Abstract
The house sparrow (Passer domesticus) is a gregarious generalist species, which makes it a good model for studying play. However, play has not been described for this species so far. We describe play behaviour in house sparrows for the first time, quantifying all play and play-related behaviours, searching for differences between the different sexes and ages, the possible association with reproductive success and the diffusion of this behaviour in the population. All behaviours were recorded from the end of 2018 breeding season to the start of the new one in 2019. Behaviours were classified into four levels of interaction of increasing complexity and intensity. Results showed that play behaviour was restricted to the breeding season, adult males played more often than the rest of the groups, and their behaviours correlated with the number of recruits they produced. Moreover, “Maximum Level” of play of the mothers significantly and positively correlated with that of their offspring, and the “Maximum Level” of an individual with the proportion of playing siblings. Despite the limitations of the present study, our results point out the existence of benefits for the reproductive success of playing individuals.
Keywords: Object play behaviour, House sparrow Passer domesticus, Reproductive success, Captivity
Introduction
The house sparrow (Passer domesticus, hereafter sparrow) is a small passerine common in cities around the world. Their strong gregarious foraging and breeding habits make it a good model species for studying play, as gregariousness promotes social learning and increases innovative problem-solving (Morand-Ferron and Quinn 2011). Play can be learned in social contexts, as it can sometimes be performed by imitation and may even be considered contagious (Ficken 1997; Kaplan 2024). Observing one individual play makes others feel playful (Stöwe et al. 2006; Osvath and Sima 2014; Schwing et al. 2017; Kaplan 2024). In the case of sparrows, they tend to follow their brood siblings when initiating activities (Tóth et al. 2009), and parents play an important role in fledgling learning (Truskanov and Lotem 2015). Sparrows are more prone to explore in the presence of kin (Tuliozi et al. 2018) and learn to exploit novel food sources by observing others (Anderson 2006). Moreover, behaviours towards new food are strongly influenced by the presence of conspecifics (Fryday and Greig-Smith 1994).
Play is a widespread behaviour observed across diverse taxonomic groups, from fish and all tetrapod lineages (Diamond et al. 2006; Burghardt 2015; Sharpe 2018) to invertebrates, including cephalopods and insects (Kuba et al. 2003; Dapporto et al. 2006; Galpayage Dona et al. 2022).
To our knowledge play has not been described in sparrows, although it has been extensively documented among birds. Play has been reported in 13 out of 27 orders (Emery and Clayton 2015; Kaplan 2020), typically more among those with well-developed cognitive abilities and large brains relative to their size, as corvids and psittacines (Ortega and Bekoff 1987; Olkowicz et al. 2016; Kaplan 2020). Locomotor play is the most common type of play in birds and has been reported in many species (Kaplan 2024). Object play is the second most frequent category of play in birds (Ortega and Bekoff 1987; Diamond and Bond 2003; Kaplan 2024), being observed in fewer species than locomotor play and is more commonly found in juveniles (Ficken 1997).
To help differentiate play from other similar behaviours, especially in species phylogenetically distant from us, Burghardt (2010) stablished a set of five criteria used successfully in several unrelated taxa (e.g., Kuba et al. 2003; Barabanov et al. 2015; Galpayage Dona et al. 2022): (1) play is incompletely functional in the context in which it appears; (2) it is spontaneous, pleasurable, rewarding, or voluntary; (3) it differs from other more serious behaviours in form or timing; (4) it is repeated, but not in an abnormal and unvarying stereotypic form; and (5) it is initiated in the absence of severe stress.
The occurrence of play has been linked to exploratory behaviour, innovation and ecological generality (Bond and Diamond 2004). For this reason, it is sometimes difficult to distinguish between object play and object exploration (Greenberg 2003; O’Hara and Auersperg 2017), despite they are functionally distinct (Auersperg et al. 2015).
Predators usually play with objects in a similar way to how they hunt, which has been interpreted as a way of enhancing physical, perceptual and physiological traits that will give them advantage confronting life challenges (Hall 1998; Graham and Burghardt 2010; Gray 2018; Burghardt et al. 2024). Examples among birds include Neotropic cormorants (Phalacrocorax brasialianus), green herons (Butorides striata) (Sazima 2008), anhingas (Anhinga anhinga), Australasian darters (A. novaehollandiae) (Sazima 2019) and Montagu’s harriers (Circus pygargus) (Kitowski 2005).
Object play can also be social (Kaplan 2024). Social play may have a role in the formation and maintenance of social bonds, including mate choice (Bekoff 1984; Heinrich and Smolker 1998; Bugnyar et al. 2007; Chick et al. 2012; Palagi 2023). Additionally, play seems to contribute to making the individual more resilient to stress, so that it acts as a way of coping with unexpected and stressful situations as may be social interactions (Palagi 2006; Norscia and Palagi 2011; Kaplan 2020; Gabrielle et al. 2022; Francesconi et al. 2024). This category of play has been found in hornbills (Bycanistes brevis and Bucorvus leadbeateri) (Diamond and Bond 2003), Eurasian (Turdoides striatus) and Arabian babblers (T. squamiceps) (Diamond and Bond 2003; Zahavi et al. 2004), Australian magpies (Gymnorhina tibicen) (Pellis 1981) and striated caracaras (Phalcoboenus australis) (Harrington and Lambert 2024). Keas (Nestor notabilis) are known to engage in much social object play even as adults (Diamond and Bond 2003; Bond and Diamond 2004). Corvids, which exhibit the most complex plays (Ficken 1997; Brazil 2002), also engage in social object play (Heinrich and Smolker 1998; Diamond and Bond 2003; Osvath and Sima 2014; Osvath et al. 2014).
If play is a way of enhancing abilities or learning to cope with social expectations, these would explain why the majority of play behaviours are found in young individuals (Bekoff 1984; Bekoff and Byers 1998; Burghardt 2005; Sharpe 2018; Gray 2018). The form and frequency of play change along animal age (Owens 1975; Bond and Diamond 2004; Foroud et al. 2004; Nahallage and Huffman 2007; O’Hara and Auersperg 2017). In this sense, adult play has often been more problematic to tackle, as it cannot be explained as a practice of skills that they should have already mastered, and its infrequency has resulted in less study (Hall 1998; Norscia and Palagi 2011; Kaplan 2024).
It is possible that adult object play is associated with behavioural flexibility (Hall 1998), which would explain why it is more associated with opportunistic species (O’Hara and Auersperg 2017). It has been suggested that exploration triggers adult object play behaviour (Kuba et al. 2006), allowing the individual to get used to novel stimuli that may initially cause fear. Those animals that play more may adjust their behaviour to novelties in the environment (Hall 1998). It is likely that individuals that play more as juveniles retain this play behaviour as adults (O’Meara et al. 2015).
One last possibility is that play appears as a way of using surplus energy or resources in contexts in which animals are in perfect nutritional and physiological condition, which is known as the surplus resource theory (Hall 1998; Graham and Burghardt 2010). However, this explanation lacks an evolutionary understanding of play.
