Abstract
Glioblastoma (GBM) is an aggressive brain tumor characterized by cellular and molecular diversity. This diversity presents significant challenges for treatment and leads to poor prognosis. Surgery remains the primary treatment of choice for GBMs, but it often results in tumor recurrence due to complex interactions between GBM cells and the peritumoral brain zone. Phytochemicals have shown promising anticancer activity in in-vitro studies and are being investigated as potential treatments for various cancers, including GBM. However, some phytochemicals have failed to translate their efficacy to pre-clinical studies due to limited penetration into the tumor microenvironment, leading to high toxicity. Thus, combining phytochemicals with nanotechnology has emerged as a promising alternative for treating GBM. This review explores the potential of utilizing specific nanoparticles to deliver known anticancer phytochemicals directly to tumor cells. This method has demonstrated potential in overcoming the challenges of the complex GBM microenvironment, including the tight blood–brain barrier while minimizing damage to healthy brain tissue. Therefore, employing this interdisciplinary approach holds significant promise for developing effective phyto-nanomedicines for GBM and improving patient outcomes.
Keywords: Glioblastoma, Phytochemicals, Nanoparticles, Blood–brain barrier, Curcumin, Resveratrol, Quercetin, Luteolin
Introduction
Glioblastoma (GBM) as recently classified in the 2021 World Health Organization (WHO) Classification of Tumours of the Central Nervous System-fifth edition applies only to isocitrate dehydrogenase (IDH)–wild-type tumours, WHO grade 4, while tumours previously diagnosed as IDH-mutated glioblastomas are now classified as astrocytomas IDH mutated, WHO grade 4 [1, 2]. For newly diagnosed GBM patients, the Stupp protocol, which includes maximal safe resection, radiotherapy with concurrent and adjuvant temozolomide (TMZ) treatment became the standard of care (SOC) with evidence of improved median overall survival from 12.1 to 14.6 months and increase 2-year survival rate 10–27%. Furthermore, the addition of tumor treating fields (TTFields) to the SOC therapy has improved the median overall survival from to 20.5 months with no negative effect on the quality of life [3]. The main driver of GBM aggressiveness is attributed to its cellular and molecular heterogeneity, which significantly influences its biological behaviour [4]. GBMs have aggressive infiltrative growth into adjacent normal brain tissue which in turn affects surgical excision of the tumor with clear margins. There is an intimate relationship between the tumor and the peritumor brain zone (PBZ) which contributes to its infiltrative growth. The PBZ consists of a heterogeneous composition of cells, including inflammatory cells (such as tumor-associated macrophages, myeloid-derived suppressor cells, dendritic cells, and neutrophils), stromal cells, vascular cells, reactive astrocytes, and infiltrative tumor cells. Together, these cells protect the tumor core and contribute to poor treatment outcomes [5, 6]. Glial cells are responsible for several regulatory functions including the maintenance of ionic balance, the blood–brain barrier (BBB), redox potential, the gliotransmitter, synapses, immunoregulation [7], and ensuring a toxin and debris-free cerebrospinal fluid (CSF) [8].
GBM tumor cells retained the same properties as glial cells, thus, any therapeutic targets for treatment should ideally be able to distinguish and permeate the BBB tumor microenvironment and the tumor core. The current standard treatment for GBM involves surgery followed by chemotherapy and radiotherapy. However, challenges in treating GBM include the difficulty of achieving complete tumor resection due to the presence of GBM cells localized at the tumor margins [9]. The current first line chemotherapeutic treatment for primary GBM is TMZ and more recently the United States Food and Drug Administration (FDA) approved anti-vascular endothelial growth factor (VEGF) antibody bevacizumab, although still lacking strong beneficial evidence for GBM treatment, it is effective in reducing peritumour oedema [6, 10]. TMZ effectiveness depends on the methylation status of O-6 methylguanine-DNA methyltransferase (MGMT) and patients with methylated MGMT benefit from this treatment, whereas those without methylation do not benefit [11]. It is therefore evident that current onco-therapies do not address some of the mechanisms of cancer evasion, which is the main cause of mortality and drug resistance [12]. Natural plant products have gained attention as promising alternative anti-cancer treatment strategies with phytochemicals such as terpenoids, coumarins, flavonoids, taxanes, lignins, alkaloids, stilbenes, and saponins targeting tumor through various signaling mechanisms [13]. Phytochemicals target GBM cancer stem cells which are known to be responsible for tumor relapse, drug resistance, and tumor recurrence following treatments [14]. Furthermore, phytochemicals also inhibit angiogenesis, which plays a role in tumor metastasis [15] by downregulating signaling mechanisms involved in neovessel generation [16]. Phytochemicals impede GBM cell migration and invasion by downregulating matrix metalloproteins (MMPs) expression. They also have the ability to modulate cell cycle progression, autophagy, apoptosis, and cell proliferation [16].
The clinical application of phytochemicals faces challenges due to poor water solubility, rapid metabolism, and issues with bioavailability as well as low chemical stability [13]. These challenges can potentially be overcome through the use of nanoparticles (NPs). The most commonly studied NPs include liposomes, lipid-based nanosystems, micelles, polymeric NPs, niosomes, and nanosponges [13]. These NPs can carry phytochemicals either by conjugation or encapsulation. The utilization of NPs can overcome the issues of drug solubility, stability, non-specific targeting, and lack of drug retention at the tumor site. The BBB, which is the protective layer of the CNS restricts the entry of drugs into the brain thereby reducing their therapeutic efficacy [17]. Blood–brain tumor barrier (BBTB) is highly heterogeneous with increased permeability in bulky tumors while it remains highly intact in invasive tumors [18]. However, NPs can cross the BBTB and enhance the therapeutic efficacy of drugs [19]. Although several phytochemicals with anti-GBM activity have been reported, this review will focus on the most commonly studied phytochemicals with known mechanistic action in GBM and in combination with NPs, to enhance specificity and improve targeted therapy by delivering the phytochemicals directly to the tumor site.
Methodology
A literature search was conducted across multiple electronic databases, including Web of Science, PubMed, Scopus, and Google Scholar. The search was performed using a combination of keywords related to GBM, phytochemicals, nanoparticles, and their various synonyms and medical subject headings (MeSH terms). The search strategy aimed to identify all relevant studies published up to 2024. Studies were included if they met the following criteria: published in the English language, published in peer-reviewed journals, investigated the effects of phytochemicals on GBM, investigated the use of nanoparticles for delivering phytochemicals in GBM treatment, and reported potential pros and cons of phyto-nanomedicine. Studies were excluded if they were not published in English or peer-reviewed journals, did not focus on GBM or phytochemicals unless they provided background knowledge that formed the basis of a particular concept and did not involve the use of nanoparticles for drug delivery in GBM treatment. Study selection and data extraction was performed by two independent reviewers who screened the titles and abstracts of all identified studies to assess their eligibility based on the inclusion and exclusion criteria. Full-text articles of potentially eligible studies were retrieved and assessed for final inclusion. Any discrepancies were resolved through consensus or consultation with a third reviewer. A data collection sheet was used to extract data required for inclusion in the review. Extracted data included study characteristics (e.g., study design), participant characteristics (e.g., study population, sample type), intervention details (e.g., type of phytochemical, type of nanoparticles), and outcomes of interest (e.g., tumor regression, survival rates, potential adverse effects).
Known phytochemicals with anticancer properties
Curcumin
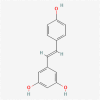
Curcumin is a commonly studied phenolic extract of the Curcuma longa possessing anti-inflammatory, anti-cancer, and reactive oxidative species (ROS) scavenging properties [20, 21]. It can perform these functions due to its unique and intricate structure with the chemical name 1,7-bis (4-hydroxy-3-methoxyphenyl)-1,6-hepadiene-3,5-dione. It has three functional groups namely; two phenolic groups responsible for its multiple functions and 1,3-diketone moiety [21]. Curcumin targets molecular signaling pathways associated with GBM tumor growth, proliferation, and migration [20]. These include but are not limited to Janus kinase (JAK)-signal transducer and activator of transcription (STAT). Curcumin inhibits the activity of JAK upstream of STAT thereby disrupting the JAK/STAT pathway (Table 1) [22]. Of all the STAT family, STAT3 is regarded as the master regulator in glioma and thus targeted due to its tumor initiating capacity and ability to invade the normal brain tissue. The active form of STAT3 was shown to be highly expressed in glioma cells as compared to normal cells [22].
Table 1.
The origin of phytochemicals and their mechanism of action in GBM
| Phytochemical and structure | Origin | Mechanism of action | References |
|---|---|---|---|
|
Curcumin
Molecular formular: C21H20O6 PubChem CID: 969516 [26] |
Curcuma longa | Promotes cell cycle arrest, impedes STAT3 activation, invasiveness, and migratory potential of glioma cell lines | [22] |
| Downregulates GBM cell proliferation, migration, and invasion through p-Akt/mTOR suppression | [27] | ||
|
Luteolin
Molecular formula: C15H10O6 PubChem CID: 5280445 [26] |
Common fruits, vegetables and herbs | Suppresses IL-1β-induced phosphorylation of JNK, ERK, IκB, and NF-κB | [28] |
| Inhibits cell migration and epithelial mesenchymal transition (EMT) by disrupting the activation of p-IGF-1R/PI3K/Akt/mTOR signaling pathway | [29] | ||
| Reduces cell proliferation by inhibiting the Akt and MAPK signaling pathways | [30] | ||
|
Zerumbone
Molecular formula: C15H22O PubChem CID: 5470187 [26] |
Zingiber zerumbet Smith | Exerts cytotoxic effects by generating ROS in malignant GBM cells | [31] |
| Inhibits invasion and metastasis by downregulating the expression of MMPs | [32] | ||
|
Quercetin
Molecular formula: C15H10O7 PubChem CID: 5280343 [26] |
Fruits, vegetables and in Chinese medicine | Inhibits EMT by downregulating the GSK-3β/β-catenin/ZEB1 pathway | [33] |
| Inhibits the activation of glycoprotein 130 (gP130), JAK1 and STAT3 mediated by IL-6 | [34] | ||
| Inhibits cell proliferation and migration by downregulating the expression of Cyclin D1 and MMP-2 | |||
| Inhibits GBM cell migration and angiogenesis at low concentration by down regulating MMPs and VEGF expression respectively | [35] | ||
|
Resveratrol
Molecular formula: C14H12O3 PubChem CID: 445154 [26] |
Fruits, vegetable, Chinese and Japanese traditional medicine | Targets the Wnt signaling pathway | [36] |
| Inhibits EMT and its associated molecules such as Twist and Snail | |||
| Other phytochemicals with anti-GBM activity | |||
|
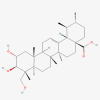
Asiatic acid
Molecular formula: C30H48O5 Pubchem CID: 119034 [26] |
Centella asiatica | Inhibits angiogenesis by suppressing the release of VEGF by LN18 and U87-MG cells | [37] |
|
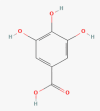
Gallic acid
Molecular formula: C7H6O5 Pubchem CID: 370 [26] |
Chinese gall | Inhibits invasion by suppressing the expression and activity of ADAM17, and downregulating Ras/MAPK and PI3K/Akt signaling pathways | [38] |
|
Methyl gallate
Molecular formula: C8H8O5 Pubchem CID: 7428 [26] |
Acer barbinerve | Inhibits glioma cell migration by suppressing the phosphorylation of paxillin, Akt and ERK1/2 | [39] |
|
Nobiletin
Molecular formula: C21H22O8 Pubchem CID: 72344 [26] |
Shiikuwasa, oranges and lemons | Inhibits tumor cell proliferation and migration by suppressing MAPK and Akt pathways | [40] |
| Citrus fruits | Inhibits cell proliferation by downregulating the Ras and MEK/ERK pathways | [41] | |
| Inhibits invasion by downregulating AKT/GSK3β/β-catenin pathways | [42] | ||
|
Fucoxanthin
Molecular formula: C42H58O6 Pubchem CID: 5281239 [26] |
Seaweed | Inhibits invasion and migration by downregulating p38/MMP-2/9 pathways | [43] |
IL-1β, Interleukin 1beta; mTOR, Mammalian target of rapamycin; p-Akt, Phosphorylated protein kinase B; JNK, Jun N-terminal kinase; ERK, Extracellular signal-regulated protein kinases; IκB, Inhibitor of nuclear factor kappa B; NF-κB, Nuclear factor kappa B; pIGF1R, Phosphorylated insulin like growth factor 1 receptor; PI3K, Phosphoinositide 3-kinase; MAPK, Mitogen-activated protein kinase; GSK-3β, Glycogen synthase kinase-3 beta; ZEB1, Zinc-finger E-box-binding homeobox 1; GP130, Glycoprotein; IL-6, Interleukin 6; VEGF, Vascular endothelial growth factor; EMT, Epithelial mesenchymal transition; ADAM17, A disintegrin and metalloproteinase 17; Wnt, Wingless related integrated site; Ras, Rat sarcoma virus and MEK, Mitogen activated protein kinase kinase
Furthermore, JAK/STAT signaling pathways are implicated in GBM hallmarks such as tumor invasion, migration and progression. This was shown by a study that found IL-8 to induce STAT/hypoxia-inducible factor-1α (HIF-1α)/Snail pathway, resulting in tumor cell epithelial-mesenchymal transition (EMT), invasion and migration [23]. EMT is a reversible process of transforming epithelial cells into a mesenchymal phenotype with an enhanced cell mobility potential, which is known to play a role in metastasis [24]. Curcumin was shown to downregulate in a dose-dependent manner STAT target genes including cellular myelocytomatosis oncogene (c-Myc), matrix metalloproteinase-9 (MMP-9), Snail, and Twist, as well as glioma cells migratory and invasive behaviour, thereby suggesting curcumin can provide a beneficial effect for treating GBM [22]. Furthermore, Curcumin decreased the proliferation of GBM stem cells (GSC), their colony formation and sphere forming potential, induced the activation of mitogen-activated protein kinase (MAPK) and the downregulation of STAT3 through dephosphorylation at Tyr705 and increasing phosphorylation at Ser727 in an ROS dependent manner [25]. Table 1 presents the chemical structures of various phytochemicals and provides a summarised description of their modes of action. It includes information on how these phytochemicals interact with cellular pathways, their potential therapeutic effects, and the specific mechanisms through which they inhibit GBM cell proliferation, migration, and invasion.
