Abstract
Stroke is a major cause of death and disability worldwide, with the majority of cases resulting from ischemic events due to arterial occlusion. Current therapeutic approaches focus on rapid reperfusion through intravenous thrombolysis and intravascular thrombectomy. Although these interventions can mitigate long-term disability, reperfusion itself may induce neuronal injury. The exact mechanisms underlying neuronal damage following cerebral ischemia have yet to be reported. Recent research suggests that ferroptosis may play a significant role in post-ischemic neuronal death, which can be targeted to prevent neuronal loss. This review explores the three essential hallmarks of ferroptosis: the presence of redox-active iron, the peroxidation of polyunsaturated fatty acid-containing phospholipids, and the loss of lipid peroxide repair capacity. The involvement of ferroptosis in neuronal injury following ischemic stroke is also explored, along with an overview of ferroptosis-associated changes in different ischemic stroke animal models. Furthermore, recent therapeutic interventions targeting the ferroptosis pathway, as well as the opportunities, difficulties, and future directions of ferroptosis-targeted therapies in ischemic stroke, are discussed.
Keywords: Ischemic stroke, Ferroptosis, Animal models, Iron deposition, Lipid peroxidation
INTRODUCTION
Stroke is the second leading cause of death and third leading cause of disability among adults worldwide ( Campbell & Khatri, 2020), affecting approximately 15 million people each year ( GBD 2019 Stroke Collaborators, 2021). Strokes are classified as ischemic or hemorrhagic, with ischemic stroke resulting from the obstruction of cerebral blood vessels, restricting blood flow to the brain. Ischemic stroke accounts for approximately 71% of all strokes globally ( Campbell et al., 2019). Given the restrictions of obtaining brain tissue from ischemic stroke patients, various experimental models, including cell lines, tissue cultures, and animal models, have been developed to study ischemic neuronal injury. These models allow differentiation based on the size of the ischemic region (focal or global), duration of ischemic event (transient or permanent), degree of blood flow decline, and extent of oxygen and glucose deficiency ( Lipton, 1999). In addition, the complex architecture of the cerebral vasculature leads to differences in the incidence and severity of ischemic events across brain regions, influencing susceptibility to ischemic stroke ( Tuo et al., 2022b). Clarifying the pathological mechanisms underlying ischemic stroke is essential for developing preventative strategies and identifying effective therapeutic targets.
Ferroptosis is a distinct form of regulated cell death, mediated by iron-dependent lipid peroxidation and characterized by the accumulation of toxic lipid reactive oxygen species (ROS) ( Fearnhead et al., 2017). Unlike the three main types of programmed cell death, namely apoptosis, necroptosis, and pyroptosis, no specific regulatory genes or proteins have been identified for ferroptosis. Additionally, it does not involve the formation of proactive signaling complexes such as apoptosomes, necrosomes, or inflammasomes, which are critical for the initiation of apoptosis, necroptosis, and pyroptosis, respectively ( Berndt et al., 2024). The discovery of ferroptosis emerged from research conducted by the Stockwell laboratory in 2012, where small molecule compounds erastin and RSL3 were identified during anti-cancer drug screening. Notably, the morphological features of cell death induced by these compounds were distinct from any previously recognized form of cell death. Furthermore, it was observed that the lethality of erastin and RSL3 could be inhibited by iron chelators, indicating that their effects were contingent upon iron availability. Consequently, this mode of cell death was defined as ferroptosis, with subsequent investigations identifying lipid peroxide accumulation as a critical executor of this process ( Dixon et al., 2012). The initiation and execution of ferroptosis are intricately linked to iron metabolism, lipid metabolism, and lipid peroxidation. Ferroptosis has also been implicated in various diseases, particularly neurological disorders ( Luoqian et al., 2022; Tang et al., 2023; Tuo et al., 2022a; Yan et al., 2021). Thus, ferroptosis represents a highly adaptable form of cell death, regulated by many functional metabolites and proteins ( Dixon & Pratt, 2023).
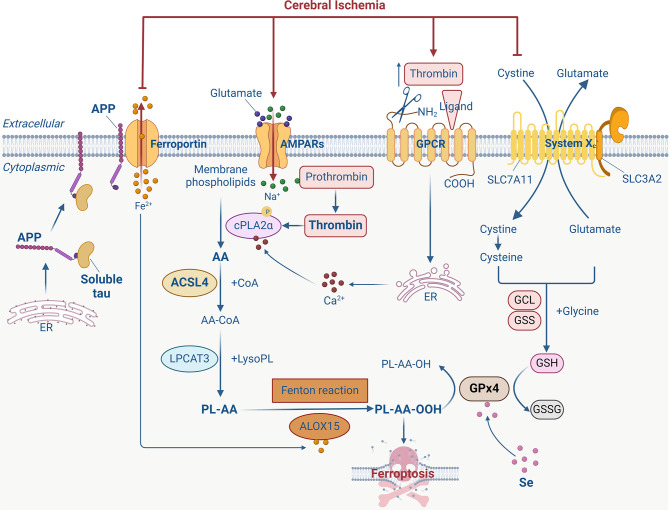
Lipids and their intermediates are important components of brain structure and function. Lipid abundance in the brain is second only to adipose tissue, accounting for 50% of dry weight ( Yoon et al., 2022). However, unlike adipose tissue, the brain primarily utilizes acylated lipids for the synthesis of phospholipids, which are integral components of cellular membranes ( Manni et al., 2018). Given the high oxygen and energy demands of the brain, it is extremely sensitive to ischemic and hypoxic conditions ( Lee et al., 2000; Li et al., 2023; Yan et al., 2020). Experimental models of cerebral ischemia have demonstrated a marked increase in iron accumulation within brain tissue ( Tuo et al., 2017), along with elevated levels of arachidonic acid (AA) released from phospholipids and disrupted lipid metabolism ( Tuo et al., 2022a). These models have also revealed a decline in lipid peroxidation repair ability, largely due to excessive consumption of glutathione peroxidase 4 (GPx4), a critical antioxidant enzyme ( Tuo et al., 2021a). Ultimately, the convergence of these factors—elevated iron levels, lipid dysregulation, and impaired lipid peroxide detoxification—may trigger ferroptosis in neurons following ischemic stroke ( Figure 1).
Figure 1.
