ABSTRACT
BACKGROUND
Many patients who get discharged from the intensive care unit experience physical dysfunction that persists even after discharge. Physical dysfunction is associated with skeletal muscle atrophy and accompanying intensive care unit-acquired weakness in the early stages of intensive care unit admission, and early diagnosis and prevention with early mobilization are crucial. However, the amount of physical activity required for early mobilization remains controversial in critically ill patients. This study aims to reveal the optimal mobilization quantification score dose associated with physical dysfunction after hospital discharge.
METHODS
This is a multicenter prospective cohort study planned in 22 facilities; all consecutive patients admitted to the participating facilities between June 2024 and May 2025 will be included. Adult patients on ventilator management for at least 2 days and who will consent to this study will be included. Patients’ mobility level and duration will be documented by the mobilization quantification score during their intensive care unit stay, and physical dysfunction will be assessed using muscle mass changes from day one to seven with ultrasonography and the Short-Form 12 Health Survey at 3 months after hospital discharge. The primary outcome is physical dysfunction at 3 months.
RESULTS AND CONCLUSION
Mobilization quantification score dose and muscle mass evaluation with ultrasonography will enable the quantification of the early mobilization intervention. This study will lay the foundation for future randomised studies.
Keywords: mobilization quantification score (MQS) dose, post intensive care syndrome (PICS), muscle mass atrophy, rectus femoris ultrasonography, Short Form 12 items (SF-12)
INTRODUCTION
Although advances in the medical field have significantly improved the survival rate of patients admitted to the intensive care unit (ICU), 40–70% of ICU survivors continue to suffer from physical dysfunction1), with many of them having long-term impairment in activities of daily living and reduced health-related quality of life (HRQoL)2). Diffuse symmetrical muscle weakness that develops after ICU admission is called ICU-acquired muscle weakness (ICU-AW) and is the most important part of the physical category of the post-intensive care syndrome, a long-term physical, mental, and cognitive dysfunction after ICU discharge3). Skeletal muscle atrophy progresses by 2% per day in the first week after ICU admission and muscle mass decreases by 12% from the first day in the ICU4,5). These muscle atrophies are associated with ICU-AW, which necessitates early diagnosis and prevention6,7).
Currently, adherence to the ABCDEF bundle and the implementation of early mobilization (EM) are attracting attention for preventing ICU-AW and improving short-term physical dysfunction8–10). EM for critically ill patients is effective in reducing ICU length of stay, ventilation duration, and muscle atrophy10). Studies on the impact of EM on short- and long-term patient outcomes have evaluated the outcomes based on patient backgrounds (disease, age, device, etc.), intensity (achieved mobility level), duration (number of minutes per day), frequency (number of interventions per day), and timing (time to first intervention) in detail10–14). Earlier timing with 24–72 h of intervention is more effective than with 72 h or more11,14). A frequency of at least once daily, with a medium-to-high frequency of at least three days weekly, improves physical dysfunction10,15). Regarding the studies of background factors, in critically ill patients, the individualization of patient disease and the classification of patient characteristic categories have helped identify those who are more likely or less likely to benefit from EM16,17).
However, intensity and frequency remain controversial as several randomized controlled trials on high-intensity or high-intensity and long-term intensive interventions have shown no significant differences in outcomes11,18). The timing, intensity, and frequency of EM in the control group were not mentioned in detail, and this was mentioned as a limitation of those studies18). Furthermore, rather than investigating intensity and duration separately, investigating both as doses of physical activity can help quantify rehabilitation interventions and optimize their effectiveness12,19–21). We hypothesized that optimised combination of intensity and duration of physical activity in the ICU would contribute most to HRQoL20,21). To verify this hypothesis, we planned this study; Evaluating optimal rehabilitation strategies in ICU: study protocol for a multicentre cohort study to assess physical activity dosing, muscle mass, and physical outcomes (IPAMICS study). This study will use mobilization quantification score (MQS) dose and SF-12. The MQS dose accounts for the achieved mobility level (intensity) and duration (duration and frequency). SF-12 includes physical and mental status and widely used for HRQoL22,23). In addition, this study focuses on musle mass atrophy to quantify ICU-AW, as well as other evaluating tools. Through this study, we aim to disseminate standard and optimized interventions to participating facilities and investigate the superiority of the MQS dose and its correlation with physical outcomes, which will lay the foundation for future randomised contorol trials.
