Abstract
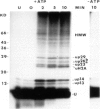
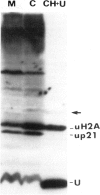
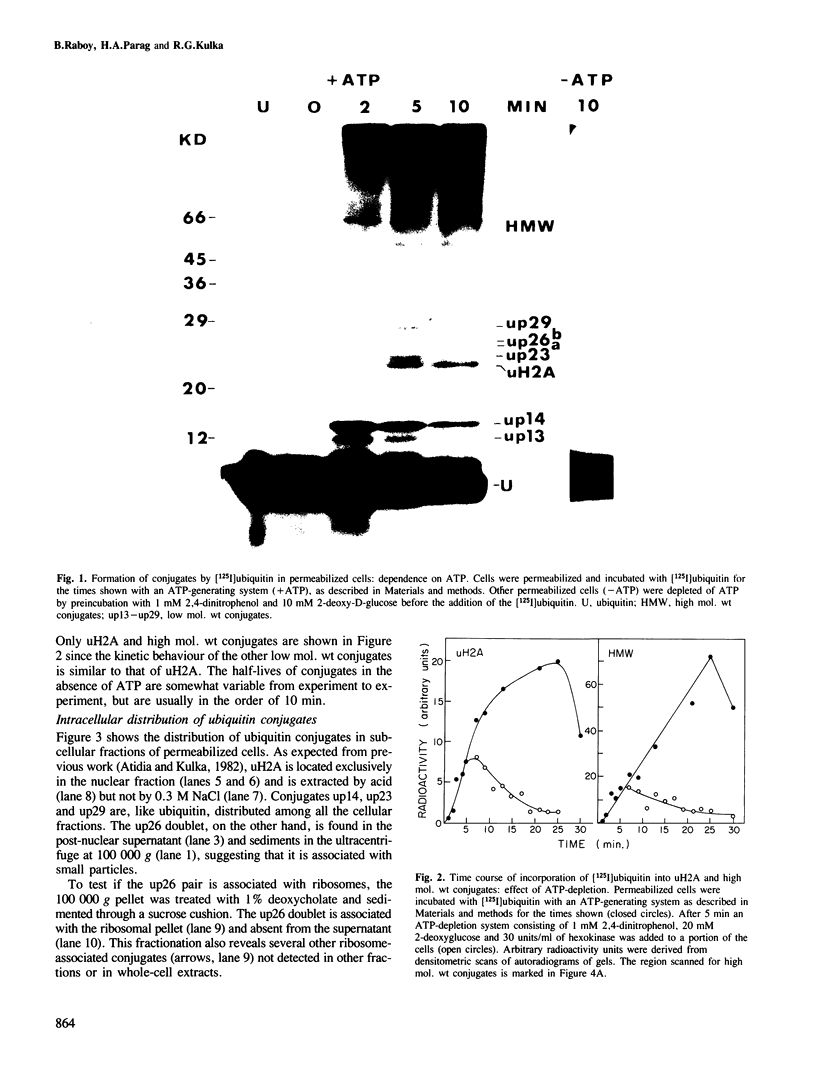
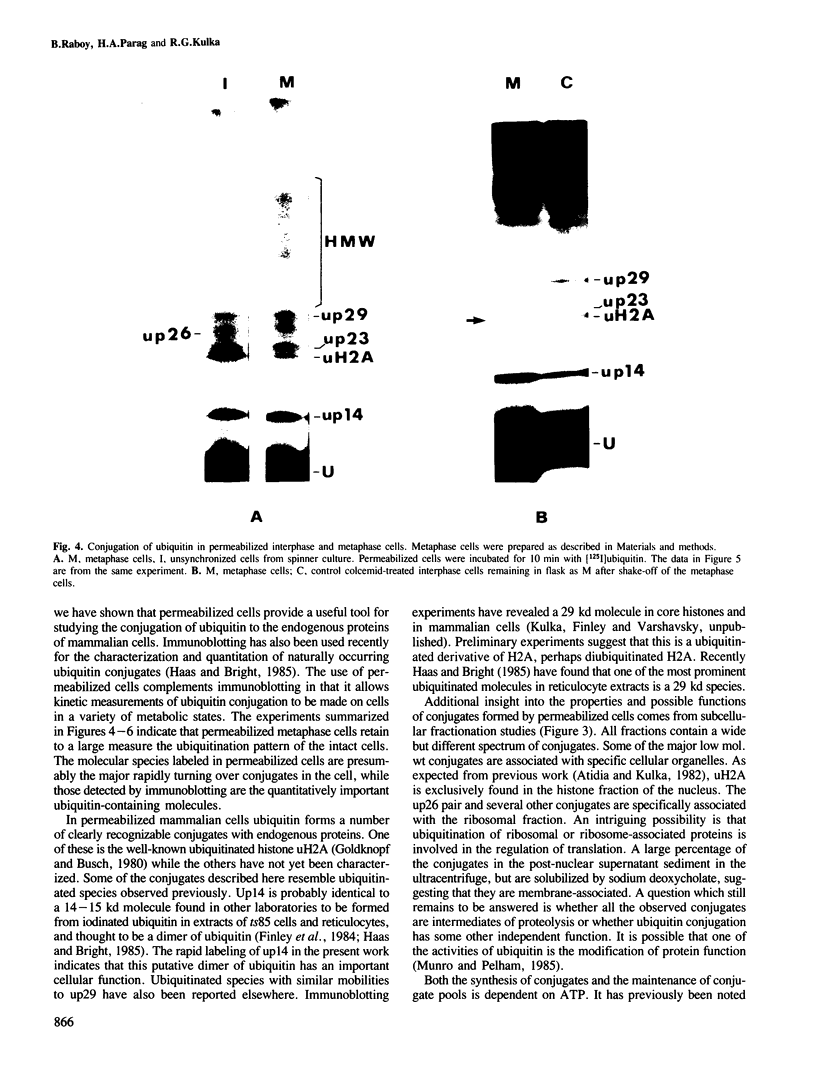
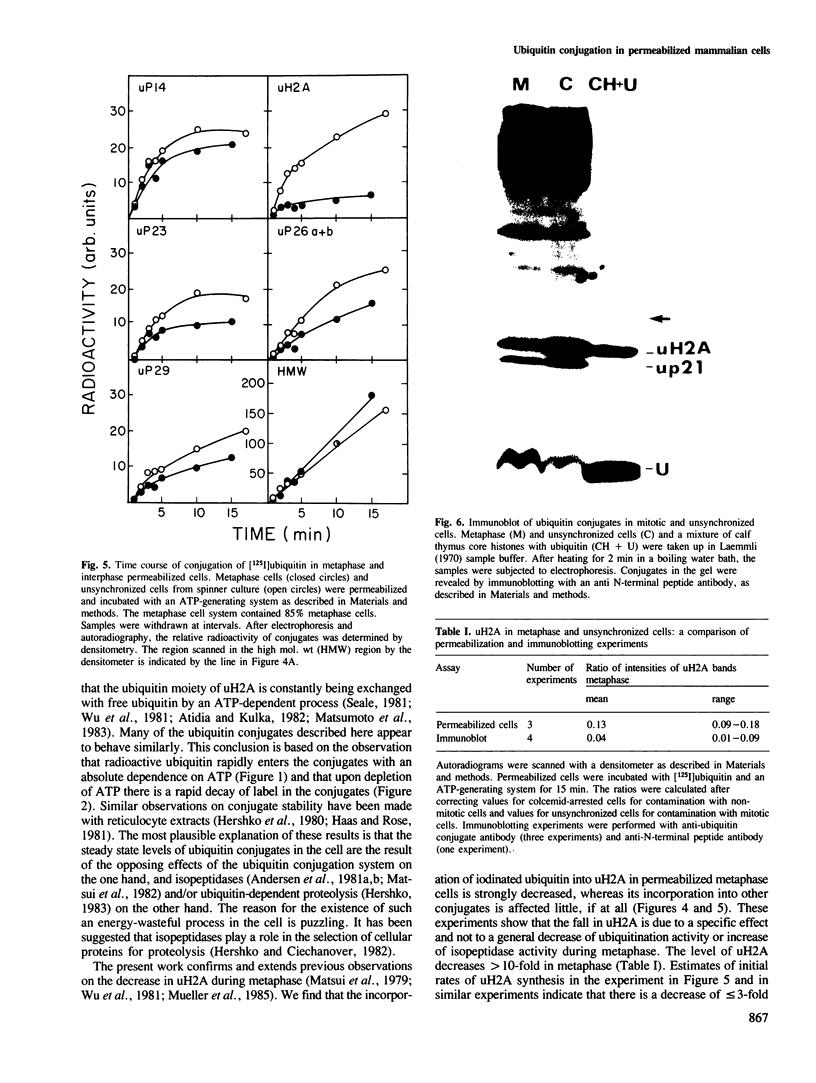
[125I]Ubiquitin introduced into permeabilized hepatoma tissue culture (HTC) cells rapidly forms conjugates with endogenous proteins. A characteristic pattern of low mol. wt conjugates is obtained which includes the ubiquitinated histone, uH2A, and unknown molecular species with MrS of 14, 23, 26 (two bands) and 29 kd. A broad spectrum of higher mol. wt conjugates is also produced. The formation of all conjugates is absolutely dependent on ATP, and upon depletion of ATP they are rapidly broken down. The 14, 23 and 29 kd species are found in all subcellular fractions examined. uH2A is located exclusively in the nuclear fraction. The pair of 26 kd bands is specifically associated with the ribosome fraction. A considerable percentage of the higher mol. wt conjugates sediments with the small particle (100,000 g) fraction in the ultracentrifuge but is solubilized with deoxycholate, indicating that there are many membrane-associated conjugates. The pattern of ubiquitin conjugation in interphase and metaphase cells was compared. The incorporation of ubiquitin into uH2A was markedly reduced in metaphase cells whereas its incorporation into other low mol. wt conjugates and into high mol. wt conjugates was affected slightly, if at all. This shows that the known decrease of uH2A levels in metaphase is due to a specific effect on histone ubiquitination and not to a general decrease in ubiquitination activity or increase of isopeptidase activity. Changes in the levels of uH2A during mitosis measured by immunoblotting were similar to those estimated in permeabilized cells. These experiments indicate that permeabilized cells provide a useful approach to the study of rapidly turning over ubiquitin conjugates in mammalian cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen M. W., Ballal N. R., Goldknopf I. L., Busch H. Protein A24 lyase activity in nucleoli of thioacetamide-treated rat liver releases histone 2A and ubiquitin from conjugated protein A24. Biochemistry. 1981 Mar 3;20(5):1100–1104. doi: 10.1021/bi00508a009. [DOI] [PubMed] [Google Scholar]