Finally, for the maintenance of play, it must have beneficial impact on the reproduction of individuals which outweigh any possible costs (e.g., Martin 1984; Harcourt 1991; Siviy and Atrens 1992; Caro 1995; De Oliveira et al. 2003; Yanagi and Berman 2018). However, few works have found evidence of positive effects of play on reproductive success, or even on reproduction in general. In Belding’s ground squirrels (Urocitellus beldingi), the weaning success of the yearling females is predicted by rates of social play as juveniles (Nunes et al. 2004). This may affect long-term reproductive success, although the association is somewhat limited (Nunes 2014). Another case is the American mink (Neovison vison), where play-fighting during the juvenile period predicts sexual behaviour in males, although how this affects their reproductive success is unknown (Dallaire and Mason 2017). Finally, in brown bears (Ursus arctos) playful behaviour was found to predict juvenile survival (Fagen and Fagen 2004).
During the 2018 breeding season, a previously unrecorded play behaviour involving a rope was observed in a sparrow population kept in captivity at the University of Granada. This behaviour disappeared after the end of the season until the following spring. The aim of this work is to characterise this new behaviour, to analyse its origins, its relationship with the breeding season and reproductive success, and its transmission through the population.
Materials and methods
Captivity conditions
The study was carried out in the aviary of the Faculty of Sciences of the University of Granada, a 375 m3 open-air space where a population of about 130 individuals was maintained at the beginning of the study, consisting of 50 original breeding pairs and their offspring born that summer. All sparrows were marked with a unique combination of coloured leg rings for individual identification.
Diet consisted mainly of birdseeds and bread. Sparrows also had fruit paste (Bogena), containing cereal flour, dried fruit and honey, an important source of vitamins. This food was supplied ad libitum. The diet was supplemented daily with fresh fruit (apple and pear), lettuce and hard-boiled eggs, the shells of which is a good source of calcium. Growing chicks require protein-rich food, so fly larvae (Family Calliphoridae, asticot) were also provided throughout the breeding season, being useful as dietary supplement for the adults. Food was distributed throughout the aviary in several feeders to minimise competition between individuals, ensuring that all birds had easy access to food. There were also two watering troughs in opposite sides of the aviary, and, in summer, a tap was left slightly open so it formed a little stream and a puddle where they could drink fresh water and bathe.
For nesting, 71 holes were available in the wall, the end of which opened into nest boxes located inside the laboratory, so researchers could access them directly, allowing easy handling of the nests and causing minimal stress to the birds. The number of potential nests was higher than the number of pairs, which minimised competition for nesting sites.
The rope
Some of the behaviours discovered and analysed in this work could be consider object play carried out with a rope. This was an old, frayed rope of about 40 cm, with several knots and ends (Fig. 1). The rope was hanging from a metal bar that was part of the bird perches. The rope had been there for at least three years before the first behaviour was observed.
Fig. 1.

The rope that house sparrows (Passer domesticus) used for object play as it was present in the aviary
The rope was close to the last nest hole (number 71), although far enough away so it did not completely enter the territory defended by the pair that nested in that hole in 2018. However, in 2019 a particularly dominant male nesting in the that nest box kept some control over the rope area, although it did not occupy it completely. Regardless of how much control that 71st male could exert over the rope, sparrows tended to fight over control or access to the rope. Some sparrows did not interact until the last remaining individual had left the bar. These confrontations were brief and were deeply marked by hierarchy. The mere display of an aggressive posture was enough to immediately dispel rivals (personal observations).
Recording and data collection
First recordings were filmed on August 14th, 2018. In the present work, we mainly analysed those behaviours corresponding to the first month, from August 14th to September 16th, 2018.
The recordings were performed by placing a camera (Panasonic HC-V130 HD) in the morning, recording from one of the observation windows of the laboratory. Recordings lasted between one to six hours per day, totalling up to 479.48 h of video.
Each interaction has been classified into categories called levels. These categories range from 1 to 4 and imply an increase in the complexity and intensity of the interaction with the rope (Table 1). Those sparrows that were never seen interacting with the rope were assigned as level 0. The level to which an interaction belongs is determined by the presence of specific behaviours (Table 1), following Kuba et al. (2003):
Table 1.
Different rope-related behaviours of house sparrow (Passer domesticus) involved in each play level. Level 1: brief contact; level 2: prolonged contact; level 3: basic play; level 4: acrobatic play
| NON-PLAY | PLAY | ||
|---|---|---|---|
| Level 1 | Level 2 | Level 3 | Level 4 |
| Light pecking | Pecking | Use several angles | Bungee jumping |
| Exploratory touch | Chewing | Catch at flight | Hung spin |
| Pulling once | Swallowing | Upside-down touch | Rappelling |
| Pulling several times | Raise the rope | ||
| Drag the rope | |||
Level 1 (brief contact) consisted of brief exploratory approaches (see Online Resource 1). These sparrows were probably individuals looking for nest materials, and it is possible that this behaviour was behind the origin of the most complex ones.
Level 2 (prolonged contact) was characterised by a genuine interest in the object. These interactions were longer (the longest one observed corresponded to this level). It is worth noting some odd behaviours: swallowing large portions of rope, regurgitating it and swallowing it again; and the “chewing”, which was similar to the way in which sparrows open birdseeds (see Online Resources 2 and 3).
Level 3 (basic play) was an increase in complexity and intensity. There was less pecking and more pulling and pulling was stronger. Sparrows used several angles to approach the rope (see Online Resource 4). As it was not always easy to reduce complex behaviours to discrete categories, to distinguish level 2 from level 3 at least two level 3 behaviours were required to consider the interaction as level 3.
Level 4 (acrobatic play) behaviours were so evident and clear that there was no room for doubt. Most of the sparrows performed only one of the possible level 4 behaviours: bungee jumping. They grabbed the rope with their beaks and jumped into the air, so that they ended up hanging from the rope. Some took advantage of this movement to swing and returned to the bar, usually to start again (see Online Resources 5 and 6). Other behaviours were descents through the rope and spins hanging from the tail of the rope.
Levels 1 and 2 do not meet clearly Burghardt criteria 3 and 4, so they cannot be considered play behaviours with certainty. Level 1 is impossible to distinguish from exploration or searching for nest materials, and it is unclear whether some of the level 2 behaviours are not stereotypies. Some individuals looked anxious, and the duration of the behaviour and the fixed repetitive patterns raise some doubts about that possibility. However, levels 3 and 4 meet all Burghardt’s criteria and therefore can be considered unambiguously play. Level 3 is a clear case of object play, while level 4 is a mix of object and locomotor play.
The individual’s ability to interact with the rope is best measured by the Maximum Level (ML), which we defined as the higher level of interaction an individual was able to perform during the studied period. Initially, we considered using average level of interaction, but sparrows do not always perform at the highest complexity of which they are capable, making it inferior when measuring ability.