METHODS
The protocol is described according to the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) checklist for clinical trials (Additional file 1)
STUDY DESIGN
This multicenter observational prospective cohort study will use data from a follow-up assessment conducted 3 months after hospital discharge. This study will begin when the first patient is enrolled and will continue until the 3-month follow-up of the last discharged patient is completed. Twenty-two ICUs from facilities nationwide will participate in this study (Additional file 11). Enrollment will be initiated in June 2024 and is intended to continue for 12 months.
APPROVALS
The IPAMICS study protocol was approved by the Ethics Committee of the Nagoya Medical Center (No 2023-007). The central institution of this study and all participating facilities received approval from local ethics committees before enrolling patients. This study is being conducted in accordance with the Declaration of Helsinki and the Ethical Policy published by the Japanese Government. Written informed consent will be obtained from all patients or their designated representatives, such as close relatives, if they cannot provide consent at the time of ICU admission. The study registration was conducted at the University Hospital Medical Information Network (UMIN) 000051582.
STUDY SETTING
Twenty-two ICUs from nationwide facilities will participate in this study (Additional file 11). Of the 22 facilities, 15 (68%) are local hospitals and the remaining 7 are university/university-affiliated hospitals. Most (90%) of these ICUs are mixed medical and surgical ICUs. Background information for each hospital and ICU (presence of unique protocols, nurse-to-patient ratio, presence of intensivists and other ICU specialists) will be obtained before study initiation and will not change throughout the patient enrollment period. Protocols for ICU care at participating facilities are not unified or shared but are based on recent standard guidelines such as the 2018 Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Guidelines24), Nutrition Guidelines25), and Ventilator Management Guidelines26).
All patients admitted to the participating ICUs will be screened within the first 24 h after ICU admission; screening will be performed by investigators and the departments’ research rehabilitation teams during working days. If the eligibility conditions are met, participants (if awake and able to collaborate) or family members will be approached to provide informed consent within the first 24 h, after which data will be collected (Table 1).
Table 1 . Schedule of enrolment, interventions, and assessments.
| TIMEPOINT** | Enrolment ICU admission T0 | Allocation Day 1 | Post-allocation | Close-out After 3 Months T4 | ||
|---|---|---|---|---|---|---|
| Day 7 T1 | ICU discharge T2 | Hospital discharge T3 | ||||
| ENROLMENT | ||||||
| Eligibility screen | × | |||||
| Informed consent | × | |||||
| Allocation | × | |||||
| ASSESSMENTS: | ||||||
| Baseline variables (listed in Table 1) | × | |||||
| Muscle cross-sectional area muscle thickness | × | × | ||||
| Physical functions (Clinical Frail Scale) | × | × | × | |||
| Physical functions (FSS-ICU, Barthel index) | × | × | ||||
| Muscle strength (MRC, Handgrip) | × | × | ||||
| SF-12 (PCS, MCS) | × | × | ||||
ICU; Intensive care unit, FSS-ICU; Functional status score, MRC; Medical research council, SF-12; Short form 12, PCS; Physical component summary, MCS; Mental component summary,
TIMELINE
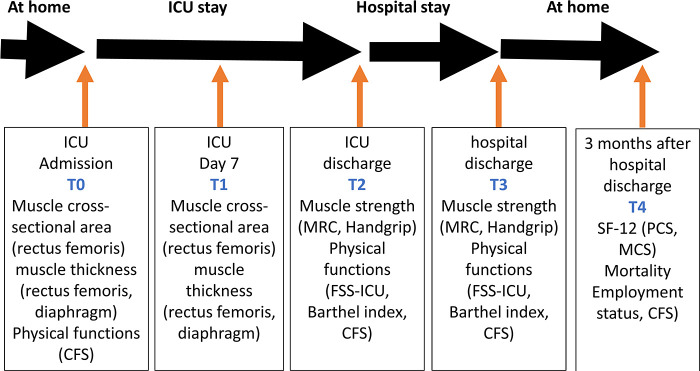
Once enrolled this cohort study, the participants will remain in the cohort until they withdraw or complete follow-up 3 months after hospital discharge. Data will be collected (1) at enrollment, (2) 7 days after ICU admission, (3) at ICU discharge, (4) at hospital discharge, and (5) 3 months after hospital discharge (Table 1, Fig. 1).
Fig. 1 . Flowchart of performed questionnaires during the study.
Intensive care unit, ICU; Clinical frailty scale, CFS; Medical research council, MRC; Short Form Survey-12, SF-12; Health related quality of life, HRQoL; Functional status score for the Intensive Care Unit, FSS-ICU; Physical component summary, PCS; Mental component summary, MCS.