Possible ferroptosis signaling pathway after cerebral ischemia
After cerebral ischemia, energy deficiency leads to impaired clearance of excitatory neurotransmitters in synaptic gaps, increasing the concentration of neurotransmitters such as glutamate, stimulating AMPA receptor activation, and mediating Na + influx. The increase in intracellular Na + leads to the conversion of prothrombin into active thrombin, thereby inducing the phosphorylation and activation of calcium-dependent cytosolic phospholipase A2α (cPLA2α). In addition, prothrombin in the blood may enter the brain through blood-brain barrier (BBB) disruption and be converted into thrombin, mediating cPLA2α activation by increasing cytoplasmic free Ca 2+. cPLA2α can cleave arachidonic acid (AA) at the sn-2 position of glycerophospholipids in the cell membrane. Subsequently, AA is converted to AA-CoA under catalysis of acyl-CoA synthetase long-chain family member 4 (ACSL4) and immediately incorporated into membrane phospholipids by lysophosphatidylcholine acyltransferase 3 (LPCAT3) to form AA-containing phospholipids (PL-AA). Under the catalysis of arachidonate 15-lipoxygenase (ALOX15) and Fenton reaction, PL-AA undergoes oxidation to produce biologically active lipid peroxides, which ultimately participate in ferroptosis. Glutathione peroxidase 4 (GPx4) can reduce lipid hydroperoxides (L-OOH) to lipid alcohols (L-OH). High concentrations of extracellular glutamate inhibit cysteine uptake, thereby limiting the biosynthesis of glutathione (GSH). GPx4 is inactivated due to a lack of necessary substrates, further leading to the accumulation of toxic lipid peroxides and ferroptosis. In addition, the decrease in soluble tau protein after cerebral ischemia inhibits the transport of amyloid precursor protein (APP) to the surface of neuronal membranes, blocking the interaction between APP and ferroportin (Fpn), and prevents the neuronal transfer of Fe 2+, leading to toxic intracellular accumulation of Fe 2+ and ultimately ferroptosis. (Created with BioRender.com). GCL: glutamate-cysteine ligase. GSS: glutathione synthetase. GSSG: Oxidized glutathione. GPCR: G protein-coupled receptor. ER: Endoplasmic reticulum.
Ferroptosis-specific inhibitors, such as ferrostatin-1 and liproxstatin-1, have exhibited efficacy in reversing neuronal damage in animal models of ischemic stroke ( Fang et al., 2021; Li et al., 2021, 2024; Liang et al., 2022; Liu et al., 2023; Tuo et al., 2017; Yang et al., 2021). Furthermore, inhibition of acyl-CoA synthetase long-chain family member 4 (ACSL4), a key enzyme in lipid metabolism, has been shown to improve neurological outcomes in mouse models of ischemic stroke ( Chen et al., 2021b; Cui et al., 2021; Tuo et al., 2022a). Selenium-containing compounds have been reported to reduce cerebral infarct volume by enhancing GPx4 expression and inhibiting ferroptosis ( Alim et al., 2019; Tuo et al., 2021a). These findings suggest that ferroptosis may be a critical signaling pathway driving neuronal death following ischemic stroke.
This review aims to provide a comprehensive summary of the role and molecular mechanisms of ferroptosis in ischemic neuronal injury, evaluate the changes in ferroptosis observed in different ischemic stroke models, and discuss recent therapeutic interventions targeting ferroptosis. The insights gathered may pave the way for novel approaches to understanding the pathological mechanisms of ischemic stroke and developing targeted pharmacological treatments.
MOLECULAR MECHANISMS OF FERROPTOSIS AND ITS ROLE IN ISCHEMIC STROKE
Iron deposition
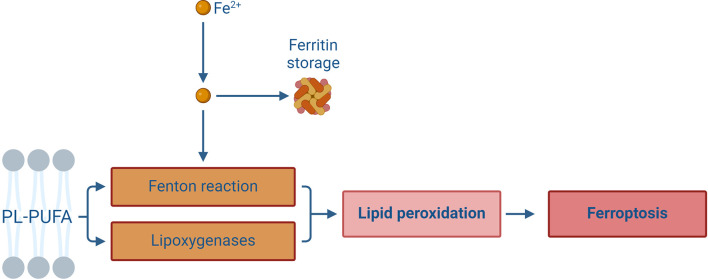
Iron plays a central role in ferroptosis, particularly through its involvement in lipid peroxidation. Free iron or iron-containing lipoxygenase (LOX) is responsible for oxidizing polyunsaturated fatty acid-containing phospholipids (PL-PUFAs), leading to the generation of lipid ROS and further inducing lipid peroxidation ( Figure 2). This process is pivotal in ferroptotic cell death ( Jiao et al., 2022; Yan et al., 2021, 2024). Thus, the availability of sufficient free iron and PL-PUFAs within cells is an essential prerequisite for the initiation of ferroptosis, and both iron transport mechanisms and intracellular iron storage can affect ferroptosis sensitivity.
Figure 2.
Redox-active iron participates in the lipid peroxidation process
In addition to being stored in the form of ferritin, intracellular free iron can also catalyze the oxidation reaction of polyunsaturated fatty acid-containing phospholipids (PL-PUFAs) via the Fenton reaction and lipoxygenase activation, thereby producing a large number of lipid peroxides and further participating in the execution of ferroptosis. (Created with BioRender.com)
Clinical research has demonstrated that elevated levels of ferritin, the protein responsible for iron storage, in plasma and cerebrospinal fluid are associated with early neurological deterioration following ischemic stroke. Increased systemic iron storage exacerbates stroke progression, leading to poorer clinical outcomes ( Carbonell & Rama, 2007; Dávalos et al., 2000; Millan et al., 2007). T 2-weighted magnetic resonance imaging (MRI) studies have also revealed increased iron deposition in the basal ganglia, thalami, and white matter of children following severe ischemic-anoxic events ( Dietrich & Bradley, 1988). This accumulation may result from disruptions in axonal iron transportation after anoxic-ischemic injury, causing iron uptake in regions such as the basal ganglia and white matter. Furthermore, iron deposition may increase under direct damage from iron-catalyzed lipid peroxidation degradation products ( Dietrich & Bradley, 1988). Animal experiments support these findings, indicating that cerebral ischemia alters brain iron metabolism, leading to iron deposition, lipid peroxidation, and neuronal death in ischemic regions ( Kondo et al., 1995, 1997; Li et al., 2009; Oubidar et al., 1994; Park et al., 2011; Tuo et al., 2017). Ferritin, serving as a source of iron after ischemic injury ( Guo et al., 2023), significantly increases in parallel with iron deposition after focal cerebral ischemia/reperfusion, with a strong correlation observed, especially in necrotic tissue areas ( Chi et al., 2000). Various therapeutic interventions targeting iron metabolism have shown promise in animal models of ischemic stroke, including iron chelators and transport facilitators, shown to reduce neuronal injury and improve neurological function ( Oubidar et al., 1994; Park et al., 2011; Prass et al., 2002; Ryan et al., 2018; Tuo et al., 2017). In summary, iron plays a critical role in driving neuronal damage in ischemic stroke, highlighting its potential as a key target for therapeutic intervention.
The microtubule-associated protein tau is a critical factor in the pathology of neurodegenerative disorders, such as Alzheimer’s disease (AD) and various tauopathies ( Ballatore et al., 2007; Chen et al., 2023; Ding et al., 2021, 2024; Kwapong et al., 2024; Lei & Ayton, 2023; Wu et al., 2023). Recent studies have indicated that tau also plays an important role in peripheral tissues, regulating insulin secretion by inhibiting microtubule dissociation in pancreatic β-islet cells ( Mangiafico et al., 2023). Furthermore, tau is implicated in the transport of amyloid precursor protein (APP) to the neuronal surface, where APP interacts with ferroportin (Fpn) to facilitate the export of ferrous iron (Fe 2+) from neurons ( Duce et al., 2010). A deficiency in tau disrupts this interaction, resulting in impaired Fe 2+ transport and increased iron accumulation ( Lei et al., 2012). In the context of transient focal cerebral ischemia, soluble tau protein levels are significantly decreased, contributing to iron accumulation and subsequent ferroptotic neuronal death ( Tuo et al., 2017).