Another pathway targeted by curcumin is protein kinase B/Mammalian target of rapamycin (Akt/mTOR) pathway [27] with evidence demonstrating this pathway to be stimulated in approximately 90% of GBM [44]. GBM patients with activated phosphoinositide 3-kinase (PI3K)/Akt/mTOR pathway have been shown to have poor prognosis [45]. Active Akt activates the mTORC1 through the inhibition of tuberous sclerosis 1/2 (TSC1/2) [46] leading to mTORC1 mediated protein, ribosome biogenesis and cell growth. mTOR can function as an upstream regulator and as a downstream effector. It comprises mTORC1 and 2 which are functionally and structurally distinct complexes. mTORC2 is associated with cell survival, proliferation, metabolism, and cytoskeletal organization [47]. The cytoskeletal organization pathway is crucial for achieving stem cell migration resulting in aggressive invasion in GBM [48]. Curcumin targets the Akt/mTOR pathway by downregulating the expression of phospho-Akt and phospho-mTOR protein resulting in a reduction in GBM cell proliferation, invasion, and migration (Table 1) [27]. Furthermore, curcumin in combination with temozolomide generated ROS, which were associated with a decrease in AKT/mTOR phosphorylation leading to the suppression of AKT/mTOR signaling. This resulted in GBM cell death [49]. It is therefore evident that curcumin targets both the JAK/STAT and Akt/mTOR signalling pathways to execute its anti-cancer effect, thus indicating a potential application of curcumin in developing therapies for combating GBM (Fig. 1).
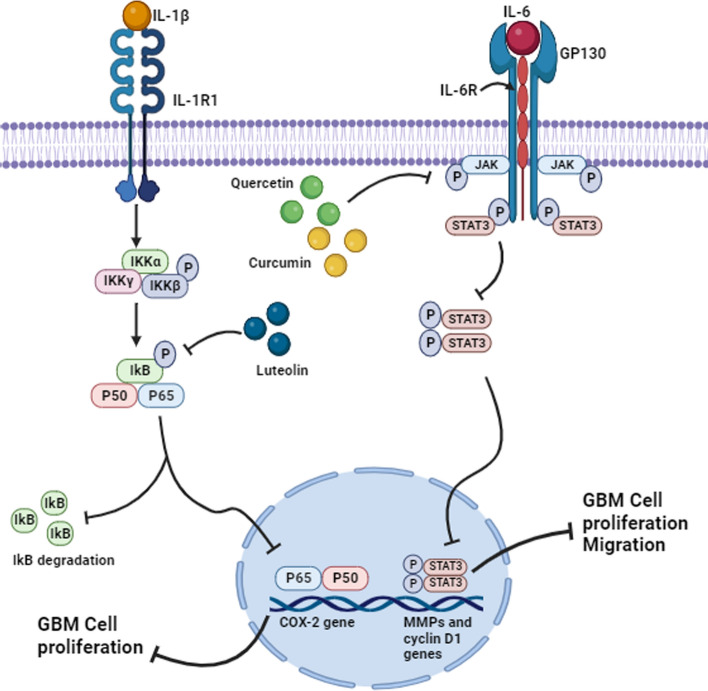
Fig. 1.
Pathways targeted by luteolin, quercetin and curcumin in GBM. Luteolin inhibits IL-1β mediated phosphorylation of IkB and NF-kB complex resulting in the inhibition of the gene expression of COX-2. This suppressed GBM cell proliferation. Quercetin and curcumin inhibited the phosphorylation of JAK as a result inhibiting the activation of other molecules downstream of JAK. Consequently, this downregulates the gene expression of MMPs and cyclin D1 suppressing GBM cell proliferation and migration. Image created with Biorender.com, accessed on 07 November 2023
Luteolin
Luteolin (3′,4′,5,7-tetrahydroxyflavone) is a commonly known flavonoid present in many plant types and possess anti-cancer, neuroprotective, and anti-inflammatory properties [28]. Luteolin occurs in high levels in common herbs, fruits, and vegetables [28, 50]. It has been shown to act as an anti-cancer agent in multiple cancers such as colon, lung, prostate, pancreatic, breast, and GBM. Luteolin impedes tumor development both in-vitro and in-vivo by blocking tumor cell proliferation, promoting cell cycle arrest and apoptosis by targeting the signaling pathways involved [50]. One such pathway is the interleukin 1 beta/Nuclear factor kappa B/Cyclooxygenase-2 (IL-1β/NF-kB/COX-2) signaling pathway (Fig. 1). IL-1β normally binds to its type 1 receptor (IL-1R1) and phosphorylates a cascade of transcription factors that leads to nuclear factor kappa-light-chain-enhancer of activated B (NF-κB) activation. This is followed by translocation of the p65 and p50 subunits of an activated NF-κB to the nucleus and ultimately binding to the promoter region of the cyclooxygenase-2 (COX-2) gene to promote its expression. Upregulated levels of COX-2 have been detected in several malignancies including GBM and are associated with increased tumor proliferation and shorter patient survival rates (Table 1) [28].
Another study showed that luteolin promotes apoptosis by inhibiting autophagy which is reported to be a survival strategy for GBM cells. However, the authors suggested that these findings need to be investigated further [51]. Treatment of GBM cell lines with luteolin blocked EMT and downregulated the expression of EMT associated proteins such as β-catenin, N-cadherin, and Vimentin while upregulating E-cadherin [29]. E-cadherin is usually downregulated in cancer because it is associated with a fully differentiated epithelium since it functions to sustain cell–cell junctions thereby inhibiting abnormal proliferation and migration however, its loss is associated with tumor invasiveness [52].
Furthermore, luteolin downregulated the expression of MMPs while upregulating the expression of inhibitors of the MMPs known as tissue inhibitor of metalloproteinase (TIMPs). Luteolin was shown to inhibit GBM cell migration by downregulating the phosphorylated insulin-like growth factor-1 receptor (p-IGF-1R)/PI3K/Akt/mTOR signaling pathway (Table 1) [29]. The p-IGF-1R/PI3K/Akt/mTOR signaling pathway is induced and activated by the binding of IGF-1R to its ligand leading to autophosphorylation of insulin receptor substrate 1 (IRS-1) [53]. Subsequently, PI3K is activated through the attachment of its regulatory subunit via the SH2 domain to the IRS-1 resulting in increased phosphatidylinositol 3,4,5-trisphosphate (PIP3) [54]. IGF-1R is upregulated in most cancer types and its inhibition is associated with tumor regression due to its involvement in the activation of PI3K/Akt/mTOR pathway that is associated with tumor cell migration [29]. An overexpression of epidermal growth factor receptor (EGFR) has been reported in many malignancies including GBM. EGFR modulates receptor tyrosine kinases (RTK)/RAS/PI3K activation. These pathways are involved in the regulation of angiogenesis, cell proliferation, and invasion. The treatment of GBM cell lines with luteolin promoted apoptosis and reduced EGFR-induced cell proliferation as well as the Akt and MAPK signaling pathways downstream of EGFR [30]. In summary, there is substantial evidence demonstrating luteolin acts through multiple pathways such as RTK/RAS/PI3K, IL-1β/NF-kB/COX-2, and p-IGF-1R/PI3K/Akt/mTOR to inhibit GBM cell proliferation and migration while promoting apoptosis, thereby making it a good candidate drug for GBM treatment.
Zerumbone
The rhizomes of Zingiber zerumbet Smith contain a sesquiterpenoid compound called zerumbone, also known as 2,6,9,9-tetramethyl-[2E,6E,10E-cycloundeca-2,6,10-trien-1-one] [31]. This compound possesses antioxidant, immunomodulatory, anti-microbial [32], anti-inflammatory, and anti-tumor properties [55]. Zerumbone has been shown to promote cytotoxicity through the generation of ROS in GBM cell line, Table 1 [31]. Zerumbone plays an important role in suppressing GBM cell migration and invasion. Its mechanism of action targets pathways leading to the expression of the MMPs, Table 1, which are proteolytic enzymes that degrade extracellular matrix during invasion and metastasis in other solid tumors such as breast, liver, and cervical cancer [56–58]. Targeted molecules involved in this process includes Erk1/Erk2 and Akt, which are the transcription promoters of MMP-2 and -9 via the MAPK signaling pathway. Zerumbone targets Erk1/Erk2 and Akt to downregulate the expression of MMPs and ultimately tumor invasion [32]. Other targeted molecules include IL-1β and monocyte chemoattractant protein-1 (MCP-1), which are associated with MMP expression inhibited by zerumbone [32].
Quercetin
Quercetin is a flavonol commonly found in various fruits, vegetables, and in certain Chinese medicines. It can suppress cell viability and inhibit invasion and migration by blocking EMT process via downregulation of the glycogen synthase kinase-3 beta (GSK-3β)/β-catenin/Zinc finger E-box binding homeobox 1 (ZEB1) signaling pathway in-vitro and in-vivo (Table 1) [33]. Furthermore, quercetin inhibited angiogenesis and cell migration at low concentrations in human GBM cells which was associated with the downregulation of VEGF and, MMP-2 and -9 (Table 1). VEGF is a potent angiogenic factor and MMPs are responsible for dissociating the ECM leading to cell migration [35]. Exposure of T98G and U87 GBM cell lines to quercetin inhibited IL-6, which is overexpressed in many tumors including GBM and also regulates various pathways involved in cell proliferation, invasion, and migration. Quercetin targets most of the molecules involved in the IL-6 signaling pathways including GP130 and JAK1, which may potentially affect their downstream signaling molecules in GBM cell lines (Table 1). Under normal physiological conditions, the binding of IL-6 to IL-6Rα induces glycoprotein 130 (GP130) [34]. This leads to the phosphorylation of GP130 followed by JAK activation. These events stimulate various pathways such as STAT, Ras-MAPK, and PI3K. STAT3 inhibits apoptosis and promotes immune evasion, cell cycle progression, cell migration, angiogenesis, and invasion. Thus, the inhibition of STAT3 phosphorylation by quercetin suppressed cell proliferation and migration through the downregulation of cyclin D1 and MMP-2 expression (Table 1) (Fig. 1) [34].
Resveratrol
Resveratrol also known as 3,4′,5-stilbenetriol, (E)-5-(7-hydroxystyryl) resorcinol, (E)-5-(4-hydroxystyryl) benzene-1,3-diol is a polyphenolic phytoalexin found in fruits, and some Japanese and Chinese traditional medicine [59] and vegetables. This polyphenol possesses many health benefits such as antioxidant, anti-inflammatory, and anti-cancer properties. Its lipophilic structure allows it to pass through the BBB via simple diffusion [60]. The therapeutic potential of resveratrol has been demonstrated in the 3 stages of tumorigenesis namely initiation, promotion, and progression. Additionally, other studies have demonstrated its anti-cancer effects such as anti-proliferative, pro-apoptotic, and anti-migratory in various cancer cells [36]. Resveratrol can impact aberrantly activated self-renewal pathways of cancer stem cells directly or indirectly. It has also been indicated that some cancer incidences are reduced in people consuming high amounts of this dietary phytoestrogen [36]. This compound is harmless to normal cells or tissues of the CNS however, it’s not commonly used in practice due to its low bioavailability [60]. Resveratrol can reduce GBM stem cell viability, motility, and proliferation and also targets the Wnt signaling pathway (Table 1) to reduce nuclear β-catenin and c-Myc protein levels although, it can also induce transcriptional upregulation of MYC [36]. MYC pathway is generally initiated by the binding of the Wnt protein ligands to the frizzled (FZD) and low-density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor (LRP) families on the cell surface. This results in the dissociation of AXIN, adenomatous polyposis coli (APC), and GSK3β complex, which stabilizes β-catenin. Subsequently, β-catenin migrates into the nucleus where it interacts with T-cell factor/lymphoid enhancer factor (TCF/LEF) and promotes transcription of target genes including MYC (Fig. 2). Wnt is aberrantly activated in GBM and regulates self-renewal of GBM stem cells, differentiation and migration [61], which are associated with tumor initiation, progression and relapse [36, 61]. Moreover, resveratrol supresses Twist1 and Snail proteins (Table 1) [36], which are crucial for EMT activation that is linked to tumor invasiveness and metastatic capacity [62]. Therefore, the ability of resveratrol to cross the BBB and specifically target the tumor microenvironment makes it a potential anti-GBM therapeutic agent. Other phytochemicals with demonstrated therapeutic effect against GBM have been listed in Table 1.
Fig. 2.
Schematic illustration of the potential nano-delivery of resveratrol to GBM cells to modulate the Wnt pathway. The administrated pep-1 modified polyethylene glycol-b-polycaprolactone (PEG-b-PCL; PP) containing resveratrol (Pep-PP@Res) interacts with GBM cells expressing IL-13Rα2 via its pep-1 component, a ligand for IL-13Rα2 thus, increasing specificity. Thereafter, resveratrol exerts its effects on GBM cells by modulating the Wnt pathway. This pathway initiates by the binding of Wnt protein ligands to FZD and LRP receptor resulting in the dissociation of AXIN, APC, and GSK3β complex, which stabilizes β-catenin. The presence of resveratrol results in a reduced nuclear β-catenin, and the downregulation of MYC transcription and c-Myc protein levels, which inhibits EMT and cell migration. Image created with Biorender.com, accessed on 06 November 2023
Asiatic acid
Asiatic acid (AA), derived from the tropical medicinal plant, Centella asiatica, is a pentacyclic triterpenoid with a widely reported anti-cancer effect. Treatment of malignant glioma cells with AA induces apoptosis through an increase in intracellular calcium release [63]. Furthermore, AA inhibits the pro-angiogenic effect of human glioma cells by inhibiting tube formation induced by glioma conditioned media and invasiveness in endothelial cells [37]. AA significantly decreases cell viability of U87-MG grade IV glioblastoma cells in a time and concentration-dependent manner under normoxic (21% O2) conditions and when compared to cisplatin which showed no changes in cell cycle progression or proliferation. Furthermore, AA induced apoptosis in a greater number of cells compared to cisplatin under hypoxia conditions demonstrating a strong cytotoxic effect on glioma cells [64]. Also, AA administered orally to nude mice with orthotopic U87MG xenograft growth strongly decreased tumor volume in-vivo. Liquid chromatography-mass spectrometry (LC/MS)-MS was used to confirm the presence of AA in brain tissue, demonstrating it crosses the BBB to exhibit its effect [65]. In this model, AA was shown to induce apoptosis by modulating the expression of caspases, Bcl-2 family members, and survivin, as well as inducing endoplasmic reticulum stress. These combined effects highlight the effectiveness of AA against GBM [65] and prompt its consideration as an alternative treatment.