PARTICIPANTS
Inclusion criteria
1. Patients admitted to the participating ICUs for the first time.
2. Patients expected to have >2 days of mechanical ventilation.
3. Patients aged ≥18 years at the time of admission to the ICUs.
4. Patients who will consented.
Exclusion criteria
1. Patients unable to walk even with the use of assistive devices before admission (use of care level 3 or higher services: guidelines).
2. Patients who are considered end of life/terminal care cases by their doctor-in-charge, according to those terms’ definitions (27), Additional file 3), their disease severity and their difficulty in aggressive treatment. That is because they might receive less or different degree of ICU care than usual.
3. Patients expected to be restricted to bed for a long time owing to severe trauma, including multiple unstable fractures, burns, and limb amputation.
OUTCOME MEASURES
Primary outcome
The primary outcome is the physical component summary of Short-Form 12 items (SF-12) at 3 months after hospital discharge28), which is important and easy-to-use scale for measuring HRQoL22,23). Using this scale for primary outcome, we believe the optimal MQS dose without much loss to follow-up. HRQoL is defined as a physical component summary score of <50 points at the 3-month follow-up29) (Table 2).
Table 2 . Details of outcome measures at follow-up.
| Variable | Description |
|---|---|
| Survival | If a patient dies during follow-up, date of death is recorded |
| Employment status | Whether the patient/family has a job at follow-up (full time or part time) and whether the job is the same as before ICU admission |
| General information | Readmission to hospital or ICU during follow-up |
| Health-related quality of life | |
| SF-12 | Three months after discharge, the evaluator will administer the SF-12 questionnaire to the patient or proxy by telephone. SF-12 questionnaire is a comprehensive 12-item survey of HRQOL with two summary scales, PCS and MCS, with scores ranging from 0 to 100. A higher score indicates better physical and mental functions. |
| Physical function/activities of daily living | |
| Clinical frail scale | The Clinical Frailty Scale is a judgement-based frailty tool that evaluates specific domains including comorbidity, function, and cognition to generate a frailty score ranging from 1 (very fit) to 9 (terminally ill). |
| Nutrition assessment | |
| MUST | The MUST score is based on three items regarding BMI (score 0–2), weight loss (score 0–2), and no nutritional intake due to acute disease (score 0–2). Based on the MUST score, patients were categorized as having a low risk (MUST score 0), medium risk (MUST score 1), or high risk (MUST score ≥2) of malnutrition [[13]]. |
| Family- Mental component summary (SF-12) | HRQOL with two summary scales, MCS, with scores ranging from 0 to 100. |
ICU; Intensive care unit, SF-12; Short Form Survey, HRQOL, Quality of life, PCS; Physical component summary, MCS; Mental component summary, MUST; Malnutrition Universal Screening Tool.
Secondary outcomes
As secondary outcomes, first we chose mental component summary score of the SF-1229) after hospital discharge. That will indicate us the optimal MQS dose for mental outcome. Second, we chose those items, Barthel Index, Medical Research Council score3), functional status score for the ICU30), grip strength31), clinical frailty scale32), and muscle atrophy changes using ultrasonography5,6). Investigating these items, we aim to quantify ICU-AW from multiple perspectives. Data will also be collected on the incidence of post intensive care syndrome-family, defined as family mental health disorders after a patient’s hospital discharge33) (Table 2).
BASELINE CHARACTERISTICS AND TREATMENT
The baseline characteristics of enrolled patients will be prospectively collected, including basic components such as age, height, weight, type of admission, employment status, pre-existing comorbidity (Charlson comorbidity index), status of activities of daily living (Barthel index)34), and frailty32); illness components such as Acute Physiology and Chronic Health Evaluation II and Sequential Organ Failure Assessment score; and a nutrition component, a Malnutrition Universal Screening Tool at ICU admission35). The Sequential Organ Failure Assessment score will be recorded at the maximum time during the ICU stay and at discharge. The details of ICU care and treatment that could influence outcomes will also be prospectively collected, including surgical infection source control; use of neuromuscular blockade, sedation agents, corticosteroids, and vasopressors; non-invasive ventilation, mechanical ventilation, extracorporeal membrane oxygenation, and continuous or intermittent renal replacement treatment.