Hepcidin, a peptide hormone, plays a crucial role in regulating iron homeostasis ( Ganz, 2011). Animal studies have shown that hepcidin expression increases markedly in the ischemic cortex, hippocampus, and striatum following cerebral ischemia. Hepcidin knockout models have demonstrated that the absence of this hormone prevents the increase in L-ferritin and decrease in Fpn typically observed after cerebral ischemia/reperfusion, suggesting that hepcidin may be a key regulator of post-ischemic iron deposition ( Ding et al., 2011). Mitochondrial ferritin (FtMt), a critical mitochondrial iron storage protein, plays a vital role in maintaining iron homeostasis and redox balance ( Wang et al., 2022; Wu et al., 2019). Experimental evidence suggests that FtMt levels are up-regulated in ischemic brain regions, potentially serving a protective function. Mice lacking FtMt experience more severe brain damage and neurological deficits after ischemic injury, as FtMt deletion promotes the hepcidin-mediated reduction of Fpn, resulting in elevated levels of both total and chelatable iron. Conversely, FtMt overexpression has been shown to attenuate brain damage and neurological deficits after cerebral ischemia/reperfusion ( Wang et al., 2021). These findings suggest that FtMt may also be a major regulator of iron deposition after cerebral ischemia. These observations provide further insights into how iron accumulates during cerebral ischemia/reperfusion injury.
Oxidation of AA-containing phospholipids
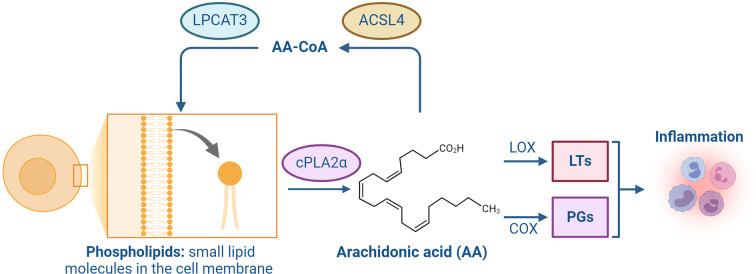
As the most abundant and widely distributed ω-6 polyunsaturated fatty acid in the human body, AA is predominantly found within cell membrane phospholipids, including phosphatidylethanolamine (PE), phosphatidylcholine (PC), and phosphatidylinositol (PI), which are essential for preserving membrane structure and function ( Tallima & El Ridi, 2018). However, the structural nature of AA, notably the presence of four cis-double bonds, renders it highly susceptible to oxidative degradation, leading to the loss of normal physiological functions ( Tuo et al., 2022b). AA also plays a key role in the deacylation-reacylation cycle of membrane phospholipids and is primarily released by calcium-dependent cytosolic phospholipase A2α (cPLA2α), which cleaves glycerophospholipids at the sn-2 position ( Tuo et al., 2022b). Free AA can be transformed into arachidonoyl-CoA (AA-CoA) via ACSL4 and subsequently re-acylated into membrane phospholipids through the action of lysophosphatidylcholine acyltransferase 3 (LPCAT3) ( Stockwell et al., 2017). In quiescent cells, the reacetylation process predominates, with most intracellular AA remaining esterified within membrane phospholipids. However, in activated cells, cPLA2α-mediated deacylation predominates, leading to a significant release of free AA and the synthesis of biologically active eicosanoids through the LOX and cyclooxygenase (COX) pathways ( Bermúdez et al., 2021; Buczynski et al., 2009; Funk, 2001), which participate in inflammatory responses ( Figure 3).
Figure 3.
AA participates in the deacylation/reacylation cycle of membrane phospholipids
cPLA2α cleaves glycerophospholipid molecules at the sn-2 position to produce AA. Free AA can be converted to AA-CoA through ACSL4 and immediately re-acylated into membrane phospholipids through LPCAT3. Conversely, bioactive eicosanoids such as leukotrienes (LTs) and prostaglandins (PGs) are synthesized through the lipoxygenase (LOX) and cyclooxygenase (COX) pathways, which participate in the inflammatory response. (Created with BioRender.com). ACSL4: acyl-CoA synthetase long-chain family member 4. LPCAT3: lysophosphatidylcholine acyltransferase 3. AA-CoA: arachidonoyl-CoA.
In activated cells, the majority of AA released by cPLA2α during phospholipid cleavage is rapidly re-acylated into membrane phospholipids, with only a small fraction utilized for eicosanoid synthesis ( Pérez-Chacón et al., 2009). Multiple studies have shown that the incorporation of AA into phospholipids significantly increases under stress conditions ( Balsinde et al., 1992; Fonteh & Chilton, 1992; Nieto et al., 1991; Reinhold et al., 1989; Tou, 1989), suggesting that this process replenishes intracellular AA levels ( Pérez-Chacón et al., 2009). Notably, genetic deletion of ACSL4 and LPCAT3—key enzymes responsible for esterifying AA into membrane phospholipids—can effectively prevent ferroptosis, highlighting the importance of PL-PUFAs in the occurrence of this form of cell death ( Dixon et al., 2015; Doll et al., 2017; Kagan et al., 2017; Yuan et al., 2016). This also indicates that free PUFAs do not directly promote ferroptosis but must be esterified into membrane phospholipids to participate in this lethal process. Unlike saturated and monounsaturated fatty acids, PUFAs contain bis-allylic hydrogen atoms that are highly sensitive to oxidation by free radicals or enzymatic activity ( Gardner, 1989; Gaschler & Stockwell, 2017). Upon cellular stimulation, PL-PUFA molecules can adopt non-bilayer arrangements ( Van Den Brink-Van Der Laan et al., 2004), exposing these bis-allylic hydrogen atoms and facilitating oxidation by free radicals or enzymes.
As a non-heme iron-containing dioxygenase, LOX catalyzes the oxidation of PL-PUFAs to produce biologically active lipid peroxides, which ultimately participate in the execution of ferroptosis ( Yang & Stockwell, 2016). It has been proposed that LOX isoforms should be uniformly named based on their encoding genes, such as 12/15-lipoxygenase (12/15-LOX) orthologs from different species standardized under the name arachidonate 15-lipoxygenase (ALOX15) ( Singh & Rao, 2019). In human ischemic stroke patients, immunofluorescence analysis of autopsy brain tissues has revealed a significant up-regulation of 12/15-LOX expression in ischemic regions ( Jung et al., 2015). Additionally, in vitro and in vivo studies have indicated that both the expression and activity of ALOX15 are significantly elevated, accompanied by increased lipid peroxide formation in neurons subjected to oxygen-glucose deprivation (OGD) and in animal models of ischemic brain injury ( Cui et al., 2010; Han et al., 2015; Jung et al., 2015; Van Leyen et al., 2006). Inhibiting ALOX15 has been shown to reduce lipid peroxide levels, improve neurological symptoms, and reduce infarct volume in ischemic stroke animal models ( Tuo et al., 2017; Van Leyen et al., 2006; Yigitkanli et al., 2013). Knockout of ALOX15 shows similar protective effects, while ALOX15 inhibitors exert no further synergistic effects in ALOX15 knockout models ( Van Leyen et al., 2006). In summary, ALOX15-mediated lipid peroxidation may be a key link in triggering ischemic brain injury.
