Abstract
Purpose
The reduced cardiorespiratory fitness (CRF) and functional capacity following surgical procedures and during cancer treatments is a major risk factor for morbidity and mortality among patients with cancer. We aimed to assess the impact of endurance and combined resistance exercise interventions during the postoperative rehabilitation period for patients with colorectal, breast, and prostate cancer.
Methods
A systematic search was conducted in MEDLINE Pubmed, Web of Science, and Cochrane Library until October 2023 for randomized controlled trials that assessed exercise interventions (aerobic/endurance; resistance or combined training) on postoperative patients with cancer. The trials evaluated the change in oxygen uptake (VO2max), six-minute walking distance (6MWD), quality of life (QoL), and fatigue.
Results
Twelve studies, including 1298 patients, were part of this systematic review, and ten studies were included in the meta-analysis. Postoperative exercise interventions led to improvements in CRF and functional capacity (VO2max: MD 1.46 ml/kg/min; 95%-CI 0.33, 2.58; p = 0.01; 6MWD: MD 63.47 m; 95%-CI 28.18, 98.76; p = 0.0004, respectively) as well as QoL (0.91; 95%-CI 0.06, 1.76; p = 0.04). The quality of evidence was moderate to low.
Conclusion
Postoperative exercise interventions could effectively improve CRF, functional capacity and QoL as shown in this meta-analysis. However, there is a lack of high-quality trials with a higher number of participants examining the effects of postoperative exercise in patients with colorectal, breast, and prostate cancer. There is an obvious need for long-term, cancer-specific exercise therapies and their evaluation in cancer care.
Keywords: Breast cancer, Colorectal cancer, Functional capacity maximum oxygen uptake, Morbidity, Physical exercise, Postoperative rehabilitation, Prostate cancer, Quality of life
Objectives
Each year, nearly 20 million people worldwide are newly diagnosed with cancer (Bray et al. 2024; Sung et al. 2021). In Germany, more than 4.5 million people currently live with or have survived a cancer diagnosis, with half of all cancers in Germany being breast, prostate, or colon carcinoma (Arndt et al. 2021). Early diagnosis and advancements in treatment have improved prognoses, creating a growing need to address unique health issues for cancer survivors (Campbell et al. 2019). Physical function plays an important role because cancer is strongly associated with aging. Those affected must deal not only with the effects of cancer treatment and its aftermath, such as the risk of developing heart disease that can accompany a cancer diagnosis but also with the aging effects of the organism (Curigliano et al. 2016; Arndt et al. 2021; Campbell et al. 2019; Scott et al. 2018; Miller et al. 2019). There are clear indications that regular physical exercise is an important additional component of cancer treatment to improve cancer-related health outcomes, particularly physical function or cardiorespiratory fitness (CRF), in secondary and tertiary prevention (Campbell et al. 2019). Friedenreich et al. (2017) proposed several potential beneficial biological mechanisms through which exercise, might delay tumor growth, lower the risk of metastatic disease, and enhance treatment efficacy. There are indications that exercise training or physical activity, which enhances CRF after a cancer diagnosis, is beneficial for overall survival and may help prevent a recurrence (Patel et al. 2019). This benefit varies based on the intensity levels of physical activity following the diagnosis. Overall, evidence from observational trials across diseases indicates that CRF and positive changes in CRF are inversely associated with the risk of all-cause mortality (Laukkanen et al. 2022; Kokkinos et al. 2023).
However, the most important measure of CRF, the oxygen uptake, is often calculated rather than directly measured, or just functional capacity measures are used. Published reviews and meta-analyses in this area have covered a variety of time periods in cancer treatment (e.g. prehabilitation or rehabilitation), outcomes, and exercise protocols (Cheng et al. 2017; Batalik et al. 2021; Baumann et al. 2012; Buffart et al. 2017; Courneya 2001; Cramer et al. 2014; Falz et al. 2022; Hilfiker et al. 2018; Kampshoff et al. 2014; McGettigan et al. 2020; Speck et al. 2010; Spence et al. 2010; Sweegers et al. 2018; Thomson et al. 2021). The wide range of findings makes it challenging to provide specific exercise recommendations (Cramer et al. 2014; Baumann et al. 2012; Lahart et al. 2018). Several reviews have concentrated on cancer-specific quality of life, surgical outcomes, as well as particular symptoms such as lymphedema, limited range of motion, or incontinence (McNeely et al. 2010; Hasenoehrl et al. 2020; Baumann et al. 2012; Thomson et al. 2021). A primary aim of an exercise intervention for cancer patients, however, is to effectively enhance CRF, as it is directly linked to improved morbidity and mortality outcomes. In summary, the basis for recommendations is limited, with the initial development of German guidelines for physical activity in patients with cancer currently underway (German Cancer Society, 2019; German Cancer Society, 2021; German Cancer Society, 2024).
Currently, there is no meta-analysis available that examines the impact of aerobic and combined aerobic and resistance exercise interventions on objectively measured CRF, as indicated by oxygen uptake, during the postoperative period in patients with colorectal, breast, and prostate cancer. Therefore, this systematic review and meta-analysis aim to evaluate the effect of postoperative exercise interventions on CRF by measuring oxygen uptake and functional capacity evaluated by the six-minute walk test (6MWD). Additionally, we focused on the three most common cancer entities, which show significant evidence of exercise effects on mortality and morbidity (Patel et al. 2019; Arndt et al. 2021). We also assessed quality of life and changes in fatigue.
Methods
Search strategy
This review was conducted and recorded in accordance with the Cochrane systematic review guidelines and Preferred Reporting Items for Systematic Reviews and Meta-Analysis Checklist (PRISMA) (Page et al. 2021). It was also prospective registered with the International Prospective Register of Systematic Reviews (PROSPERO 2022; CRD42022355287). Two of the authors (CB, MB) performed a systematic literature search within the electronic databases PubMed (NCBI; all fields), Cochrane Library (Wiley; all fields), and Web of Sciences (https://www.webofscience.com/;all fields) initially on 1 April 2022 and rerun on 1 October 2023. The search included terms such as ‘breast cancer postoperative exercise rehabilitation’ OR ‘prostate cancer postoperative exercise rehabilitation’ OR ‘colorectal cancer postoperative exercise rehabilitation’ OR ‘colon cancer postoperative exercise rehabilitation’ OR ‘rectal cancer postoperative exercise rehabilitation’, while excluding ‘review’ and ‘meta-analysis’, without any limits. Additionally, we screened also studies through the reference lists in relevant articles and reviews. We did not search for grey literature or seek additional studies by contracting authors.
Study selection
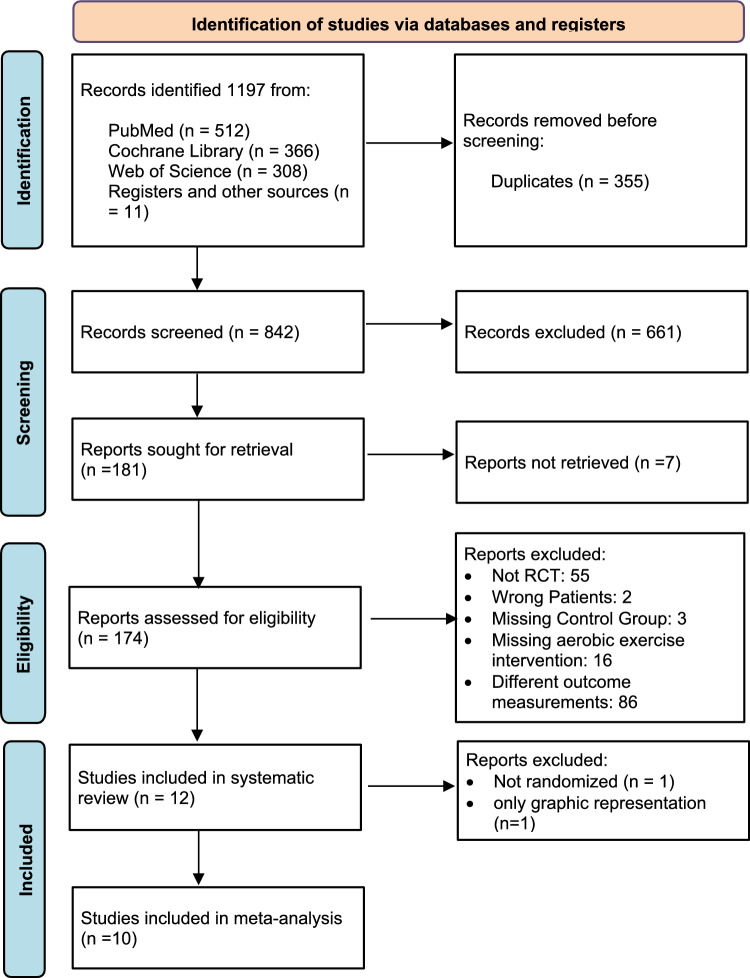
Three independent reviewers (CB, MB, JL) screened potentially eligible articles after removing duplicates and reviewing for our set inclusion criteria. Disagreements were resolved by consensus. This review included RCTs and prospective controlled trials that examined the functional outcome effects (VO2max, 6MWT, 12MWT) of an exercise intervention on adults with resected colorectal, breast, or prostate cancer. Detailed inclusion criteria are found in Table 1. Our systematic literature search process is depicted in Fig. 1.