- Andersen M. W., Goldknopf I. L., Busch H. Protein A24 lyase is an isopeptidase. FEBS Lett. 1981 Sep 28;132(2):210–214. doi: 10.1016/0014-5793(81)81162-3. [DOI] [PubMed] [Google Scholar]
- Atidia J., Kulka R. G. Formation of conjugates by 125I-labelled ubiquitin microinjected into cultured hepatoma cells. FEBS Lett. 1982 Jun 1;142(1):72–76. doi: 10.1016/0014-5793(82)80222-6. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Cande W. Z. Nucleotide requirements for anaphase chromosome movements in permeabilized mitotic cells: anaphase B but not anaphase A requires ATP. Cell. 1982 Jan;28(1):15–22. doi: 10.1016/0092-8674(82)90370-1. [DOI] [PubMed] [Google Scholar]
- Chin D. T., Kuehl L., Rechsteiner M. Conjugation of ubiquitin to denatured hemoglobin is proportional to the rate of hemoglobin degradation in HeLa cells. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5857–5861. doi: 10.1073/pnas.79.19.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groot N., Yuli I. J., Czosnek H., Shiklosh Y., Hochberg A. A. Studies on the amino acid-incorporating activity of native rat liver rough membrane and that reconstituted in vitro. Biochem J. 1976 Jul 15;158(1):23–31. doi: 10.1042/bj1580023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley D., Ciechanover A., Varshavsky A. Thermolability of ubiquitin-activating enzyme from the mammalian cell cycle mutant ts85. Cell. 1984 May;37(1):43–55. doi: 10.1016/0092-8674(84)90299-x. [DOI] [PubMed] [Google Scholar]
- Goldknopf I. L., Busch H. Isopeptide linkage between nonhistone and histone 2A polypeptides of chromosomal conjugate-protein A24. Proc Natl Acad Sci U S A. 1977 Mar;74(3):864–868. doi: 10.1073/pnas.74.3.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldknopf I. L., Busch H. N-Bromosuccinimide fragments of protein A24 (uH2A): an implication that ubiquitin is the precursor of conjugation in vivo. Biochem Biophys Res Commun. 1980 Oct 31;96(4):1724–1731. doi: 10.1016/0006-291x(80)91373-x. [DOI] [PubMed] [Google Scholar]