The phospholipase A2 (PLA2) superfamily currently includes six major families of isoenzymes: secretory PLA2 (sPLA2), cytosolic PLA2 (cPLA2), Ca 2+-independent PLA2 (iPLA2), lipoprotein-associated PLA2 (Lp-PLA2), lysosomal PLA2 (LPLA2), and adipose tissue-specific PLA2 (AdPLA2) ( Khan & Ilies, 2023). cPLA2α, a Ca 2+-sensitive member of the PLA2 family of enzymes, regulates membrane phospholipid metabolism by controlling the generation of free AA ( Dennis, 1994). In animal models of ischemic stroke, both cPLA2α expression and activity are significantly increased in ischemic brain tissue, and inhibition of cPLA2α can significantly reduce infarct volume after cerebral ischemia ( Adibhatla & Hatcher, 2003; Phillis & O'Regan, 2003; Ugidos et al., 2017; Zhang et al., 2012). iPLA2β (encoded by PLA2G6) can cleave the acyl tails from the glycerol backbone of lipids and release oxidized fatty acids from phospholipids ( Sun et al., 2021). Even in GPx4-deficient cells, iPLA2β-mediated detoxification of lipid peroxides is sufficient to inhibit p53-driven ferroptosis under ROS-induced stress ( Chen et al., 2021a). This suggests that iPLA2β acts as a major ferroptosis suppressor in a GPx4-independent manner. Research has shown that selenoprotein I (SELENOI) can prevent ferroptosis by maintaining ether lipid homeostasis ( Huang et al., 2024). SELENOI deficiency can also lead to a significant decrease in ether-linked phosphatidylethanolamine (ePE) and a significant increase in ether-linked phosphatidylcholine (ePC). Imbalance between ePE and ePC leads to the up-regulation of PLA2G2A (Encoding sPLA2), PLA2G5 (Encoding sPLA2), and ALOX15, resulting in excessive lipid peroxidation. Knockout of PLA2G2A, PLA2G5, or ALOX15 can reverse the ferroptosis phenotype, indicating that they are downstream effectors of SELENOI ( Huang et al., 2024). In mouse ischemic stroke models, PLA2G2E (encoding sPLA2) expression in the infarct penumbra area is significantly increased, while PLA2G2E knockout significantly aggravates cerebral ischemia/reperfusion injury ( Nakamura et al., 2023). At the same time, as observed via immunohistochemical staining, PLA2G2E is expressed in surviving neurons around the infarct area of ischemic stroke patients but not in neurons of normal brain areas ( Nakamura et al., 2023). This suggests that PLA2G2E may be the basis of brain self-repair after ischemic stroke. Research has shown that Lp-PLA2 can control intracellular phospholipid metabolism and help combat ferroptosis. Darapladib, an inhibitor of Lp-PLA2, can synergistically induce ferroptosis in the presence of a GPx4 inhibitor ( Oh et al., 2023). Clinical studies have also shown that higher levels of Lp-PLA2 during the acute period of ischemic stroke are associated with an increased short-term risk of recurrent vascular events in stroke patients ( Lin et al., 2015), as well as a markedly increased risk of death within one year ( Han et al., 2017).
Thrombin is a Na +-activated serine protease that typically exists as its inactive form of prothrombin in tissues ( Krishnaswamy, 2013). Prothrombin in plasma can enter the brain and be converted into thrombin due to blood-brain barrier (BBB) disruption ( Ye et al., 2021). Thrombin facilitates the activation of cPLA2α by promoting intracellular free Ca 2+ or inducing cPLA2α phosphorylation, thereby enhancing the release of AA ( Gluck et al., 2008; Kramer et al., 1993, 1996; Sergeeva et al., 2002). In animal models, thrombin levels are significantly elevated in ischemic brain regions where vascular disruption is severe, compared to non-ischemic areas without vascular disruption. Immunohistochemical analysis has shown that approximately 65% of neurons in ischemic areas co-express thrombin, indicating its association with neuronal damage. Furthermore, intra-arterial infusion of thrombin during ischemia has been shown to exacerbate brain injury ( Chen et al., 2012). One hour after the onset of cerebral ischemia in rats, thrombin expression is significantly up-regulated in the affected brain tissue ( Tuo et al., 2022a). Notably, intracerebroventricular administration of hirudin, a specific thrombin inhibitor, before global cerebral ischemia significantly increases neuronal survival in the hippocampal CA1 region of gerbils ( Striggow et al., 2000). Additionally, both thrombin inhibitors and silencing of the thrombin receptor PAR-1 have been shown to protect neuronal activity after OGD, improve neurological symptoms, and reduce infarct volume in ischemic stroke models ( Chen et al., 2012; Denorme et al., 2016; Lyden et al., 2014; Mao et al., 2010; Rajput et al., 2014; Tuo et al., 2022a). Collectively, this evidence highlights the critical role of thrombin in mediating neuronal damage following cerebral ischemia.
Proteomic and lipid metabolomic analyses have revealed a substantial increase in thrombin levels in ischemic brain tissue during the early stages of ischemic stroke in model mice. This elevation triggers cPLA2α activation, leading to an increase in the generation of free AA, which then participates in neuronal ferroptosis through ACSL4-mediated membrane phospholipid remodeling ( Tuo et al., 2022a; Xu et al., 2024). These studies offer insights into the molecular mechanism by which thrombin drives neuronal injury after ischemic stroke and suggest that the thrombin-ACSL4 axis may be a key therapeutic target to ameliorate ferroptotic neuronal injury during ischemic stroke. Dabigatran and argatroban, two thrombin inhibitors, have advanced to phase III (NCT03961334) and phase IV (NCT03740958) clinical trials, respectively. Their clinical application is based on the inhibition of thrombin activity, preventing the conversion of fibrinogen to fibrin via thrombin-mediated proteolytic cleavage. This effectively disrupts the coagulation cascade, reducing blood clot formation during ischemia. However, in animal models of middle cerebral artery occlusion (MCAO), where mechanical blockage induces ischemia without directly engaging the coagulation system ( Tuo et al., 2021b), dabigatran still provides neuroprotection against ischemia/reperfusion injuries, suggesting that inhibition of thrombin-mediated ferroptosis may also be therapeutically beneficial.