Table 1.
Inclusion criteria according to PICOS schema for systematic review and meta-analysis
| Category | Description |
|---|---|
| Population | adults diagnosed with colorectal, breast, or prostate cancer resection less than 24 months before intervention start |
| Intervention | aerobic/endurance or combined cardiovascular and resistance exercise ≥ 2 weeks |
| Comparison | at least one comparison group receiving usual/regular care |
| Outcome |
Primary: measured VO2max or 6MWD or 12MWD Secondary: quality of life or fatigue |
| Publication language | English |
| Study design | RCTs for meta-analysis, as well as Quasi-randomized trials for systematic review |
Fig. 1.
PRISMA flow chart of included and excluded studies in this systematic review and meta-analysis (Page et al. 2021)
Data extraction
Study inclusion was initially decided by CB and MB and discussed with the senior author RF. The selected studies were organized into narrative analysis in Tables 2 and 3 based on the functional outcome measured. These tables contain details from the selected studies, including sample size, cancer entity, type of postoperative exercise intervention, details of the exercise intervention (such as training frequency, session time, and intensity), age of participants, duration of intervention, adherence to the intervention, and main results.
Table 2.
Summary of study characteristics and outcomes regarding postoperative rehabilitation programs involving exercise interventions
| Study | Cancer | Additional treatments/influential factors | Study design | Sample size (IG/CG) |
Age, years IG vs. CG |
Exercise intervention/ control | Drop-outs IG vs. CG |
Objectvely measured outcomes IG vs. CG |
|---|---|---|---|---|---|---|---|---|
|
Anderson et al. |
Breast | Lymph-edema education | RCT | 104 (52/52) | m (R) 53.6 (32–82) | Specific lymphedema intervention + 60 min combined moderate aerobic and resistance training two times per week vs. usual care + information about preventing lymphedema, nutrition and physical activity | 9 vs.13 |
∆6MWD [m]B: 54.0 ± 183.43 vs. 20.9 ± 165.78 (p = 0.0098) |
|
Bøhn et al. 2021* |
Ductal carcinoma in situ grade 3, Breast stage I, II | RCT |
55 (29/26) |
55.7 ± 7.8 54.3 ± 7.7 |
Group training sessions (moderate to high intensity aerobic endurance training) for 60 min two times per week + 120 min exercise at home vs. standard care + voluntary exercise | 6 vs. 3 | Significant change in mean VO2max [ml/kg/min] between the groups. Data only reported graphically | |
| Cantarero-Villanueva et al. 2016 | Colon | RCT | 46 (23/23) |
57.5 ± 8.0 62.3 ± 7.9 |
Three sessions 90 min Core stabilization exercises weekly | 9 vs. 8 |
∆6MWD [m]: 79.7 ± 106.33A vs. 4.9 ± 106.02A |
|
| Courneya et al. 2003 | Breast | Post-menopausal patients | RCT |
50 (24/26) |
59 ± 5 58 ± 6 |
Supervised aerobic exercise vs. no training during intervention period | 1 vs. 2 |
∆VO2max[ml/kg/min]: + 2.7 ± 2.6 vs. −0.6 ± 1.7 (p < 0.001) ∆PPO [w]: + 14.2 ± 18.7 vs. −16.5 ± 18.6 (p < 0.001) |
| Courneya et al. 2007 | Breast |
Undergoing chemotherapy 2 intervention groups |
RCT |
242 (78/82/ 82) (AET/ RET/CG) |
m ® AET: 49.5 (25–76) RET: 49 (30–75) CG: 49 (26–78) |
Aerobic exercise AET vs. resistance exercise RET vs. no newly initiated exercise program CG |
AET: 7 RET: 5 CG: 9 |
∆VO2max[ml/kg/min]B: AET 0.2 ± 14.16 vs. RET − 1.4 ± 11.83 vs. CG −1.6 ± 11.25 (AET vs. CG: p = 0.004; AET vs. RET: p = 0.031) |
| Falz et al. 2023 | Colorectal, Breast, Prostate | RCT | 122 (62/60) |
54.4 ± 11 54.6. ± 12 |
Home-based body-weight strength-endurance training vs. general information about lifestyle changes and physical activity and wearables for activity tracking | 14 vs. 12 |
∆VO2max[ml/min/kg]: 1.82 ± 2.71 vs. 0.66 ± 3.5 ∆PPO [w]: 10.2 ± 16.2 vs. 6.3 ± 18.7 |
|
| Lee et al. 2017 | Colorectal | RCT | 123 (62/61) |
56.3 ± 9.7 56.3 ± 9.9 |
Daily home-based endurance and resistance activity vs. maintaining usual daily activity | 11 vs. 13 |
∆6MWD [m]B: 25.2 ± 149.60 vs. −9.2 ± 142.91 |
|
| Lin et al. 2013* | Colorectal, stage II - III | Undergoing chemo-therapy | Controlled trial |
45 (21/24) |
59.0 ± 9.5 54.3 ± 10.6 |
Moderate aerobic/resistance exercise for 60 min two times per week vs. usual care | 1 vs. 5 |
∆6MWD [m] mean (95% CI): 58.93 (40.59–77.27) vs. 44.60 (19.73–69.48) (p = 0.353) ∆Physical activity [MET] mean (95% CI): 1996.74 (328.96–3664.52) vs. −266.96 (−1030.57–496.66) (p = 0.01) |
| Murtezani et al. 2014 | Breast | RCT | 73 (37/36) |
53 ± 11 51 ± 11 |
Supervised moderate intensity aerobic exercise vs. sedentary lifestyle | 7 vs. 4 |
∆12MWD[m]B: 75.5 ± 164.35 vs. 9.1 ± 168.51 |
|
| Mutrie et al. 2007 | Breast | RCT | 201 (99/102) |
51.3 ± 10.3 51.8 ± 8.7 |
45 min combined exercise three times per week vs. usual care + information about exercise after cancer diagnosis | 19 vs. 7 |
∆12MWD[m]B: 138 ± 343.62 vs. 9 ± 442.12 |
|
|
Travier et al. |
Breast | During adjuvant treatment | RCT | 204 (102/102) |
49.7 ± 8.2 49.5 ± 7.9 |
Supervised aerobic and strength exercise intervention | 15 vs. 25 |
∆VO2max[ml/kg/min]: −2.8 ± 4.38A vs. −3.2 ± 5.15A |
| Van Vulpen et al. 2016 | Colon | Undergoing Chemotherapy | RCT | 33 (17/16) |
58.1 ± 10.3 58.1 ± 9.6 |
Supervised aerobic and resistance exercise vs. usual care | 2 vs. 3 |
Male Participants: ∆VO2max[ml/kg/min]: −0.7 ± 3.7A vs. 0.5 ± 3.5A Female Participants: ∆VO2max[ml/kg/min]: 0.7 ± 3.35A vs. −1.0 ± 3.22A |
Mean m and standard deviation SD are presented. Other data (median MD, 95% Confidence interval 95%CI) are marked. Order of groups in the columns: Sample size; Age and Main Outcomes: IG vs.CG
IG intervention group, CG control group, RCT randomized controlled trial, 6MWD six minute walk distance, VO2 oxygen uptake, PPO peak power output, MET metabolic equivalent of task, EORTC QLQ-C30 European Organization for Research and Treatment of Cancer Core Quality of Life Questionnaire
*Excluded from meta-analysis
A SD calculated by 95% CI
B mean calculated by post-pre + SD calculated by (√(SDbase2+SDfinal2+2*0,88*SDbase*SDfinal))
Table 3.
