013.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y., Choksi S., Chen K., Pobezinskaya Y., Linnoila I., Liu Z.-G. ROS Play a Critical Role in the Differentiation of Alternatively Activated Macrophages and the Occurrence of Tumor-Associated Macrophages. Cell Research . 2013;23(7):898–914. doi: 10.1038/cr.2013.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu J., Xu Z., Chen X., et al. Parasitic Antigens Alter Macrophage Polarization During Schistosoma Japonicum Infection in Mice. Parasites & Vectors . 2014;7(1):1–9. doi: 10.1186/1756-3305-7-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdallahi O. M. S., Hanna Séphane, De Reggi M., Gharib B. Visualization of Oxygen Radical Production in Mouse Liver in Response to Infection With Schistosoma mansoni. Liver . 1999;19(6):495–500. doi: 10.1111/j.1478-3231.1999.tb00082.x. [DOI] [PubMed] [Google Scholar]
- 35.Mccormick M. L., Metwali A., Railsback M. A., Weinstock J. V., Britigan B. E. Eosinophils From Schistosome-Induced Hepatic Granulomas Produce Superoxide and Hydroxyl Radical. The Journal of Immunology . 1996;157(11):5009–5015. doi: 10.4049/jimmunol.157.11.5009. [DOI] [PubMed] [Google Scholar]
- 36.Esparza I., Ruppel A., Mestan J., Krammer P. H. Preactivation of Macrophages in Mice Acutely Infected With Schistosoma Mansoni. Immunobiology . 1988;177(2):105–119. doi: 10.1016/S0171-2985(88)80032-9. [DOI] [PubMed] [Google Scholar]
- 37.Pelletier M., Lepow T. S., Billingham L. K., Murphy M. P., Siegel R. M. Seminars in Immunology . 6. Vol. 24. Elsevier; 2012. New Tricks From an Old Dog: Mitochondrial Redox Signaling in Cellular Inflammation; pp. 384–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goldring O. L., Clegg J. A., Smithers S. R., Terry R. J. Acquisition of Human Blood Group Antigens by Schistosoma mansoni. Clinical and Experimental Immunology . 1976;26(1):181–187. [PMC free article] [PubMed] [Google Scholar]
- 39.Skelly P. J., Alan Wilson R. Making Sense of the Schistosome Surface. Advances in Parasitology . 2006;63:185–284. doi: 10.1016/S0065-308X(06)63003-0. [DOI] [PubMed] [Google Scholar]
- 40.Braschi S., Wilson R. A. Proteins Exposed at the Adult Schistosome Surface Revealed by Biotinylation. Molecular & Cellular Proteomics . 2006;5(2):347–356. doi: 10.1074/mcp.M500287-MCP200. [DOI] [PubMed] [Google Scholar]
- 41.Mkoji G. M., Smith J. M., Prichard R. K. Antioxidant Systems in Schistosoma mansoni: Correlation Between Susceptibility to Oxidant Killing and the Levels of Scavengers of Hydrogen Peroxide and Oxygen Free Radicals. International Journal for Parasitology . 1988;18(5):661–666. doi: 10.1016/0020-7519(88)90101-4. [DOI] [PubMed] [Google Scholar]
- 42.Nare B., Smith J. M., Prichard R. K. Schistosoma mansoni: Levels of Antioxidants and Resistance to Oxidants Increase During Development. Experimental Parasitology . 1990;70(4):389–397. doi: 10.1016/0014-4894(90)90122-S. [DOI] [PubMed] [Google Scholar]
- 43.Mei H., LoVerde P. T. Schistosoma mansoni:The Developmental Regulation and Immunolocalization of Antioxidant Enzymes. Experimental Parasitology . 1997;86(1):69–78. doi: 10.1006/expr.1997.4150. [DOI] [PubMed] [Google Scholar]