- Haas A. L., Bright P. M. The immunochemical detection and quantitation of intracellular ubiquitin-protein conjugates. J Biol Chem. 1985 Oct 15;260(23):12464–12473. [PubMed] [Google Scholar]
- Haas A. L., Rose I. A. Hemin inhibits ATP-dependent ubiquitin-dependent proteolysis: role of hemin in regulating ubiquitin conjugate degradation. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6845–6848. doi: 10.1073/pnas.78.11.6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko A., Ciechanover A., Heller H., Haas A. L., Rose I. A. Proposed role of ATP in protein breakdown: conjugation of protein with multiple chains of the polypeptide of ATP-dependent proteolysis. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1783–1786. doi: 10.1073/pnas.77.4.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko A., Ciechanover A. Mechanisms of intracellular protein breakdown. Annu Rev Biochem. 1982;51:335–364. doi: 10.1146/annurev.bi.51.070182.002003. [DOI] [PubMed] [Google Scholar]
- Hershko A. Ubiquitin: roles in protein modification and breakdown. Cell. 1983 Aug;34(1):11–12. doi: 10.1016/0092-8674(83)90131-9. [DOI] [PubMed] [Google Scholar]
- Kulka R. G., Tokins G. M., Crook R. B. Clonal differences in glutamine synthetase activity of hepatoma cells. Effects of glutamine and dexamethasone. J Cell Biol. 1972 Jul;54(1):175–179. doi: 10.1083/jcb.54.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsui S. I., Seon B. K., Sandberg A. A. Disappearance of a structural chromatin protein A24 in mitosis: implications for molecular basis of chromatin condensation. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6386–6390. doi: 10.1073/pnas.76.12.6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui S., Sandberg A. A., Negoro S., Seon B. K., Goldstein G. Isopeptidase: a novel eukaryotic enzyme that cleaves isopeptide bonds. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1535–1539. doi: 10.1073/pnas.79.5.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y., Yasuda H., Marunouchi T., Yamada M. Decrease in uH2A (protein A24) of a mouse temperature-sensitive mutant. FEBS Lett. 1983 Jan 10;151(1):139–142. doi: 10.1016/0014-5793(83)80359-7. [DOI] [PubMed] [Google Scholar]