KEY DATA COLLECTION: MQS DOSE (ACHIEVED MOBILIZATION LEVEL AND DURATION)
In the IPAMICS study, the physical activity dose is defined using the MQS, which will be measured by a physiotherapist using the ICU mobility scale36). Although MQS has been shown its validity and effectiveness to quantify the ICU physical activity19), it uses English, and the calculation way does not fit in Japanese rehabilitation culture. So, before this study, we made a prospective cohort study to investigate the validation and effectiveness of MQS Japanese version, and both of them were proven38). To calculate the MQS dose (achieved mobilization level and duration), each mobilization level will be assigned a duration (min) to define one unit of MQS. The daily MQS obtained from the nursing and physiotherapy data will be totaled throughout the ICU stay and then divided by the duration of the ICU stay to obtain the average daily MQS (average daily MQS = total MQS during the ICU/ICU length of stay). The rehabilitation duration will be measured using the ICU mobility scale level to quantify the dose of physical activity performed in the ICU. The actual duration of each rehabilitation intervention (from start to finish) will be measured in seconds using a stopwatch. The time required for rehabilitation preparation, rest, assessment, and measurement will be excluded from the activity time. This will ensure that only the actual physical activity time is recorded. Even if one unit of activity time at that level is shorter than the specified time, the activity time will be rounded up to one unit.
DATA SOURCE/MEASUREMENTS
At the time points mentioned above, we will assess the rectus femoris cross-sectional area, muscle thickness, and diaphragm muscles using ultrasonography (Additional file 4, 5). While intubated and ventilated, the patient’s airway occlusion pressure will be monitored and recorded using the ventilator. All collected data will be prepared according to published standard protocols in the field and will be analyzed by a team experienced in muscle ultrasonography (a physiotherapist and/or intensivist, both currently working in the ICUs).
REHABILITATION PROTOCOL
Study participants will receive the usual rehabilitation at their respective facilities. Treatment will be directed by the treating medical team, except when another medical specialist is required. We aim to mobilize all participants equally and daily under a five-level protocol (level 1: passive range of motion and respiratory physical therapy; level 2: active range of motion; level 3: sitting exercise; level 4: standing exercise; and level 5: walking exercise) tailored for each participating hospital (Additional file 6)37,38). All participating ICUs provide patients with standardized EM according to the 2023 guidelines of the Japanese Society of Intensive Care Medicine39). To ensure the safe implementation of EM, the early mobilization protocol describes the step-up criterion as level 3 or higher (Additional file 6). In addition, adverse events during implementation will be indicated with appropriate values in the categories of medical, cardiovascular, respiratory, and neurological problems (Additional file 7). If any deviation from these values occurs, we will immediately the patient make rest, and will record as an adverse event (Additional file 8). After ICU discharge, physical or occupational therapists will provide rehabilitation, such as muscle strengthening, balance, walking, and stair exercises, for more than 20 min on weekdays to each patient, according to the rehabilitation policy in the general ward of each hospital.
SAMPLE SIZE
There is no maximum sample size for this study; however, the outcome may be subject to targets or maximum sample sizes, which will be specified in the relevant sub-protocol.
The sample size is calculated based on the assumed survival rate at three months after hospital discharge. Assuming that the survival rate at 3 months is 80% with a 95% confidence interval width of 10%, the calculated sample size required is 194 patients41). Considering that approximately 20% of the patients are lost to follow-up, 242 patients will be sufficient. Collecting a sample of this calculated size is considered feasible because each participating site should be able to enroll at least one patient per month based on the number of patients admitted to the ICU at each site in the past.
PATIENT RETENTION AND WITHDRAWAL
The IPAMICS study will be conducted in compliance with the Guidelines for the Appropriate Handling of Personal Information by Medical and Nursing Care Providers and will be conducted with the utmost care in the handling of patients’ personal information40). Once patients are enrolled, they will be managed anonymously using an electronic data capture system created for the study by a data management and clinical research support company (TXP Medical, Tokyo, Japan; Additional file 10) and will be followed during hospitalisation and after hospital discharge with every effort over the entire study period (Fig. 1). Deaths during the study period will be recorded as deaths. Participants will be able to complete questionnaires with the help of family members, if necessary, and this will be recorded, including whether they answer the questionnaires themselves or with the help of family members. If there are significant deviations from the study design, if the participant or their family cannot be contacted after several phone calls, or if the individual withdraws their consent to the study, the participant is considered as lost to follow-up and excluded from the analysis. Participants who withdraw from the study may be allowed to retain data and samples obtained up to the point of withdrawal for analysis. Participants who are discontinued during the study are not compensated, and sample size calculations indicate a loss of up to 3 months follow-up of 20% and therefore is not likely to jeopardise the study’s power of detection. Cases that are lost to follow-up are assessed for bias. However, missing data points may raise issues with the internal validity of the results. Efforts to minimise loss to follow-up include respecting participants’ time constraints, formal follow-up procedures such as multiple contact methods, strong interpersonal skills of study member and flexible testing times.