Loss of lipid peroxide repair capacity mediated by GPx4
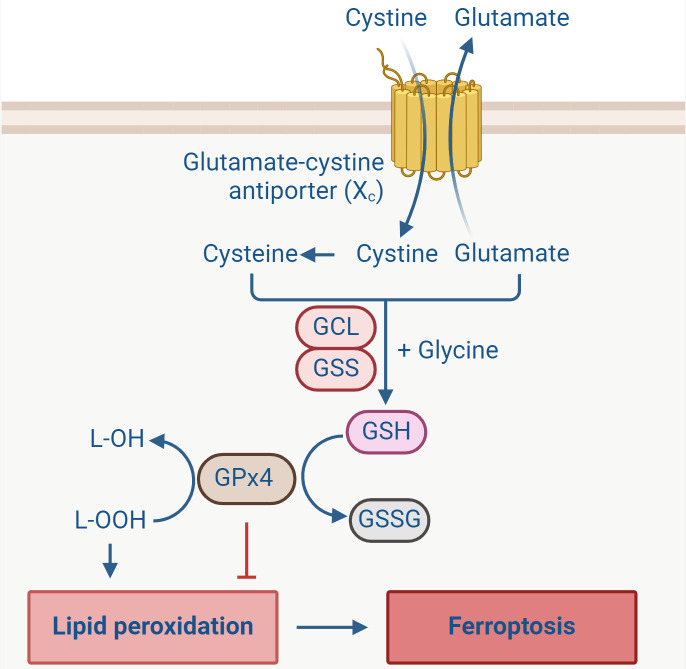
The catalytic oxidation of PL-PUFAs by free iron or iron-containing LOX is highly toxic and typically kept under strict control to prevent cellular damage. GPx4, a crucial selenoenzyme, plays a pivotal role in mitigating this toxicity by reducing lipid hydroperoxides (L-OOH) to lipid alcohols (L-OH) in lipid bilayers ( Bayır et al., 2020; Yan et al., 2021). As a necessary selenoprotein for normal mammalian development, deletion of GPx4 is embryonically lethal ( Friedmann Angeli et al., 2014; Seiler et al., 2008). Selenium, as well as selenium-containing compounds, can inhibit ferroptosis by up-regulating GPx4 activity or expression ( Alim et al., 2019; Ingold et al., 2018; Tuo et al., 2021a). In addition, glutathione (GSH), also known as γ-L-glutamyl-L-cysteinylglycine, serves as a substrate for GPx4 in its lipid peroxide reduction reaction, thereby regulating GPx4 activity ( Jiang et al., 2021). As an essential intracellular antioxidant, reduced GSH plays a vital role in lipid peroxide repair ( Stockwell et al., 2017; Wu et al., 2018). GSH is synthesized through a two-step, ATP-dependent process involving the glutamate-cysteine ligase (GCL) and glutathione synthetase (GSS) enzymes, utilizing glutamate, cysteine, and glycine as precursors ( Figure 4). During the reduction of L-OOH to L-OH, GPx4 converts reduced GSH into its oxidized form (GSSG) ( Bayır et al., 2020; Yang & Stockwell, 2016). The availability of cysteine, a key component for GSH synthesis, directly limits the rate of GSH production. Consequently, cysteine deprivation can impair GSH synthesis, leading to a further decrease in GPx4 activity and lipid peroxide accumulation, ultimately resulting in ferroptosis ( Fujii et al., 2020; Sun et al., 2018; Yu & Long, 2016). The intracellular level of cysteine is largely dependent on the import of extracellular cystine, the oxidized form of cysteine, via the Xc − glutamate-cystine antiporter system, composed of the light chain subunit SLC7A11 and heavy chain subunit SLC3A2 ( Jiang et al., 2021).
Figure 4.
GPx4 counteracts lipid peroxidation
Extracellular cystine is transferred into the cell through the action of the cell membrane glutamate-cystine antiporter system Xc −, then converted to cysteine. Synthesis of reduced glutathione (GSH) from glutamate, cysteine, and glycine is catalyzed by GCL and GSS. GPx4 converts reduced GSH into oxidized glutathione (GSSG), while reducing lipid hydroperoxides (L-OOH) to lipid alcohols (L-OH), preventing the accumulation of lipid peroxides and inhibiting ferroptosis. (Created with BioRender.com). GCL: glutamate-cysteine ligase. GSS: glutathione synthetase. GPx4: glutathione peroxidase 4.
In ischemic brain tissue, the expression levels of SLC7A11 and GPx4 are significantly diminished compared to those observed in sham-operated rats, indicating a disruption in antioxidant defense mechanisms ( Lan et al., 2020). The selenocysteine-containing Tat-linked SelP peptide (Tat SelPep) can reduce infarct volume following acute ischemic stroke by up-regulating GPx4 expression through the activation of transcription factors TFAP2c and Sp1 ( Alim et al., 2019). Similarly, in mouse models of ischemic stroke, GPx4 expression is markedly decreased in affected brain tissue, while selenium-containing compounds such as methylselenocysteine or selenocystamine offer neuroprotection by enhancing GPx4 expression or activity, thereby minimizing neuronal damage ( Tuo et al., 2021a). GSH levels are also significantly reduced in ischemic brain tissue, with L-2-oxothiazolidine-4-carboxylic acid (OTC), a precursor of cysteine, found to reduce neuronal damage by restoring depleted GSH levels after cerebral ischemia ( Liu et al., 2020). In summary, the loss of lipid peroxide repair capacity mediated by GPx4 is a key factor triggering neuronal ferroptosis after ischemic stroke.
ALTERATIONS IN FERROPTOSIS IN ISCHEMIC STROKE ANIMAL MODELS
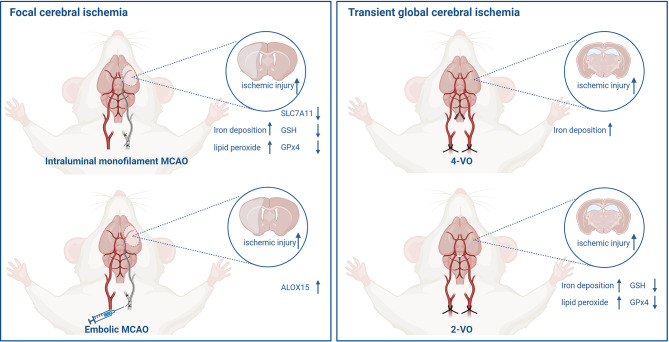
Animal models are valuable tools for exploring the molecular mechanisms, behavioral functions, and therapeutic strategies to diseases. Cerebral ischemia occurs when the brain experiences a reduced or interrupted blood supply, leading to a state of cerebral ischemia and hypoxia ( Walter, 2022). This condition can manifest as either acute or chronic cerebral ischemia. Acute cerebral ischemia, which is the focus of this discussion, can be divided into focal and global cerebral ischemia. Focal ischemia is characterized by the occlusion of the internal carotid arterial branch (usually the middle cerebral artery) that supplies blood to the brain, resulting in localized blood flow interruption to the brain region supplied by the affected artery. In contrast, global cerebral ischemia involves the obstruction of blood flow to the entire brain, typically caused by the occlusion of multiple major blood vessels, leading to a complete cessation of cerebral circulation ( Traystman, 2003). These distinct ischemic models present unique alterations in key ferroptosis regulators ( Figure 5), which vary depending on the type and severity of ischemia. The complexity of ischemic stroke etiology arises from multiple influencing factors, including the size of the affected ischemic area, duration of ischemic events, degree of blood flow decline, and level of oxygen and glucose deficiency. Each of these variables can significantly impact the activation of ferroptotic pathways. While existing animal models of ischemic stroke offer valuable insights, they come with inherent limitations ( Table 1), and none can fully replicate the clinical progression and complexity of human ischemic stroke. Consequently, it is essential for researchers to select the most appropriate animal models based on their specific experimental purposes.
Figure 5.
Alterations in key factors of ferroptosis in different ischemic stroke animal models
Focal cerebral ischemia models constructed by intraluminal monofilament or homologous blood clots (left). Transient global cerebral ischemia models constructed by 4-VO and 2-VO, respectively (right). (Created with BioRender.com). MCAO: middle cerebral artery occlusion. 4-VO: four-vessel occlusion. 2-VO: two-vessel occlusion. GSH : glutathione. GPx4: glutathione peroxidase 4. ALOX15: arachidonate 15-lipoxygenase.