Gallic acid
Gallic acid commonly found in Chinese gall (gallnut) is known to show selective cytotoxicity against certain cancer cells was used in a study to treat U251n glioma cells and showed dose and time-dependent inhibition of cell viability [38]. Furthermore, it inhibited tube formation in endothelial cells of the mouse brain and significantly decreased the proliferation of glioma cells. The gallic acid suppressed the expression of a disintegrin and metalloprotease 17 (ADAM17), p-Akt and p-Erk in glioma cells, suggesting the inhibition of Ras/MAPK and PI3K/Akt signaling pathways may be a possible mechanism through which gallic acid decreases GBM invasiveness [38]. Gallic acid is also shown to inhibit the proliferation of T98G human glioblastoma cell line in a dose-dependent manner mediated by miRNA expression [66]. Gallic acid reduced tumor volume by 90% in preclinical model of GBM demonstrating a decrease in oxidative damage induced by the tumor in-vivo. Furthermore, it also showed a decrease in oxidative stress on rat GBM (C6) cell line in-vitro [67].
Development of specific nanotechnologies with effective delivery systems and targeted specificity
Brain tumors are hard-to-treat cancers owing to the low efficacy of the current drugs, difficulties in surgical resection, and high recurrence rates [68]. The BBB poses a major challenge for the treatment of GBM due to its high selectivity. The BBB is a semi-permeable defensive membrane separating the brain from the circulatory system [69] and protecting the brain from internal and external molecules [68]. Its selectivity is due to tight junctions created by endothelial cells’ efflux transporter system that actively removes molecules from the brain and cerebrospinal fluid into the circulation [69, 70] and metabolizing enzymes which inactivate drugs before they can even reach the tumor site [70]. The tight protein junctions restrict passage to only a few hydrophilic small molecules whereas other macromolecular chemotherapeutics are unable to pass through and instead end up in untargeted tissues thereby damaging healthy cells [68]. Only a few administered drugs can reach the brain. An attempt to increase the transport of drugs to the brain was through the increased dosage of drugs which led to unpleasant side effects [69]. In addition to the normal BBB, other factors acting as obstacles to chemotherapeutic agents include the heterogeneity of GBM tumor BBB, the presence of cancer stem cells as well as the BBTB [71]. The BBTB in low-grade tumors may be intact but is compromised in high-grade tumors as it becomes leakier. It remains intact in invaded regions to protect the newly formed tumor from foreign molecules such as therapeutic agents. This fortification negatively impacts GBM treatment efficacy as these areas are unresectable and may result in tumor recurrence [18].
Therefore, nano-delivery systems offer a wide range of advantages enabling drug delivery to target tissues with high specificity and reduced side effects [69]. Additional benefits of nanocarriers include enhanced solubility, increased drug bioactivity, sustained drug release, BBB penetrability, and biosafety. Nanocarriers can be classified based on their method of preparation into NPs, nanocapsules, and nanospheres. NPs are increasingly being explored and used in the treatment of GBMs [70]. NPs are classified according to their composition into organic NPs example, peptide-based [72], polymeric NPs including polylactic acid (PLA), PEG, and poly (D, l -lactic-co-glycolic acid (PLGA) [73]. Dendrimers include polyamidoamine (PAMAM), polypropylene Imine (PPI), and polyester dendrimers [74, 75]. Lipid NPs are liposomes, micelles, solid lipid nanoparticles (SLNPs) [73, 76], and niosomes [73]. In addition to organic NPs, inorganic NPs include metal-based, metal oxide [72, 73], ceramic, and semiconductor [73], carbon-based NPs namely fullerenes, graphene, carbon nanotubes, and carbon nanofiber [72, 77], and lastly composites consisting of more than one type of NPs [72, 78].
Nano-delivery of curcumin
Curcumin has a limited therapeutic potential owing to its poor solubility and bioavailability in aqueous solutions [79]. NPs have been used to overcome these challenges due to their physicochemical properties [80]. PLGA NPs hold great potential in drug delivery applications due to their biodegradability, biocompatibility, and versatility properties [79]. PLGA is a United States Food and Drug Administration (FDA) approved biodegradable polymer used to formulate NPs that are utilized in targeted drug delivery. The metabolites generated from the breakdown of PLGA NPs include glycolic acid and lactic acid endogenous to the body which minimises its toxicity [81]. The use of anti-epidermal growth factor receptor variant III (EGFRvIII) monoclonal antibody targeted PLGA NPs (anti-EGFRvIII-PLGA) delivered curcumin with an increased specificity and enhanced its concentration in EGFRvIII overexpressing cells [79]. Other PLGA-based NPs utilised for delivery of curcumin include PLGA-1,2-distearoyl-glycerol-3-phospho-ethanolamine-N- [methoxy (PEG)-2000] ammonium salt (DSPE-mPEG), which consists of PLGA and DSPE bound PEG [82]. PEG was approved by the FDA as a vehicle in pharmaceutical preparations. PEGylation enhances drug circulation time and stability [83]. Curcumin-loaded PLGA-DSPE-mPEG NPs were shown to have a cytotoxic effect on rat glioma cells. They also attenuated tumor growth and significantly reduced its size as compared to curcumin alone and untreated control groups after intra tumoral administration [82]. Senturk et al. synthesized magnetic polymeric NPs (mPNPs) consisting of superparamagnetic iron oxide NPs (SPIONs), which were coated with PLGA-PEG di-block copolymer. These NPs released approximately 70% of curcumin loaded in a controlled manner during a 72-h period. The conjugation of these NPs with glycine-arginine-glycine-aspartic acid-serine (GRGDS) enhanced cellular uptake of the NPs by interacting with specific cell receptors as a result augmenting the bioavailability of curcumin. The treatment of GBM cells with these NPs loaded with curcumin demonstrated an impressive cytotoxicity which was reported to be enhanced by the presence of GRGD [84]. Another method of delivering curcumin is through the utilization of dodecamer peptide-functionalized polydopamine-coated zein (CUR-ZpD-G23 NPs). Zein is an alcohol-soluble protein that has emerged as an ideal drug delivery system due to its biocompatibility and biodegradability. The coating of zein with polydopamine layer increased its stability, hydrophilicity, and reactivity with nucleophilic compounds. They were further functionalized with the G23 dodecamer peptide capable of facilitating the crossing of BBB by RNA-binding proteins, polymersomes, and iron oxide NPs. The NPs enhanced uptake and high penetration of curcumin into the 3D tumor spheroids of C6 glioma cells compared to free curcumin. NPs-coated curcumin inhibited cell migration, decreased ROS generation, and induced apoptosis. The G23-functionalized NPs enhanced transcytosis across BBB in an in-vitro model and also exhibited the ability to circulate which is a requirement for utilization of targeted drug delivery (Fig. 3) [85].
Fig. 3.
A schematic illustration showing NPs and the roles of phytochemicals in targeting GBM. Curcumin acts as an inhibitor of cell migration, proliferation, and apoptosis in GBM. NPs that have demonstrated a potential as carriers of curcumin include Anti-EGFRvIII-PLGA, PLGA-DSPE-mPEG, mPNPs, ZpD-G23, mPEG-PCL, and micellar curcumin. Dextran-coated SPIONs, polymeric mixed micelles (PMMs), PLGA NPs, freeze-dried micelles, and quercetin NPs (qNPs) shuttles quercetin and downregulates angiogenesis, cell migration and proliferation within GBM tumor micro environment (TME). Luteolin is delivered by folic acid-modified PEG-PCL (Fa-PEG-PCL) and mPEG-PCL to the TME where it inhibits EMT, cancer cell migration and proliferation. Resveratrol targets EMT and its nanocarriers are Pep-PP, transferrin modified PEGylated liposomes (Tf-PEG-Lip), mPEG-PCL, Tf-PEG-PLA, SPIONs-3-chloropropyltriethoxysilane (CPTES), and solid lipid NPs (SLNPs). AA inhibits angiogenesis and promotes apoptosis within GBM TME and its potential carriers to the GBM TME include PCL, glyceryl monostereate (MS)-SLNPs, Tf-PLGA and Arginyl-glycyl-aspartic acid (RGD)-SLNPs. Gallic acid inhibits GBM cell proliferation and promote apoptosis. Its nanocarriers include gold nanoparticles (GNPs), iron–gallic acid/bovine serum albumin (Fe-GA/BSA), and dextran-coated magnetic nanoparticles (MNPs). Image created with Biorender.com, accessed on 08 June 2024
mPEG-PCL are nanoscale self-assembled spherical structures with PEG as the hydrophilic outer segment and PCL as the hydrophobic inner core. The lipophilic drugs are embedded within the PCL which has an excellent drug encapsulation capacity and PEG protects the NPs from scavenging activity of the reticuloendothelial systems. PEG is suitable for use as an outer component due to its good biocompatibility, hydrophilicity, reduced toxicity, and low immunogenicity [86]. The use of mPEG-PCL offers an improved strategy to enhance the efficacy of curcumin. mPEG-PCL encapsulated curcumin has a high drug-loading content and encapsulation efficiency. This could be due to the high binding affinity between curcumin and the PCL. Furthermore, 20% of curcumin load burst from the NPs within the first 10 h and 80% slowly dissociated from the NPs in the remaining hours 120 h of the study period providing a more sustained release [86]. This is very beneficial since curcumin metabolizes faster [87] and this will allow for a prolonged release of curcumin into the target cells at a controlled rate [88]. The oral administration of micellar curcumin in pre-operative GBM patients over 4 days (3 times per day) resulted in low bioavailability of curcumin in tumor area as compared to serum levels. These levels of curcumin were insufficient to generate short-term effects but seemed to be high enough to generate long-term antitumor effects (Fig. 3) (Table 2) [89].
Table 2.
Examples of potential nano-delivery systems for various phytochemicals in the treatment of GBM
| Phytochemical | Nano delivery system | Properties | References |
|---|---|---|---|
| Curcumin | Anti-EGFRvIII-PLGA | Biocompatible, biodegradable, and versatile with high specificity towards EGFRvIII overexpressing cells | [79, 82, 84] |
| PLGA-DSPE-mPEG | Narrow particle size distribution and provides prolonged curcumin release (168 h) | [82] | |
| m-PNPs | Controlled curcumin release and can be conjugated with GRGDS for enhanced bioavailability of curcumin | [84] | |
| mPEG-PCL | High drug encapsulation efficiency, high drug loading content, slow curcumin release (120 h for sustained drug exposure, with approximately 20% burst from the NP surface within the first 10 h | [86] | |
| Micellar NPs | Low bioavailability of curcumin in tumor and high serum curcumin concentrations | [89] | |
| ZpD-G23 NPs | Biocompatible, biodegradable, stable with an increased hydrophilicity, reactivity and ability to circulate and, can cross the BBB via trans endocytosis | [85] | |
| Chitosan coated mesoporous silica NPs | Nontoxic, biocompatible, and biodegradable. Chitosan coating is pH sensitive, making it suitable for controlled drug delivery | [112] | |
| Niosome NPs | Safe, biocompatible, biodegradable, non-immunogenic, able to cross the BBB with high curcumin encapsulation efficiency and release drugs in a sustained manner | [87] | |
| PBAE (poly(beta-amino ester) | Highly selective and suitable for the delivery of drugs in a steady, controlled manner | [113] | |
| Luteolin | mPEG-PCL | Stable, biodegradable, soluble in aqueous solution, improves drug bioavailability, retention at tumor site and selectivity | [92] |
| Fa-PEG-PCL | Slow drug release to maintain the desirable drug concentration at tumor region. Smaller diameter to facilitate active diffusion through tumor cell intercellular junctions | [91] | |
| Quercetin | Dextran coated SPIONs | Less toxic, suitable for targeted drug delivery systems due to their magnetic properties, increased quercetin bioavailability in brain. Biodistribution of quercetin can be regulated with the aid of an external magnet | [94] |
| PMMs | Stable for over 60 days, have a high encapsulation capacity, release quercetin in a sustained manner and have an enhanced safety and efficacy | [95] | |
| PLGA NPs | High cellular uptake, small in size and improves solubility and bioavailability of quercetin | [96] | |
| Freeze-dried Polymeric micelles | Released quercetin in a sustained drug release pattern, increased the concentration of quercetin at primary tumor tissues and its cellular uptake | [97] | |
| Resveratrol | Pep-PP | Highly selective for IL-13Rα2 overexpressing GBM cells, specific and enhances the intracellular retention of resveratrol | [98] |
| mPEG-PCL | Encapsulation efficiency of over 90% and a sustained drug release | [99] | |
| Tf-PEG-Lip | Selective for transferrin overexpressing tumor cells, able to cross the BBB, stable, easily scalable, have a sustained drug release pattern and a good drug loading capacity | [101] | |
| Tf-PEG-PLA | Cross the brain through Tf mediated transcytosis, increases resveratrol’s cellular uptake and have less toxicity | [102] | |
| SPIONs-CPTES | Small in size, high surface to volume ratio, high magnetic moment, biocompatible and surface coating consisting of HAPtS and CPTES enhances passage via the BBB barrier | [103] | |
| SLNPs | Small sized, biodegradable, biocompatible, able to cross the BBB, released resveratrol in a sustained drug release pattern and enhanced resveratrol’s brain concentration | [104] | |
| Asiasic acid | PCL | Sustained drug release pattern and effective at low half-maximal inhibitory concentration (IC50) | [105] |
| MS-SLNPs | Small in size, high drug loading capacity and reduced toxicity | [106] | |
| Tf-PLGA | High selectivity, cellular intake, encapsulation efficiency and slow drug release | [107] | |
| RGD-SLNPs | High selectivity and penetration capacity in spheroid models | [108] | |
| Gallic acid | GNPs | Small in size, biocompatible, low biotoxicity and easy to be synthesized | [109] |
| Fe–GA/BSA | pH-responsiveness, good biocompatibility and low toxicity | [110] | |
| Dextran-coated MNPs | Enhanced stability, superparamagnetism and increased cellular internalization | [111] |
EGFRvIII, Epidermal growth factor receptor variant III; PLGA, Poly (D, l-lactic-co-glycolic acid); DSPE-mPEG, 1,2-Distearoyl-glycerol-3-phospho-ethanolamine-N-[methoxy (polyethylene glycol)-2000] ammonium salt; mPNPs, Magnetic polymeric nanoparticles; ZpD-G23, Dodecamer peptide-functionalized polydopamine-coated zein; PCL, Polycaprolactone; SPIONs, Superparamagnetic iron oxide nanoparticles; PMM, Polymeric mixed micelles; qNPs, Quercetin nanoparticles; Fa, Iron; Pep-PP, Pep 1-PEG-b-PCL; lip, Liposomes; Tf, Transferrin; CPTES, 3-Chloropropyltriethoxysilane; SLNPs, Solid lipid NPs; MS, Glyceryl monostereate; RGD, Arginyl-glycyl-aspartic acid; GNPs, Gold nanoparticles; GA/BSA, Gallic acid/bovine serum albumin; PLA, Poly lactic acid and MNPs, Magnetic nanoparticles
Nano-delivery of luteolin
Luteolin has many health benefits which are limited because of low oral bioavailability and water solubility due to its hydrophobic nature [90]. It can cross the BBB and reach the target areas. However, there is difficulty in maintaining its effective concentration for inhibiting or killing tumor cells [91]. The encapsulation of luteolin in mPEG-PCL micelles (Lu-mPEG-PCL) enhanced its solubility and allowed its dispersion in water due to the presence of the hydrophilic outer part of the micelle. In addition, the gradual degradation of mPEG-PCL micelles allows for luteolin to be released slowly thereby improving its bioavailability at tumor sites. The 36 nm size of the self-synthesized mPEG-PCL micelles permits their diffusion into tumor area with ease since it is smaller than the size of the interendothelial junctions [92]. Another study has looked at the nano-delivery of luteolin using folic acid-modified PEG-PCL (Fa-PEG-PCL) NPs in-vitro and in-vivo. The folic acid used to modify PEG-PCL assisted in achieving enhanced targeted therapy through the recognition of its folate receptor, which is highly expressed in many tumors. Furthermore, this NP is 34.7 nm in diameter and the tumor intercellular junctions are usually 40 to 80 nm making it easier for their passage through the BBB via diffusion. Fa-PEG-PCL sustained luteolin drug release, demonstrated a greater inhibitory effect on cell proliferation and angiogenesis, and significantly induced cell apoptosis (Fig. 3) (Table 2) [91].