DATA MANAGEMENT
Data collected at each centre are promptly entered into electronic data capture system, anonymised and stored with a separate study ID for each participant. Only the principal investigators (YM and SW) and committee group members can check the entry status of all participating patients via the database and, if necessary, request data entry from collaborators. The database is protected by standard internet security and sufficient data are only provided for analysis plans with appropriate authorisation from the principal investigator. These arrangements are described in the study protocol and explanatory documents, using simplified terminology, and information is always available to participating centres and interested parties through the study protocol, and to patients and their families through the explanatory documents. In addition, eligible patients and their families can contact to the principal investigators or each investigator at their respective centres at any time. The information obtained in this study may be used to conduct new research beyond the original purpose (secondary use), including outside the research group. In such cases, a new research protocol will be drawn up and implemented after primary review by the research office, secondary review by the ethics review committee of the institution where the secondary use is intended, and approval by the head of the research organisation at each institution.
STATISTICAL METHODS
Continuous variables will be summarised as mean and standard deviation for symmetric distributions and median and interquartile range for asymmetric distributions. Continuous variables, categorical variables and time versus event endpoints will be assessed using standard statistical analysis approaches (e.g. chi-square, Fisher exact, Student t-test, Mann-Whitney U test). Categorical variables will be summarised at each level as frequencies and proportions. Predictors of rehabilitation outcomes will be assessed using logistic regression methods for binary, continuous and time-to-event endpoints, as appropriate. There will also be a sensitivity analysis including the untraceable group in physical impairment. Safety analyses will compare the proportion of adverse event criteria (Additional file 8) met after the initiation of rehabilitation. All summary statistics, analyses and data visualisation will be performed using JMP (version 13.0; SAS Institute, Cary, NC, USA) and IBM SPSS software (version 23.0; IBM, Armonk, NY, USA) for statistical calculations.
DISCUSSION
This multicenter prospective cohort study investigates the association between the MQS dose during ICU stays and skeletal muscle atrophy, ICU-AW, and HRQoL 3 months after hospital discharge in mechanically ventilated patients in twenty-two ICUs, including both medical and surgical settings. This study overcomes the limitations of the currently available data. This study reports evidence based on EM practice, and reinforced previous EM findings13). EM is recommended for incorporation into practice in current clinical guidelines39). HRQoL in critically ill patients shows the greatest decline at 3 months and shows gradual improvement, but it is still below the average at one year after hospital discharge42). Reassessing safety and identifying the optimal EM intervention of ventilated patients have a significant impact on their HRQoL11,43). This study aims to determine the optimal MQS dose that correlates with HRQoL, and with ICU-AW from multiple perspective. The importance of adherence to the ABCDEF bundle is emphasized for ICU-AW and post intensive care syndrome prevention, and EM is attempted daily with shallow sedation management; however, in practice, there are many barriers to implementing EM, including prolonged disturbance of consciousness or vital instability8,44). Some important studies have shown that premature initiation timing and high-intensity interventions did not achieve significant results, rather they increased the number of adverse events11,43). The limitations of these studies include the inability to adequately define the level and duration of mobilization in the control group and the low follow-up rate at 6 months after hospital discharge11). This study used the MQS dose to quantify physical activity, including frequency, duration, and intensity19). Previous reports using the MQS score have shown that the optimal dose correlates with the outcome of independence at discharge without increasing adverse events, even in stroke and surgical ICU settings19,27). We also used a telephone-based questionnaire, the SF-12, to assess HRQoL at 3 months post-discharge. This SF-12 is a brief, non-inferior instrument to the SF-36 items, which is widely used to assess HRQoL that employs a telephone-based questionnaire7,22,23). In addition, a three-month period was chosen to improve the follow-up rates. Combining these measures allows for the assessment of optimal quantitative interventions for physical outcomes while reducing the loss of follow-up. Furthermore, the data from this study will be used to develop a simple scale of physical activity that can be used in ICUs and is directly related to outcomes.