Table 1. Characteristics of ischemic stroke models.
| Model | Advantages | Limitations | Animals |
| MCAO: Middle cerebral artery occlusion. 4-VO: Four-vessel occlusion. 2-VO: Two-vessel occlusion. | |||
| Focal ischemic stroke | |||
| Intraluminal filament MCAO | Easy manipulation; controllable reperfusion;
ischemic penumbra |
Not suitable for thrombolysis | Rats, mice |
| Embolic MCAO | Investigate thrombolytic processes | Poor reproducibility; spontaneous recirculation | Rats, rabbits |
| Global ischemic stroke | |||
| 4-VO | Not strain-dependent; high reproducibility | Two-stage surgical procedure; permanent occlusion
of vertebral arteries; high mortality |
Rats |
| 2-VO | One-stage surgical procedure; controllable
recirculation; lower mortality |
Strain-dependent | Gerbils |
Focal cerebral ischemia
Acute focal cerebral ischemia models can be constructed using various techniques, with the most common being intraluminal monofilament occlusion and homologous blood clot embolization.
Intraluminal monofilament MCAO: This widely used animal model involves the insertion of a silicon-coated monofilament into the common carotid artery (CCA) and advancing it through the internal carotid artery (ICA) to block the middle cerebral artery (MCA) at the Circle of Willis ( Engel et al., 2011). The duration of cerebral ischemia can be precisely controlled by adjusting the residence time of the monofilament in the ICA, usually between 30–120 minutes. Reperfusion is achieved by removing the monofilament, while leaving it in place for 24 hours without reperfusion represents permanent cerebral ischemia.
Studies have shown that cerebral ischemia/reperfusion leads to iron deposition and lipid peroxide accumulation in the brain, while iron chelators and ferroptosis-specific inhibitors (ferrostatin-1 or liproxstatin-1) can significantly improve neurological deficits and reduce infarct volume in intraluminal monofilament MCAO model mice ( Tuo et al., 2017, 2022b). However, ferroptosis-specific inhibitors cannot effectively reverse ischemic brain injury in mice with permanent MCAO ( Tuo et al., 2022b), indicating that reperfusion may be necessary for initiating ferroptosis in neurons.
In intraluminal monofilament MCAO mice and rats, SLC7A11 and GPx4 levels in ischemic brain tissue significantly decrease after reperfusion compared with the non-ischemic side ( Lan et al., 2020; Tuo et al., 2021a). Furthermore, up-regulating GPx4 expression or activity has been shown to significantly reduce infarct volume ( Alim et al., 2019; Tuo et al., 2021a). In addition, as a reactive substrate of GPx4, depletion of GSH in ischemic brain tissue can be mitigated by OTC, a cysteine precursor, reducing neuronal damage ( Liu et al., 2020).
The intraluminal monofilament MCAO model is ideal for studying the molecular mechanisms of ferroptosis in ischemic stroke. Its advantages include high reproducibility, precise control of ischemia/reperfusion time, and focal brain injury similar to that seen in human ischemic stroke, without requiring craniotomy ( Menzies et al., 1992). However, it does not fully replicate the thrombotic processes in human strokes and is unsuitable for studying anticoagulant mechanisms or thrombolysis effects on brain tissue.
Embolic MCAO: Thrombolytic therapy using recombinant tissue-type plasminogen activator (r-tPA) remains the primary treatment for acute ischemic stroke. However, its effectiveness is constrained by a narrow therapeutic window and an increased risk of hemorrhagic transformation ( Hacke et al., 2008). Therefore, the development of more accurate stroke models is essential for studying new thrombolytic agents and exploring advanced treatment strategies for ischemic stroke. Although the intraluminal monofilament MCAO model is widely used to investigate neuronal injury mechanisms, it cannot fully replicate clinical conditions and is unsuitable for thrombolytic studies ( Jin et al., 2014). The embolic MCAO model, which employs homologous blood clots, better simulates the pathophysiology of human ischemic stroke, making it suitable for preclinical studies of thrombolysis.
The embolic MCAO model involves advancing a PE-50 catheter from the external carotid artery (ECA) into the ICA until it reaches the origin of the MCA, where a homologous blood clot is delivered ( Jin et al., 2014). This model has been used to assess neuroprotective agents, such as the ALOX15-specific inhibitor ML351, shown to significantly reduce the risk of hemorrhagic transformation while preserving the thrombolytic efficacy of r-tPA and improving neurological deficits in embolic MCAO rats ( Cheng et al., 2021). The seleno-organic antioxidant ebselen has also been shown to mimic GPx activity ( Kil et al., 2017; Yamaguchi et al., 1998). Notably, studies have demonstrated that the combination of ebselen and r-tPA provides synergistic neuroprotection in embolic MCAO model rabbits ( Lapchak & Zivin, 2003).
In brief, embolic MCAO is an ideal model for evaluating the neuroprotective effects of ferroptosis-targeting interventions in ischemic stroke. It closely mimics the human ischemic stroke process and allows for the study of therapeutic interventions combined with thrombolytic agents, providing a more realistic basis for preclinical drug efficacy evaluations. However, one limitation of this model is its inability to precisely control the duration of ischemia, resulting in variability in the extent of injury, which can complicate comparisons of neuroprotective drug efficacy.
Additional focal cerebral ischemia models: Apart from the aforementioned focal cerebral ischemia models, two other commonly employed models for studying ischemic stroke include the distal MCAO (dMCAO) model and the photothrombotic stroke model. The dMCAO model, first described by Robinson et al. ( 1975) in their study on the effects of experimental cerebral infarction on catecholamines and behavior in rats, can induce either permanent or transient MCAO. Permanent MCAO is typically achieved through electrocauterization of the artery, while transient MCAO is achieved through the application of ligatures or microclips, which can later be removed for reperfusion ( Buchan et al., 1992). This model allows for accurate observation of successful occlusion and provides flexibility in inducing either transient or permanent ischemia. A recent study applied the dMCAO mouse model, established by electrocoagulation, to explore the potential neuroprotective effects of rosmarinic acid (RosA) encapsulated in nanoliposomes (RosA-LIP) on ischemic stroke, which inhibited ferroptosis by improving mitochondrial abnormalities, increasing GPx4 levels, and reducing ACSL4/LPCAT3/LOX-dependent lipid peroxidation ( Jia et al., 2024). However, the invasive nature of this model, which requires direct brain tissue manipulation and exposure, makes it less reflective of human disease, as it can induce intracranial inflammation, alter intracranial pressure, and compromise BBB function ( Sokolowski et al., 2023).
The photothrombotic stroke model involves transcranial illumination of the brain following systemic administration of the photosensitive dye Rose Bengal, which results in localized coagulation of the irradiated brain tissue ( Watson et al., 1985). This model offers several advantages, including precisely targeting predefined ischemic areas with stereotactic accuracy, making it ideal for focusing on specific cortical regions. Studies using this model have shown significant increases in free iron levels in the ischemic core and penumbra compared to the non-ischemic side, with ischemic injury being markedly reduced by the intraperitoneal administration of a lipid-soluble iron chelator, 2,2’-bipyridyl, within an hour of stroke onset ( Millerot-Serrurot et al., 2008). However, photothrombotic injury differs from human ischemic stroke as it typically involves more blood vessels in the illuminated regions, in contrast to human ischemia, in which blood flow is typically interrupted in a single terminal artery. Moreover, photocoagulation also leads to severe vascular damage and early vasogenic edema, features that are not typically observed in human ischemic stroke ( Labat-Gest & Tomasi, 2013). These studies suggest that both the dMCAO and photothrombotic stroke models can be used to study the mechanisms of neurological damage caused by ferroptosis in ischemic stroke. However, due to inherent limitations in their construction, these models deviate from the underlying pathophysiology of clinical ischemic stroke, which may affect the translatability of findings to human disease.