Characteristics of exercise program in included RCT´s
| Study | Description of exercise intervention | Intervention start post-surgery | Duration of rehabilitation | Training frequency [per week] | Session duration | Overall training sessions | Intensity / control of intensity | Adherence in training sessions / (serious) Adverse events |
|---|---|---|---|---|---|---|---|---|
|
Anderson et al. |
a) supervised strength and cardiovascular training b) supervised and home-based strength and cardiovascular training c) voluntarily supervised training + home-based training |
a) 4–12 weeks b) after a c) after b |
a) 12 weeks b) 12 weeks c) 24 weeks |
a) 2x b) > 1x c) voluntarily |
65 min | a) Resistance training: 50% established 1RM weekly increased by 1–5lbs; cardiovascular training: 14–16 RPE | 71.2% R:0–97 | |
|
Bøhn et al. 2021* |
a) Group-based moderate to high aerobic and strength exercise b) home-based exercise |
21–28 days | 48 weeks |
a) 2x b) 120 min |
a) 60 min | VO2max not described in detail | ||
| Cantarero-Villanueva et al. 2016 | Core stabilization exercises |
12.0 ± 7.4 months 14.6 ± 10.0 months |
8 weeks | 3x | 20–30 min | 22.0 ± 1.1 | RPE | 88.36% / IG: 2, CG: 1 |
|
Courneya et al. |
Supervised cycle ergometry sessions |
IG: 14 ± 6 months CG: 14 ± 7 months |
15 weeks | 3x |
Wk1-3: 25 min; increased by 5 min every third week |
44.3 | 5 min warm-up + cool-down 50% of VO2max; training intensity at 70–75% of VO2max |
98.4% / Adverse events: IG: 20,8% CG: 7.1% |
|
Courneya et al. |
a) supervised aerobic exercise (AET) b) supervised resistance exercise (RET) − 9 exercises á 2 sets á 8–12 reps |
Not given | Mean: 17 weeks | 3x |
a) Wk1-3: 25 min; increased by 5 min every third week |
a) 60% of VO2max wk1-6, 70% of VO2max wk 7–12, 89% of VO2max wk > 12 b) 60–70% of 1RM increased by 10% if 12 reps completed |
AET: 72% RET: 68.2% / Adverse events: 2 |
|
| Falz et al. 2023 | Home-based, bodyweight strength-endurance training | Not given | 24 weeks | 2-3x | 30 min | 1.5 sessions per week (36 overall sessions) | Entity-specific, individually performance adapted and heart rate limited; targeted 5–8 CR10 scale |
56.4% Adverse events: 18 |
| Lee et al. 2017 |
a) home based endurance exercise b) home based resistance exercise |
IG: 10.7 ± 8.8 CG: 8.8 ± 7.2 |
12 weeks | Daily | b) 30 min | 3000 steps with HR > 65% of estimated HRmax, 18–27 MET-hours per week |
> 18 MET-hours: 86.3% 27 MET-hours: 74.5% |
|
| Lin et al. 2013* | Group based supervised combined aerobic and resistance exercise | 37.8 (16.4) days | 12 weeks | 2x | 40–60 min | 24 | Increasingly from 40–75 of HRmax | 73% (17.3) |
|
Murtezani et al. |
supervised group moderate intensity aerobic exercise program |
19.0 ± 6.9 vs. 19.1 ± 4.8 |
10 weeks | 3x | 25 min | Increasingly from 50–75 of estimated HRmax and from 25 min to 45 min | ||
|
Mutrie et al. |
a) group based supervised diverse exercise b) home based exercise session |
Not given | 12 weeks |
a) 2x b) 1x |
45 min | 50–72% of age adjusted HRmax | ||
| Travier et al. 2015 | supervised combined aerobic and muscle strength training | within 10 weeks post diagnosis | 18 weeks | 2x | 60 min | different aerobic methods according to HR on ventilatory threshold + strength exercises between 45–75% of 1RM (increasing repetitions or intensity) | 83% (IQR: 69–91%) | |
| Van Vulpen et al.2016 | supervised combined aerobic and muscle strength training | within 10 weeks post diagnosis | 18 weeks | 2x | 60 min | different aerobic methods according to HR on ventilatory threshold + strength exercises between 45–75% of 1RM (increasing repetitions or intensity) | 89% (IQR: 72–97%) |
Mean and standard deviation are presented. Other data (median MD, 95% Confidence interval 95%CI; interquartile range IQR; Range R) are marked
HR max maximal reached heart rate, 1RM one repetition maximum, RPE rating of perceived exertion, CR-10 Category-Ratio Scale anchored at 10, MET metabolic equivalent of task, MAP maximal aerobic power
*Excluded for meta-analysis
Quality assessment (risk of bias and quality of evidence)
The methodological quality of each study was assessed independently by two authors (CB and MB) using the Cochrane risk of bias tool ROB2 (Higgins et al. 2011). Five components of bias were evaluated: bias arising from the randomization process; bias due to deviations from intended interventions: bias due to missing outcome data; bias in the measurement of the outcome; and bias in the selection of the reported result. The tools evaluate criteria such as randomization method; allocation concealment; baseline comparability of study groups; blinding and completeness of follow-up. Trials were categorized as having low (green circle), high (red circle), or unclear (yellow circle) risk of bias. Publication bias was assessed visually and with a funnel graph.
The Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach was used to interpret and evaluate the quality of evidence (Guyatt et al. 2011a). The overall quality of evidence for each pooled estimate was initially considered “high”, and could be downgraded by 1 level for each of the following 5 criteria: risk of bias (any of the trials included in the analysis showed “high” or “unsure”) (Guyatt et al. 2011f), inconsistency (large heterogeneity among trials, I2 > 50%) (Guyatt et al. 2011d), imprecision of evidence (< 400 participants for each comparison) (Guyatt et al. 2011c), indirectness of effect estimates (indirectness of population, outcomes or intervention) (Guyatt et al. 2011b), and potential reporting bias (which was assessed by an asymmetry in the funnel plot) (Guyatt et al. 2011e).
Data synthesis and statistical analysis
The quantitative synthesis was performed using RevMan 5 (Review Manager 5 software, Version 5.4, The Cochrane Collaboration, 2020). In cases of missing data, study authors were contacted. Continuous outcomes were analyzed using the random-effects model to calculate the weighted mean difference and 95% confidence interval, which were visualized in forest plots. We selected the random model due to the expected heterogeneity from the varying exercise interventions. The functional outcome effects were determined by extracting data directly from the included study or calculating them from the mean and 95% confidence intervals. In cases where the mean and standard deviation of the change from baseline were not reported in the papers, we used specific equations to calculate them or reached out to the authors for the original database. The correlation coefficient was calculated as described by Higgins et al. (2024).
Meanchance = Meanendpoint - Meanbaseline.
SDchange =
The quality of life (QoL) and fatigue variables were calculated using standardized mean difference with standard error, based on the diversity of questionnaire surveys used. QoL data were extracted from the Functional Assessment of Cancer Therapy General Scale (FACT-G), the Functional Assessment of Cancer Therapy-Anemia Scale (FACT-AN), or the subscale of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C30 (EORTC QLQ-C30). The fatigue variable was derived from the fatigue subscales of the FACT survey or from the EORTC QLQ-C30’s fatigue subscale.
I2 was used to assess statistical heterogeneity. We categorized the results as follows: less than 25% - low heterogeneity; to 25% and 75% - potentially moderate heterogeneity; over 75% - considerable heterogeneity. Random effects models were employed to calculate overall effects, and forest plots to depict estimates.
For all statistical analyses, p < 0.05 was considered statistically significant.
Results
In October 2023, the search identified 1187 papers. Among these, 355 were duplicates and removed before the initial screening. Eleven additional papers were obtained from other sources, such as citations in screened publications. This brought the total number of articles and reports screened to 842. At first glance, 12 papers met our inclusion criteria (Anderson et al. 2012; Bøhn et al. 2021; Cantarero-Villanueva et al. 2016; Courneya et al. 2003, 2007; Falz et al. 2023; Lee et al. 2017; Lin et al. 2013; Murtezani et al. 2014; Mutrie et al. 2007; Travier et al. 2015; Van Vulpen et al. 2016). One of these 12 studies only described their functional outcome graphically. After consulting with the authors about the original dataset, it was decided to exclude this study (Bøhn et al. 2021). Another study was excluded due to the lack of a randomization process (Lin et al. 2013). In total, the meta-analysis included ten studies with a combined total of 1198 randomly assigned study participants (including dropouts). Half of the included studies measured maximum oxygen uptake (VO2max) directly during incremental exercise tests. Three trials used the 6-minute walking distance (6MWD) as a measure, and two trials used the 12-minute walking distance (12MWD) as functional outcome for cardiovascular assessment. The characteristics and main outcome of the studies included in our systematic review are summarized in Table 2. Studies excluded from the meta-analysis are indicated.
Study characteristics
Seven of the twelve remaining studies focused on patients with breast cancer only (Anderson et al. 2012; Bøhn et al. 2021; Courneya et al. 2003, 2007; Murtezani et al. 2014; Mutrie et al. 2007; Travier et al. 2015). Two studies assessed patients with colon cancer (Cantarero-Villanueva et al. 2016; van Vulpen et al. 2016), and two studies examined patients with colorectal cancer (Lee et al. 2017; Lin et al. 2013). Only one study assessed the effectiveness of a postoperative training intervention in mixed patients with colorectal, breast and prostate cancer (Falz et al. 2023). In our meta-analysis, patients with colon and colorectal cancer are evaluated together and summarized as ‘colorectal´. The analysis examined a total of 1013 postoperative patients with breast cancer, including dropouts, with 546 undergoing some sort of exercise intervention. In the collective group of colorectal patients, there were 266 individuals, including dropouts, of whom 133 participated in the intervention groups. 45 patients with prostate cancer were examined by Falz et al. (2023), 23 of whom underwent an exercise intervention.