- 44.Oliveira M. P., Correa Soares J. B. R., Oliveira M. F., Liesa M. Sexual Preferences in Nutrient Utilization Regulate Oxygen Consumption and Reactive Oxygen Species Generation in Schistosoma mansoni: Potential Implications for Parasite Redox Biology. PLOS ONE . 2016;11(7) doi: 10.1371/journal.pone.0158429.e0158429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oliveira M. F., Timm B. L., Machado E. A., et al. On the pro-Oxidant Effects of Haemozoin. FEBS Letters . 2002;512(1–3):139–144. doi: 10.1016/S0014-5793(02)02243-3. [DOI] [PubMed] [Google Scholar]
- 46.Oliveira M. F., d’Avila J. C. P., Tempone Aônio J., et al. Inhibition of Heme Aggregation by Chloroquine Reduces Schistosoma mansoni Infection. The Journal of Infectious Diseases . 2004;190(4):843–852. doi: 10.1086/422759. [DOI] [PubMed] [Google Scholar]
- 47.Oliveira M. F., D’avila J. C., Torres C. R., et al. Haemozoin in Schistosoma mansoni. Molecular and Biochemical Parasitology . 2000;111(1):217–221. doi: 10.1016/S0166-6851(00)00299-1. [DOI] [PubMed] [Google Scholar]
- 48.Seed J. L., Boff M., Bennett J. L. Phenol Oxidase Activity: Induction in Female Schistosomes by In vitro Incubation. The Journal of Parasitology . 1978;64(2):283–289. doi: 10.2307/3279674. [DOI] [PubMed] [Google Scholar]
- 49.SChiller E. L., Bueding E., Turner V. M., Fisher J. Aerobic and Anaerobic Carbohydrate Metabolism and Egg Production of Schistosoma mansoni in Vitro. The Journal of Parasitology . 1975;61(3):385–389. [PubMed] [Google Scholar]
- 50.Coles G. Oxidative Phosphorylation in Adult Schistosoma mansoni. Nature . 1972;240:488–489. doi: 10.1038/240488a0. [DOI] [PubMed] [Google Scholar]
- 51.Huang S. C.-C., Freitas T. C., Amiel E., et al. Fatty Acid Oxidation Is Essential for Egg Production by the Parasitic Flatworm Schistosoma mansoni. PLoS Pathogens . 2012;8(10) doi: 10.1371/journal.ppat.1002996.e1002996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mei H., Thakur A., Schwartz J., Lo Verde P. T. Expression and Characterization of Glutathione Peroxidase Activity in the Human Blood Fluke Schistosoma mansoni. Infection and Immunity . 1996;64:4299–4306. doi: 10.1128/iai.64.10.4299-4306.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alger H. M., Sayed A. A., Stadecker M. J., Williams D. L. Molecular and Enzymatic Characterisation of Schistosoma mansoni Thioredoxin. International Journal for Parasitology . 2002;32:1285–1292. doi: 10.1016/s0020-7519(02)00108-x. [DOI] [PubMed] [Google Scholar]
- 54.Kwatia M. A., Botkin D. J., Williams D. L. Molecular and Enzymatic Characterization of Schistosoma mansoni Thioredoxin Peroxidase. Journal of Parasitology . 2000;86(5):908–915. doi: 10.1645/0022-3395(2000)086[0908:MAECOS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 55.Sayed A. A., Williams D. L. Biochemical Characterization of 2-Cys Peroxiredoxins From Schistosoma mansoni. Journal of Biological Chemistry . 2004;279(25):26159–26166. doi: 10.1074/jbc.M401748200. [DOI] [PubMed] [Google Scholar]
- 56.Sayed A. A., Cook S. K., Williams D. L. Redox Balance Mechanisms in Schistosoma mansoni Rely on Peroxiredoxins and Albumin and Implicate Peroxiredoxins as Novel Drug Targets. Journal of Biological Chemistry . 2006;281(25):17001–17010. doi: 10.1074/jbc.M512601200. [DOI] [PubMed] [Google Scholar]
- 57.Zelck U., Von Janowsky B. Antioxidant Enzymes in Intramolluscan Schistosoma mansoni and ROS-Induced Changes in Expression. Parasitology . 2004;128:493–501. doi: 10.1017/s0031182004004895. [DOI] [PubMed] [Google Scholar]
