Abstract
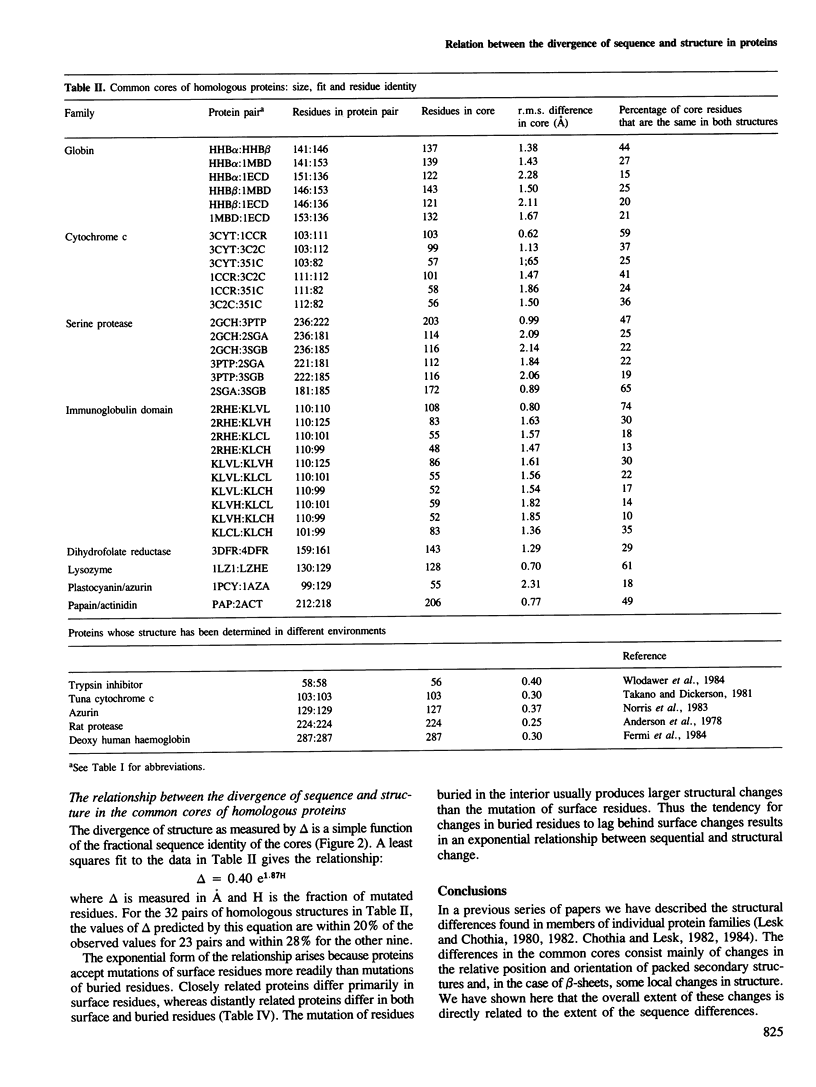
Homologous proteins have regions which retain the same general fold and regions where the folds differ. For pairs of distantly related proteins (residue identity approximately 20%), the regions with the same fold may comprise less than half of each molecule. The regions with the same general fold differ in structure by amounts that increase as the amino acid sequences diverge. The root mean square deviation in the positions of the main chain atoms, delta, is related to the fraction of mutated residues, H, by the expression: delta(A) = 0.40 e1.87H.
Full text
PDF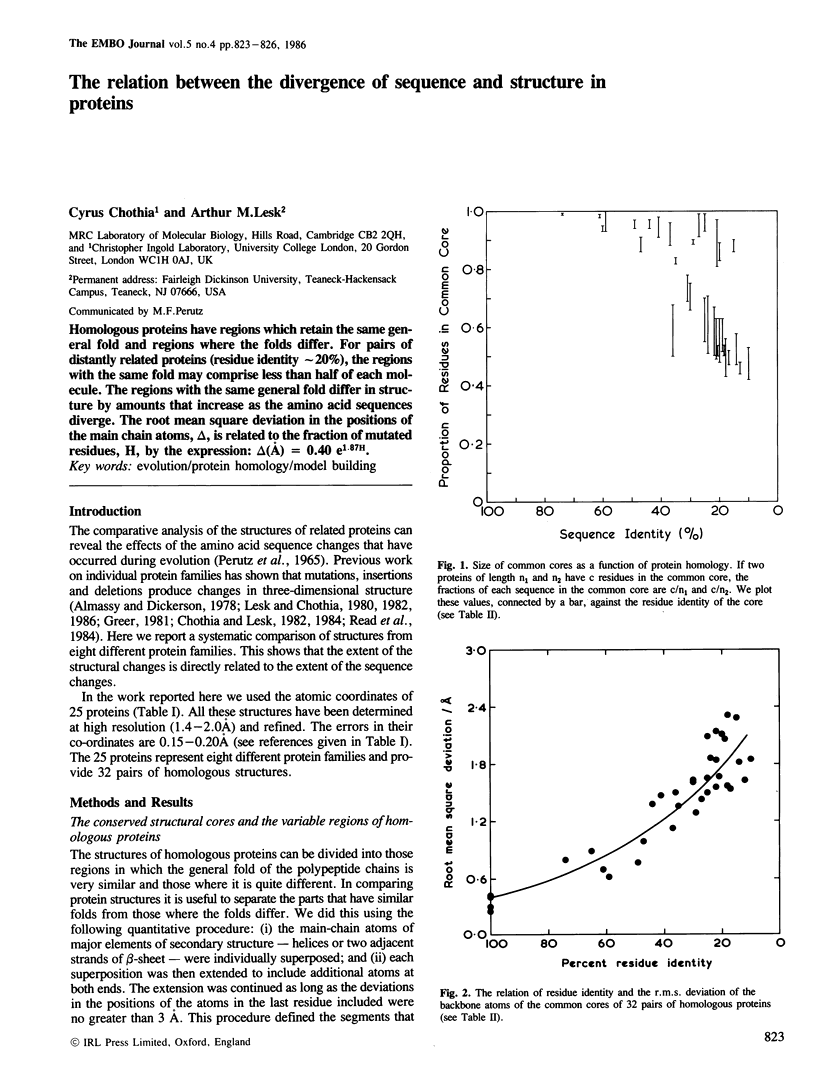


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almassy R. J., Dickerson R. E. Pseudomonas cytochrome c551 at 2.0 A resolution: enlargement of the cytochrome c family. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2674–2678. doi: 10.1073/pnas.75.6.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artymiuk P. J., Blake C. C. Refinement of human lysozyme at 1.5 A resolution analysis of non-bonded and hydrogen-bond interactions. J Mol Biol. 1981 Nov 15;152(4):737–762. doi: 10.1016/0022-2836(81)90125-x. [DOI] [PubMed] [Google Scholar]
- Baker E. N. Structure of actinidin, after refinement at 1.7 A resolution. J Mol Biol. 1980 Aug 25;141(4):441–484. doi: 10.1016/0022-2836(80)90255-7. [DOI] [PubMed] [Google Scholar]
- Bernstein F. C., Koetzle T. F., Williams G. J., Meyer E. F., Jr, Brice M. D., Rodgers J. R., Kennard O., Shimanouchi T., Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1977 May 25;112(3):535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- Bolin J. T., Filman D. J., Matthews D. A., Hamlin R. C., Kraut J. Crystal structures of Escherichia coli and Lactobacillus casei dihydrofolate reductase refined at 1.7 A resolution. I. General features and binding of methotrexate. J Biol Chem. 1982 Nov 25;257(22):13650–13662. [PubMed] [Google Scholar]
- Chothia C., Lesk A. M. Evolution of proteins formed by beta-sheets. I. Plastocyanin and azurin. J Mol Biol. 1982 Sep 15;160(2):309–323. doi: 10.1016/0022-2836(82)90178-4. [DOI] [PubMed] [Google Scholar]
- Chothia C., Lesk A. M. Helix movements and the reconstruction of the haem pocket during the evolution of the cytochrome c family. J Mol Biol. 1985 Mar 5;182(1):151–158. doi: 10.1016/0022-2836(85)90033-6. [DOI] [PubMed] [Google Scholar]