The primary outcomes focused on improving CRF or functional capacity, which was measured in all studies using oxygen uptake and the 6MWD/12MWD. Some studies also reported other outcomes such as arm volume or lymphedema (Anderson et al. 2012; Courneya et al. 2007), blood parameters related to hemostasis, inflammation or hormones (Bøhn et al. 2021; Falz et al. 2023; Lee et al. 2017), different strength and mobility parameters (Cantarero-Villanueva et al. 2016; Lin et al. 2013; Mutrie et al. 2007), anthropometric or body composition data (Cantarero-Villanueva et al. 2016; Courneya et al. 2007; Falz et al. 2023; Murtezani et al. 2014; Mutrie et al. 2007; Van Vulpen et al. 2016), various questionnaires or subscales (Courneya et al. 2007; Lin et al. 2013; Mutrie et al. 2007; Travier et al. 2015; Van Vulpen et al. 2016), chemotherapy completion rate (Courneya et al. 2007; Van Vulpen et al. 2016) cardiovascular parameters (Falz et al. 2023), and overall physical activity (Falz et al. 2023; Lin et al. 2013; Mutrie et al. 2007). Subjects in four studies (Courneya et al. 2007; Lin et al. 2013; Travier et al. 2015; Van Vulpen et al. 2016) were undergoing adjuvant chemo- and/or radiotherapy treatment during the intervention. Surgical procedures are reported in nine of the 12 studies (Anderson et al. 2012; Bøhn et al. 2021; Cantarero-Villanueva et al. 2016; Courneya et al. 2003, 2007; Murtezani et al. 2014; Mutrie et al. 2007; Travier et al. 2015; Van Vulpen et al. 2016), but the categories vary across the trials, and subgroup evaluations are not included.
Cantarero-Villanueva et al. (2016) and Courneya et al. (2003) reported adverse events by groups, and the absolute numbers of adverse events in the intervention groups were twice as high in Cantarero-Villanueva et al. (2016) (IG: 2 vs. CG: 1) and 20.8% vs. 7.1% IG vs. CG in Courneya et al. (2003). Moreover, two trials reported on overall numbers of adverse events (Courneya et al. 2007; Falz et al. 2023). Reasons for adverse events included postoperative ventral hernias (Cantarero-Villanueva et al. 2016), lymphedema, other medical complications not related to the intervention, or accidents (Courneya et al. 2003; Falz et al. 2023) and minor medical problems from exercise testing (Courneya et al. 2007). Overall dropouts were very similar across groups (106 IG vs. 104 CG).
Exercise interventions
The exercise interventions varied in terms of types and prescribed intensity. Six trials involved combined aerobic and resistance exercises (Anderson et al. 2012; Cantarero-Villanueva et al. 2016; Courneya et al. 2007; Lee et al. 2017; Mutrie et al. 2007; van Vulpen et al. 2016). Four trials focused solely on aerobic exercise (Bøhn et al. 2021; Courneya et al. 2003; Murtezani et al. 2014; Travier et al. 2015). Falz et al. (2023) used exclusively body-weight strength-endurance training. The duration of the different interventions varied ranging from two months (Cantarero-Villanueva et al. 2016) to one year (Anderson et al. 2012). Home-based training sessions ranged from two to three times per week (Falz et al. 2023) and daily exercising (Lee et al. 2017). Six trials had supervised training sessions two to three times per week (Cantarero-Villanueva et al. 2016; Courneya et al. 2003, 2007; Murtezani et al. 2014; Travier et al. 2015; van Vulpen et al. 2016). Anderson et al. (2012) and Mutrie et al. (2007) combined supervised and home-based sessions three times per week.
The intensity of aerobic exercise was monitored through heart rate ranges that are individually defined by maximum heart rate or the VO2max (Bøhn et al. 2021; Courneya et al. 2003, 2007; Lee et al. 2017; Lin et al. 2013; Murtezani et al. 2014; Mutrie et al. 2007; Travier et al. 2015; Van Vulpen et al. 2016). The intensity specifications for resistance training reveal different percentage ranges of a single repetition maximum (Anderson et al. 2012; Courneya et al. 2007; Travier et al. 2015; Van Vulpen et al. 2016). Other scales, such as the rating of perceived exertion (RPE), Borg Category-Ratio scale (CR10), and targeted metabolic equivalent of task (MET) values, were also used (Anderson et al. 2012; Cantarero-Villanueva et al. 2016; Falz et al. 2023; Lee et al. 2017).
The results of eight of the twelve trials included information about adherence, with varying methods of measurement. Some trials measured participation rate in the exercise classes offered (Anderson et al. 2012; Cantarero-Villanueva et al. 2016; Courneya et al. 2003, 2007; Travier et al. 2015; Van Vulpen et al. 2016), while others measured the percentage of patient’s rate fulfilling100% of the recommendations (Falz et al. 2023; Lee et al. 2017). Due to these diverse measurement methods, it wasn´t possible to accurately assess differences. However, it was noted that 56.4% (Falz et al. 2023) and 86.2% (Lee et al. 2017) of patients completed all recommended exercises. Additionally, the intervention participation rate varied from 71.2% (Anderson et al. 2012) to 98.4% (Courneya et al. 2003).
Control groups
A total of 610 patients were assigned to control groups (CG). These patients received standard care in three studies (Lee et al. 2017; Travier et al. 2015; van Vulpen et al. 2016). In addition, they were provided with extra information about physical activity during cancer rehabilitation in three other trials (Anderson et al. 2012; Cantarero-Villanueva et al. 2016; Mutrie et al. 2007). Both groups used technical devices to monitor daily activities, and were given access to an informational platform about physical activity during postoperative cancer treatment in Falz et al. (2023). The remaining three trials instructed their CG participants not to initiate new exercises during the intervention period (Courneya et al. 2003, 2007; Murtezani et al. 2014).
Risk of Bias and quality of evidence for each outcome measure considered following GRADE assessment
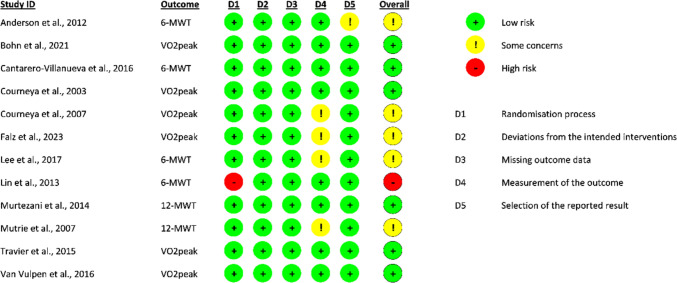
In our assessment of bias, six studies (Bøhn et al. 2021; Cantarero-Villanueva et al. 2016; Courneya et al. 2003; Murtezani et al. 2014; Travier et al. 2015; van Vulpen et al. 2016) were found to have a low risk of bias. Five studies raised concerns about bias related to how outcomes were measured (Courneya et al. 2007; Falz et al. 2023; Lee et al. 2017; Mutrie et al. 2007) or the reported results (Anderson et al. 2012) (Fig. 2). Only one study was excluded from meta-analysis due to a high risk of bias in the randomization process (Lin et al. 2013), as was the study by Bøhn et al. (2021) due to missing result data.
Fig. 2.
Cochrane risk of bias assessment of randomized controlled trials included in meta-analysis
The GRADE assessment for the quality of evidence showed low quality of evidence for VO2max and QoL (downgraded due to risk of bias, inconsistency) and moderate quality of evidence for 6-MWD and Fatigue (downgraded due to risk of bias) Table 4. As none of the comparisons included 10 or more studies, publication bias could only assessed in Funnel plots visually.
Table 4.
GRADE assessment for the certainty of evidence
| No trials | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | No of patients (IG/CG) |
Effect SMD (95% CI) | quality | |
|---|---|---|---|---|---|---|---|---|---|
| VO2max | 6 | serious | Serious | Not serious | Not serious | Undetected | 283/288 | 0.42 higher (0.04–0.79 higher) | Low |
| 6-MWD | 5 | serious | Not serious | Not serious | Not serious | Undetected | 273/274 | 0.44 higher (0.27–0.61 higher) | Moderate |
| QoL | 6 | serious | Serious | Not serious | Not serious | Undetected | 320/324 | 0.91 higher (0.06–1.76 higher) | Low |
| Fatigue | 4 | serious | Not serious | Not serious | Not serious | Undetected | 221/228 | 0.22 higher (− 0.07 lower–0.59 higher) | moderate |
VO2max maximal oxygen uptake; 6 or 12-MWD 6- or 12-minute walk distance
QoL quality of life, IG intervention group, CG control group, SMD standard mean difference, CI confidence interval
Meta-analysis of main outcome parameters
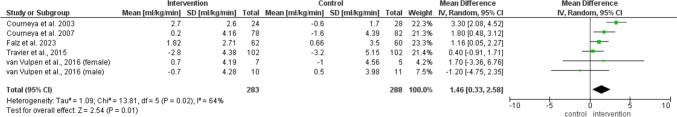
Five studies measured VO2max (Courneya et al. 2003, 2007; Falz et al. 2023; Travier et al. 2015; van Vulpen et al. 2016. The analysis of mean change in VO2max showed a significantly higher improvement (MD 1.46 ml/kg/min; 95% CI 0.33, 2.58; p = 0.01; I2 = 64%) in the IG (Fig. 3).