To assess ICU-AW from multiple perspectives, Medical Research Council score3), functional status score for the ICU30), grip strength31), frailty32), and muscle atrophy with ultrasonography5,6) were selected based on previous reports. Among others, the changes in muscle atrophy correlate with the duration of ventilation, ICU stay, and hospital length of stay5,6) and complement the shortcoming of the diagnosis of ICU-AW by Medical Research Council score, which depends on the patients’ awakening status3,45). Using ultrasonography, the cross-sectional area and muscle thickness of the rectus femoris are recognized as methods of muscle mass assessment. Focusing on the rapidity, versatility, and repeatability of ultrasonography, it can be used by physiotherapists as well as doctors46,49) and is comparable to computed tomography for muscle mass assessment47). In general, ultrasonography and muscle mass evaluation are influenced by the practioners’ experience and skill. We created a detailed manual specifying the patient’s position, drawing method, and probe use (angle, position, strength, and number of times). To simplify the assessment, we focus on cross-sectional area and muscle thickness of the rectus femoris muscle45) and limited the assessment days to the first and seventh day of the ICU stays5,48). In addition, ultrasound workshops at the site will be held at multiple locations to ensure the uniformity of assessment, and video materialswill be prepared for repeated reviewing. Participating facilities are required to attend these workshops and view the video materials to ensure a uniform evaluation. In a previous study, an ultrasound educational program enabled physiotherapists to assess ultrasonography at the same level as a doctor49).
STRENGTH AND LIMITATION
The IPAMICS study will be the first large cohort study to enroll more than 400 ICU patients and examine the association among physical activity volume, muscle mass atrophy, and physical impairment36,46). This study can help clarify the predominance of the MQS dose in correlation with physical outcomes and disseminate the method of measurement of muscle mass to physicians and physiotherapists in the participating facilities49). This study has several limitations. The first limitation is loss to follow-up after discharge. If loss to follow-up is excluded from the primary analysis, it can introduce significant bias in the outcomes18). To decrease loss to follow-up, we decided to evaluate primary outcome only one point, with easy-to-evaluate scale. Second, ultrasonographic assessment of muscle mass will depend on the skill or condition of each participating member. To reduce this risk, we created detailed manual and video materials on how to implement ultrasonography and conducted site-based ultrasound workshops several times. Then, the participating members will be able to improve their skills and provide a certain level of assessment. Finally, we cannot adjust for unmeasured and unknown confounding factors or draw causal inferences because of the study design. However, we believe that this study will bring effective rehabilitation cultures to the ICUs of participating facilities and provide a foundation for future randomised contorol studies.
ACKNOWLEDGMENTS
We wish to thank the members of the study committee office: Shizuka Watanabe helped conduct this project smoothly. We thank all the collaborators from the participating sites listed in Additional file 10.
This project was funded by a grant from Japan Society for the Promotion of Science
(JSPS) KAKENHI. Grant number is 24K14242. JSPS had no role in the design of this study or writing of this manuscript.
TRIAL STATUS
Ethical approval was obtained in September 2023 (protocol version 2; 15 February 2024). Facility recruitment started in September 2023 and is still ongoing. Facility recruitment is expected to be completed in May 2024.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest in relation to the work presented in the manuscript.
Supplementary Material
Supplemental Appendix
REFERENCES
- 1.Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomized controlled trial. Lancet. 2009;373:1874–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cuthbertson BH, Roughton S, Jenkinson D, et al. Quality of life in the five years after intensive care: a cohort study. Crit Care. 2010;14:R6:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stevens RD, Marshall SA, Cornblath DR, et al. A framework for diagnosing and classifying intensive care unit-acquired weakness. Crit Care Med. 2009;37:S299–S308. [DOI] [PubMed] [Google Scholar]
- 4.Inoue S, Hatakeyama J, Kondo Y, et al. Post-intensive care syndrome: its pathophysiology, prevention, and future directions. Acute Med Surg. 2019;6:233–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puthucheary ZA, Rawal J, McPhail M, et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310:1591–1600. [DOI] [PubMed] [Google Scholar]