We also considered the physical condition of individuals, as it is an important variable that influences the behaviours and their reproductive success of birds (see below).
Individual and kinship identification
Adults carried four colour rings, two on each leg, with a unique colour combination that allowed individuals to be identified in the recordings and/or from the laboratory observation windows with binoculars. The fourth ring had a year-specific colour and a number. Nestlings were ringed only with this last ring on the fifth day after hatching. Later, at the end of December, when all individuals were captured, juveniles were ringed with the other three colour rings. The identification of juveniles was thus dependent on the ring number. In some cases, identification was not possible due to the difficulty of observing the ring number with the binoculars or on recordings. A total of 147 of 1180 interactions (12.46%) were performed by unidentified sparrows. Most of them were juvenile individuals (124 interactions) in brief interactions of low level, with only one interaction above level 2. Since all juveniles have a ML of at least 2, at most one individual ML is underestimated. Thus, this circumstance should have no significant impact in the analysis using the ML.
After identifying the individuals who interacted, kinship relationships were reconstructed, even of those individuals which were not seen around the rope. The result was a family tree going back nine generations connecting most of the individuals, although there was only data on play behaviour for the younger generations. This tree is not shown due to the difficulty of its representation. Unfortunately, several old data were not systematized and some information, especially of the first generations of the tree, was not collected. Moreover, it should be noted that no molecular analysis of parentage was carried out, so that each individual is considered to be a biological offspring of the pair that raised it. However, it is well known that, in natural conditions, sparrows engage in extra-pair copulation (around 14%; Anderson 2006) and intraspecific brood parasitism (8,5% of nests; Kendra et al. 1988).
Statistical analysis
First, 2 × 2 χ2 tests were carried out for exploring differences in the percentage of individuals interested in the rope by sex (male and female) and age (juvenile and adult).
Second, variables did not meet the requirements of normality or homoscedasticity, nor did the residuals reached normality, so Kruskal-Wallis tests were conducted to explore differences in the number of times that an individual interacted with the rope within each level, between sexes and ages. The levels of the independent variable were adult male, juvenile male, adult female and juvenile female.
Third, regarding the relationship between interaction levels and different reproductive parameters, we used Spearman’s correlations to explore the relationship between ML and different reproductive parameters such as number of clutches during the breeding season, fledgling production (total number of fledglings produced) and recruit production (total number of fledglings still alive in December).
Fourth, in relation to learning and diffusion of play as an innovative behaviour, evidence for both vertical (from parents to offspring) and horizontal (between brothers) transmission has been sought. For the vertical transmission, we used Spearman’s correlations to explore the association between the ML of the individual and the ML of the father or the mother, analysing both sexes separately. The relationship between the ML of each sex within couples was also explored with Spearman’s correlations. The ML of each parent and the number of visits to the rope (independent variables) were correlated with the percentage of playing offspring (those individuals that reach level 3 or 4) and interested offspring in the rope (every level apart from 0).
Horizontal transmission was studied considering the percentage of playing siblings or interested siblings (those who share mother and father) explaining the ML of an individual. These analyses were performed for siblings in general, as well as for same-brood siblings and non-brood siblings separately.
Finally, we analysed the influence of physical condition on the play abilities, separately for adults and juveniles. Physical condition was estimated following Peig and Green (2009), which considers condition as the residuals of the correlation between the logarithm of weight against the logarithm of tarsus.
All statistical analysis were carried out with STATISTICA 10 (StatSoft, Inc, Tulsa, OK, USA).
Results
Differences among sexes and ages
Seventy-five individuals were identified while interacting with the rope (59.5% of the total aviary population), with 51 not seen interacting during the 2018 breeding season. Of these 75 individuals, 31 were adult females (41.3%), 20 adult males (26.7%), 14 juvenile females (18.7%) and 10 juvenile males (13.3%). Juvenile females were the most involved in levels 1, 2 and 3 (Table 2). Females, regardless of the age, showed a significant decrease from level 2 to level 3 and from level 3 to level 4 (Table 2). Adult males were the most involved in level 4 (Table 2).
Table 2.
Percentages of the entire aviary population for each group of house sparrows involved in each level of play according to sex and age. Level 1: brief contact; level 2: prolonged contact; level 3: basic play; level 4: acrobatic play
| Male | Female | ||||
|---|---|---|---|---|---|
| Juvenile | Adult | Juvenile | Adult | ||
| Level 1 | 53.3 | 41.3 | 68.8 | 46.9 | |
| Level 2 | 66.7 | 34.8 | 81.3 | 42.9 | |
| Level 3 | 33.3 | 26.1 | 43.8 | 16.3 | |
| Level 4 | 13.3 | 21.7 | 12.5 | 2.0 | |
| Total | 66.7 | 43.5 | 87.5 | 63.3 | |
Percentage of individuals involved at levels 1, 2 and 3 did not differ significantly between ages and sexes (level 1, χ²= 1.29, P = 0.256; level 2, χ²= 0.70, P = 0.402; level 3, χ²= 0.74, P = 0.391). However, the percentage of individuals showing level 4 differed significantly between sexes and ages (level 4, χ² = 19.14, P < 0.001). Adult females showed the lowest level 4 play and adult males the highest (Table 2). Juveniles showed intermediate level 4 play (Table 2).
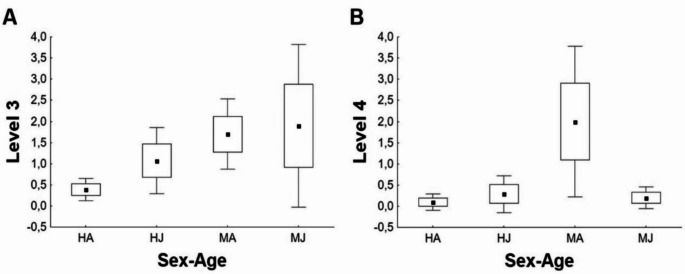
When we considered the number of times an individual interacted with the rope within each level, the number of times that adult males interacted with the rope at level 3 was significantly higher than adult females. At level 4, adult males showed significantly more interactions than any other group (Table 3; Fig. 2).
Table 3.
Kruskal-Wallis non-parametric tests showing differences in the number of interactions for each play level in each sex and age of house sparrows (Passer domesticus). Level 1: brief contact; level 2: prolonged contact; level 3: basic play; level 4: acrobatic play
| Level | H | Degrees of freedom | P |
|---|---|---|---|
| 1 | 2.83 | 3,75 | 0.418 |
| 2 | 2.41 | 3,75 | 0.492 |
| 3 | 9.03 | 3,75 | 0.029 |
| 4 | 16.70 | 3,75 | < 0.001 |
Fig. 2.