Fig. 3.
Meta-analysis of the change in VO2max after postoperative exercise
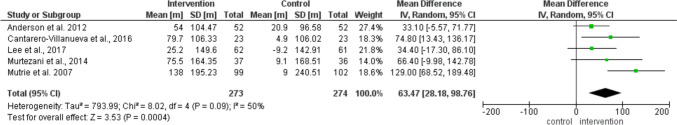
The evaluation of the five studies examining 6MWD as a functional capacity marker (Anderson et al. 2012; Courneya et al. 2003; Cantarero-Villanueva et al. 2016; Lee et al. 2017; Murtezani et al. 2014; Mutrie et al. 2007) confirms the positive effect. The IG increased their walking distance significantly more than the CG (MD 63.47 m; 95% 28.18, 98.76; p = 0.0004; I2 = 50%) (Fig. 4).
Fig. 4.
Meta-analysis of the change in walking distance in 6MWT or 12MWT after postoperative exercise
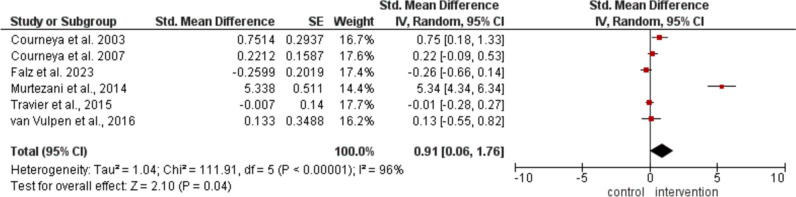
Quality of life was assessed in six of the included trials (Falz et al. 2023; Courneya et al. 2003, 2007; Murtezani et al. 2014; Travier et al. 2015; van Vulpen et al. 2016), where the IG demonstrated a significantly higher increase compared to the CG (MD 0.91; 95% CI 0.06, 1.76; p = 0.04; I2 = 96%) (Fig. 5).
Fig. 5.
Meta-analysis of the change in quality of life after postoperative exercise
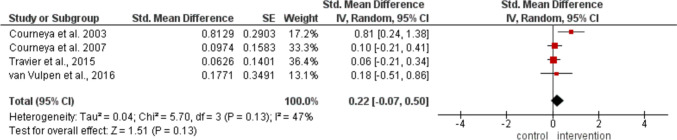
Regarding fatigue during the intervention period, no significant differences were observed between groups in the four trials (Courneya et al. 2003, 2007; Travier et al. 2015; van Vulpen et al. 2016) (MD 0.22; 95% CI −0.07, 0.50; p = 0.13; I2 = 47%) as shown in Fig. 6.
Fig. 6.
Meta-analysis of the change in fatigue after postoperative exercise
Discussion
The current systematic review included 12 studies that investigate the impact of exercise interventions on patients with colorectal, breast, and prostate cancer within two years after cancer surgery. The reported findings yield evidence of the positive impact of exercise interventions on CRF in patients during postoperative rehabilitation. These results underline the latest research, which suggests that encouraging patients with cancer to engage in active exercise programs can improve their CRF and overall well-being. Considering the strong evidence about the inverse effect of CRF on the relative risk of all-cause and cancer-cause mortality, even small improvements are beneficial, particularly for patients with colorectal, breast, and prostate cancer (Jensen et al. 2017; Laukkanen et al. 2022; Kokkinos et al. 2023; Patel et al. 2019; Schmid and Leitzmann 2015).
The 6MWD is a common method to assess functional capacity in patients with heart and lung diseases. Studies have shown that clinically significant improvements in these patients typically range from 14 to 42 m (Moutchia et al. 2023; Bohannon and Crouch 2017; Granger et al. 2015). However, it´s worth noting that while these trials demonstrate an important increase, they do not provide information on patients with colorectal, breast, and prostate cancer. Further research is necessary to validate these findings in those specific patient populations.
The small number of trials in our review prevented us from conducting a subgroup analysis of results. Research interest in exercise intervention trials for patients with cancer and survivors has increased over the last three decades, resulting in the identification of 842 trials in the systematic literature search. However, we had to exclude most of these trials for various reasons, including the lack of randomization or a high risk of bias. However, is that other meta-analyses evaluating cancer rehabilitation interventions did not exclude such trials (Høeg et al. 2019; Bradt et al. 2011). Additionally, while predicted CRF is frequently reported, the trials had to be excluded (Bourke et al. 2011; Daley et al. 2007; De Luca et al. 2016; Nusca et al. 2021; Pinto et al. 2005) whereas directly measured VO2max is rarely assessed.
CRF is not closely associated with the acute symptoms in postoperative patients with colorectal, breast, and prostate cancer, such as incontinence or reduced range of shoulder motion. Numerous trials focus on short-term side effects, leading to the exclusion of 86 trials (e.g., Wennerberg et al. 2023; Schrempf et al. 2023; Min et al. 2023; Shu et al. 2023; Park et al. 2023) However, the long-term benefits of improved CRF should not be neglected, indicating the need for further research. Some trials were excluded due to missing information about the medical history of the subjects, lack of randomization, and incompletely reported outcome measures (Alibhai et al. 2019; Battaglini et al. 2007; Leclerc et al. 2017; Schwartz and Winters-Stone 2009; Segal et al. 2009). These methodological differences may explain variations between some trials.
Courneya et al. (2003) found that the most significant improvements in VO2max in patients with breast cancer were achieved through individually tailored aerobic exercise using cycle ergometry after completing chemotherapy or radiation therapy. This contrasted with other trials where a smaller effect was observed, possibly due to the exclusive use of an aerobic cycle ergometry exercise instead of combined aerobic and resistance exercise, which was sometimes performed during chemotherapy. The results of the only colon cancer trial included in this evaluation (Van Vulpen et al. 2016) show differences between female and male patients. Surprisingly, male patients experienced decreased VO2max during the intervention period, a phenomenon the authors could not explain.
Only one of the six trials assessing quality of life reported a conspicuous effect (Murtezani et al. 2014). The methods used in that trial, such as patient characteristics or assessments, are comparable to those of the other trials, and that difference is not explained.
We found that the overall effects on quality of life and fatigue were lower than anticipated. Most published reviews of comprehensive programs for patients with cancer and survivors reported significant positive effects on quality of life or fatigue (Baumann et al. 2012; Buffart et al. 2017; Cheng et al. 2017; Hilfiker et al. 2018; Speck et al. 2010; Sweegers et al. 2018). However, some reviews yielded ambiguous results on fatigue and quality of life (Batalik et al. 2021; Spence et al. 2010) and even indicated no significant effect of exercise interventions on patients with colorectal cancer (Cramer et al. 2014). The methods in the mentioned reviews vary, and there is still significant research interest in the effects of exclusive physical exercise interventions on fatigue and quality of life. Further high-quality prospective randomized trials with adequate participant numbers are urgently needed to address these two relevant outcome parameters.
We were surprised that the time, duration, intensity, and frequency variations across the trials did not seem to noticeably impact results. The shortest interventions (Cantarero-Villanueva et al. 2016: 8 weeks; Murtezani et al. 2014: 10 weeks) showed a similar increase in 6MWD compared to the longer interventions (Anderson et al. 2012: 48 weeks), despite no disparities in the intensity and frequency of the exercises. However, a meta-analysis must confirm this observation, including more trials than ours. The level of adherence we noted in the trials included in our review did not differ from that observed in other meta-analyses (Batalik et al. 2021; Falz et al. 2022). The number of trials reporting (serious) adverse events is too limited to conduct robust evaluations or draw definitive conclusions. The author describes the highest absolute number of reported serious adverse events as unrelated to the intervention (Falz et al. 2023).
Limitations
One major limitation of this meta-analysis is the absence of RCTs investigating exercise training during postoperative periods in patients with cancer. This limitation is consistent with those mentioned in other reviews (Batalik et al. 2021; Cheng et al. 2017; Cramer et al. 2014; Spence et al. 2010). Second, the methodological quality of trials has not shown improvement, and there has been persistent insufficient reporting of exercise interventions, according to Spence et al. (2010). Other notable issues include inconsistent reporting of Inclusion criteria, missing or inadequately described patient characteristics, and insufficient information on chemotherapy or radiotherapy during the intervention period, outcome measurement methods, and results. Third, comparing trial results becomes much more difficult when interventions and subjects are very specific. For example, when only post-menopausal or anemic patients with breast cancer or combined pharmacological and exercise interventions are considered (Courneya et al. 2008; Dieli-Conwright et al. 2018). Fourth, it was impossible to conduct subgroup analyses targeting exercise duration, training intensity, or type of exercise (such as aerobic vs. resistance training; supervised vs. non-supervised) due to the lack of differentiated patient groups or insufficient available data.