Transient global cerebral ischemia
The transient global cerebral ischemia model is frequently used to study the occurrence of cerebral ischemic injury during cardiac arrest and resuscitation. The two primary methods for constructing this model are four-vessel occlusion (4-VO) and two-vessel occlusion (2-VO) ( Tuo et al., 2021b). The 4-VO model is constructed by permanently blocking bilateral vertebral arteries through electrocoagulation, followed by transient occlusion of the bilateral common carotid arteries (BCCA) with arterial clips ( Kim et al., 2023). Research has demonstrated that 5 minutes of 4-VO can induce substantial neuronal death in the hippocampal CA1 region ( Wang et al., 2003), accompanied by iron deposition ( Kondo et al., 1995). However, the complexity of this approach—requiring exposure and permanent occlusion of the bilateral vertebral arteries via electrocoagulation—demands a highly skilled experimental operator, limiting its broader promotion and application in research settings.
The 2-VO model, also known as bilateral common carotid artery occlusion (BCCAO), is a more feasible alternative. It is primarily used in Mongolian gerbils, which lack a complete cerebral Circle of Willis, making them particularly susceptible to global cerebral ischemia when their BCCA is occluded ( Martínez et al., 2012). However, this method requires careful control, as occlusion duration must not exceed 20 minutes to avoid high mortality rates in the experimental animals ( Lee et al., 2019). Research using the 2-VO method has revealed significant changes in key indicators of ferroptosis, including enhanced iron deposition, reduced GSH levels, down-regulated GPx4 expression, and increased lipid peroxide accumulation in the ischemic hippocampal region, while drug interventions have been shown to up-regulate GPx4 or reduce lipid peroxides ( Guan et al., 2019; Gupta & Sharma, 2006). However, this model is limited by its reliance on Mongolian gerbils, which lack a complete Circle of Willis, making them difficult to source in large quantities for experimental use.
TARGETING FERROPTOSIS IN ISCHEMIC STROKE TREATMENT
The neuroprotective potential of ferroptosis-specific inhibitors in experimental ischemic stroke models has been widely studied ( Fang et al., 2021; Li et al., 2021, 2024; Liang et al., 2022; Liu et al., 2023; Tuo et al., 2017; Yang et al., 2021), leading to a renewed focus on iron as an therapeutic target. Notably, the phase II clinical trial “Thrombolysis and Deferoxamine in Middle Cerebral Artery Occlusion (TANDEM-1)” (ClinicalTrials.gov Identifier: NCT00777140) was conducted to evaluate the safety, tolerability, and therapeutic efficacy of the iron chelator deferoxamine mesylate (DFO) in ischemic stroke patients. In 2021, the study reported no significant difference in adverse effects between the placebo and DFO-treated groups, suggesting that DFO is safe for clinical use. Additionally, a higher proportion of DFO-treated patients showed improved outcomes based on the National Institutes of Health Stroke Scale (NIHSS) evaluation at day 90 of treatment ( Millán et al., 2021). Despite these promising findings, direct chelation of iron can interfere with normal function of the body, potentially triggering undesirable side effects. An alternative strategy involves the regulation of iron homeostasis, which may offer a safer therapeutic option. Animal studies have demonstrated that proteins involved in iron regulation, such as ceruloplasmin or APP, can alleviate brain injury in ischemic stroke model mice, providing protection comparable to iron chelation without the associated risks ( Tuo et al., 2017).
ALOX15 plays a critical role in catalyzing the oxidation of PL-PUFAs, generating biologically active lipid peroxides that participate in the execution of ferroptosis. Conversely, GPx4 acts as a key protective enzyme, effectively mitigating the toxic effects of PL-PUFA oxidation and inhibiting ferroptosis ( Ingold et al., 2018). Consequently, targeting ALOX15 to block the production of toxic substances that induce ferroptosis or enhancing GPx4 expression or activity to accelerate lipid peroxide metabolism represent promising therapeutic strategies for the development of ferroptosis-targeting drugs ( Table 2). Currently, the intraluminal monofilament MCAO model is most often employed in ischemic stroke research involving ferroptosis-targeting drugs, with no reported application of the embolic MCAO model. Notably, in the 2-VO method, carvacrol—a natural monoterpenoid phenol—has been shown to protect hippocampal neurons from ischemia/reperfusion injury in gerbils by up-regulating GPx4 expression ( Guan et al., 2019). However, further studies are needed to determine whether these strategies can be advanced to clinical trials.
Table 2. Summary of ferroptosis-stimulating agents with therapeutic potential in ischemic stroke animal models.
| Compound | Mechanism of action | Pharmacological activity | Ischemic stroke animal model | Reference |
| ALOX15: Arachidonate 15-lipoxygenase. OTC: L-2-oxothiazolidine-4-carboxylic acid. Tat SelPep: Tat-linked SelP Peptide. GSH: Glutathione. GPx4: Glutathione peroxidase 4. | ||||
| Liproxstatin-1 | Inhibiting lipid peroxidation | Reduced infarct volume and improved behavioral outcomes | Intraluminal filament MCAO (mice) | Li et al., 2021; Tuo et al., 2017 |
| Ferrostatin-1 | Inhibiting lipid peroxidation | Reduced infarct volume and improved behavioral outcomes | Intraluminal filament MCAO (mice, rats) | Fang et al., 2021; Li et al., 2024;
Liang et al., 2022; Liu et al., 2023; Tuo et al., 2017; Yang et al., 2021 |
| Ceruloplasmin | Regulating iron homeostasis | Inhibiting loss of neurons in ischemic brain areas, reducing infarct volume, and improving behavioral outcomes | Intraluminal filament MCAO (mice) | Tuo et al., 2017 |
| ML351 | Inhibiting ALOX15 | Reduced infarct volume and improved behavioral outcomes | Intraluminal filament MCAO (mice) | Liu et al., 2017; Tuo et al., 2017 |
| OTC | Increasing GSH levels | Reduced infarct volume and improved behavioral outcomes | Intraluminal filament MCAO (mice) | Liu et al., 2020 |
| Tat SelPep | Driving GPx4 expression | Reduced infarct volume | Intraluminal filament MCAO (mice) | Alim et al., 2019 |
| Selenocystamine/
Methylselenocysteine |
Increasing GPx4 expression and activity | Reduced infarct volume and improved behavioral outcomes | Intraluminal filament MCAO (mice) | Tuo et al., 2021a |
| Carvacrol | Increasing GPx4 expression | Reduced neuronal death in hippocampus
CA1 region and ameliorated cognitive impairment |
2-VO model (gerbils) | Guan et al., 2019 |
DISCUSSION
The development of effective treatments for ischemic stroke remains a major challenge in both clinical and research settings. Despite considerable efforts from basic and clinical researchers to develop novel therapeutic agents, few drugs, apart from r-tPA, have demonstrated clear efficacy in clinical practice. However, recent advancements in understanding the mechanisms of cell death forms have provided new insights into neuronal death after ischemic stroke, especially through the concept of ferroptosis. This emerging cell death pathway offers a promising new direction to combat ischemic stroke. The brain, with its rich phospholipid content and substantial energy and oxygen demands, is especially vulnerable to disruptions in phospholipid metabolism during ischemia and hypoxia. These conditions can lead to the accumulation of lipid peroxides, which drives ferroptotic cell death. The brain also contains extremely complex signaling pathways, requiring the involvement of multiple pathways to effectively respond to abnormal stimuli. However, the majority of studies have not yet investigated the interactions between different cell death pathways.