- 58.Facundo H. T. F., Brandt C. T., Owen J. S., Lima V. L. M. Elevated Levels of Erythrocyte-Conjugated Dienes Indicate Increased Lipid Peroxidation in Schistosomiasis Mansoni Patients. Brazilian Journal of Medical and Biological Research . 2004;37(7):957–962. doi: 10.1590/S0100-879X2004000700003. [DOI] [PubMed] [Google Scholar]
- 59.Eboumbou C., Steghens J.-P., Abdallahi O. M. S., et al. Circulating Markers of Oxidative Stress and Liver Fibrosis in Sudanese Subjects at Risk of Schistosomiasis and Hepatitis. Acta Tropica . 2005;94(2):99–106. doi: 10.1016/j.actatropica.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 60.Aragon A. D., Imani R. A., Blackburn V. R., Cunningham C. Microarray Based Analysis of Temperature and Oxidative Stress Induced Messenger RNA in Schistosoma mansoni. Molecular and Biochemical Parasitology . 2008;162(2):134–141. doi: 10.1016/j.molbiopara.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Messerli S. M., Morgan W., Birkeland S. R., et al. Nitric Oxide-Dependent Changes in Schistosoma mansoni Gene Expression. Molecular and Biochemical Parasitology . 2006;150:p. 367. doi: 10.1016/j.molbiopara.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tripathi T., Chetri P. B. Potent Inhibitors of Thioredoxin Glutathione Reductase: Grail of Anti-Schistosome Drug Within Reach? ACS Infectious Diseases . 2020;6(5):893–895. doi: 10.1021/acsinfecdis.0c00072. [DOI] [PubMed] [Google Scholar]
- 63.Sun Q.-A., Kirnarsky L., Sherman S., Gladyshev V. N. Selenoprotein Oxidoreductase With Specificity for Thioredoxin and Glutathione Systems. Proceedings of the National Academy of Sciences; 2001; pp. 3673–3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alger H. M., Williams D. L. The Disulfide Redox System of Schistosoma mansoni and the Importance of a Multifunctional Enzyme, Thioredoxin Glutathione Reductase. Molecular and Biochemical Parasitology . 2002;121(1):129–139. doi: 10.1016/S0166-6851(02)00031-2. [DOI] [PubMed] [Google Scholar]
- 65.Angelucci F., Sayed A. A., Williams D. L., et al. Inhibition of Schistosoma mansoni Thioredoxin-Glutathione Reductase by Auranofin. Journal of Biological Chemistry . 2009;284(42):28977–28985. doi: 10.1074/jbc.M109.020701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang H.-H., Rigouin C., Williams D. L. The Redox Biology of Schistosome Parasites and Applications for Drug Development. Current Pharmaceutical Design . 2012;18(24):3595–3611. doi: 10.2174/138161212801327220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ewuzie A., Wilburn L., Thakrar D. B., Roberts N., Malouf R., Chami G. F. medRxiv; 2024. Mekongi Infection Status and Intensity With Periportal Fibrosis: A Systematic Review and Meta-Analysis. [DOI] [PubMed] [Google Scholar]
- 68.Silva P. C. V., Leal T. V., Domingues A. L. C. Treatment and Education Reduce the Severity of Schistosomiasis Periportal Fibrosis. Revista DA Sociedade Brasileira De Medicina Tropical . 2013;46(4):472–477. doi: 10.1590/0037-8682-0110-2013. [DOI] [PubMed] [Google Scholar]