Nano-delivery of quercetin
The NPs that can carry up to 73% payload quercetin capable of recognising tumor vasculature with high specificity and efficiency without the need for a modification have been utilized in glioma mouse models. These qNPs disrupt existing tumor vasculature after administration in glioma mouse models, enhancing drug delivery to brain tumors and improving the survival of mice. This strategy may assist in addressing quercetin’s clinical limitations such as low water solubility, poor bioavailability, rapid clearance, enzymatic degradation, and metabolism [93]. SPIONs could be used as targeted delivery systems for transporting drugs to the brain with the help of an external magnet. Dextran-coated SPIONs augment the bioavailability and delivery of the poorly water-soluble quercetin to the brain. The shape, size, structure, and coating of the iron oxide NPs have a significant effect on cellular uptake, cytotoxicity, distribution, and clearance [94]. Paranthaman and colleagues synthesized PMMs consisting of soluplus, poloxamer 407 and Vitamin-E polyethyleneglycol-1000 succinate (E-TPGS) using the single emulsion-solvent evaporation method. Soluplus is a biocompatible copolymer comprising of a hydrophilic backbone namely PEG and a lipophilic sidechain called PCL-PVA. E-TPGS is known for enhancing drug solubility, cellular uptake, and systemic residence. Poloxamer 407 is a copolymer comprising ethylene oxide and propylene oxide which can improve the bioavailability of quercetin when used in combination with soluplus. The quercetin-encapsulated PMMs prepared in this study exhibited a sustained release of quercetin as well as improved safety and efficacy [95]. PLGA NPs were also used as nanocarriers of quercetin, which improved its solubility and bioavailability. Furthermore, quercetin-loaded PLGAs have exhibited a higher cell proliferation inhibitory effect on C6 glioma cells compared to free quercetin. This study also revealed that the cellular uptake of quercetin can be enhanced by the NP size [96]. Polymeric micelles have also attracted attention as promising drug delivery systems due to their high drug encapsulation efficiency, high drug retention capacity, biocompatibility, high stability, longevity, and ability to effectively cross the BBB. The nano-delivery of quercetin by freeze-dried polymeric micelles generated a sustained release pattern and increased its concentration at primary tumor tissues and its cellular uptake. Furthermore, this quercetin-loaded NP suppressed tumor growth and improved survival rate in-vivo (Fig. 3) (Table 2) [97].
Nano-delivery of resveratrol
Lin et al. synthesized an NP using PEG-b-PCL modified with Pep-1 for targeted delivery of resveratrol to GBM tumor cells. Pep-1 is an oligo peptide consisting of nine amino acids capable of binding GBM xenografts expressing IL-13Rα2. The resveratrol-loaded Pep-PP@Res exhibited cytotoxic effects on brain tumor cells with minimal toxicity on the normal brain cells. IL-13Rα2 which is overexpressed in GBM cell lines is rarely expressed or absent in normal cells and the presence of Pep-1 helps to create an active target for the NP since Pep-1 can act as a ligand for IL-13Rα2 and thereby increase the specificity of this nano-delivery construct for GBM cells. Moreover, this NP was shown to significantly enhance the release and intracellular retention time of resveratrol, (Fig. 2) [98]. Resveratrol loaded mPEG-PCL also exhibited a higher cytotoxicity than that of the same dosage of resveratrol alone, which was associated with an accumulation of ROS intracellularly, (Fig. 3) [99]. Resveratrol was also co-encapsulated with temolozomide in mPEG-PCL nano-delivery system which enhanced their cytotoxicity and also improved their stability [100]. Another study reported the use of Tf-PEG-Liposomes to deliver resveratrol to GBM cells. Transferrin is a serum glycoprotein that helps to transfer iron into growing cells via the transferrin receptor by clathrin-mediated endocytosis. This receptor is overexpressed in cancer cells to meet the iron demands of the rapidly growing tumor and thus is targeted for selective drug delivery to cancer cells. Transferrin can cross the BBB and enter the brain through receptor mediated endocytosis. Modifying the NP with transferrin allows the NP to reach and interact directly with the tumor cells and ensure effective internalisation into tumor cells as compared to normal cells that do not overexpress transferrin. Moreover, this NP is characterised by stability, prolonged drug release in-vitro, good drug loading capacity and is easily scalable. Resveratrol-loaded Tf-PEG-Lip has significantly produced a great therapeutic response compared to free resveratrol or untargeted PEG-Lip [101]. Guo et al. modified PLA NPs with transferrin (Tf-PLA) for targeted delivery of resveratrol. The treatment of C6 glioma cells with resveratrol-loaded Tf-PEG-PLA NPs resulted in increased cellular uptake of resveratrol by C6 glioma cells compared to cells treated with free resveratrol or PEG-PLA NPs indicating the possible role of Tf in resveratrol cellular uptake. Furthermore, the concentration of resveratrol was higher in tumor tissues as compared to normal tissues of glioma bearing rat treated with resveratrol-loaded PEG-PLA NPs. These resveratrol NPs significantly decreased the tumor size compared to resveratrol only or PEG-PLA NPs and prolonged C6 bearing glioma rat survival [102]. Sallem et al. utilized SPIONs to deliver resveratrol derivative namely 4’-hydroxy-4-(3-aminopropoxy) trans-stilbene (HAPtS) to glioma cells. They synthesized SPIONs and modified their surface using HAPtS with 3-chloropropyltriethoxysilane (CPTES) as a coupling agent. Surface modification of SPIONs enhances their ability to cross BBB as the molecules on the surface of SPIONs interact with endothelial cells. These SPIONs-HAPtS-CPTES significantly inhibited C6 glioma cell proliferation by damaging plasma membrane [103]. SLNPs are 100 to 400 nm sized colloidal NPs consisting of a lipid matrix that is biocompatible, biodegradable and solid at physiological body temperature. SLNPs were synthesised by combining glyceryl behenate as a lipid core with Tween 80 and polyvinyl alcohol as shell materials which were then loaded with resveratrol. Cytotoxicity analysis using 4,5-(dimethylthiazol-2-yl) 2,5-diphenyl-tetrazolium bromide (MTT) assay on C6 glioma cell lines demonstrated that resveratrol retained its anti-glioma activity when loaded in SLNPs since its cytotoxic effect was equivalent to that of free resveratrol. NPs alone did not exhibit any cytotoxic effect. Furthermore, these NPs are able to cross the BBB and in-vivo studies demonstrated an increased concentration of resveratrol in brain tumors which could be attributed to the presence of Tween 80 as it can mimic the endogenous low-density lipoprotein receptors (LDL) and enhance uptake by endothelial cells within the BBB. A sustained drug release pattern was observed in resveratrol-loaded SLNs and was suggested to be dependent on the lipid component of the nanoformulations as resveratrol is soluble in glyceryl behenate and is homogeneously distributed within the lipid matrix (Fig. 3) (Table 2) [104].
Nano-delivery of Asiatic acid
PCL NP loaded with AA showed sustained release of AA in phosphate buffered saline over a 24-h period. Treatment of U87-MG cells with this AA loaded NP showed an IC50 values of 7 mM compared to 50 mM with cisplatin loaded PCL [105], demonstrating a potential use of this NPs for AA delivery for GBM treatment. MS, glyceryl distearate (DS) and glyceryl tristearate (TS) are some of the solid lipids used to produce SLNPs for delivery of AA to glioma cells [106]. AA-loaded MS-SLNPs (AA-MS-SLNPs) showed higher cytotoxicity in U87 MG cells with a favourable drug release profile compared to the other AA-loaded SLNPs. The cytotoxic activity of AA-MS-SLNPs was shown to be significantly higher towards U87 MG cells compared to the normal human foetal glial cells (SVG P12). In addition, the apoptotic activity of this AA-MS-SLNPs on glioma cells was observed to be concentration-dependent [106]. PLGA modified with Tf is another class of NPs with higher encapsulation efficiency for AA showing a slow and controlled release of the phytochemical and is being explored for the delivery of AA to glioma cells [107]. The authors showed that PLGA-loaded AA was able to maintain the anti-glioma activity. The study showed that its modification with Tf increased cellular uptake by glioma cells demonstrating enhanced selectivity for GBM treatment [107]. Arginyl-glycyl-aspartic acid (RGD)-conjugated SLNPs (RGD-SLNPs) have been used for selective targeting of glioma cells by AA [108]. AA-loaded RGD-SLNPs showed higher cytotoxicity, better control of tumor growth with greater penetration in U87-MG spheroid model compared to AA alone or non-RGD-containing SLNPs [108] demonstrating the potential of RGD-SLNPs in improving the efficacy of AA as targeted therapy for GBM (Fig. 3) (Table 2).
Nano-delivery of gallic acid
Gallic acid-loaded gold nanoparticles (GA-GNPs) demonstrated a significant cytotoxic effect on U251 GBM cells and further enhanced cell death when combined with radiation. This combination treatment arrested cells in the S and G2/M phases, thereby triggering apoptosis. This was confirmed by a decrease in BCL-2 and an increase in BAX protein levels post-treatment [109]. This demonstrates the potential of GA-GNPs in delivering GA to glial cells and rendering them sensitive to radiation, thereby suggesting a combination of GA-GNPs and radiation as a possible GBM treatment option. The combination polymer of Fe–gallic acid (Fe–GA) nanosphere prepared with bovine serum albumin (BSA) referred to as Fe–GA/BSA has been used as a drug delivery system because of its pH-responsiveness, good biocompatibility, and minimal side effects [110]. Furthermore, a combination of doxorubicin (DOX), an anti-cancer drug with Fe–GA/BSA (Fe–GA/BSA@DOX), showed an effective anti-tumor effect in glioma-bearing mice [110]. Dextran-coated MNPs have been used to deliver gallic acid into LN-229 cells (human glioma cells). The treatment of LN-229 cells with GA-MNPs showed concentration dependent increase in cell-associated MNPs, demonstrating the importance of using MNPs for gallic acid delivery in the GBM treatment (Fig. 3) (Table 2) [111]. In summary, these studies demonstrate the benefits of using nanoparticles for the delivery of phytochemicals to GBM TME. The encapsulation of phytochemicals in NPs protects the drugs from the external environment, retains the amount of drug loaded, and selectively delivers to GBM tumor cells with an increased bioavailability prolonging the antitumor effect of the phytochemicals.
Decrease in toxicity and prevention of cancer metastasis and recurrence using phyto nanomedicine
GBM is characterized by an aggressive infiltration into surrounding areas [114]. It commonly spreads to adjacent brain tissues [115].There is a high recurrence rate of GBM within 7 months post-diagnosis [10] and even following surgical resections[116].Currently, there are no treatment strategies that target invasive tumor cells [114]. Most phytochemicals have demonstrated the ability to mitigate GBM metastasis in pre-clinical studies [16]. However, their efficacy in-vivo is hindered by their poor physicochemical properties [13]. The use of NPs have demonstrated their potential in carrying these phytochemicals across BBB into GBM TME [19]. However, the effect of phyto-NPs on GBM metastasis is not well studied as there are few pre-clinical studies that have reported this matter (which are discussed below) according to the literature consulted.
Targeting GBM cell migration using phyto-NPs
Targeting the migratory potential of GBM cells may help reduce its recurrence [117] and prolong patient survival [118]. Mesoporous silica nanoparticles possess great physicochemical properties such as low toxicity [119], modifiable pore size, biocompatibility, relatively high surface area, adjustable surface coatings, and excellent stability and as a result, they have been studied as promising NPs for the delivery of drugs at various target sites within the body [120]. Pre-clinical studies in breast cancer cells have reported that mesoporous silica nanoparticles modified with hyaluronic acid or PLGA for the delivery of curcumin inhibited tumor cell migration [121, 122]. Due to the potential anti-migratory effects of curcumin-NPs demonstrated in these studies, future research should focus on testing these formulations in GBM in-vitro models and other phyto-NP combinations on the migratory potential of GBM cells.
Targeting GBM stem cells using phyto-NPs
GBM stem cells have gained attention as targets in brain tumor therapies [123] due to their migration, self-renewal, and treatment resistance properties, implicating them in tumor metastasis and recurrence [124, 125]. The administration of curcumin embedded within niosome NPs (Cur-niNP) significantly reduced GBM stem cell viability, proliferation, and migration through multiple molecular mechanisms such as inducing ROS generation, cell cycle arrest, and apoptosis as compared to free curcumin. In addition, the invasiveness of GBM stem cells was also inhibited possibly due to the downregulation of MCP-1 mediated pathways. Furthermore, the Cur-niNP was shown to have minimal effect on normal cells [87]. Poly(beta-amino ester (PBAE) are less toxic NPs as they have high specificity for brain tumor cells over normal brain cells, thereby minimising the side effects on untargeted healthy cells [126]. Wattamwar et al. reported that PBAE can shuttle both quercetin and curcumin [127], which are known to have anti-cancer effects against brain tumor stem cells [113]. Quercetin alone has a safety window with a TC50 value of 0.23. This demonstrates the potential of combining PBAE with curcumin or quercetin for targeting GBM stem cells and thus should be further studied to determine the overall response and toxicity (Fig. 4). In summary, these treatments are promising in circumventing disease recurrence. This indicates the need to further explore phyto-nanomedicine for effective GBM treatment.