Conclusions
Based on the available evidence from RCTs, this meta-analysis demonstrated post-operative exercise interventions in patients with cancer cardiorespiratory fitness, functional capacity, and quality of life. The period after surgery seems to be a feasible time for exercise interventions to support recovery and enhance patient outcomes. The potential to enhance patients’ cardiorespiratory fitness and functional capacity may lower morbidity and overall mortality. However, there is a need for high-quality postoperative exercise trials to analyze different types of interventions, such as home-based or supervised exercise and aerobic or resistance training. Evidence from these studies could help develop specific exercise guidelines for patients with cancer during and after surgical, pharmacological, and/or radiation therapy, as modern tumor therapy often involves multimodal treatments.
Author contributions
The conceptual design, the main idea, and research design were made by RF and IG. Writing by MB, CB, and RF. Data collection by MB, CB and JL. Data analysis by MB and CB. Data interpretation by MB, CB, JL and RF. All authors contributed to the critical review of the manuscript before submission.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Conflict of interest
The authors have no conflicts of interest to report.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alibhai SMH, Mina S, Ritvo D, Tomlinson P, Sabiston G, Krahn C, Durbano M, Matthew S, Warde A, O’Neill P, Timilshina M, Segal N, R., Culos-Reed N (2019) A phase II randomized controlled trial of three exercise delivery methods in men with prostate cancer on androgen deprivation therapy. BMC Cancer 19(1):2. 10.1186/s12885-018-5189-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RT, Kimmick GG, McCoy TP, Hopkins J, Levine E, Miller G, Ribisl P, Mihalko SL (2012) A randomized trial of exercise on well-being and function following breast cancer surgery: the RESTORE trial. J cancer Survivorship: Res Pract 6(2):172–181. 10.1007/s11764-011-0208-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt V, Dahm S, Kraywinkel K (2021) Krebsprävalenz in Deutschland 2017. Onkologe 27:717–723. 10.1007/s00761-021-00988-7 [Google Scholar]
- Batalik L, Winnige P, Dosbaba F, Vlazna D, Janikova A (2021) Home-based aerobic and resistance exercise interventions in cancer patients and survivors: a systematic review. Cancers 13(8):1915. 10.3390/cancers13081915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglini C, Bottaro M, Dennehy C, Rae L, Shields E, Kirk D, Hackney AC (2007) The effects of an individualized exercise intervention on body composition in breast cancer patients undergoing treatment. Sao Paulo Med J = Revista paulista de Med 125(1):22–28. 10.1590/s1516-31802007000100005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann FT, Zopf EM, Bloch W (2012) Clinical exercise interventions in prostate cancer patients–a systematic review of randomized controlled trials. Supp care cancer: Off J Multinatl Assoc Supp Care Cancer 20(2):221–233. 10.1007/s00520-011-1271-0 [DOI] [PubMed] [Google Scholar]
- Bohannon RW, Crouch R (2017) Minimal clinically important difference for change in 6-minute walk test distance of adults with pathology: a systematic review. J Eval Clin Pract 23(2):377–381. 10.1111/jep.12629 [DOI] [PubMed] [Google Scholar]
- Bøhn SK, Thune I, Flote VG, Frydenberg H, Bertheussen GF, Husøy A, Fjeldheim F, Brunvoll SH, Hjartåker A, Mowinckel MC, Sandset PM, Iversen PO (2021) Effects of a 1-Year physical activity intervention on markers of hemostasis among breast cancer survivors: a randomized controlled trial. TH open: Companion J Thromb Haemost 5(1):e14–e23. 10.1055/s-0040-1721782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke L, Thompson G, Gibson DJ, Daley A, Crank H, Adam I, Shorthouse A, Saxton J (2011) Pragmatic lifestyle intervention in patients recovering from colon cancer: a randomized controlled pilot study. Arch Phys Med Rehabil 92(5):749–755. 10.1016/j.apmr.2010.12.020 [DOI] [PubMed] [Google Scholar]
- Bradt J, Goodill SW, Dileo C (2011) Dance/movement therapy for improving psychological and physical outcomes in cancer patients. Cochrane Database Syst Rev 10:CD007103. 10.1002/14651858.CD007103.pub2 [DOI] [PubMed] [Google Scholar]
- Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A (2024) Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin 74(3):229–263. 10.3322/caac.21834 [DOI] [PubMed] [Google Scholar]
- Buffart LM, Kalter J, Sweegers MG, Courneya KS, Newton RU, Aaronson NK, Jacobsen PB, May AM, Galvão DA, Chinapaw MJ, Steindorf K, Irwin ML, Stuiver MM, Hayes S, Griffith KA, Lucia A, Mesters I, van Weert E, Knoop H, Goedendorp MM, Brug J (2017) Effects and moderators of exercise on quality of life and physical function in patients with cancer: an individual patient data meta-analysis of 34 RCTs. Cancer Treat Rev 52:91–104. 10.1016/j.ctrv.2016.11.010 [DOI] [PubMed] [Google Scholar]
- Campbell KL, Winters-Stone KM, Wiskemann J, May AM, Schwartz AL, Courneya KS, Zucker DS, Matthews CE, Ligibel JA, Gerber LH, Morris GS, Patel AV, Hue TF, Perna FM, Schmitz KH (2019) Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc 51(11):2375–2390. 10.1249/MSS.0000000000002116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantarero-Villanueva I, Sánchez-Jiménez A, Galiano-Castillo N, Díaz-Rodríguez L, Martín-Martín L, Arroyo-Morales M (2016) Effectiveness of lumbopelvic exercise in colon cancer survivors: a randomized controlled clinical trial. Med Sci Sports Exerc 48(8):1438–1446. 10.1249/MSS.0000000000000917 [DOI] [PubMed] [Google Scholar]
- Cheng KKF, Lim YTE, Koh ZM, Tam WWS (2017) Home-based multidimensional survivorship programmes for breast cancer survivors. Cochrane Database Syst Rev 8(8):CD011152. 10.1002/14651858.CD011152.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courneya KS (2001) Exercise interventions during cancer treatment: biopsychosocial outcomes. Exerc Sport Sci Rev 29(2):60–64. 10.1097/00003677-200104000-00004 [DOI] [PubMed] [Google Scholar]
- Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS (2003) Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: cardiopulmonary and quality of life outcomes. J Clin Oncol: Off J Am Soc Clin Oncol 21(9):1660–1668. 10.1200/JCO.2003.04.093 [DOI] [PubMed] [Google Scholar]
- Courneya KS, Segal RJ, Mackey JR, Gelmon K, Reid RD, Friedenreich CM, Ladha AB, Proulx C, Vallance JK, Lane K, Yasui Y, McKenzie DC (2007) Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol: Official J Am Soc Clin Oncol 25(28):4396–4404. 10.1200/JCO.2006.08.2024 [DOI] [PubMed] [Google Scholar]
- Courneya KS, Jones LW, Peddle CJ, Sellar CM, Reiman T, Joy AA, Chua N, Tkachuk L, Mackey JR (2008) Effects of aerobic exercise training in anemic cancer patients receiving darbepoetin alfa: a randomized controlled trial. Oncologist 13(9):1012–1020. 10.1634/theoncologist.2008-0017 [DOI] [PubMed] [Google Scholar]
- Cramer H, Lauche R, Klose P, Dobos G, Langhorst J (2014) A systematic review and meta-analysis of exercise interventions for colorectal cancer patients. Eur J Cancer Care 23(1):3–14. 10.1111/ecc.12093 [DOI] [PubMed] [Google Scholar]
- Curigliano G, Cardinale D, Dent S, Criscitiello C, Aseyev O, Lenihan D, Cipolla CM (2016) Cardiotoxicity of anticancer treatments: epidemiology, detection, and management. CA: Cancer J Clin 66(4):309–325. 10.3322/caac.21341 [DOI] [PubMed] [Google Scholar]
- Daley AJ, Crank H, Saxton JM, Mutrie N, Coleman R, Roalfe A (2007) Randomized trial of exercise therapy in women treated for breast cancer. J Clin Oncol: Official J Am Soc Clin Oncol 25(13):1713–1721. 10.1200/JCO.2006.09.5083 [DOI] [PubMed] [Google Scholar]
- De Luca V, Minganti C, Borrione P, Grazioli E, Cerulli C, Guerra E, Bonifacino A, Parisi A (2016) Effects of concurrent aerobic and strength training on breast cancer survivors: a pilot study. Public Health 136:126–132. 10.1016/j.puhe.2016.03.028 [DOI] [PubMed] [Google Scholar]