- Cohen G. H., Silverton E. W., Davies D. R. Refined crystal structure of gamma-chymotrypsin at 1.9 A resolution. Comparison with other pancreatic serine proteases. J Mol Biol. 1981 Jun 5;148(4):449–479. doi: 10.1016/0022-2836(81)90186-8. [DOI] [PubMed] [Google Scholar]
- Fermi G., Perutz M. F., Shaanan B., Fourme R. The crystal structure of human deoxyhaemoglobin at 1.74 A resolution. J Mol Biol. 1984 May 15;175(2):159–174. doi: 10.1016/0022-2836(84)90472-8. [DOI] [PubMed] [Google Scholar]
- Furey W., Jr, Wang B. C., Yoo C. S., Sax M. Structure of a novel Bence-Jones protein (Rhe) fragment at 1.6 A resolution. J Mol Biol. 1983 Jul 5;167(3):661–692. doi: 10.1016/s0022-2836(83)80104-1. [DOI] [PubMed] [Google Scholar]
- Greer J. Comparative model-building of the mammalian serine proteases. J Mol Biol. 1981 Dec 25;153(4):1027–1042. doi: 10.1016/0022-2836(81)90465-4. [DOI] [PubMed] [Google Scholar]
- Guss J. M., Freeman H. C. Structure of oxidized poplar plastocyanin at 1.6 A resolution. J Mol Biol. 1983 Sep 15;169(2):521–563. doi: 10.1016/s0022-2836(83)80064-3. [DOI] [PubMed] [Google Scholar]
- Kamphuis I. G., Drenth J., Baker E. N. Thiol proteases. Comparative studies based on the high-resolution structures of papain and actinidin, and on amino acid sequence information for cathepsins B and H, and stem bromelain. J Mol Biol. 1985 Mar 20;182(2):317–329. doi: 10.1016/0022-2836(85)90348-1. [DOI] [PubMed] [Google Scholar]
- Lesk A. M., Chothia C. Evolution of proteins formed by beta-sheets. II. The core of the immunoglobulin domains. J Mol Biol. 1982 Sep 15;160(2):325–342. doi: 10.1016/0022-2836(82)90179-6. [DOI] [PubMed] [Google Scholar]
- Lesk A. M., Chothia C. How different amino acid sequences determine similar protein structures: the structure and evolutionary dynamics of the globins. J Mol Biol. 1980 Jan 25;136(3):225–270. doi: 10.1016/0022-2836(80)90373-3. [DOI] [PubMed] [Google Scholar]
- Marquart M., Deisenhofer J., Huber R., Palm W. Crystallographic refinement and atomic models of the intact immunoglobulin molecule Kol and its antigen-binding fragment at 3.0 A and 1.0 A resolution. J Mol Biol. 1980 Aug 25;141(4):369–391. doi: 10.1016/0022-2836(80)90252-1. [DOI] [PubMed] [Google Scholar]
- Matsuura Y., Takano T., Dickerson R. E. Structure of cytochrome c551 from Pseudomonas aeruginosa refined at 1.6 A resolution and comparison of the two redox forms. J Mol Biol. 1982 Apr 5;156(2):389–409. doi: 10.1016/0022-2836(82)90335-7. [DOI] [PubMed] [Google Scholar]