Differences between sexes and ages in the number of interactions of house sparrows with the rope at levels 3 (basic play) (A) and 4 (acrobatic play) (B). HA: adult females (N = 31); HJ: juveniles females (N = 14); MA: adult males (N = 20); MJ: juvenile males (N = 10). Black square = mean; box = mean + standard error (SE); whiskers = mean ± 1,96*SE
Phenology of the interaction with the rope
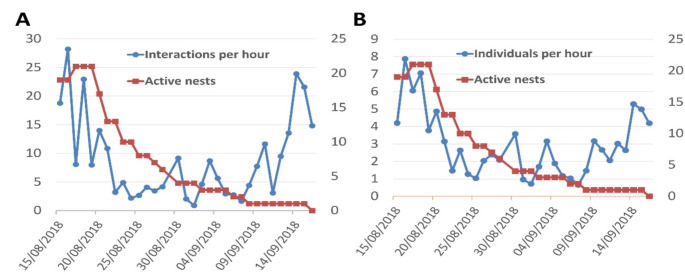
Overall activity in relation to the rope was in decline throughout the end of the breeding season, with a peak in mid-September (Fig. 3). This decline was paralleled by the decrease in the number of active nests (with eggs or nestlings) until the first week of September (red line in Fig. 3). When only true play behaviours were considered, this peak became smaller in the case of the juveniles and disappeared completely for adults (Fig. 4).
Fig. 3.
Number of interactions with the rope per hour (A) and number of house sparrows (Passer domesticus) interacting with the rope per hour (B) through the end of the breeding season, and number of active nests (with nestlings or eggs)
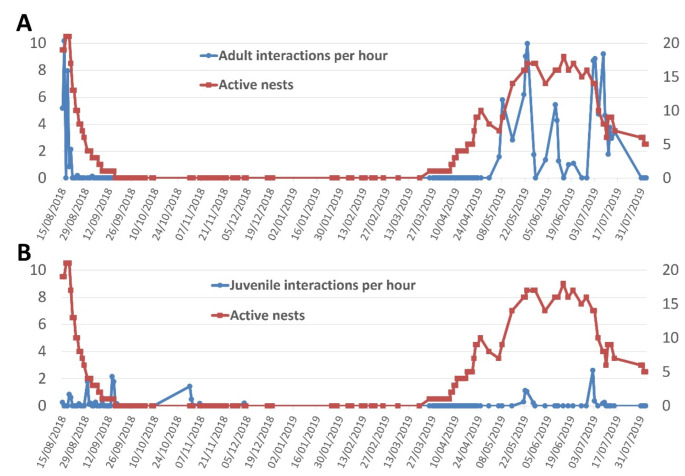
Fig. 4.
Number of level 3 and 4 interactions (true play behaviour) observed between the end of 2018’s breeding season and of 2019’s breeding season (primary axis), as well as the number of active nests (with eggs or nestlings, secondary axis) by (A) adult and (B) juvenile house sparrows (Passer domesticus)
Level 3 and 4 behaviours (those considered true play) in adults did not occur from September 2nd and in juveniles from September 16th onwards, appearing again at the beginning of next breeding season in both adults (Fig. 4A) and juveniles (Fig. 4B). Levels 1 and 2 appeared frequently until mid-September and were greatly reduced from then on. The decrease throughout August in number of total interactions and individual visits to the rope per hour was not significantly correlated with the reduction of breeding activity, but when only looking at true play behaviours correlation was close to significance (Spearman’s correlations; all levels, R2 = < 0.01, t30 = 0.09, P = 0.925; levels 3 and 4, R2 = 0.11, t30 = 1.91, P = 0.065). Juveniles maintained stable play with the rope throughout the end of the breeding season, being the only ones that played outside the breeding season (Fig. 3B). This activity was extremely reduced during the winter and disappeared after the new year.
Relationship between Maximum Level of interaction and breeding success
ML of adult males was not associated with the number of clutches or the number of fledglings, but ML of adult males positively and significantly explained the variation in the total number of recruits of the next generation (Table 4; Fig. 5). In the case of females, the ML was not significantly correlated with any of these reproductive parameters (Table 4).
Table 4.
Spearman’s correlations between Maximum Level of interaction with the rope and reproductive parameters by sex. Level 1: brief contact; level 2: prolonged contact; level 3: basic play; level 4: acrobatic play. Correlation coefficient (R) and degree of freedom (df) is shown
| Males | Females | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Reproductive parameters | R2 | df | t | P | R2 | df | t | P | |
| Nº clutches | 0.01 | 30 | 0.18 | 0.859 | 0.03 | 29 | 0.88 | 0.385 | |
| Nº fledglings | 0.02 | 30 | 0.76 | 0.451 | > 0.01 | 29 | 0.35 | 0.731 | |
| Nº recruits | 0.13 | 30 | 2.28 | 0.030 | 0.02 | 29 | 0.67 | 0.506 | |
Fig. 5.
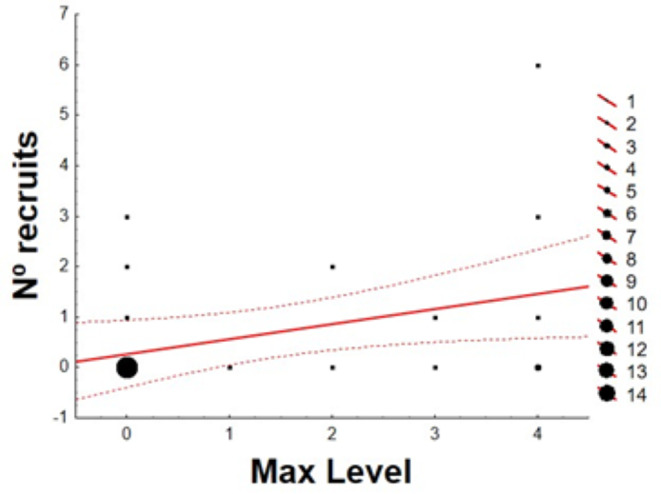
Correlation between Maximum Level of interaction with the rope reached by each adult male house sparrow (Passer domesticus) and the number of recruits for the next breeding season. Level 1: brief contact; level 2: prolonged contact; level 3: basic play; level 4: acrobatic play. Larger dots represent more individuals falling under those parameters
Relationship between the Maximum Level of interaction and kinship relationships
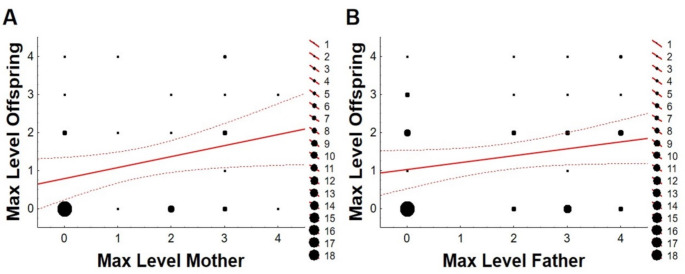
ML of parents correlated positively with ML of the offspring, although it was significant only for the mothers (Spearman’s correlations; females, R2 = 0.09, t58 = 2.41, P = 0.019; males, R2 = 0.04, t73 = 1.75, P = 0.085; Fig. 6). Furthermore, no correlation was found between the ML of the females and that of their partners (Spearman’s correlation, R2 = 0.01, t30 = 0.56, P = 0.580).