- Dieli-Conwright CM, Courneya KS, Demark-Wahnefried W, Sami N, Lee K, Sweeney FC, Stewart C, Buchanan TA, Spicer D, Tripathy D, Bernstein L, Mortimer JE (2018) Aerobic and resistance exercise improves physical fitness, bone health, and quality of life in overweight and obese breast cancer survivors: a randomized controlled trial. Breast cancer Res: BCR 20(1):124. 10.1186/s13058-018-1051-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falz R, Bischoff C, Thieme R, Lässing J, Mehdorn M, Stelzner S, Busse M, Gockel I (2022) Effects and duration of exercise-based prehabilitation in surgical therapy of colon and rectal cancer: a systematic review and meta-analysis. J Cancer Res Clin Oncol 148(9):2187–2213. 10.1007/s00432-022-04088-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falz R, Bischoff C, Thieme R, Tegtbur U, Hillemanns P, Stolzenburg JU, Aktas B, Bork U, Weitz J, Lässing J, Leps C, Voß J, Lordick F, Schulze A, Gockel I, Busse M (2023) Effect of home-based online training and activity feedback on oxygen uptake in patients after surgical cancer therapy: a randomized controlled trial. BMC Med 21(1):293. 10.1186/s12916-023-03010-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedenreich CM, Shaw E, Neilson HK, Brenner DR (2017) Epidemiology and biology of physical activity and cancer recurrence. J Mol Med 95(10):1029–1041. 10.1007/s00109-017-1558-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- German Guideline Program in Oncology (German Cancer Society, German Cancer Aid, AWMF) S3-Guideline Colorectal Cancer, long version 2.1, 2019, AWMF registrationnumber: 021-007OL, http://www.leitlinienprogramm-onkologie.de/leitlinien/kolorektales-karzinom/ [accessed: 31/07/2024]
- German Guideline Program in Oncology (German Cancer Society, German Cancer Aid, AWMF) Interdisciplinary Evidenced-based Practice Guideline for the Early Detection, Diagnosis, Treatment and Follow-up of Breast Cancer Long version 4.4, Mai 2021, AWMF Registration Number: 032/045OL, http://www.leitlinienprogramm-onkologie.de/leitlinien/mammakarzinom/ [accessed: 31/07/2024]
- German Guideline Program in Oncology (German Cancer Society, German Cancer Aid, AWMF) S3-Guideline Prostate Cancer, long version 7.0, 2024, AWMF registrationnumber: 043-022OL, http://www.leitlinienprogramm-onkologie.de/leitlinien/prostatakarzinom/ [accessed: 31/07/2024]
- Granger CL, Holland AE, Gordon IR, Denehy L (2015) Minimal important difference of the 6-minute walk distance in lung cancer. Chronic Resp Dis 12(2):146–154. 10.1177/1479972315575715 [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, DeBeer H, Jaeschke R, Rind D, Meerpohl J, Dahm P, Schünemann HJ (2011a) GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 64(4):383–394. 10.1016/j.jclinepi.2010.04.026 [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, Devereaux PJ, Montori VM, Freyschuss B, Vist G, Jaeschke R, Williams JW Jr, Murad MH, Sinclair D, Falck-Ytter Y, Meerpohl J, Whittington C, Thorlund K, Andrews J, Schünemann HJ (2011b) GRADE guidelines 6. Rating the quality of evidence–imprecision. J Clin Epidemiol 64(12):1283–1293. 10.1016/j.jclinepi.2011.01.012 [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, Alonso-Coello P, Falck-Ytter Y, Jaeschke R, Vist G, Akl EA, Post PN, Norris S, Meerpohl J, Shukla VK, Nasser M, Schünemann HJ, GRADE Working Group (2011c) GRADE guidelines: 8. Rating the quality of evidence–indirectness. J Clin Epidemiol 64(12):1303–1310. 10.1016/j.jclinepi.2011.04.014 [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, Alonso-Coello P, Glasziou P, Jaeschke R, Akl EA, Norris S, Vist G, Dahm P, Shukla VK, Higgins J, Falck-Ytter Y, Schünemann HJ, GRADE Working Group (2011d) GRADE guidelines: 7. Rating the quality of evidence–inconsistency. J Clin Epidemiol 64(12):1294–1302. 10.1016/j.jclinepi.2011.03.017 [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, Alonso-Coello P, Djulbegovic B, Atkins D, Falck-Ytter Y, Williams JW Jr, Meerpohl J, Norris SL, Akl EA, Schünemann HJ (2011e) GRADE guidelines: 5. Rating the quality of evidence–publication bias. J Clin Epidemiol 64(12):1277–1282. 10.1016/j.jclinepi.2011.01.011 [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso-Coello P, Montori V, Akl EA, Djulbegovic B, Falck-Ytter Y, Norris SL, Williams JW, Jr, Atkins D, Meerpohl J, Schünemann HJ (2011f) GRADE guidelines: 4. Rating the quality of evidence–study limitations (risk of bias). J Clin Epidemiol 64(4):407–415. 10.1016/j.jclinepi.2010.07.017 [DOI] [PubMed] [Google Scholar]
- Hasenoehrl T, Palma S, Ramazanova D, Kölbl H, Dorner TE, Keilani M, Crevenna R (2020) Resistance exercise and breast cancer-related lymphedema-a systematic review update and meta-analysis. Supp care cancer: Off J Multinatl Ass Supp Care Cancer 28(8):3593–3603. 10.1007/s00520-020-05521-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, Cochrane Bias Methods Group, Cochrane Statistical Methods Group (2011) The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ (Clin res ed.) 343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Li T, Deeks JJ (2024) Chapter 6: Choosing effect measures and computing estimates of effect [last updated August 2023]. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.5. Cochrane, 2024. www.training.cochrane.org/handbook
- Hilfiker R, Meichtry A, Eicher M, Nilsson Balfe L, Knols RH, Verra ML, Taeymans J (2018) Exercise and other non-pharmaceutical interventions for cancer-related fatigue in patients during or after cancer treatment: a systematic review incorporating an indirect-comparisons meta-analysis. Br J Sports Med 52(10):651–658. 10.1136/bjsports-2016-096422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Høeg BL, Bidstrup PE, Karlsen RV, Friberg AS, Albieri V, Dalton SO, Saltbæk L, Andersen KK, Horsboel TA, Johansen C (2019) Follow-up strategies following completion of primary cancer treatment in adult cancer survivors. Cochrane Database Syst Rev 2019(11):CD012425. 10.1002/14651858.CD012425.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MT, Holtermann A, Bay H, Gyntelberg F (2017) Cardiorespiratory fitness and death from cancer: a 42-year follow-up from the Copenhagen male study. Br J Sports Med 51(18):1364–1369. 10.1136/bjsports-2016-096860 [DOI] [PubMed] [Google Scholar]
- Kampshoff CS, Jansen F, van Mechelen W, May AM, Brug J, Chinapaw MJ, Buffart LM (2014) Determinants of exercise adherence and maintenance among cancer survivors: a systematic review. Int J Behav Nutr Phys Act 11:80. 10.1186/1479-5868-11-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokkinos P, Faselis C, Samuel IBH, Lavie CJ, Zhang J, Vargas JD, Pittaras A, Doumas M, Karasik P, Moore H, Heimal M, Myers J (2023) Changes in cardiorespiratory fitness and survival in patients with or without cardiovascular disease. J Am Coll Cardiol 81(12):1137–1147. 10.1016/j.jacc.2023.01.027 [DOI] [PubMed] [Google Scholar]
- Lahart IM, Metsios GS, Nevill AM, Carmichael AR (2018) Physical activity for women with breast cancer after adjuvant therapy. Cochrane Database Syst Rev 1(1):CD011292. 10.1002/14651858.CD011292.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laukkanen JA, Isiozor NM, Kunutsor SK (2022) Objectively assessed cardiorespiratory fitness and all-cause mortality risk: an updated Meta-analysis of 37 cohort studies involving 2,258,029 participants. Mayo Clin Proc 97(6):1054–1073. 10.1016/j.mayocp.2022.02.029 [DOI] [PubMed] [Google Scholar]
- Leclerc AF, Foidart-Dessalle M, Tomasella M, Coucke P, Devos M, Bruyère O, Bury T, Deflandre D, Jerusalem G, Lifrange E, Kaux JF, Crielaard JM, Maquet D (2017) Multidisciplinary rehabilitation program after breast cancer: benefits on physical function, anthropometry and quality of life. Eur J Phys Rehabil Med 53(5):633–642. 10.23736/S1973-9087.17.04551-8 [DOI] [PubMed] [Google Scholar]
- Lee MK, Kim JY, Kim DI, Kang DW, Park JH, Ahn KY, Yang H, Lee DH, Roh YH, Lee JW, Chu SH, Meyerhardt JA, Jones LW, Kim NK, Jeon JY (2017) Effect of home-based exercise intervention on fasting insulin and Adipocytokines in colorectal cancer survivors: a randomized controlled trial. Metab Clin Exp 76:23–31. 10.1016/j.metabol.2017.07.005 [DOI] [PubMed] [Google Scholar]