Fig. 4.
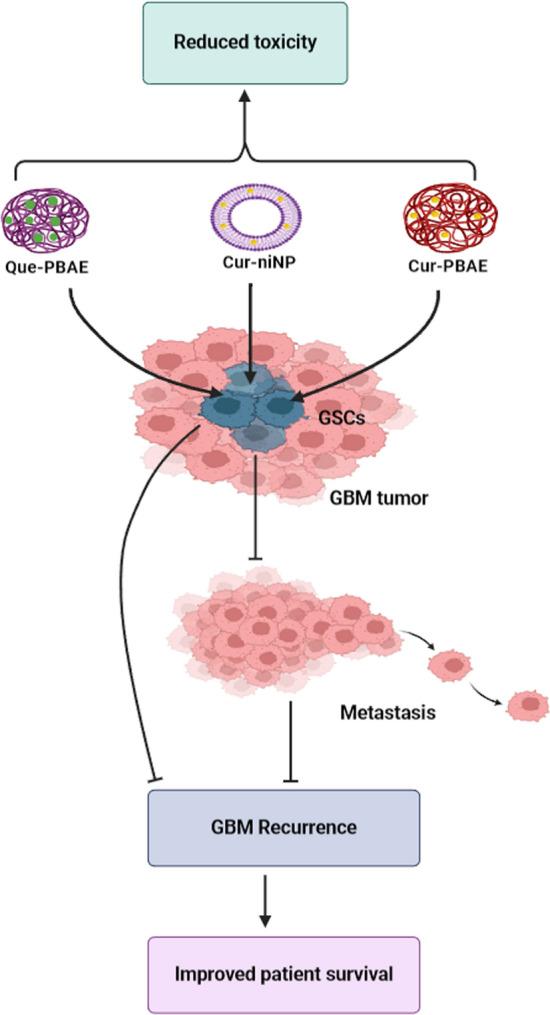
Phyto-NPs showing potential to reduce toxicity, metastasis, and recurrence of GBM. GSCs are targeted by Que-PBAE, Cur-PBAE, and Cur-niNP to prevent GBM recurrence and improve patient survival. Targeting GSCs can directly prevent tumor recurrence which is associated with resistance to therapy. The Cur-niNP and some of the other NPs combined with phytochemicals demonstrated low toxicity to normal cells. Nonetheless, more research is needed to fully establish the toxicity of these phyto-NPs. Image created with Biorender.com, accessed on 06 November 2023
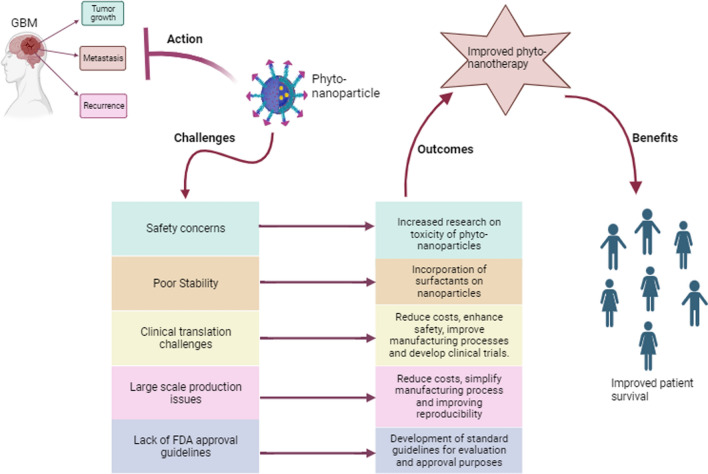
Overcoming challenges in phyto-nanomedicine for effective management of GBM through nano-delivery systems
Toxicity
Phytochemicals are natural products derived from plants which may depending on the plant species, not be abundantly available or may be restricted to certain geographical areas, thus opening up avenues for the supply of adulterated and substandard plant products, which may compromise on the quality and lead to safety concerns. Furthermore, documentation on the toxicity and potential side effects of phytochemicals is still inadequate [128]. The encapsulation of phytochemicals by NPs increases sustained drug release [129], enhances target specificity, and reduces the administered dosage, side effects [130], and toxicity [19]. However, NPs also have their toxicity limitations for example, their long-term localised or systemic effects are not known as the impact of a nanomaterial may not be quickly noticed or be immediate [131]. Furthermore, there are contradictory information regarding the toxicity of some of the NPs for example, iron oxide NPs which sometimes are regarded as toxic or non-toxic [132]. The toxicity of iron oxide may be generated by the accumulation of iron ions in tissues. The release of iron ions following the degradation of iron oxide depends on the physical or chemical properties of the coating used. These ions have the ability to generate ROS leading to oxidative destruction of the lipid bilayer membrane. Moreover, a decrease in cell viability, cell cycle alterations, cytoskeleton alteration, and mitochondrial membrane disruption have been reported after exposure to iron oxide NPs. However, studies addressing the toxicity of iron oxide NPs on human health are still limited. Therefore, further studies are required to improve knowledge of the potential long-term effects [133] and to determine the route of metabolism, metabolic products, and the destination or fate of NPs after delivering the phytochemicals at their respective targeted areas [134]. Nanotoxicology is an important research field that assesses the impacts of NPs’ interaction with biological systems and the association of chemical and physical properties of NPs with probable toxic effects [135]. The complexity of a nanocarrier may complicate its toxicity profile [136]. Thus, the physicochemical properties of NPs need to be carefully considered when synthesizing and characterizing NPs to avoid unanticipated toxicity [131]. There is a lack of data on phyto-NPs safety hence, there is a need to evaluate the potential toxicity of combining phytochemicals with NPs in GBM treatment in in-vitro and in-vivo models [137].
Stability of phytochemicals encapsulated in NPs
Phytochemicals are encapsulated within NPs to enhance their solubility, stability [129, 138], maintain their biological function as well as ensure their controlled release from the NPs [129]. The execution of these different functions may be limited by the instability of NPs which may result in aggregation or leakage of the NPs [138]. Consequently, the contents of the NPs will be released at untargeted sites and generate unwanted effects which further compromise their safety [128]. The degree of stability is crucial since the release of a phytochemical from a highly stable NP may be restricted [139]. Stability can be improved through the use of surfactants and proper manufacturing techniques [138]. One study reported an increased bioavailability and biostability of curcumin encapsulated in chitosan-coated NPs as compared to curcumin that was encapsulated in uncoated NPs [140]. Another study found that the coating of liposomes with pectin increased its stability and reduced the leakage of resveratrol [141]. Thus, finding a suitable surfactant in combination with the right manufacturing techniques can greatly increase the stability of phyto-nanomedicine for efficient clinical use.
Clinical translation
Despite the increase in research evidence supporting the potential of Phyto nanomedicine in GBM treatment, phytochemicals-loaded nanocarriers face challenges in clinical translation due to cost, safety, and large-scale production [142]. Other factors that may contribute to limited clinical translation could include complex extraction-synthesis procedure of the phytochemicals themselves, difficulty in characterization and optimization of a suitable nanomaterial, some pharmacokinetic issues such as water solubility and bioavailability, formulation issues such as instability and route of administration of the phyto-nanomedicne. Moreover, the properties of nanomaterials can have a big influence on their overall efficacy and more complex structures create manufacturing difficulties for clinical translation. Therefore, finding an appropriate technology that can manufacture and scale up production of the right nanomaterials with the required properties will serve as a strategic goal in the clinical translation of anti-tumor nanomaterials [143]. Also, more research is required to improve the cost-effectiveness and long-term safety of these nano-therapies [134].
Several studies in cancer therapy have used cell and animal models which may not reflect the actual physiological drug responses in the human body [143] as well as to recapitulate effectively the BBB and BBTB. Consequently, studies in animal models have sometimes generated contradictory results with regard to species and this may offer a finite value when translating findings into human settings [144]. Therefore, clinical trials following in-vitro and in-vivo preclinical studies in animal models of GBM are necessary to gain insights into the efficacy of these phyto-nanomedicines [145] in order to facilitate their uptake into clinical settings.
Large-scale production
The large-scale production of nanomedicine products is technically challenging [131]. Phytochemicals alone have challenges associated with large-scale production. These include complex isolation processes, low yield, high cost, and low aqueous solubility [128]. These limitations could influence the wider clinical application of phyto-nanomedicine since they are an important constituent. Various factors creating setbacks in the commercialization of NPs include the complex processes in the production of NPs according to required physicochemical properties, which may vary between batches and the high cost of sourcing the raw materials [131]. Some alternatives are promising for scaling up the production of NPs such as industrial utilization of microreactors, supercritical fluid technology, membrane extrusion, or nanotechnology. Lipid-based nanoparticles production can be scaled up using microfluidics systems by employing strategies such as pilling-up, numbering-up, or parallelization of microfluidic devices [146]. Additionally, hot melt extrusion coupled with high-pressure homogenization as well as microemulsion within temperature-regulated tank have potential for LNPs scale up production [147].
A challenge with the scaling-up of laboratory techniques is that it may result in the loss of the beneficial properties of NPs [148]. Therefore, it is important to plan the manufacturing process by first understanding the composition of the NP and how the constituents in it interact with each other at the early developmental stage before proceeding to the manufacturing process. This will help establish critical processing steps and analytical criteria which will guarantee the reproducibility of the NP. Furthermore, this planning needs to define the required standards for key NP characteristics and also identify conditions in the manufacturing processes that are crucial to obtaining these vital attributes and functions. This should be carried out on a small scale through well-designed experiments, which will provide valuable information on how production conditions may influence the physicochemical properties and other key attributes of the nanoparticle product [80].
Regulatory standards for FDA approval
The absence of FDA or any regulatory authority to set standard guidelines for products containing nanomaterials poses a great challenge in the approval of nanomedicines for clinical use. The evaluation of nanomaterials focuses mostly on the toxicities and commercial benefits that may be generated from NPs, which may delay the process of approval and consequently commercialization. Moreover, the increase in multifunctional nanoplatforms may delay the approval process due to a lack of standardization. There is a great need for the development of comprehensive guidelines for the regulatory evaluation and approval of nanotherapeutics for cancer [131]. Nonetheless, strides are being made with PLGA and PEGylated liposome already approved by the FDA for use as drug delivery systems [81, 83].
Conclusion
GBM is a highly aggressive grade IV tumor based on recent the WHO classification with a heterogeneous and tight BBB, posing a significant challenge to therapeutic interventions. Research continues to explore effective approaches to overcome the difficulty of treating GBM, aiming to minimize invasiveness, reduce disease recurrence, and improve patient survival. Natural phytochemicals exhibit promising anti-tumor properties by targeting dysregulated pathways in GBM. However, their application in GBM therapy is hampered by limitations in biocompatibility, rapid metabolism, and poor solubility. NP-based delivery systems offer a promising strategy to facilitate the delivery of phytochemicals across the BBB, enabling precise targeting of tumor cells and prolonging their cytotoxic effects. Nevertheless, the use of phyto-nanomedicine combinatorial therapy faces challenges associated with both phytochemicals and NPs, necessitating further research to overcome these limitations. Some of the key challenges include safety concerns, large-scale production, standardization, regulatory approval processes, and clinical translation. Thus, strategies for future research should focus on; (i) evaluating the long-term safety of phyto-nanomedicines, (ii) developing cost-effective and efficient manufacturing methods, (iii) establishing standardized guidelines for production, quality control, and regulatory approval, and iv) facilitating clinical trials to evaluate the efficacy and safety of phyto-nanomedicine in GBM treatment. Addressing these challenges and advancing research through clinical trials holds the potential to make phyto-nanomedicine a viable alternative treatment option for GBM (Fig. 5).
Fig. 5.
Potential anti-GBM effects of phyto-nanomedicine, challenges and future Perspectives
Phyto-nanomedicine exhibits significant anti-tumor effects, such as inhibiting tumor growth, invasion, and recurrence in glioblastoma multiforme (GBM). However, its application faces several limitations, including toxicity, poor stability, challenges in clinical translation, large-scale production issues, and the lack of FDA approval guidelines. These obstacles can be addressed by evaluating the safety of phyto-nanomedicine, enhancing large-scale production methods and reducing associated costs, developing standardized FDA review and approval guidelines, and advancing research to the clinical level. Addressing these factors could enhance the efficacy of phyto-nanomedicine therapy for GBM, potentially improving patient survival. Image created with Biorender.com, accessed on 06 November 2023.
Acknowledgements
We thank the South African Medical Research Council Self‐Initiated Research and the National Research Foundation for supporting M.A.A and B.P.D, respectively.
Author contributions
Conceptualization, BPD and MAA; writing—original draft preparation, BPD and LTM; writing—review and editing, BPD, LTM, TVM, TK, and MAA; funding acquisition, MAA and BPD. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the South African Medical Research Council Self‐Initiated Research (Grant Number A1A982), and the National Research Foundation (Grant Number 107088).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This review article does not contain any studies with human participants or animals performed by any of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Melvin Anyasi Ambele, Email: melvin.ambele@up.ac.za.
Botle Precious Damane, Email: botle.damane@up.ac.za.