Fig. 6.
Correlation between Maximum Level (ML) of interaction with the rope reached by the house sparrow (Passer domesticus) mothers with the ML of their offspring (A), and the correlation between ML of the house sparrow fathers and ML of their offspring (B). Level 1: brief contact; level 2: prolonged contact; level 3: basic play; level 4: acrobatic play. Larger dots represent more individuals falling under those parameters
ML of parents was not correlated with the proportion of playing offspring or interested offspring (Spearman’s correlations; playing offspring, R2 < 0.01, t25 = 0.10, P = 0.924; interested offspring, R2 < 0.01, t25 = 0.32, P = 0.751). These parameters were not explained by the number of visits to the rope (Spearman’s correlations; playing offspring, R2 < 0.01, t25 = 0.40, P = 0.691; interested offspring, R2 = 0.03, t25 = 0.92, P = 0.365).
When all siblings were considered, the percentage of siblings significantly explained the ML of the individual, considering both playing (Fig. 7) and interested siblings. These results were not found when considering only same-brood siblings or only non-brood siblings (Table 5).
Fig. 7.
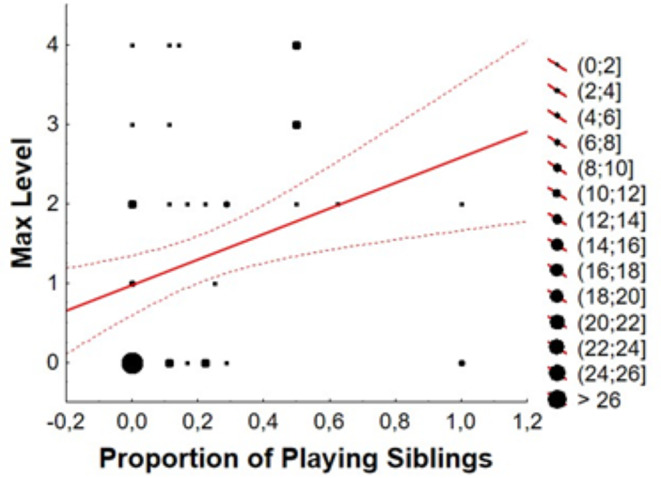
Correlation between proportion of playing siblings and Maximum Level of interaction with the rope reached by each house sparrow (Passer domesticus) individual. Larger dots represent more individuals falling under those parameters
Table 5.
Spearman’s correlation between the percentages of siblings and the Maximum Level of interaction reached by the individual, differentiating playing (levels 3 and 4), and interested (all levels) siblings and same-brood and siblings from different broods
| Variables | R 2 | df | t | P | |
|---|---|---|---|---|---|
| Playing | % siblings (general) | 0.15 | 88 | 3.95 | < 0.001 |
| % same-brood siblings | 0.08 | 43 | 1.99 | 0.052 | |
| % non-brood siblings | 0.03 | 49 | 1.14 | 0.259 | |
| Interested | % siblings (general) | 0.06 | 88 | 2.66 | 0.009 |
| % same-brood siblings | 0.01 | 43 | 0.72 | 0.477 | |
| % non-brood siblings | 0.01 | 49 | 0.63 | 0.531 |
No relation was found between physical condition and ML for either adults or juveniles (Spearman’s correlations; adults, R2 = 0.03, t70 = 1.42, P = 0.160; juveniles, R2 = 0.04, t22 = -0.97, P = 0.342).
Discussion
Play behaviour in sparrows
The behaviours displayed by our sparrows share some similarities with play behaviours observed in other avian species. Swinging and bungee jumping are the most representative play behaviours in our population. Even though we have not found any reference to bungee jumping in other species in the literature, there are some swinging reports. Swinging while hanging upside down from their feet has been described in little corellas (Cacatua sanguinea) (Kaplan 2020), and watching an individual performing this way makes others to also engage in the activity (Kaplan 2024). Swinging in social contexts has also been reported in the Arabian babbler (Zahavi et al. 2004). One babbler hung upside down from a branch and began to swing, being imitated by other babblers from other near branches, all ending in a chase. When only one place was available, they took turns to hang from it (Zahavi et al. 2004). Taking turns (or chasing away other players) was also common in our sparrows, as they had only one rope available that was highly demanded during the breeding season.
On a few occasions, one sparrow hung itself upside down from the perch just before starting to play with the rope. Despite being a very rare behaviour in our population, there are many similar reports in several corvid (Ficken 1997; Heinrich and Smolker 1998; Melletti and Mirabile 2010) and parrot species (Bond and Diamond 2004; Kaplan 2024).
Sparrows did not always engage in acrobatic play. Most of the time, sparrows merely engaged in a playful manipulation of the object, pushing and pulling it from various angles. These behaviours were sometimes difficult to differentiate from object exploration, as previously noted by other authors (Greenberg 2003; O’Hara and Auersperg 2017), because exploration may trigger object play (Kuba et al. 2006).
Play reflects ecological adaptations of species (Bond and Diamond 2004; O’Hara and Auersperg 2017), so birds play in ways related to their way of life. This seems true for parrots and corvids (O’Hara and Auersperg 2017) but also raptors (Negro et al. 1996; Kitowski 2005) and waterbirds (Sazima 2008, 2019), which play with objects similar to their preys. Auesperg et al. (2015) suggested that adult object play may affect foraging skill proficiency, allowing individuals to explore new stimuli that may represent potential food sources. In our case, where what sparrows are looking for is not food but the best materials to build the nest, this may be the ecological equivalent that explains play with the rope. We propose that the search for materials may be behind the emergence of play behaviour in our population. In other species, such as the Arabian babbler, play bouts begin with the examination of an artifact (Zahavi et al. 2004).
Play in our sparrows is limited to the breeding season. Both the number of active nests and the interactions with the rope decreased at about the same time towards the end of the season. This association disappears briefly in mid-September, when there is a peak in rope-related activity (Fig. 3). However, this increase is due to a punctual upturn in level 1 and 2 interactions at the end of August. True play behaviours (levels 3 and 4) disappear in the last third of August, when most of individuals were finishing or had already finished their reproductive activity, and, in fact, there is a marginally significant trend correlating both events. Rope-related activities ceased almost completely during autumn and winter, and true play recovery occurring around May, which coincides with the first peak of breeding activity of the year (Fig. 3). Supporting this association, the decline in activity was not caused by juveniles (Fig. 3B), but by reduced adult activity (Fig. 3A). The fact that first clutches began more than a month before play behaviours seems to discard the possibility that play have anything to do with pair choice. Seasonal effects on play behaviour have been found in other bird and mammal species (Zahavi et al. 2004). In the case of Arabian babblers, play was more frequent in summer than in winter, but this pattern in this and other cases is explained by food availability (Zahavi et al. 2004). In our case, food availability cannot be an important driver of the frequency of play behaviours as food is supplied ad libitum throughout the year. The surplus resource theory must be also discarded. If play were a way of using surplus energy and time due to captivity, one would expect it to be more frequent in autumn and winter than in spring, since during the breeding season there is a greater expenditure of energy and time cost due to breeding activities such as nest building, nest-holes defence, incubation, and food provisioning.