- 6.Mueller N, Murthy S, Tainter CR, et al. Can sarcopenia quantified by ultrasound of the rectus femoris muscle predict adverse outcome of surgical intensive care unit patients as well as frailty? a prospective, observational cohort study. Ann Surg. 2016;264:1116–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farhan H, Moreno-Duarte I, Latronico N, et al. Acquired muscle weakness in the surgical intensive care unit: nosology, epidemiology, diagnosis, and prevention. Anesthesiology. 2016;124:207–234. [DOI] [PubMed] [Google Scholar]
- 8.Ely EW. The ABCDEF bundle: science and philosophy of how ICU liberation serves patients and families. Crit Care Med. 2017;45:321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Key W. Implementation of a nurse-driven early mobility protocol in critical care to reduce intensive care unit acquired weakness (ICUAW). Doctor of Nursing Practice Projects. 2023;96. https://digitalcommons.jsu.edu/etds_nursing, Accessed 2024 April 15. [Google Scholar]
- 10.Wu RY, Yeh HJ, Chang KJ, et al. Effects of different types and frequencies of early rehabilitation on ventilator weaning among patients in intensive care units: a systematic review and meta-analysis. PloS one. 2023;18:e0284923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wright SE, Thomas K, Watson G, et al. Intensive versus standard physical rehabilitation therapy in the critically ill (EPICC): a multicentre, parallel-group, randomised controlled trial. Thorax. 2018;73:213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lorenz M, Fuest K, Ulm B, et al. The optimal dose of mobilization therapy in the ICU: a prospective cohort study. J Intensive Care. 2023;11:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lang JK, Paykel MS, Haines KJ, et al. Clinical practice guidelines for early mobilization in the ICU: a systematic review. Crit Care Med. 2020;48:e1121–e1128. [DOI] [PubMed] [Google Scholar]
- 14.Fuest K, Schaller SJ. Recent evidence on early mobilization in critical-ill patients. Curr Opin Anaesthesiol. 2018;31:144–150. [DOI] [PubMed] [Google Scholar]
- 15.Yosef-Brauner O, Adi N, Ben Shahar T, et al. Effect of physical therapy on muscle strength, respiratory muscles and functional parameters in patients with intensive care unit-acquired weakness. Clin Respir J. 2015;9:1–6. [DOI] [PubMed] [Google Scholar]
- 16.Liu K, Shibata J, Fukuchi K, et al. Optimal timing of introducing mobilization therapy for ICU patients with sepsis. J Intensive Care. 2022;10:74–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuest KE, Ulim B, Daum N, et al. Clustering of critically ill patients using an individualized learning approach enables dose optimization of mobilization in the ICU. Crit Care. 2023;27:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hodgson CL, Bailey M, Bellomo R, et al. Early active mobilization during mechanical ventilation in the ICU. N Engl J Med. 2022;387:1747–1758. [DOI] [PubMed] [Google Scholar]
- 19.Scheffenbichler FT, Teja B, Wongtangman K, et al. Effects of the level and duration of mobilization therapy in the surgical ICU on the loss of the ability to live independently: an international prospective cohort study. Crit Care Med. 2021;49:e247–e257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paton M, Lane R, Paul E, et al. Mobilization during critical illness: a higher level of mobilization improves health status at 6 months, a secondary analysis of a prospective cohort study. Crit Care Med. 2021;49:e860–e869. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe S, Morita Y, Suzuki S, et al. Effects of the intensity and activity time of early rehabilitation on activities of daily living dependence in mechanically ventilated patients. Prog Rehabil Med. 2021;6:20210054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 23.Lin Y, Yu Y, Zeng J, et al. Comparing the reliability and validity of the SF-36 and SF-12 in measuring quality of life among adolescents in China: a large sample cross-sectional study. Health Qual Life Outcomes. 2020;18:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46:e825–e873. [DOI] [PubMed] [Google Scholar]
- 25.Singer P, Blaser AR, Berger MM, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutri. 2019;38:48–79. [DOI] [PubMed] [Google Scholar]
- 26.Hashimoto S, Sanui M, Egi M, et al. The clinical practice guideline for the management of ARDS in Japan. J Intensive Care. 2017;5:1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hui D, Nooruddin Z, Didwaniya N, et al. Concepts and definitions for “actively dying,” “end of life,” “terminally ill,” “terminal care,” and “transition of care”: a systematic review. J Pain Symptom Manage. 2014;47:77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ware JE, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med care. 1996;34:220–233. [DOI] [PubMed] [Google Scholar]
- 29.Brunelli A, Socci L, Refai M, et al. Quality of life before and after major lung resection for lung cancer: a prospective follow-up analysis. Ann Thorac Surg. 2007;84:410–416. [DOI] [PubMed] [Google Scholar]