References
- 1.Park YW, Vollmuth P, Foltyn-Dumitru M, Sahm F, Ahn SS, Chang JH, Kim SH. The 2021 WHO classification for gliomas and implications on imaging diagnosis: part 1—key points of the fifth edition and summary of imaging findings on adult-type diffuse gliomas. J Magn Reson Imaging. 2023;58:677–89. 10.1002/jmri.28743. [DOI] [PubMed] [Google Scholar]
- 2.Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G, Soffietti R, von Deimling A, Ellison DW. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23:1231–51. 10.1093/neuonc/noab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Obrador E, Moreno-Murciano P, Oriol-Caballo M, López-Blanch R, Pineda B, Gutiérrez-Arroyo JL, Loras A, Gonzalez-Bonet LG, Martinez-Cadenas C, Estrela JM, Marqués-Torrejón M. Glioblastoma therapy: past, present and future. Int J Mol Sci. 2024. 10.3390/ijms25052529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Degl’Innocenti A, di Leo N, Ciofani G. Genetic hallmarks and heterogeneity of glioblastoma in the single-cell omics era. Adv Ther (Weinh). 2020;3:1900152. 10.1002/adtp.201900152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giambra M, Di Cristofori A, Valtorta S, Manfrellotti R, Bigiogera V, Basso G, Moresco RM, Giussani C, Bentivegna A. The peritumoral brain zone in glioblastoma: where we are and where we are going. J Neurosci Res. 2023;101:199–216. 10.1002/jnr.25134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hatoum A, Mohammed R, Zakieh O. The unique invasiveness of glioblastoma and possible drug targets on extracellular matrix. Cancer Manag Res. 2019;11:1843–55. 10.2147/cmar.S186142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valles SL, Singh SK, Campos-Campos J, Colmena C, Campo-Palacio I, Alvarez-Gamez K, Caballero O, Jorda A. Functions of astrocytes under normal conditions and after a brain disease. Int J Mol Sci. 2023;24:8434. 10.3390/ijms24098434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sidoryk-Wegrzynowicz M, Wegrzynowicz M, Lee E, Bowman AB, Aschner M. Role of astrocytes in brain function and disease. Toxicol Pathol. 2011;39:115–23. 10.1177/0192623310385254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atiq A, Parhar I. Anti-neoplastic potential of flavonoids and polysaccharide phytochemicals in glioblastoma. Molecules. 2020. 10.3390/molecules25214895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janjua TI, Rewatkar P, Ahmed-Cox A, Saeed I, Mansfeld FM, Kulshreshtha R, Kumeria T, Ziegler DS, Kavallaris M, Mazzieri R, Popat A. Frontiers in the treatment of glioblastoma: past, present and emerging. Adv Drug Deliv Rev. 2021;171:108–38. 10.1016/j.addr.2021.01.012. [DOI] [PubMed] [Google Scholar]
- 11.Rong L, Li N, Zhang Z. Emerging therapies for glioblastoma: current state and future directions. J Exp Clin Cancer Res. 2022;41:142. 10.1186/s13046-022-02349-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maklad A, Sharma A, Azimi I. Calcium signaling in brain cancers: roles and therapeutic targeting. Cancers (Basel). 2019. 10.3390/cancers11020145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baldassari S, Balboni A, Drava G, Donghia D, Canepa P, Ailuno G, Caviglioli G. Phytochemicals and cancer treatment: cell-derived and biomimetic vesicles as promising carriers. Pharmaceutics. 2023. 10.3390/pharmaceutics15051445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pistollato F, Bremer-Hoffmann S, Basso G, Cano SS, Elio I, Vergara MM, Giampieri F, Battino M. Targeting glioblastoma with the use of phytocompounds and nanoparticles. Target Oncol. 2016;11:1–16. 10.1007/s11523-015-0378-5. [DOI] [PubMed] [Google Scholar]
- 15.Hossain M, Banik NL, Ray SK. Synergistic anti-cancer mechanisms of curcumin and paclitaxel for growth inhibition of human brain tumor stem cells and LN18 and U138MG cells. Neurochem Int. 2012;61:1102–13. 10.1016/j.neuint.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanati M, Afshari AR, Amini J, Mollazadeh H, Jamialahmadi T, Sahebkar A. Targeting angiogenesis in gliomas: potential role of phytochemicals. J Funct Foods. 2022;96:105192. 10.1016/j.jff.2022.105192. [Google Scholar]
- 17.Tang L, Feng Y, Gao S, Mu Q, Liu C. Nanotherapeutics overcoming the blood-brain barrier for glioblastoma treatment. Front Pharmacol. 2021;12:786700. 10.3389/fphar.2021.786700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Tellingen O, Yetkin-Arik B, de Gooijer MC, Wesseling P, Wurdinger T, de Vries HE. Overcoming the blood–brain tumor barrier for effective glioblastoma treatment. Drug Resist Updates. 2015;19:1–12. 10.1016/j.drup.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Mehan S, Arora N, Bhalla S, Khan A, Rahman MU, Alghamdi BS, Zughaibi TA, Ashraf GM. Involvement of phytochemical-encapsulated nanoparticles’ interaction with cellular signalling in the amelioration of benign and malignant brain tumours. Molecules. 2022. 10.3390/molecules27113561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong SC, Kamarudin MNA, Naidu R. Anticancer mechanism of curcumin on human glioblastoma. Nutrients. 2021. 10.3390/nu13030950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryskalin L, Biagioni F, Busceti CL, Lazzeri G, Frati A, Fornai F. The multi-faceted effect of curcumin in glioblastoma from rescuing cell clearance to autophagy-independent effects. Molecules. 2020;25:4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weissenberger J, Priester M, Bernreuther C, Rakel S, Glatzel M, Seifert V, Kögel D. Dietary curcumin attenuates glioma growth in a syngeneic mouse model by inhibition of the JAK1,2/STAT3 signaling pathway. Clin Cancer Res. 2010;16:5781–95. 10.1158/1078-0432.Ccr-10-0446. [DOI] [PubMed] [Google Scholar]
- 23.Fahmideh H, Shapourian H, Moltafeti R, Tavakol C, Forghaniesfidvajani R, Zalpoor H, Nabi-Afjadi M. The role of natural products as inhibitors of JAK/STAT signaling pathways in glioblastoma treatment. Oxid Med Cell Longev. 2022;2022:7838583. 10.1155/2022/7838583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwadate Y. Epithelial-mesenchymal transition in glioblastoma progression. Oncol Lett. 2016;11:1615–20. 10.3892/ol.2016.4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gersey ZC, Rodriguez GA, Barbarite E, Sanchez A, Walters WM, Ohaeto KC, Komotar RJ, Graham RM. Curcumin decreases malignant characteristics of glioblastoma stem cells via induction of reactive oxygen species. BMC Cancer. 2017;17:99. 10.1186/s12885-017-3058-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim S, Chen J, Cheng T, Gindulyte A, He J, He S, Li Q, Shoemaker BA, Thiessen PA, Yu B, Zaslavsky L, Zhang J, Bolton EE. PubChem 2023 update. Nucleic Acids Res. 2022;51:D1373–80. 10.1093/nar/gkac956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Z, Liu F, Liao W, Yu L, Hu Z, Li M, Xia H. Curcumin suppresses glioblastoma cell proliferation by p-AKT/mTOR pathway and increases the PTEN expression. Arch Biochem Biophys. 2020;689:108412. 10.1016/j.abb.2020.108412. [DOI] [PubMed] [Google Scholar]
- 28.Lamy S, Moldovan PL, Ben Saad A, Annabi B. Biphasic effects of luteolin on interleukin-1β-induced cyclooxygenase-2 expression in glioblastoma cells. Biochim Biophys Acta. 2015;1853:126–35. 10.1016/j.bbamcr.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 29.Wang Q, Wang H, Jia Y, Ding H, Zhang L, Pan H. Luteolin reduces migration of human glioblastoma cell lines via inhibition of the p-IGF-1R/PI3K/AKT/mTOR signaling pathway. Oncol Lett. 2017;14:3545–51. 10.3892/ol.2017.6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anson DM, Wilcox RM, Huseman ED, Stump TA, Paris RL, Darkwah BO, Lin S, Adegoke AO, Gryka RJ, Jean-Louis DS, Amos S. Luteolin decreases epidermal growth factor receptor-mediated cell proliferation and induces apoptosis in glioblastoma cell lines. Basic Clin Pharmacol Toxicol. 2018;123:678–86. 10.1111/bcpt.13077. [DOI] [PubMed] [Google Scholar]
- 31.Jalili-Nik M, Sadeghi MM, Mohtashami E, Mollazadeh H, Afshari AR, Sahebkar A. Zerumbone promotes cytotoxicity in human malignant glioblastoma cells through reactive oxygen species (ROS) generation. Oxid Med Cell Longev. 2020;2020:3237983. 10.1155/2020/3237983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jalili-Nik M, Afshari AR, Sabri H, Bibak B, Mollazadeh H, Sahebkar A. Zerumbone, a ginger sesquiterpene, inhibits migration, invasion, and metastatic behavior of human malignant glioblastoma multiforme in vitro. BioFactors. 2021;47:729–39. 10.1002/biof.1756. [DOI] [PubMed] [Google Scholar]
- 33.Chen B, Li X, Wu L, Zhou D, Song Y, Zhang L, Wu Q, He Q, Wang G, Liu X, Hu H, Zhou W. Quercetin suppresses human glioblastoma migration and invasion via GSK3β/β-catenin/ZEB1 signaling pathway. Front Pharmacol. 2022;13:963614. 10.3389/fphar.2022.963614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Michaud-Levesque J, Bousquet-Gagnon N, Béliveau R. Quercetin abrogates IL-6/STAT3 signaling and inhibits glioblastoma cell line growth and migration. Exp Cell Res. 2012;318:925–35. 10.1016/j.yexcr.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y, Tang ZG, Yang JQ, Zhou Y, Meng LH, Wang H, Li CL. Low concentration of quercetin antagonizes the invasion and angiogenesis of human glioblastoma U251 cells. Onco Targets Ther. 2017;10:4023–8. 10.2147/ott.S136821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cilibrasi C, Riva G, Romano G, Cadamuro M, Bazzoni R, Butta V, Paoletta L, Dalprà L, Strazzabosco M, Lavitrano M, Giovannoni R, Bentivegna A. Resveratrol impairs glioma stem cells proliferation and motility by modulating the Wnt signaling pathway. PLoS ONE. 2017;12:e0169854. 10.1371/journal.pone.0169854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kavitha CV, Agarwal C, Agarwal R, Deep G. Asiatic acid inhibits pro-angiogenic effects of VEGF and human gliomas in endothelial cell culture models. PLoS ONE. 2011;6:e22745. 10.1371/journal.pone.0022745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu Y, Jiang F, Jiang H, Wu K, Zheng X, Cai Y, Katakowski M, Chopp M, To SS. Gallic acid suppresses cell viability, proliferation, invasion and angiogenesis in human glioma cells. Eur J Pharmacol. 2010;641:102–7. 10.1016/j.ejphar.2010.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee S-H, Kim JK, Kim DW, Hwang HS, Eum WS, Park J, Han KH, Oh JS, Choi SY. Antitumor activity of methyl gallate by inhibition of focal adhesion formation and Akt phosphorylation in glioma cells. Biochimica et Biophysica Acta (BBA) Gen Subj. 2013;1830:4017–29. 10.1016/j.bbagen.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 40.Lien L-M, Wang M-J, Chen R-J, Chiu H-C, Wu J-L, Shen M-Y, Chou D-S, Sheu J-R, Lin K-H, Lu W-J. Nobiletin, a polymethoxylated flavone, inhibits glioma cell growth and migration via arresting cell cycle and suppressing MAPK and Akt pathways. Phytother Res. 2016;30:214–21. 10.1002/ptr.5517. [DOI] [PubMed] [Google Scholar]
- 41.Aoki K, Yokosuka A, Mimaki Y, Fukunaga K, Yamakuni T. Nobiletin induces inhibitions of Ras activity and mitogen-activated protein kinase kinase/extracellular signal-regulated kinase signaling to suppress cell proliferation in C6 rat glioma cells. Biol Pharm Bull. 2013;36:540–7. 10.1248/bpb.b12-00824. [DOI] [PubMed] [Google Scholar]
- 42.Zhang X, Zheng K, Li C, Zhao Y, Li H, Liu X, Long Y, Yao J. Nobiletin inhibits invasion via inhibiting AKT/GSK3β/β-catenin signaling pathway in Slug-expressing glioma cells. Oncol Rep. 2017;37:2847–56. 10.3892/or.2017.5522. [DOI] [PubMed] [Google Scholar]
- 43.Liu Y, Zheng J, Zhang Y, Wang Z, Yang Y, Bai M, Dai Y. Fucoxanthin activates apoptosis via inhibition of PI3K/Akt/mTOR pathway and suppresses invasion and migration by restriction of p38-MMP-2/9 pathway in human glioblastoma cells. Neurochem Res. 2016;41:2728–51. 10.1007/s11064-016-1989-7. [DOI] [PubMed] [Google Scholar]
- 44.Langhans J, Schneele L, Trenkler N, von Bandemer H, Nonnenmacher L, Karpel-Massler G, Siegelin MD, Zhou S, Halatsch M-E, Debatin K-M, Westhoff M-A. The effects of PI3K-mediated signalling on glioblastoma cell behaviour. Oncogenesis. 2017;6:398. 10.1038/s41389-017-0004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barzegar Behrooz A, Talaie Z, Jusheghani F, Łos MJ, Klonisch T, Ghavami S. Wnt and PI3K/Akt/mTOR survival pathways as therapeutic targets in glioblastoma. Int J Mol Sci. 2022. 10.3390/ijms23031353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paplomata E, O’Regan R. The PI3K/AKT/mTOR pathway in breast cancer: targets, trials and biomarkers. Ther Adv Med Oncol. 2014;6:154–66. 10.1177/1758834014530023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li X, Wu C, Chen N, Gu H, Yen A, Cao L, Wang E, Wang L. PI3K/Akt/mTOR signaling pathway and targeted therapy for glioblastoma. Oncotarget. 2016;7:33440–50. 10.18632/oncotarget.7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jhanwar-Uniyal M, Gellerson O, Bree J, Das M, Kleinman G, Gandhi CD. Defining the role of mTOR pathway in the regulation of stem cells of glioblastoma. Adv Biol Regul. 2023;88:100946. 10.1016/j.jbior.2022.100946. [DOI] [PubMed] [Google Scholar]
- 49.Yin H, Zhou Y, Wen C, Zhou C, Zhang W, Hu X, Wang L, You C, Shao J. Curcumin sensitizes glioblastoma to temozolomide by simultaneously generating ROS and disrupting AKT/mTOR signaling. Oncol Rep. 2014;32:1610–6. 10.3892/or.2014.3342. [DOI] [PubMed] [Google Scholar]
- 50.Imran M, Rauf A, Abu-Izneid T, Nadeem M, Shariati MA, Khan IA, Imran A, Orhan IE, Rizwan M, Atif M, Gondal TA, Mubarak MS. Luteolin, a flavonoid, as an anticancer agent: a review. Biomed Pharmacother. 2019;112:108612. 10.1016/j.biopha.2019.108612. [DOI] [PubMed] [Google Scholar]
- 51.Lee HS, Park BS, Kang HM, Kim JH, Shin SH, Kim IR. Role of luteolin-induced apoptosis and autophagy in human glioblastoma cell lines. Medicina (Kaunas). 2021. 10.3390/medicina57090879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wendt MK, Taylor MA, Schiemann BJ, Schiemann WP. Down-regulation of epithelial cadherin is required to initiate metastatic outgrowth of breast cancer. Mol Biol Cell. 2011;22:2423–35. 10.1091/mbc.E11-04-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Denduluri SK, Idowu O, Wang Z, Liao Z, Yan Z, Mohammed MK, Ye J, Wei Q, Wang J, Zhao L, Luu HH. Insulin-like growth factor (IGF) signaling in tumorigenesis and the development of cancer drug resistance. Genes Dis. 2015;2:13–25. 10.1016/j.gendis.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vincent AM, Feldman EL. Control of cell survival by IGF signaling pathways. Growth Horm IGF Res. 2002;12:193–7. 10.1016/S1096-6374(02)00017-5. [DOI] [PubMed] [Google Scholar]