- 69.Kuntz A. N., Davioud-Charvet E., Sayed A. A., et al. Thioredoxin Glutathione Reductase From Schistosoma mansoni: An Essential Parasite Enzyme and a Key Drug Target. PLoS Medicine . 2007;4(6) doi: 10.1371/journal.pmed.0040206.e206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Higashi T., Friedman S. L., Hoshida Y. Hepatic Stellate Cells as Key Target in Liver Fibrosis. Advanced Drug Delivery Reviews . 2017;121:27–42. doi: 10.1016/j.addr.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Voieta I., De Queiroz L. C., Andrade L. M., et al. Imaging Techniques and Histology in the Evaluation of Liver Fibrosis in Hepatosplenic Schistosomiasis Mansoni in Brazil: A Comparative Study. Memórias Do Instituto Oswaldo Cruz . 2010;105(4):414–421. doi: 10.1590/S0074-02762010000400011. [DOI] [PubMed] [Google Scholar]
- 72.Aziz I. A., Yacoub M., Rashid L., Solieman A. Malondialdehyde; Lipid Peroxidation Plasma Biomarker Correlated With Hepatic Fibrosis in Human Schistosoma mansoni Infection. Acta Parasitologica . 2015;60:735–742. doi: 10.1515/ap-2015-0105. [DOI] [PubMed] [Google Scholar]
- 73.Kadry S. M., Mohamed A. M., Farrag E. M., Fayed D. B. Influence of Some Micronutrients and Citharexylum Quadrangular Extract Against Liver Fibrosis in Schistosoma mansoni Infected Mice. African Journal of Pharmacy and Pharmacology . 2013;7(38):2628–2638. doi: 10.5897/AJPP12.620. [DOI] [Google Scholar]
- 74.Halliwell B., Gutteridge J. M. Free Radicals in Biology and Medicine . USA: Oxford University Press; 2015. [Google Scholar]
- 75.Silva C. A. D., Oliveira K. F. D., Carvalho V. C. O. D., Domigues A. L. C., Brandt C. T., Lima V. L. D. M. Efeito De Tratamento Cirúrgico Sobre a Atividade DA Enzima Hepática Lecitina: Colesterol Aciltransferase (LCAT) NA Esquistossomose Mansônica. Acta Cirurgica Brasileira . 2002;17(suppl 1):28–30. doi: 10.1590/S0102-86502002000700008. [DOI] [Google Scholar]
- 76.Kryston T. B., Georgiev A. B., Pissis P., Georgakilas A. G. Role of Oxidative Stress and DNA Damage in Human Carcinogenesis. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis . 2011;711(1-2):193–201. doi: 10.1016/j.mrfmmm.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 77.Altieri F., Grillo C., Maceroni M., Chichiarelli S. DNA Damage and Repair: From Molecular Mechanisms to Health Implications. Antioxidants & Redox Signaling . 2008;10(5):891–938. doi: 10.1089/ars.2007.1830. [DOI] [PubMed] [Google Scholar]
- 78.Spassky A., Angelov D. Influence of the Local Helical Conformation on the Guanine Modifications Generated from One-Electron DNA Oxidation. Biochemistry . 1997;36(22):6571–6576. doi: 10.1021/bi962761d. [DOI] [PubMed] [Google Scholar]
- 79.Jiranusornkul S., Laughton C. A. Destabilization of DNA Duplexes by Oxidative Damage at Guanine: Implications for Lesion Recognition and Repair. Journal of the Royal Society Interface . 2008;5(suppl_3):191–198. doi: 10.1098/rsif.2008.0304.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pouget J.-P., Douki T., Richard M.-J., Cadet J. DNA Damage Induced in Cells by γ and UVA Radiation As Measured by HPLC/GC−MS and HPLC212;EC and Comet Assay. Chemical Research in Toxicology . 2000;13(7):541–549. doi: 10.1021/tx000020e. [DOI] [PubMed] [Google Scholar]
- 81.Gentile J. M., Gentile G. J. Implications for the Involvement of the Immune System in Parasite-Associated Cancers. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis . 1994;305(2):315–320. doi: 10.1016/0027-5107(94)90251-8. [DOI] [PubMed] [Google Scholar]