- Lin KY, Shun SC, Lai YH, Liang JT, Tsauo JY (2013) Comparison of the effects of a supervised exercise program and usual care in patients with colorectal cancer undergoing chemotherapy. Cancer Nurs 37(2):E21–E29. 10.1097/NCC.0b013e3182791097 [DOI] [PubMed] [Google Scholar]
- McGettigan M, Cardwell CR, Cantwell MM, Tully MA (2020) Physical activity interventions for disease-related physical and mental health during and following treatment in people with non-advanced colorectal cancer. Cochrane Database Syst Rev 5(5):CD012864. 10.1002/14651858.CD012864.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeely ML, Campbell K, Ospina M, Rowe BH, Dabbs K, Klassen TP, Mackey J, Courneya K (2010) Exercise interventions for upper-limb dysfunction due to breast cancer treatment. Cochrane Database Syst Rev 16(6):CD005211. 10.1002/14651858.CD005211.pub2 [DOI] [PubMed] [Google Scholar]
- Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, Jemal A, Kramer JL, Siegel RL (2019) Cancer treatment and survivorship statistics, 2019. CA: Cancer J Clin 69(5):363–385. 10.3322/caac.21565 [DOI] [PubMed] [Google Scholar]
- Min J, An KY, Park H, Cho W, Jung HJ, Chu SH, Cho M, Yang SY, Jeon JY, Kim NK (2023) Postoperative inpatient exercise facilitates recovery after laparoscopic surgery in colorectal cancer patients: a randomized controlled trial. BMC Gastroenterol 23(1):127. 10.1186/s12876-023-02755-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutchia J, McClelland RL, Al-Naamani N, Appleby DH, Blank K, Grinnan D, Holmes JH, Mathai SC, Minhas J, Ventetuolo CE, Zamanian RT, Kawut SM (2023) Minimal clinically important difference in the 6-minute-walk distance for patients with pulmonary arterial hypertension. Am J Respir Crit Care Med 207(8):1070–1079. 10.1164/rccm.202208-1547OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtezani A, Ibraimi Z, Bakalli A, Krasniqi S, Disha ED, Kurtishi I (2014) The effect of aerobic exercise on quality of life among breast cancer survivors: a randomized controlled trial. J Cancer Res Ther 10(3):658–664. 10.4103/0973-1482.137985 [DOI] [PubMed] [Google Scholar]
- Mutrie N, Campbell AM, Whyte F, McConnachie A, Emslie C, Lee L, Kearney N, Walker A, Ritchie D (2007) Benefits of supervised group exercise programme for women being treated for early stage breast cancer: pragmatic randomised controlled trial. BMJ (Clin Res ed.) 334(7592):517. 10.1136/bmj.39094.648553.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusca SM, Parisi A, Mercantini P, Gasparrini M, Pitasi FA, Lacopo A, Colonna V, Stella G, Cerulli C, Grazioli E, Tranchita E, Santoboni F, Latini E, Trischitta D, Vetrano M, Visco V, Pavan A, Vulpiani MC (2021) Evaluation of a post-operative rehabilitation program in patients undergoing laparoscopic colorectal cancer surgery: a pilot study. Int J Environ Res Public Health 18(11):5632. 10.3390/ijerph18115632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. 10.1136/bmj.n71PMID: 33782057; PMCID: PMC8005924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HY, Nam KE, Lim JY, Yeo SM, Lee JI, Hwang JH (2023) Real-time interactive digital health care system for postoperative breast cancer patients: a randomized controlled trial. Telemed J e-health: Off J Am Telemed Assoc 29(7):1057–1067. 10.1089/tmj.2022.0360 [DOI] [PubMed] [Google Scholar]
- Patel AV, Friedenreich CM, Moore SC, Hayes SC, Silver JK, Campbell KL, Winters-Stone K, Gerber LH, George SM, Fulton JE, Denlinger C, Morris GS, Hue T, Schmitz KH, Matthews CE (2019) American college of sports medicine roundtable report on physical activity, sedentary behavior, and cancer prevention and control. Med Sci Sports Exerc 51(11):2391–2402. 10.1249/MSS.0000000000002117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto BM, Frierson GM, Rabin C, Trunzo JJ, Marcus BH (2005) Home-based physical activity intervention for breast cancer patients. J Clin Oncol: Off J Am Soc Clin Oncol 23(15):3577–3587. 10.1200/JCO.2005.03.080 [DOI] [PubMed] [Google Scholar]
- Schmid D, Leitzmann MF (2015) Cardiorespiratory fitness as predictor of cancer mortality: a systematic review and meta-analysis. Ann Oncol 26(2):272–278. 10.1093/annonc/mdu250 [DOI] [PubMed] [Google Scholar]
- Schrempf MC, Zanker J, Arndt TT, Vlasenko D, Anthuber M, Müller G, Sommer F, Wolf S (2023) Immersive virtual reality Fitness games to improve recovery after colorectal surgery: a randomized single blind controlled pilot trial. Games Health J 12(6):450–458. 10.1089/g4h.2023.0004 [DOI] [PubMed] [Google Scholar]
- Schwartz AL, Winters-Stone K (2009) Effects of a 12-month randomized controlled trial of aerobic or resistance exercise during and following cancer treatment in women. Physician Sportsmed 37(3):62–67. 10.3810/psm.2009.10.1730 [DOI] [PubMed] [Google Scholar]
- Scott JM, Zabor EC, Schwitzer E, Koelwyn GJ, Adams SC, Nilsen TS, Moskowitz CS, Matsoukas K, Iyengar NM, Dang CT, Jones LW (2018) Efficacy of exercise therapy on cardiorespiratory fitness in patients with cancer: a systematic review and meta-analysis. J Clin Oncol: Off J Am Soc Clin Oncol 36(22):2297–2305. 10.1200/JCO.2017.77.5809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal RJ, Reid RD, Courneya KS, Sigal RJ, Kenny GP, Prud’Homme DG, Malone SC, Wells GA, Scott CG, Slovinec D (2009) Randomized controlled trial of resistance or aerobic exercise in men receiving radiation therapy for prostate cancer. J Clin Oncol: Off J Am Soc Clin Oncol 27(3):344–351. 10.1200/JCO.2007.15.4963 [DOI] [PubMed] [Google Scholar]
- Shu Q, Yang Y, Shao Y, Teng H, Liao R, Li Z, Wu G, Hou J, Tian J (2023) Comparison of rehabilitation training at different timepoints to restore shoulder function in patients with breast cancer after lymph node dissection: a randomized controlled trial. Arch Phys Med Rehabil 104(5):728–737. 10.1016/j.apmr.2023.01.021 [DOI] [PubMed] [Google Scholar]
- Speck RM, Courneya KS, Mâsse LC, Duval S, Schmitz KH (2010) An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J cancer Survivorship: Res Pract 4(2):87–100. 10.1007/s11764-009-0110-5 [DOI] [PubMed] [Google Scholar]
- Spence RR, Heesch KC, Brown WJ (2010) Exercise and cancer rehabilitation: a systematic review. Cancer Treat Rev 36(2):185–194. 10.1016/j.ctrv.2009.11.003 [DOI] [PubMed] [Google Scholar]
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin 71(3):209–249. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- Sweegers MG, Altenburg TM, Chinapaw MJ, Kalter J, Verdonck-de Leeuw IM, Courneya KS, Newton RU, Aaronson NK, Jacobsen PB, Brug J, Buffart LM (2018) Which exercise prescriptions improve quality of life and physical function in patients with cancer during and following treatment? a systematic review and meta-analysis of randomised controlled trials. Br J Sports Med 52(8):505–513. 10.1136/bjsports-2017-097891 [DOI] [PubMed] [Google Scholar]
- Thomsen SN, Mørup ST, Mau-Sørensen M, Sillesen M, Lahart I, Christensen JF (2021) Perioperative exercise training for patients with gastrointestinal cancer undergoing surgery: a systematic review and meta-analysis. Eur J Surg Oncol 47(12):3028–3039. 10.1016/j.ejso.2021.07.007 [DOI] [PubMed] [Google Scholar]
- Travier N, Velthuis MJ, Steins Bisschop CN, van den Buijs B, Monninkhof EM, Backx F, Los M, Erdkamp F, Bloemendal HJ, Rodenhuis C, de Roos MA, Verhaar M, ten Huinink B, van der Wall D, Peeters E, P. H., May AM (2015) Effects of an 18-week exercise programme started early during breast cancer treatment: a randomised controlled trial. BMC Med 13:121. 10.1186/s12916-015-0362-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vulpen JK, Velthuis MJ, Steins Bisschop CN, Travier N, Van Den Buijs BJ, Backx FJ, Los M, Erdkamp FL, Bloemendal HJ, Koopman M, De Roos MA, Verhaar MJ, Bokkel-Huinink T, Van Der Wall D, Peeters E, May AM (2016) Effects of an exercise program in colon cancer patients undergoing chemotherapy. Med Sci Sports Exerc 48(5):767–775. 10.1249/MSS.0000000000000855 [DOI] [PubMed] [Google Scholar]
- Wennerberg C, Hellström A, Schildmeijer K, Ekstedt M (2023) Effects of web-based and mobile self-care support in addition to standard care in patients after radical prostatectomy: randomized controlled trial. JMIR cancer 9:e44320. 10.2196/44320 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.