Adult males are significantly more involved in true play levels: level 3 (more than females) and level 4 (much more than any other group), although juveniles were expected to be the most playful, as is generally known to occur in object play behaviour (Hall 1998; Burghardt 2005). The fact that play is more frequent in males was more in line with previous research, as this trend that has been observed in play behaviour in primates, carnivores, rodents, even-toed ungulates and proboscideans (Vieira and Sartorio 2002; Webber and Lee 2020).
Play behaviour and breeding success
We found play behaviour to be related to male reproductive success. Males that exhibited ML 4 raised a higher number of recruits than all other males (Fig. 5). No differences were found among the females. This difference between males and females is also explained by the non-existent correlation between the ML of the pair members. This result could support two non-exclusive hypotheses related with sexual selection theory. First, that males capable of performing acrobatic play would be those with the highest quality, thus being the best parents; and second, that females would invest more when their partners show more complex play, with independence of their own play level.
The first hypothesis is not supported by the results obtained from the analysis of the physical condition. Neither the ML of adults nor the ML of juveniles is significantly explained by physical condition. However, the trend is not far from significance in adults. It is possible that the adults were more selective than the juveniles with their behaviour depending on their condition, thus showing a relationship close to significance between condition and play. However, the biometric data were collected in December, several months before the start of the breeding season, so physical condition could have changed along those six months of lag.
In the second hypothesis, play could function as a post-mating sexual selection mechanism. Play has been suggested as a method of evaluating other individuals as stabilising social interactions in both mammals and birds (Palagi 2006, 2023; Bugnyar et al. 2007) and can sometimes provide information relevant for mating (Heinrich and Smolker 1998; Chick et al. 2012). It is possible that play behaviour and playfulness function as signal of desirable male traits, or correlates with those, so that females are able to adjust their own investment based on what they see in their partners. Of course this is only a hypothesis, and more research is needed to establish which mechanism is responsible for the correlation between reproductive success and play behaviour.
Play behaviour and kinship relationships
The behaviours described in this work most likely emerged in our captive population from few individuals and were later socially transmitted to other individuals given that observation of other individuals manipulating objects or playing may be contagious (Stöwe et al. 2006; Osvath et al. 2014; Schwing et al. 2017; Kaplan 2024).
Sparrows are known to learn both from their parents and other flock members (Tóth et al. 2009; Truskanov and Lotem 2015; Tuliozi et al. 2018), so there are two possible ways of behavioural transmission in our population: vertical and horizontal. It is not possible to assure which of these options is the main way of transmission, or what influence has one over the other. However, our results suggest a kinship effect.
Vertical transmission is supported by the positive and significant correlation between the ML of adults, especially females, and the ML of their offspring (Fig. 6). Nevertheless, ML of the parents or number of interactions did not correlate with the percentage of their offspring interacting or playing with the rope. More complex or higher intensity interactions were expected to have drawn more attention from their offspring, increasing the times they would have explored the rope and eventually learning to play. It was also predicted that the more time an individual spent around the rope more individuals would be able to observe it and imitate.
The percentage of playing siblings and ML of the individual show a strong correlation (Table 5; Fig. 7). The fact that this effect is not observed when siblings from the same-brood and non-brood siblings are analysed separately is probably due to the reduction of the sample in the process of separating them. In any case, the correlation points to strong kinship effect. It may be due to horizontal transmission of the behaviour, as fledglings and juveniles spend a lot of time together and can imitate the behaviour of their sibling, but since they include non-brood siblings the results could be also explained by heritability of some characters that lead those individuals more prone to play to perform these behaviours.
This work describes for the first time play behaviour in the house sparrow and provides direct evidence of possible benefits in the reproductive success of those individuals which play best, despite obvious limitations.
Acknowledgements
We want thank the AEI, which has funded this article. We are especially grateful to all the intern students who have freely collaborated with the maintenance of the aviary and its birds during all these years. We also thank Mercedes Molina Morales for her constructive and relevant comments of an earlier version of this manuscript, and the subject editor and the reviewers of Animal Cognition, whose suggestions have significantly improved this work.
Author contributions
J.I.H-G.: wrote the manuscript. All authors commented on the manuscript. M.S.: discovered the behaviours and started recording. J.I.H-G.: recorded and watched the recordings. J.I.H-G. and J.M.P-S.: analyzed the data and prepared the figures and tables. All authors substantially contributed to the interpretation of the data. All authors read and approved the submitted manuscript.
Funding
Project PID2020-115950GB-I00 funded by MICIU/AEI/10.13039/501100011033.