- 30.Huang M, Chan KS, Zanni JM, et al. Functional Status Score for the ICU: an international clinimetric analysis of validity, responsiveness, and minimal important difference. Crit Care Med. 2016;44:e1155–e1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bragança RD, Ravetti CG, Barreto L, et al. Use of handgrip dynamometry for diagnosis and prognosis assessment of intensive care unit acquired weakness: a prospective study. Heart Lung. 2019;48:532–537. [DOI] [PubMed] [Google Scholar]
- 32.Church S, Rogers E, Rockwood K, et al. A scoping review of the Clinical Frailty Scale. BMC Geriatr. 2020;20:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elliott D, Davidson JE, Harvey MA, et al. Exploring the scope of post–intensive care syndrome therapy and care: engagement of non–critical care providers and survivors in a second stakeholders meeting. Crit Care Med. 2014;42:2518–2526. [DOI] [PubMed] [Google Scholar]
- 34.Katz for the Association of Rheumatology Health Professionals Outcomes Measures Task Force PP . Measures of adult general functional status: The Barthel index, Katz index of activities of daily living, health assessment questionnaire (HAQ), MACTAR patient preference disability questionnaire, and modified health assessment questionnaire (MHAQ). Arthritis Care Res. 2003;49:S15–S27. [Google Scholar]
- 35.Elia M. The “MUST” report the “MUST” report nutritional screening of adults: a multidisciplinary responsibility development and use of the “malnutrition universal screening tool” (‘MUST’) for adults. Malnutrition Advisory Group (MAG), a standing committee of BAPEN. 2003. [Google Scholar]
- 36.Hodgson C, Needham D, Haines K, et al. Feasibility and inter-rater reliability of the ICU Mobility Scale. Heart Lung. 2014;43:19–24. [DOI] [PubMed] [Google Scholar]
- 37.Watanabe S, Liu K, Nakamura K, et al. Association between early mobilization in the ICU and psychiatric symptoms after surviving a critical illness: a multicenter prospective cohort study. J Clin Med. 2022;11:2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watanabe S, Yamauchi K, Yasumura D, et al. Reliability and effectiveness of the Japanese version of the mobilization quantification score. Cureus. 2023;15:e43440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Unoki T, Hayashida K, Kawai Y, et al. Japanese Clinical Practice Guidelines for Rehabilitation in Critically Ill Patients 2023 (J-ReCIP 2023). J Intensive Care. 2023;11:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ministry of Health, Labour and Welfare, Japan. Personal Information Protection Commission. Guidance for the Appropriate Handling of Personal Information by Medical or Care-related Service Providers. https://www.ppc.go.jp/files/pdf/01_iryoukaigo_guidance5.pdf, Accessed 2024 Mar 29.
- 41.Schmidt K, Gensichen J, Fleischmann-Struzek C, et al. Long-term survival following sepsis: results of a single-center registry study with 4-year follow-up. Dtsch Arztebl Int. 2020;117:775–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Szymczak H, Dodoo-Schittko F, Brandstetter S, et al. Trajectories of quality of life, return to work, psychopathology, and disability in survivors of the acute respiratory distress syndrome (ARDS): a three-year prospective cohort study (DACAPO). J Crit Care. 2023;78:154356. [DOI] [PubMed] [Google Scholar]
- 43.Langhorne P, Wu O, Rodgers H, et al. Very Early Rehabilitation Trial after stroke (AVERT): a Phase III, multicentre, randomised controlled trial. Health Technol Assess. 2017;21:1–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anekwe DE, Koo KKY, de Marchie M, et al. Interprofessional survey of perceived barriers and facilitators to early mobilization of critically ill patients in Montreal, Canada. J Intensive Care Med. 2019;34:218–226. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka K, Katayama S, Okura K, et al. Skeletal muscle mass assessment in critically ill patients: method and application. Ann Cancer Res Ther. 2022;30:93–99. [Google Scholar]
- 46.Katari Y, Srinivasan R, Arvind P, et al. Point-of-care ultrasound to evaluate thickness of rectus femoris, vastus intermedius muscle, and fat as an indicator of muscle and fat wasting in critically ill patients in a multidisciplinary intensive care unit. Indian J Crit Care Med. 2018;22:781–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peres LM, Luis-Silva F, Menegueti MG, et al. Comparison between ultrasonography and computed tomography for measuring skeletal muscle mass in critically ill patients with different body mass index. Clin Nutr ESPEN. 2024;59:214–224. [DOI] [PubMed] [Google Scholar]
- 48.Mayer KP, Thompson Bastin ML, Montgomery-Yates AA, et al. Acute skeletal muscle wasting and dysfunction predict physical disability at hospital discharge in patients with critical illness. Crit Care. 2020;24:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whittaker JL, Ellis R, Hodges PW, et al. Imaging with ultrasound in physical therapy: what is the PT’s scope of practice? a competency-based educational model and training recommendations. Br J Sports Med. 2019;53:1447–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Appendix