- 55.Weng HY, Hsu MJ, Wang CC, Chen BC, Hong CY, Chen MC, Chiu WT, Lin CH. Zerumbone suppresses IKKα, Akt, and FOXO1 activation, resulting in apoptosis of GBM 8401 cells. J Biomed Sci. 2012;19:86. 10.1186/1423-0127-19-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim S, Lee J, Jeon M, Lee JE, Nam SJ. Zerumbone suppresses the motility and tumorigenecity of triple negative breast cancer cells via the inhibition of TGF-β1 signaling pathway. Oncotarget. 2016;7:1544–58. 10.18632/oncotarget.6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lv T, Zhang W, Han X. Zerumbone suppresses the potential of growth and metastasis in hepatoma HepG2 cells via the MAPK signaling pathway. Oncol Lett. 2018;15:7603–10. 10.3892/ol.2018.8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saranya J, Dhanya BP, Greeshma G, Radhakrishnan KV, Priya S. Effects of a new synthetic zerumbone pendant derivative (ZPD) on apoptosis induction and anti-migratory effects in human cervical cancer cells. Chem Biol Interact. 2017;278:32–9. 10.1016/j.cbi.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 59.Dadgostar E, Fallah M, Izadfar F, Heidari-Soureshjani R, Aschner M, Tamtaji OR, Mirzaei H. Therapeutic potential of resveratrol in the treatment of glioma: insights into its regulatory mechanisms. Mini Rev Med Chem. 2021;21:2835–47. 10.2174/1389557521666210406164758. [DOI] [PubMed] [Google Scholar]
- 60.Shu XH, Wang LL, Li H, Song X, Shi S, Gu JY, Wu ML, Chen XY, Kong QY, Liu J. Diffusion efficiency and bioavailability of resveratrol administered to rat brain by different routes: therapeutic implications. Neurotherapeutics. 2015;12:491–501. 10.1007/s13311-014-0334-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee Y, Lee J-K, Ahn SH, Lee J, Nam D-H. WNT signaling in glioblastoma and therapeutic opportunities. Lab Investig. 2016;96:137–50. 10.1038/labinvest.2015.140. [DOI] [PubMed] [Google Scholar]
- 62.Wang Y, Liu J, Ying X, Lin PC, Zhou BP. Twist-mediated epithelial-mesenchymal transition promotes breast tumor cell invasion via inhibition of hippo pathway. Sci Rep. 2016;6:24606. 10.1038/srep24606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cho CW, Choi DS, Cardone MH, Kim CW, Sinskey AJ, Rha C. Glioblastoma cell death induced by asiatic acid. Cell Biol Toxicol. 2006;22:393–408. 10.1007/s10565-006-0104-2. [DOI] [PubMed] [Google Scholar]
- 64.Thakor FK, Wan KW, Welsby PJ, Welsby G. Pharmacological effects of asiatic acid in glioblastoma cells under hypoxia. Mol Cell Biochem. 2017;430:179–90. 10.1007/s11010-017-2965-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kavitha CV, Jain AK, Agarwal C, Pierce A, Keating A, Huber KM, Serkova NJ, Wempe MF, Agarwal R, Deep G. Asiatic acid induces endoplasmic reticulum stress and apoptotic death in glioblastoma multiforme cells both in vitro and in vivo. Mol Carcinog. 2015;54:1417–29. 10.1002/mc.22220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Paolini A, Curti V, Pasi F, Mazzini G, Nano R, Capelli E. Gallic acid exerts a protective or an anti-proliferative effect on glioma T98G cells via dose-dependent epigenetic regulation mediated by miRNAs. Int J Oncol. 2015;46:1491–7. 10.3892/ijo.2015.2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pedra NS, Bona NP, de Aguiar MSS, Spohr L, Alves FL, Santos F, Saraiva JT, Stefanello FM, Braganhol E, Spanevello RM. Impact of gallic acid on tumor suppression: modulation of redox homeostasis and purinergic response in in vitro and a preclinical glioblastoma model. J Nutr Biochem. 2022;110:109156. 10.1016/j.jnutbio.2022.109156. [DOI] [PubMed] [Google Scholar]
- 68.Wei D, Zhang N, Qu S, Wang H, Li J. Advances in nanotechnology for the treatment of GBM. Front Neurosci. 2023;17:1180943. 10.3389/fnins.2023.1180943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ayub A, Wettig S. An overview of nanotechnologies for drug delivery to the brain. Pharmaceutics. 2022. 10.3390/pharmaceutics14020224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hsu JF, Chu SM, Liao CC, Wang CJ, Wang YS, Lai MY, Wang HC, Huang HR, Tsai MH. Nanotechnology and nanocarrier-based drug delivery as the potential therapeutic strategy for glioblastoma multiforme: an update. Cancers (Basel). 2021. 10.3390/cancers13020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu D, Dai X, Tao Z, Zhou H, Hong W, Qian H, Cheng H, Wang X. Advances in blood–brain barrier-crossing nanomedicine for anti-glioma. Cancer Nanotechnol. 2023;14:58. 10.1186/s12645-023-00211-9. [Google Scholar]
- 72.Mobeen H, Safdar M, Fatima A, Afzal S, Zaman H, Mehdi Z. Emerging applications of nanotechnology in context to immunology: a comprehensive review. Front Bioeng Biotechnol. 2022. 10.3389/fbioe.2022.1024871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vassal M, Rebelo S, Pereira ML. Metal oxide nanoparticles: evidence of adverse effects on the male reproductive system. Int J Mol Sci. 2021. 10.3390/ijms22158061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Odaudu OR, Akinsiku AA. Toxicity and cytotoxicity effects of selected nanoparticles: a review. IOP Conf Ser Earth Environ Sci. 2022;1054:012007. 10.1088/1755-1315/1054/1/012007. [Google Scholar]
- 75.Wang J, Li B, Qiu L, Qiao X, Yang H. Dendrimer-based drug delivery systems: history, challenges, and latest developments. J Biol Eng. 2022;16:18. 10.1186/s13036-022-00298-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dattani S, Li X, Lampa C, Lechuga-Ballesteros D, Barriscale A, Damadzadeh B, Jasti BR. A comparative study on micelles, liposomes and solid lipid nanoparticles for paclitaxel delivery. Int J Pharm. 2023;631:122464. 10.1016/j.ijpharm.2022.122464. [DOI] [PubMed] [Google Scholar]
- 77.Khan Y, Sadia H, Ali Shah SZ, Khan MN, Shah AA, Ullah N, Ullah MF, Bibi H, Bafakeeh OT, Khedher NB, Eldin SM, Fadhl BM, Khan MI. Classification, synthetic, and characterization approaches to nanoparticles, and their applications in various fields of nanotechnology: a review. Catalysts. 2022;12:1386. [Google Scholar]
- 78.Mekuye B, Abera B. Nanomaterials: an overview of synthesis, classification, characterization, and applications. Nano Sel. 2023;4:486–501. 10.1002/nano.202300038. [Google Scholar]
- 79.Jamali Z, Khoobi M, Hejazi SM, Eivazi N, Abdolahpour S, Imanparast F, Moradi-Sardareh H, Paknejad M. Evaluation of targeted curcumin (CUR) loaded PLGA nanoparticles for in vitro photodynamic therapy on human glioblastoma cell line. Photodiagn Photodyn Ther. 2018;23:190–201. 10.1016/j.pdpdt.2018.06.026. [DOI] [PubMed] [Google Scholar]
- 80.Desai N. Challenges in development of nanoparticle-based therapeutics. Aaps j. 2012;14:282–95. 10.1208/s12248-012-9339-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alvi M, Yaqoob A, Rehman K, Shoaib SM, Akash MSH. PLGA-based nanoparticles for the treatment of cancer: current strategies and perspectives. AAPS Open. 2022;8:12. 10.1186/s41120-022-00060-7. [Google Scholar]
- 82.Orunoğlu M, Kaffashi A, Pehlivan SB, Şahin S, Söylemezoğlu F, Oğuz KK, Mut M. Effects of curcumin-loaded PLGA nanoparticles on the RG2 rat glioma model. Mater Sci Eng C Mater Biol Appl. 2017;78:32–8. 10.1016/j.msec.2017.03.292. [DOI] [PubMed] [Google Scholar]
- 83.Taher M, Susanti D, Haris MS, Rushdan AA, Widodo RT, Syukri Y, Khotib J. PEGylated liposomes enhance the effect of cytotoxic drug: a review. Heliyon. 2023;9:e13823. 10.1016/j.heliyon.2023.e13823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Senturk F, Cakmak S, Kocum IC, Gumusderelioglu M, Ozturk GG. GRGDS-conjugated and curcumin-loaded magnetic polymeric nanoparticles for the hyperthermia treatment of glioblastoma cells. Colloids Surf A. 2021;622:126648. 10.1016/j.colsurfa.2021.126648. [Google Scholar]
- 85.Zhang H, van Os WL, Tian X, Zu G, Ribovski L, Bron R, Bussmann J, Kros A, Liu Y, Zuhorn IS. Development of curcumin-loaded zein nanoparticles for transport across the blood–brain barrier and inhibition of glioblastoma cell growth. Biomater Sci. 2021;9:7092–103. 10.1039/D0BM01536A. [DOI] [PubMed] [Google Scholar]
- 86.Shao J, Zheng D, Jiang Z, Xu H, Hu Y, Li X, Lu X. Curcumin delivery by methoxy polyethylene glycol-poly(caprolactone) nanoparticles inhibits the growth of C6 glioma cells. Acta Biochim Biophys Sin (Shanghai). 2011;43:267–74. 10.1093/abbs/gmr011. [DOI] [PubMed] [Google Scholar]
- 87.Sahab-Negah S, Ariakia F, Jalili-Nik M, Afshari AR, Salehi S, Samini F, Rajabzadeh G, Gorji A. Curcumin loaded in niosomal nanoparticles improved the anti-tumor effects of free curcumin on glioblastoma stem-like cells: an in vitro study. Mol Neurobiol. 2020;57:3391–411. 10.1007/s12035-020-01922-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Alasvand N, Urbanska AM, Rahmati M, Saeidifar M, Gungor-Ozkerim PS, Sefat F, Rajadas J, Mozafari M. Chapter 13—Therapeutic nanoparticles for targeted delivery of anticancer drugs. In: Grumezescu AM, editor. Multifunctional systems for combined delivery, biosensing and diagnostics. Elsevier; 2017. p. 245–59. [Google Scholar]
- 89.Dützmann S, Schiborr C, Kocher A, Pilatus U, Hattingen E, Weissenberger J, Geßler F, Quick-Weller J, Franz K, Seifert V, Frank J, Senft C. Intratumoral concentrations and effects of orally administered micellar curcuminoids in glioblastoma patients. Nutr Cancer. 2016;68:943–8. 10.1080/01635581.2016.1187281. [DOI] [PubMed] [Google Scholar]
- 90.Deogratias G, Shadrack DM, Munissi JJE, Kinunda GA, Jacob FR, Mtei RP, Masalu RJ, Mwakyula I, Kiruri LW, Nyandoro SS. Hydrophobic π-π stacking interactions and hydrogen bonds drive self-aggregation of luteolin in water. J Mol Graph Model. 2022;116:108243. 10.1016/j.jmgm.2022.108243. [DOI] [PubMed] [Google Scholar]
- 91.Wu C, Xu Q, Chen X, Liu J. Delivery luteolin with folacin-modified nanoparticle for glioma therapy. Int J Nanomed. 2019;14:7515–31. 10.2147/ijn.S214585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zheng S, Cheng Y, Teng Y, Liu X, Yu T, Wang Y, Liu J, Hu Y, Wu C, Wang X, Liu Y, You C, Gao X, Wei Y. Application of luteolin nanomicelles anti-glioma effect with improvement in vitro and in vivo. Oncotarget. 2017;8:61146–62. 10.18632/oncotarget.18019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu F, Peng B, Li M, Ma J, Deng G, Zhang S, Sheu WC, Zou P, Wu H, Liu J, Chen AT, Mohammed FS, Zhou J. Targeted disruption of tumor vasculature via polyphenol nanoparticles to improve brain cancer treatment. Cell Rep Phys Sci. 2022;3:100691. 10.1016/j.xcrp.2021.100691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Enteshari Najafabadi R, Kazemipour N, Esmaeili A, Beheshti S, Nazifi S. Using superparamagnetic iron oxide nanoparticles to enhance bioavailability of quercetin in the intact rat brain. BMC Pharmacol Toxicol. 2018;19:59. 10.1186/s40360-018-0249-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Paranthaman S, Uthaiah CA, Osmani RAM, Hani U, Ghazwani M, Alamri AH, Fatease AA, Madhunapantula SV, Gowda DV. Anti-proliferative potential of quercetin loaded polymeric mixed micelles on rat C6 and human U87MG glioma cells. Pharmaceutics. 2022. 10.3390/pharmaceutics14081643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ersoz M, Erdemir A, Derman S, Arasoglu T, Mansuroglu B. Quercetin-loaded nanoparticles enhance cytotoxicity and antioxidant activity on C6 glioma cells. Pharm Dev Technol. 2020;25:757–66. 10.1080/10837450.2020.1740933. [DOI] [PubMed] [Google Scholar]
- 97.Wang G, Wang JJ, Chen XL, Du L, Li F. Quercetin-loaded freeze-dried nanomicelles: improving absorption and anti-glioma efficiency in vitro and in vivo. J Control Release. 2016;235:276–90. 10.1016/j.jconrel.2016.05.045. [DOI] [PubMed] [Google Scholar]
- 98.Lin XM, Shi XX, Xiong L, Nie JH, Ye HS, Du JZ, Liu J. Construction of IL-13 receptor α2-targeting resveratrol nanoparticles against glioblastoma cells: therapeutic efficacy and molecular effects. Int J Mol Sci. 2021;2:2. 10.3390/ijms221910622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shao J, Li X, Lu X, Jiang C, Hu Y, Li Q, You Y, Fu Z. Enhanced growth inhibition effect of Resveratrol incorporated into biodegradable nanoparticles against glioma cells is mediated by the induction of intracellular reactive oxygen species levels. Colloids Surf B. 2009;72:40–7. 10.1016/j.colsurfb.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 100.Xu H, Jia F, Singh PK, Ruan S, Zhang H, Li X. Synergistic anti-glioma effect of a coloaded nano-drug delivery system. Int J Nanomed. 2017;12:29–40. 10.2147/ijn.S116367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jhaveri A, Deshpande P, Pattni B, Torchilin V. Transferrin-targeted, resveratrol-loaded liposomes for the treatment of glioblastoma. J Control Release. 2018;277:89–101. 10.1016/j.jconrel.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Guo W, Li A, Jia Z, Yuan Y, Dai H, Li H. Transferrin modified PEG-PLA-resveratrol conjugates: in vitro and in vivo studies for glioma. Eur J Pharmacol. 2013;718:41–7. 10.1016/j.ejphar.2013.09.034. [DOI] [PubMed] [Google Scholar]
- 103.Sallem F, Haji R, Vervandier-Fasseur D, Nury T, Maurizi L, Boudon J, Lizard G, Millot N. Elaboration of trans-resveratrol derivative-loaded superparamagnetic iron oxide nanoparticles for glioma treatment. Nanomaterials. 2019;9:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jose S, Anju SS, Cinu TA, Aleykutty NA, Thomas S, Souto EB. In vivo pharmacokinetics and biodistribution of resveratrol-loaded solid lipid nanoparticles for brain delivery. Int J Pharm. 2014;474:6–13. 10.1016/j.ijpharm.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 105.Thakor F, Welsby PJ, Wan KW and Welsby G. P51: Preparation of Asiatic acid loaded nanoparticles for delivery across the blood brain barrier and its effects on glioblastoma cells in vitro.