- Norris G. E., Anderson B. F., Baker E. N. Structure of azurin from Alcaligenes denitrificans at 2.5 A resolution. J Mol Biol. 1983 Apr 15;165(3):501–521. doi: 10.1016/s0022-2836(83)80216-2. [DOI] [PubMed] [Google Scholar]
- Ochi H., Hata Y., Tanaka N., Kakudo M., Sakurai T., Aihara S., Morita Y. Structure of rice ferricytochrome c at 2.0 A resolution. J Mol Biol. 1983 May 25;166(3):407–418. doi: 10.1016/s0022-2836(83)80092-8. [DOI] [PubMed] [Google Scholar]
- Phillips S. E. Structure and refinement of oxymyoglobin at 1.6 A resolution. J Mol Biol. 1980 Oct 5;142(4):531–554. doi: 10.1016/0022-2836(80)90262-4. [DOI] [PubMed] [Google Scholar]
- Read R. J., Brayer G. D., Jurásek L., James M. N. Critical evaluation of comparative model building of Streptomyces griseus trypsin. Biochemistry. 1984 Dec 18;23(26):6570–6575. doi: 10.1021/bi00321a045. [DOI] [PubMed] [Google Scholar]
- Read R. J., Fujinaga M., Sielecki A. R., James M. N. Structure of the complex of Streptomyces griseus protease B and the third domain of the turkey ovomucoid inhibitor at 1.8-A resolution. Biochemistry. 1983 Sep 13;22(19):4420–4433. doi: 10.1021/bi00288a012. [DOI] [PubMed] [Google Scholar]
- Sielecki A. R., Hendrickson W. A., Broughton C. G., Delbaere L. T., Brayer G. D., James M. N. Protein structure refinement: Streptomyces griseus serine protease A at 1.8 A resolution. J Mol Biol. 1979 Nov 15;134(4):781–804. doi: 10.1016/0022-2836(79)90486-8. [DOI] [PubMed] [Google Scholar]
- Steigemann W., Weber E. Structure of erythrocruorin in different ligand states refined at 1.4 A resolution. J Mol Biol. 1979 Jan 25;127(3):309–338. doi: 10.1016/0022-2836(79)90332-2. [DOI] [PubMed] [Google Scholar]
- Takano T., Dickerson R. E. Conformation change of cytochrome c. II. Ferricytochrome c refinement at 1.8 A and comparison with the ferrocytochrome structure. J Mol Biol. 1981 Nov 25;153(1):95–115. doi: 10.1016/0022-2836(81)90529-5. [DOI] [PubMed] [Google Scholar]
- Wlodawer A., Walter J., Huber R., Sjölin L. Structure of bovine pancreatic trypsin inhibitor. Results of joint neutron and X-ray refinement of crystal form II. J Mol Biol. 1984 Dec 5;180(2):301–329. doi: 10.1016/s0022-2836(84)80006-6. [DOI] [PubMed] [Google Scholar]
- Woodbury R. G., Katunuma N., Kobayashi K., Titani K., Neurath H., Anderson W. F., Matthews B. W. Covalent structure of a group-specific protease from rat small intestine. Appendix: crystallographic data for a group specific protease from rat intestine. Biochemistry. 1978 Mar 7;17(5):811–819. doi: 10.1021/bi00598a010. [DOI] [PubMed] [Google Scholar]