- 82.Ma N., Thanan R., Kobayashi H., et al. Nitrative DNA Damage and Oct3/4 Expression in Urinary Bladder Cancer With Schistosoma Haematobium Infection. Biochemical and Biophysical Research Communications . 2011;414(2):344–349. doi: 10.1016/j.bbrc.2011.09.073. [DOI] [PubMed] [Google Scholar]
- 83.Wamachi A. N., Mayadev J. S., Mungai P. L., et al. Increased Ratio of Tumor Necrosis Factor013;α to Interleukin-10 Production Is Associated With Schistosoma haematobium –Induced Urinary-Tract Morbidity. The Journal of Infectious Diseases . 2004;190(11):2020–2030. doi: 10.1086/425579. [DOI] [PubMed] [Google Scholar]
- 84.Ghosh S., Das A. K., Sarkar P., Sil P. C. Oxidative Stress in Schistosomiasis, Echinococcosis, and Trypanosomiasis: A Therapeutic Approach. In: Brahmachari G., editor. Discovery and Development of Therapeutics From Natural Products Against Neglected Tropical Diseases . Elsevier; 2019. pp. 219–239. [Google Scholar]
- 85.Carvalho-Queiroz C., Nyakundi R., Ogongo P., et al. Protective Potential of Antioxidant Enzymes as Vaccines for Schistosomiasis in a Non-Human Primate Model. Frontiers in Immunology . 2015;6 doi: 10.3389/fimmu.2015.00273.273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.De Oliveira R. B., Senger M. R., Vasques L. M., et al. Schistosoma mansoni Infection Causes Oxidative Stress and Alters Receptor for Advanced Glycation Endproduct (RAGE) and Tau Levels in Multiple Organs in Mice. International Journal for Parasitology . 2013;43(5):371–379. doi: 10.1016/j.ijpara.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 87.Felix A. S., Soliman A. S., Khaled H., et al. The Changing Patterns of Bladder Cancer in Egypt Over the Past 26 Years. Cancer Causes & Control . 2008;19(4):421–429. doi: 10.1007/s10552-007-9104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Al-Olayan E. M., El-Khadragy M. F., Alajmi R. A., et al. Ceratonia siliqua Pod Extract Ameliorates Schistosoma mansoni-Induced Liver Fibrosis and Oxidative Stress. BMC Complementary and Alternative Medicine . 2016;16(1) doi: 10.1186/s12906-016-1389-1.434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.El-Sokkary G. H., Omar H. M., Hassanein A.-F. M. M., Cuzzocrea S., Reiter R. J. Melatonin Reduces Oxidative Damage and Increases Survival of Mice Infected With Schistosoma mansoni. Free Radical Biology and Medicine . 2002;32(4):319–332. doi: 10.1016/S0891-5849(01)00753-5. [DOI] [PubMed] [Google Scholar]
- 90.Matés J. M., Pérez-Gómez C., Núñez de Castro I. Antioxidant Enzymes and Human Diseases. Clinical Biochemistry . 1999;32(8):595–603. doi: 10.1016/s0009-9120(99)00075-2. [DOI] [PubMed] [Google Scholar]
- 91.Noda N., Wakasugi H. Cancer and Oxidative Stress. Japan Medical Association Journal . 2001;44:535–539. [Google Scholar]
- 92.Rambau P. F., Chalya P. L., Jackson K. Schistosomiasis and Urinary Bladder Cancer in North Western Tanzania: A Retrospective Review of 185 Patients. Infectious Agents and Cancer . 2013;8(1):1–6. doi: 10.1186/1750-9378-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Abol-enein H. Infection: Is It a Cause of Bladder Cancer? Scandinavian Journal of Urology and Nephrology . 2008;42(sup218):79–84. doi: 10.1080/03008880802325309. [DOI] [PubMed] [Google Scholar]