Data availability
The database used for the analysis and the supplementary material (Online Resources 1–6) are available at https://figshare.com/s/182cd9beaf592284be55 (DOI: 10.6084/m9.figshare.27247353)
Declarations
Ethical approval
All studied specimens were handled in accordance with the Spanish (Real Decreto 53/2013) and European Union Guidelines (Directive 2010/63/UE) for the use of animals in research.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors have no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Anderson TR (2006) Biology of the ubiquitous house sparrow: from genes to populations. Oxford University Press, New York [Google Scholar]
- Auersperg AMI, van Horik JO, Bugnyar T et al (2015) Combinatory actions during object play in psittaciformes (Diopsittaca nobilis, Pionites melanocephala, Cacatua goffini) and corvids (Corvus corax, C. monedula, C. Moneduloides). J Comp Psychol 129:62–71. 10.1037/a0038314 [DOI] [PubMed] [Google Scholar]
- Barabanov V, Gulimova V, Berdiev R, Saveliev S (2015) Object play in thick-toed geckos during a space experiment. J Ethol 33:109–115. 10.1007/s10164-015-0426-8 [Google Scholar]
- Bekoff M (1984) Social Play Behavior. Bioscience 34:228–233. 10.2307/1309460 [Google Scholar]
- Bekoff M, Byers JA (1998) Animal play: Evolutionary, comparative and ecological perspectives. Cambridge University Press, Cambridge [Google Scholar]
- Bond A, Diamond J (2004) Social play in kaka (Nestor meridionalis) with comparisons to kea (Nestor notabilis). Behaviour 141:777–798. 10.1163/1568539042265680 [Google Scholar]
- Brazil M (2002) Common raven Corvus corax at play; records from Japan. Ornithological Sci 1:150–152. 10.2326/osj.1.150 [Google Scholar]
- Bugnyar T, Schwab C, Schloegl C et al (2007) Ravens Judge competitors through experience with play caching. Curr Biol 17:1804–1808. 10.1016/j.cub.2007.09.048 [DOI] [PubMed] [Google Scholar]
- Burghardt GM (2005) The genesis of animal play: testing the limits. MIT Press, Cambridge, Massachusetts [Google Scholar]
- Burghardt GM (2010) Defining and recognizing play. In: Nathan P, Pellegrini AD (eds) The Oxford Handbook of the development of play. Oxford University Press
- Burghardt GM (2015) Play in fishes, frogs and reptiles. Curr Biol 25:R9–R10. 10.1016/j.cub.2014.10.027 [DOI] [PubMed] [Google Scholar]
- Burghardt GM, Pellis SM, Schank JC et al (2024) Animal play and evolution: seven timely research issues about enigmatic phenomena. Neurosci Biobehav Rev 160:105617. 10.1016/j.neubiorev.2024.105617 [DOI] [PubMed] [Google Scholar]
- Caro TM (1995) Short-term costs and correlates of play in cheetahs. Anim Behav 49:333–345. 10.1006/anbe.1995.9999 [Google Scholar]
- Chick G, Yarnal C, Purrington A (2012) Play and mate preference: testing the Signal Theory of Adult Playfulness. Am J Play 4:407–440 [Google Scholar]
- Dallaire JA, Mason GJ (2017) Juvenile rough-and-tumble play predicts adult sexual behaviour in American mink. Anim Behav 123:81–89. 10.1016/j.anbehav.2016.10.023 [Google Scholar]
- Dapporto L, Turillazzi S, Palagi E (2006) Dominance Interactions in Young Adult Paper Wasp (Polistes dominulus) Foundresses: A Playlike Behavior? J Comp Psychol (Washington, DC: 1983) 120:394–400. 10.1037/0735-7036.120.4.394 [DOI] [PubMed]
- De Oliveira CR, Ruiz-Miranda CR, Kleiman DG, Beck BB (2003) Play Behavior in Juvenile Golden Lion tamarins (Callitrichidae: Primates): Organization in relation to costs. Ethol 109:593–612. 10.1046/j.1439-0310.2003.00901.x [Google Scholar]
- Diamond J, Bond AB (2003) A comparative analysis of Social Play in Birds. Behav 140:1091–1115 [Google Scholar]
- Emery NJ, Clayton NS (2015) Do birds have the capacity for fun? Curr Biol 25:R16–R20. 10.1016/j.cub.2014.09.020 [DOI] [PubMed] [Google Scholar]
- Fagen R, Fagen J (2004) Juvenile survival and benefits of play behaviour in brown bears, Ursus arctos. Evol Ecol Res 6:89–102 [Google Scholar]
- Ficken MS (1997) Avian play. Auk 94:573–578. 10.1093/auk/94.3.573 [Google Scholar]
- Foroud A, Whishaw IQ, Pellis SM (2004) Experience and cortical control over the pubertal transition to rougher play fighting in rats. Behav Brain Res 149:69–76. 10.1016/S0166-4328(03)00230-4 [DOI] [PubMed] [Google Scholar]
- Francesconi M, Pedruzzi L, Bagnato S et al (2024) Social play and affiliation as possible coping strategies in a group of Maremmana beef cattle. J Ethol 42:41–52. 10.1007/s10164-023-00801-5 [Google Scholar]
- Fryday SL, Greig-Smith PW (1994) The effects of Social Learning on the Food Choice of the House Sparrow (Passer domesticus). Behav 128:281–300 [Google Scholar]
- Gabrielle L, Rebecca O, Louise H et al (2022) Play ontogeny in young chickens is affected by domestication and early stress. Sci Rep 12:13576. 10.1038/s41598-022-17617-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galpayage Dona HS, Solvi C, Kowalewska A et al (2022) Do bumble bees play? Anim Behav 194:239–251. 10.1016/j.anbehav.2022.08.013 [Google Scholar]
- Graham KL, Burghardt GM (2010) Current perspectives on the Biological Study of Play: signs of Progress. Q Rev Biol 85:393–418. 10.1086/656903 [DOI] [PubMed] [Google Scholar]
- Gray P (2018) Evolutionary functions of play: practice, Resilience, Innovation, and Cooperation. In: Smith PK, Roopnarine JL (eds) The Cambridge Handbook of Play, 1st edn. Cambridge University Press, pp 84–102
- Greenberg R (2003) The role of Neophobia and Neophilia in the development of innovative behaviour of birds. In: Reader SM, Laland KN (eds) Animal Innovation. Oxford University Press, pp 175–196
- Hall SL (1998) Object play by adult animals. In: Bekoff M, Byers JA (eds) Animal play, 1st edn. Cambridge University Press, pp 45–60
- Harcourt R (1991) Survivorship costs of play in the south American fur seal. Anim Behav 42:509–511. 10.1016/S0003-3472(05)80055-7 [Google Scholar]
- Harrington KJ, Lambert ML (2024) Object play in Wild Striated Caracaras (Falconidae). J Raptor Res 58:212–220. 10.3356/JRR-23-19 [Google Scholar]
- Heinrich B, Smolker R (1998) Play in common ravens (Corvus corax). In: Bekoff M, Byers JA (eds) Animal play, 1st edn. Cambridge University Press, pp 27–44
- Kaplan G (2020) Play behaviour, not tool using, relates to brain mass in a sample of birds. Sci Rep 10:20437. 10.1038/s41598-020-76572-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan G (2024) The evolution of social play in songbirds, parrots and cockatoos - emotional or highly complex cognitive behaviour or both? Neurosci Biobehav Rev 161:105621. 10.1016/j.neubiorev.2024.105621 [DOI] [PubMed] [Google Scholar]
- Kitowski I (2005) Play behaviour and active training of Montagu’s harrier (Circus pygargus) offspring in the post-fledging period. J Ethol 23:3–8. 10.1007/s10164-004-0120-8 [Google Scholar]