- 106.Garanti T, Stasik A, Burrow AJ, Alhnan MA, Wan KW. Anti-glioma activity and the mechanism of cellular uptake of asiatic acid-loaded solid lipid nanoparticles. Int J Pharm. 2016;500:305–15. 10.1016/j.ijpharm.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 107.Ramalho MJ, Bravo M, Loureiro JA, Lima J, Pereira MC. Transferrin-modified nanoparticles for targeted delivery of Asiatic acid to glioblastoma cells. Life Sci. 2022;296:120435. 10.1016/j.lfs.2022.120435. [DOI] [PubMed] [Google Scholar]
- 108.Garanti T, Alhnan MA, Wan KW. RGD-decorated solid lipid nanoparticles enhance tumor targeting, penetration and anticancer effect of asiatic acid. Nanomedicine (Lond). 2020;15:1567–83. 10.2217/nnm-2020-0035. [DOI] [PubMed] [Google Scholar]
- 109.Jing Z, Li M, Wang H, Yang Z, Zhou S, Ma J, Meng E, Zhang H, Liang W, Hu W, Wang X, Fu X. Gallic acid-gold nanoparticles enhance radiation-induced cell death of human glioma U251 cells. IUBMB Life. 2021;73:398–407. 10.1002/iub.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu C, Li C, Jiang S, Zhang C, Tian Y. pH-responsive hollow Fe-gallic acid coordination polymer for multimodal synergistic-therapy and MRI of cancer. Nanoscale Adv. 2021;4:173–81. 10.1039/d1na00721a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cheng MC, Lu YC, Wu J, Ma YH. Gallate-induced nanoparticle uptake by tumor cells: structure-activity relationships. Colloids Surf B Biointerfaces. 2019;179:28–36. 10.1016/j.colsurfb.2019.03.048. [DOI] [PubMed] [Google Scholar]
- 112.Ahmadi Nasab N, Hassani Kumleh H, Beygzadeh M, Teimourian S, Kazemzad M. Delivery of curcumin by a pH-responsive chitosan mesoporous silica nanoparticles for cancer treatment. Artif Cells Nanomed Biotechnol. 2018;46:75–81. 10.1080/21691401.2017.1290648. [DOI] [PubMed] [Google Scholar]
- 113.Zhang I, Beus M, Stochaj U, Le PU, Zorc B, Rajić Z, Petrecca K, Maysinger D. Inhibition of glioblastoma cell proliferation, invasion, and mechanism of action of a novel hydroxamic acid hybrid molecule. Cell Death Discov. 2018;4:41. 10.1038/s41420-018-0103-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vollmann-Zwerenz A, Leidgens V, Feliciello G, Klein CA, Hau P. Tumor cell invasion in glioblastoma. Int J Mol Sci. 2020. 10.3390/ijms21061932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Song Z, Liu T, Chen T. Overcoming blood–brain barrier by HER2-targeted nanosystem to suppress glioblastoma cell migration, invasion and tumor growth. J Mater Chem B. 2018;6:568–79. 10.1039/C7TB02677C. [DOI] [PubMed] [Google Scholar]
- 116.Vaz-Salgado MA, Villamayor M, Albarrán V, Alía V, Sotoca P, Chamorro J, Rosero D, Barrill AM, Martín M, Fernandez E, Gutierrez JA, Rojas-Medina LM, Ley L. Recurrent glioblastoma: a review of the treatment options. Cancers (Basel). 2023. 10.3390/cancers15174279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schiapparelli P, Guerrero-Cazares H, Magaña-Maldonado R, Hamilla SM, Ganaha S, Goulin Lippi Fernandes E, Huang CH, Aranda-Espinoza H, Devreotes P, Quinones-Hinojosa A. NKCC1 regulates migration ability of glioblastoma cells by modulation of actin dynamics and interacting with cofilin. EBioMedicine. 2017;21:94–103. 10.1016/j.ebiom.2017.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li J, Feng L, Lu Y. Glioblastoma multiforme: diagnosis, treatment, and invasion. J Biomed Res. 2022;37:47–58. 10.7555/jbr.36.20220156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ghosh S, Dutta S, Sarkar A, Kundu M, Sil PC. Targeted delivery of curcumin in breast cancer cells via hyaluronic acid modified mesoporous silica nanoparticle to enhance anticancer efficiency. Colloids Surf B. 2021;197:111404. 10.1016/j.colsurfb.2020.111404. [DOI] [PubMed] [Google Scholar]
- 120.Djayanti K, Maharjan P, Cho KH, Jeong S, Kim MS, Shin MC, Min KA. Mesoporous silica nanoparticles as a potential nanoplatform: therapeutic applications and considerations. Int J Mol Sci. 2023;24:6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ghosh S, Dutta S, Sarkar A, Kundu M, Sil PC. Targeted delivery of curcumin in breast cancer cells via hyaluronic acid modified mesoporous silica nanoparticle to enhance anticancer efficiency. Colloids Surf B Biointerfaces. 2021;197:111404. 10.1016/j.colsurfb.2020.111404. [DOI] [PubMed] [Google Scholar]
- 122.Mohebian Z, Babazadeh M, Zarghami N, Mousazadeh H. Anticancer efficiency of curcumin-loaded mesoporous silica nanoparticles/nanofiber composites for potential postsurgical breast cancer treatment. J Drug Deliv Sci Technol. 2021;61:102170. 10.1016/j.jddst.2020.102170. [Google Scholar]
- 123.Tzeng SY, Green JJ. Therapeutic nanomedicine for brain cancer. Ther Deliv. 2013;4:687–704. 10.4155/tde.13.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Abdalla Y, Luo M, Mäkilä E, Day BW, Voelcker NH, Tong WY. Effectiveness of porous silicon nanoparticle treatment at inhibiting the migration of a heterogeneous glioma cell population. J Nanobiotechnol. 2021;19:60. 10.1186/s12951-021-00798-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jackson M, Hassiotou F, Nowak A. Glioblastoma stem-like cells: at the root of tumor recurrence and a therapeutic target. Carcinogenesis. 2015;36:177–85. 10.1093/carcin/bgu243. [DOI] [PubMed] [Google Scholar]
- 126.Kozielski KL, Ruiz-Valls A, Tzeng SY, Guerrero-Cázares H, Rui Y, Li Y, Vaughan HJ, Gionet-Gonzales M, Vantucci C, Kim J, Schiapparelli P, Al-Kharboosh R, Quiñones-Hinojosa A, Green JJ. Cancer-selective nanoparticles for combinatorial siRNA delivery to primary human GBM in vitro and in vivo. Biomaterials. 2019;209:79–87. 10.1016/j.biomaterials.2019.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wattamwar PP, Biswal D, Cochran DB, Lyvers AC, Eitel RE, Anderson KW, Hilt JZ, Dziubla TD. Synthesis and characterization of poly(antioxidant β-amino esters) for controlled release of polyphenolic antioxidants. Acta Biomater. 2012;8:2529–37. 10.1016/j.actbio.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 128.Chavda VP, Vihol D, Mehta B, Shah D, Patel M, Vora LK, Pereira-Silva M, Paiva-Santos AC. Phytochemical-loaded liposomes for anticancer therapy: an updated review. Nanomedicine. 2022;17:547–68. 10.2217/nnm-2021-0463. [DOI] [PubMed] [Google Scholar]
- 129.Bartosz T, Irene T. Polyphenols encapsulation—application of innovation technologies to improve stability of natural products. Phys Sci Rev. 2016. 10.1515/psr-2015-0005. [Google Scholar]
- 130.Wang S, Su R, Nie S, Sun M, Zhang J, Wu D, Moustaid-Moussa N. Application of nanotechnology in improving bioavailability and bioactivity of diet-derived phytochemicals. J Nutr Biochem. 2014;25:363–76. 10.1016/j.jnutbio.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Navya PN, Kaphle A, Srinivas SP, Bhargava SK, Rotello VM, Daima HK. Current trends and challenges in cancer management and therapy using designer nanomaterials. Nano Converg. 2019;6:23. 10.1186/s40580-019-0193-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Anwar M, Asfer M, Prajapati AP, Mohapatra S, Akhter S, Ali A, Ahmad FJ. Synthesis and in vitro localization study of curcumin-loaded SPIONs in a micro capillary for simulating a targeted drug delivery system. Int J Pharm. 2014;468:158–64. 10.1016/j.ijpharm.2014.04.038. [DOI] [PubMed] [Google Scholar]
- 133.Valdiglesias V, Kiliç G, Costa C, Fernández-Bertólez N, Pásaro E, Teixeira JP, Laffon B. Effects of iron oxide nanoparticles: cytotoxicity, genotoxicity, developmental toxicity, and neurotoxicity. Environ Mol Mutagen. 2015;56:125–48. 10.1002/em.21909. [DOI] [PubMed] [Google Scholar]
- 134.Lushchak O, Strilbytska O, Koliada A, Zayachkivska A, Burdyliuk N, Yurkevych I, Storey KB, Vaiserman A. Nanodelivery of phytobioactive compounds for treating aging-associated disorders. Geroscience. 2020;42:117–39. 10.1007/s11357-019-00116-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Caruso G, Merlo L, Tot E, Pignataro C, Caffo M. Chapter 6—Nanotechnology and the new frontiers of drug delivery in cerebral gliomas. In: Grumezescu AM, editor. Nano- and microscale drug delivery systems. Elsevier; 2017. p. 95–112. [Google Scholar]
- 136.Maniam G, Mai C-W, Zulkefeli M, Dufès C, Tan DM-Y, Fu J-Y. Challenges and opportunities of nanotechnology as delivery platform for tocotrienols in cancer therapy. Front Pharmacol. 2018. 10.3389/fphar.2018.01358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gupta P, Authimoolam SP, Hilt JZ, Dziubla TD. Quercetin conjugated poly(β-amino esters) nanogels for the treatment of cellular oxidative stress. Acta Biomater. 2015;27:194–204. 10.1016/j.actbio.2015.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhou F, Peterson T, Fan Z, Wang S. The commonly used stabilizers for phytochemical-based nanoparticles: stabilization effects, mechanisms, and applications. Nutrients. 2023. 10.3390/nu15183881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pasarin D, Ghizdareanu A-I, Enascuta CE, Matei CB, Bilbie C, Paraschiv-Palada L, Veres P-A. Coating materials to increase the stability of liposomes. Polymers. 2023;15:782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cuomo F, Cofelice M, Venditti F, Ceglie A, Miguel M, Lindman B, Lopez F. In-vitro digestion of curcumin loaded chitosan-coated liposomes. Colloids Surf B. 2018;168:29–34. 10.1016/j.colsurfb.2017.11.047. [DOI] [PubMed] [Google Scholar]
- 141.Shao P, Wang P, Niu B, Kang J. Environmental stress stability of pectin-stabilized resveratrol liposomes with different degree of esterification. Int J Biol Macromol. 2018;119:53–9. 10.1016/j.ijbiomac.2018.07.139. [DOI] [PubMed] [Google Scholar]
- 142.Das A, Adhikari S, Deka D, Baildya N, Sahare P, Banerjee A, Paul S, Bisgin A, Pathak S. An updated review on the role of nanoformulated phytochemicals in colorectal cancer. Medicina. 2023;59:685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cheng Z, Li M, Dey R, Chen Y. Nanomaterials for cancer therapy: current progress and perspectives. J Hematol Oncol. 2021;14:85. 10.1186/s13045-021-01096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Shreffler JW, Pullan JE, Dailey KM, Mallik S, Brooks AE. Overcoming hurdles in nanoparticle clinical translation: the influence of experimental design and surface modification. Int J Mol Sci. 2019. 10.3390/ijms20236056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Solanki R, Jodha B, Prabina KE, Aggarwal N, Patel S. Recent advances in phytochemical based nano-drug delivery systems to combat breast cancer: a review. J Drug Deliv Sci Technol. 2022;77:103832. 10.1016/j.jddst.2022.103832. [Google Scholar]
- 146.Maeki M, Uno S, Niwa A, Okada Y, Tokeshi M. Microfluidic technologies and devices for lipid nanoparticle-based RNA delivery. J Control Release. 2022;344:80–96. 10.1016/j.jconrel.2022.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.John R, Monpara J, Swaminathan S, Kalhapure R. Chemistry and art of developing lipid nanoparticles for biologics delivery: focus on development and scale-up. Pharmaceutics. 2024. 10.3390/pharmaceutics16010131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Herdiana Y, Wathoni N, Shamsuddin S, Muchtaridi M. Scale-up polymeric-based nanoparticles drug delivery systems: development and challenges. OpenNano. 2022;7:100048. 10.1016/j.onano.2022.100048. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.