- 94.Mungadi I. A., Malami S. A. Urinary Bladder Cancer and Schistosomiasis in North-Western Nigeria. West African Journal of Medicine . 2007;26(3):226–229. doi: 10.4314/wajm.v26i3.28315. [DOI] [PubMed] [Google Scholar]
- 95.Zaghloul M. S., Nouh A., Moneer M., El-Baradie M., Nazmy M., Younis A. Time-Trend in Epidemiological and Pathological Features of Schistosoma-Associated Bladder Cancer. Journal of the Egyptian National Cancer Institute . 2008;20:168–174. [PubMed] [Google Scholar]
- 96.Rosin M. P., El Din Zaki S. S., Ward A. J., Anwar W. A. Involvement of Inflammatory Reactions and Elevated Cell Proliferation in the Development of Bladder Cancer in Schistosomiasis Patients. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis . 1994;305(2):283–292. doi: 10.1016/0027-5107(94)90248-8. [DOI] [PubMed] [Google Scholar]
- 97.Hicks R. M., Ismail M. M., Walters C. L., Beecham P. T., Rabie M. F., El Alamy M. A. Association of Bacteriuria and Urinary Nitrosamine Formation With Schistosoma Haematobium Infection in the Qalyub Area of Egypt. Transactions of the Royal Society of Tropical Medicine and Hygiene . 1982;76(4):519–527. doi: 10.1016/0035-9203(82)90153-5. [DOI] [PubMed] [Google Scholar]
- 98.Risch H. A. Etiology of Pancreatic Cancer, With a Hypothesis concerning the Role of N-Nitroso Compounds and Excess Gastric Acidity. Journal of the National Cancer Institute . 2003;95:948–960. doi: 10.1093/jnci/95.13.948. [DOI] [PubMed] [Google Scholar]
- 99.FRIPP P. J. Schistosomiasis and Urinary β-Glucuronidase Activity. Nature . 1960;188(4749):507–508. doi: 10.1038/188507a0. [DOI] [PubMed] [Google Scholar]
- 100.Hammam O. A., Aziz A. A., Roshdy M. S., Abdel Hadi A. M. Possible Role of Cyclooxygenase-2 in Schistosomal and Non-Schistosomal-Associated Bladder Cancer. Medscape Journal of Medicine . 2008;10(3):60–60. [PMC free article] [PubMed] [Google Scholar]
- 101.Oshima M., Dinchuk J. E., Kargman S. L., et al. Suppression of Intestinal Polyposis in ApcΔ716 Knockout Mice by Inhibition of Cyclooxygenase 2 (COX-2) Cell . 1996;87(5):803–809. doi: 10.1016/S0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- 102.Cheever A. W. Schistosomiasis and Neoplasia. JNCI: Journal of the National Cancer Institute . 1978;61(1):13–18. doi: 10.1093/jnci/61.1.13. [DOI] [PubMed] [Google Scholar]
- 103.Lucas S. B. Squamous Cell Carcinoma of the Bladder and Schistosomiasis. East African Medical Journal . 1982;59(5):345–351. [PubMed] [Google Scholar]
- 104.El-Tonsy M. M., Hussein H. M., Helal T. E.-S., Tawfik R. A., Koriem K. M., Hussein H. M. Human Schistosomiasis Mansoni Associated With Hepatocellular Carcinoma in Egypt: Current Perspective. Journal of Parasitic Diseases . 2016;40(3):976–980. doi: 10.1007/s12639-014-0618-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Toda K. S., Kikuchi L., Chagas A. L., et al. Hepatocellular Carcinoma Related to Schistosoma mansoni Infection: Case Series and Literature Review. Journal of Clinical and Translational Hepatology . 2015;3(4):p. 260. doi: 10.14218/JCTH.2015.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Roderfeld M., Padem S., Lichtenberger J., et al. Schistosoma mansoni Egg–Secreted Antigens Activate Hepatocellular Carcinoma–Associated Transcription Factors c-Jun and STAT3 in Hamster and Human Hepatocytes. Hepatology . 2020;72(2):626–641. doi: 10.1002/hep.30192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