- Kuba M, Meisel DV, Byrne RA et al (2003) Looking at play in Octopus vulgaris. Berliner Paläontol Abh 3:163–169 [Google Scholar]
- Kuba MJ, Byrne RA, Meisel DV, Mather JA (2006) When do octopuses play? Effects of repeated testing, object type, age, and food deprivation on object play in Octopus vulgaris. J Comp Psychol 120:184–190. 10.1037/0735-7036.120.3.184 [DOI] [PubMed] [Google Scholar]
- Martin P (1984) The time and energy costs of Play Behaviour in the cat. Z Tierpsychol 64:298–312. 10.1111/j.1439-0310.1984.tb00365.x [Google Scholar]
- Melletti M, Mirabile M (2010) Hanging behavior of the hooded crow (Corvus cornix). Wilson J Ornithol 122:183–185. 10.1676/09-041.1 [Google Scholar]
- Morand-Ferron J, Quinn JL (2011) Larger groups of passerines are more efficient problem solvers in the wild. Proc Natl Acad Sci USA 108:15898–15903. 10.1073/pnas.1111560108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahallage CAD, Huffman MA (2007) Age-specific functions of Stone Handling, a solitary-object play behavior, in Japanese macaques (Macaca fuscata). Am J Primatol 69:267–281. 10.1002/ajp.20348 [DOI] [PubMed] [Google Scholar]
- Negro JJ, Bustamante J, Milward J, Bird DM (1996) Captive fledgling American kestrels prefer to play with objects resembling natural prey. Anim Behav 52:707–714. 10.1006/anbe.1996.0215 [Google Scholar]
- Norscia I, Palagi E (2011) When play is a family business: adult play, hierarchy, and possible stress reduction in common marmosets. Primates 52:101–104. 10.1007/s10329-010-0228-0 [DOI] [PubMed] [Google Scholar]
- Nunes S (2014) Juvenile social play and yearling behavior and reproductive success in female Belding’s ground squirrels. J Ethol 32:145–153. 10.1007/s10164-014-0403-7 [Google Scholar]
- Nunes S, Muecke E-M, Lancaster LT et al (2004) Functions and consequences of play behaviour in juvenile Belding’s ground squirrels. Anim Behav 68:27–37. 10.1016/j.anbehav.2003.06.024 [Google Scholar]
- O’Hara M, Auersperg AM (2017) Object play in parrots and corvids. Curr Opin Behav Sci 16:119–125. 10.1016/j.cobeha.2017.05.008 [Google Scholar]
- O’Meara BC, Graham KL, Pellis SM, Burghardt GM (2015) Evolutionary models for the retention of adult–adult social play in primates: the roles of diet and other factors associated with resource acquisition. Adapt Behav 23:381–391. 10.1177/1059712315611733 [Google Scholar]
- Olkowicz S, Kocourek M, Lučan RK et al (2016) Birds have primate-like numbers of neurons in the forebrain. Proc Natl Aca Sci 113:7255–7260. 10.1073/pnas.1517131113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega JC, Bekoff M (1987) Avian play: comparative evolutionary and developmental trends. Auk 104:338–341. 10.1093/auk/104.2.338 [Google Scholar]
- Osvath M, Sima M (2014) Sub-adult Ravens synchronize their play: a case of emotional contagion? Anim Behav Cogn 2:197–205. 10.12966/abc.05.09.2014 [Google Scholar]
- Osvath M, Osvath H, Bååth R (2014) An exploration of play behaviors in Raven Nestlings. 10.12966/abc.05.06.2014. Anim Behav Cogn 2:
- Owens NW (1975) Social play behaviour in free-living baboons, Papio anubis. Anim Behav 23:387–408. 10.1016/0003-3472(75)90087-1 [DOI] [PubMed] [Google Scholar]
- Palagi E (2006) Social play in bonobos (Pan paniscus) and chimpanzees (Pan troglodytes): implications for natural social systems and interindividual relationships. Am J Phys Anthropol 129:418–426. 10.1002/ajpa.20289 [DOI] [PubMed] [Google Scholar]
- Palagi E (2023) Adult play and the evolution of tolerant and cooperative societies. Neurosci Biobehav Rev 148:105124. 10.1016/j.neubiorev.2023.105124 [DOI] [PubMed] [Google Scholar]
- Pellis SM (1981) A description of social play by the Australian Magpie Gymnorhina tibicen based on Eshkol-Wachman notation. Bird Behav 3:61–79. 10.3727/015613881791560685 [Google Scholar]
- Sazima I (2008) Playful birds: cormorants and herons play with objects and practice their skills. Biota Neotrop 8:259–264. 10.1590/S1676-06032008000200025 [Google Scholar]
- Sazima I (2019) Playful waterbird: Australasian Darter (Anhinga novaehollandiae) plays with sticks. Rev Bras Ornitol 27:56–58. 10.1007/BF03544448 [Google Scholar]
- Schwing R, Nelson XJ, Wein A, Parsons S (2017) Positive emotional contagion in a New Zealand parrot. Curr Biol 27:R213–R214. 10.1016/j.cub.2017.02.020 [DOI] [PubMed] [Google Scholar]
- Sharpe L Fun, FurFuture Fitness. The Evolution of Play in Mammals. In: The Cambridge handbook of play: Developmental and disciplinary perspectives, Smith PK (2018) & J. L. Roopnarine. Cambridge University Press, pp 49–66
- Siviy SM, Atrens DM (1992) The energetic costs of rough-and-tumble play in the juvenile rat. Dev Psychobiol 25:137–148. 10.1002/dev.420250206 [DOI] [PubMed] [Google Scholar]
- Stöwe M, Bugnyar T, Heinrich B, Kotrschal K (2006) Effects of Group size on Approach to Novel objects in Ravens (Corvus corax). Ethol 112:1079–1088. 10.1111/j.1439-0310.2006.01273.x [Google Scholar]
- Tóth Z, Bókony V, Lendvai ÁZ et al (2009) Whom do the sparrows follow? The effect of kinship on social preference in house sparrow flocks. Behav Processes 82:173–177. 10.1016/j.beproc.2009.06.003 [DOI] [PubMed] [Google Scholar]
- Truskanov N, Lotem A (2015) The importance of active search for effective social learning: an experimental test in young passerines. Anim Behav 108:165–173. 10.1016/j.anbehav.2015.07.031 [Google Scholar]
- Tuliozi B, Fracasso G, Hoi H, Griggio M (2018) House sparrows’ (Passer domesticus) behaviour in a novel environment is modulated by social context and familiarity in a sex-specific manner. Front Zool 15:1–14. 10.1186/s12983-018-0267-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira ML, Sartorio R (2002) Análise motivacional, causal e funcional Da brincadeira em duas espécies de roedores. Estud Psicol (Natal) 7:189–196. 10.1590/S1413-294X2002000100020 [Google Scholar]
- Webber CE, Lee PC (2020) Play in Elephants: Wellbeing, Welfare or Distraction? Animals 10:305. 10.3390/ani10020305 [DOI] [PMC free article] [PubMed]
- Yanagi A, Berman C (2018) Non-human Primate Social Play: coping with costs. In: Roopnarine JL, Smith PK (eds) The Cambridge Handbook of Play: developmental and disciplinary perspectives. Cambridge University Press, Cambridge, pp 67–83 [Google Scholar]
- Zahavi A, Zahavi A, Pozis-Francois O (2004) Social Play in Arabian babblers. Behav 141:425–450. 10.1163/156853904323066720 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The database used for the analysis and the supplementary material (Online Resources 1–6) are available at https://figshare.com/s/182cd9beaf592284be55 (DOI: 10.6084/m9.figshare.27247353)