- Miller M. R., Castellot J. J., Jr, Pardee A. B. A permeable animal cell preparation for studying macromolecular synthesis. DNA synthesis and the role of deoxyribonucleotides in S phase initiation. Biochemistry. 1978 Mar 21;17(6):1073–1080. doi: 10.1021/bi00599a021. [DOI] [PubMed] [Google Scholar]
- Mueller R. D., Yasuda H., Hatch C. L., Bonner W. M., Bradbury E. M. Identification of ubiquitinated histones 2A and 2B in Physarum polycephalum. Disappearance of these proteins at metaphase and reappearance at anaphase. J Biol Chem. 1985 Apr 25;260(8):5147–5153. [PubMed] [Google Scholar]
- Munro S., Pelham H. What turns on heat shock genes? Nature. 1985 Oct 10;317(6037):477–478. doi: 10.1038/317477a0. [DOI] [PubMed] [Google Scholar]
- Schliwa M., van Blerkom J., Porter K. R. Stabilization and the cytoplasmic ground substance in detergent-opened cells and a structural and biochemical analysis of its composition. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4329–4333. doi: 10.1073/pnas.78.7.4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale R. L. Rapid turnover of the histone-ubiquitin conjugate, protein A24. Nucleic Acids Res. 1981 Jul 10;9(13):3151–3158. doi: 10.1093/nar/9.13.3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. O., Kornberg R. D. An octamer of histones in chromatin and free in solution. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2626–2630. doi: 10.1073/pnas.72.7.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West M. H., Bonner W. M. Histone 2B can be modified by the attachment of ubiquitin. Nucleic Acids Res. 1980 Oct 24;8(20):4671–4680. doi: 10.1093/nar/8.20.4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R. S., Kohn K. W., Bonner W. M. Metabolism of ubiquitinated histones. J Biol Chem. 1981 Jun 10;256(11):5916–5920. [PubMed] [Google Scholar]