There are inherent connections between various cell death signaling pathways, which become particularly relevant in the context of ischemic stroke. Recent studies have found that receptor-interacting serine/threonine-protein kinase 1 (RIPK1) plays a crucial role in mediating both cell apoptosis and necroptosis following ischemic events ( Naito et al., 2020). RIPK1 mediates apoptosis when sufficient ATP is available but shifts toward mediating necroptosis under ATP deficient conditions ( Degterev et al., 2019). Ferroptosis has also emerged as an autophagy-dependent form of cell death, where knockout or knockdown of autophagy-related genes Atg5 and Atg7 can limit erastin-induced ferroptosis by reducing intracellular iron levels and lipid peroxidation ( Zhou et al., 2020). Recent findings also suggest that neuronal cells first undergo ferroptosis and necroptosis, followed by further apoptosis after cerebral ischemia/reperfusion ( Du et al., 2024). Interestingly, ferroptosis-specific inhibitors can prevent the occurrence of necroptosis, while necroptosis-specific inhibitors also inhibit the expression of proteins involved in the activation of ferroptosis. Iron appears to act as a key mediator between these pathways, as iron chelators can inhibit both ferroptosis and necroptosis in neuronal cells during cerebral ischemia/reperfusion. Excessive iron disrupts redox homeostasis, leading to necroptosis activation and increased sensitivity to ferroptosis. Furthermore, cerebral ischemia can significantly reduce Fpn expression in ischemic brain tissue ( Ding et al., 2011). Fpn knockout mice exhibit hallmark characteristics of ferroptosis in the hippocampus, including mitochondrial shrinkage in hippocampal neurons, accumulation of lipid peroxides, reduced expression of GPx4, and increased mRNA levels of ferroptosis-related genes PTGS2 and IREB2 ( Bao et al., 2021). Overexpression of Fpn not only effectively reduces the protein levels of cleaved caspase 3 and apoptosis signaling, but also significantly inhibits the expression of ferroptosis-related genes PTGS2 and IREB2 ( Bao et al., 2020). This suggests that Fpn may be a key factor mediating the interaction between neuronal ferroptosis and apoptosis during cerebral ischemia/reperfusion. Given these complex interactions, it is essential to conduct further in-depth research on the similarities, differences, and connections between different forms of cell death, to further explore more effective and easily convertible intervention targets, and to enhance our understanding of the molecular mechanisms of neuronal injury after ischemic stroke.
The function of the mitochondrial respiratory chain is critically reliant on oxygen and glucose. During cerebral ischemia, the deprivation of these key resources in brain tissue results in mitochondrial dysfunction, which, in turn, triggers various forms of cell death, including apoptosis, necroptosis, pyroptosis, autophagy, and ferroptosis ( Bock & Tait, 2020). Mitochondrial shrinkage and increased membrane density are specific morphological features of ferroptosis ( Dixon et al., 2012), highlighting mitochondria as a potential focal point for identifying key intervention targets.
Animal models serve as an indispensable bridge between basic research and clinical drug development ( Fluri et al., 2015). Both focal and global cerebral ischemia models exhibit similar neuropathologies observed in ischemic stroke patients ( Bacigaluppi et al., 2010), providing valuable platforms for mechanistic and therapeutic investigations of ferroptosis in ischemic stroke. However, ischemic stroke in humans is a highly heterogeneous condition with complex pathophysiological processes ( Kuriakose & Xiao, 2020), and as reviewed here, no single animal model can fully replicate the clinical progression and variability of human ischemic stroke. As a result, researchers must carefully select appropriate animal models that align with the specific objectives of their studies. In addition, the majority of current research is conducted on young animals without any comorbidities, which contrasts with the typical human ischemic stroke population—elderly people often burdened with various cerebrovascular risk factors ( Candelario-Jalil & Paul, 2021). Therefore, selecting the most suitable ischemic stroke model and optimizing the research design of preclinical trials may increase the translational potential of drug candidates.
In the present era, advancements in intravenous thrombolysis and thrombectomy have significantly improved the success rates of reperfusing ischemic brain tissue, presenting new opportunities for neuroprotective agents to prevent reperfusion injuries. Multiple clinical trials have shown that neuroprotective agents with antioxidant properties, such as edaravone (ClinicalTrials.gov Identifier: NCT02430350) and butylphthalide (ClinicalTrials.gov Identifier: NCT03539445), combined with reperfusion therapy, can effectively ameliorate deleterious consequences post-reperfusion, including reperfusion injury and BBB disruption. Patients receiving these combined treatments have shown marked improvements in functional outcomes following acute ischemic stroke ( Lochhead et al., 2024; Savitz et al., 2019; Wang et al., 2023; Xu et al., 2021). Theoretically, antioxidants have the capacity to inhibit phospholipid peroxidation and may function as potential ferroptosis inhibitors. However, whether these antioxidants exert neuroprotective effects by blocking ferroptosis needs further experimental verification. Nevertheless, the promising potential of ferroptosis inhibition as a therapeutic strategy for ischemic stroke should be further investigated clinically.
Acknowledgments
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
Q.Z.T. wrote the first draft of the manuscript. P.L. and Q.Z.T. contributed to the conception, design, and revision of the manuscript. All authors read and approved the final version of the manuscript.
Funding Statement
This work was supported by the National Key Research and Development Program of China (2021YFC2500100), Major Science & Technology Program of Sichuan Province (2022ZDZX0021), National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University (Z2024JC007), Sichuan Science and Technology Program (2024YFHZ0010), and West China Hospital 1.3.5 Project for Disciplines of Excellence (ZYYC23016)
Contributor Information
Qing-Zhang Tuo, Email: qing-zhang.tuo@scu.edu.cn.
Peng Lei, Email: peng.lei@scu.edu.cn.
References
- Adibhatla RM, Hatcher JF Citicoline decreases phospholipase A 2 stimulation and hydroxyl radical generation in transient cerebral ischemia . Journal of Neuroscience Research. 2003;73(3):308–315. doi: 10.1002/jnr.10672. [DOI] [PubMed] [Google Scholar]
- Alim I, Caulfield JT, Chen YX, et al. 2019. Selenium drives a transcriptional adaptive program to block ferroptosis and treat stroke. Cell, 177(5): 1262–1279. e25.
- Bacigaluppi M, Comi G, Hermann DM Animal models of ischemic stroke. Part two: modeling cerebral ischemia. The Open Neurology Journal. 2010;4:34–38. doi: 10.2174/1874205X01004020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballatore C, Lee VMY, Trojanowski JQ Tau-mediated neurodegeneration in Alzheimer's disease and related disorders. Nature Reviews Neuroscience. 2007;8(9):663–672. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- Balsinde J, Fernández B, Solís-Herruzo JA, et al Pathways for arachidonic acid mobilization in zymosan-stimulated mouse peritoneal macrophages. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research. 1992;1136(1):75–82. doi: 10.1016/0167-4889(92)90087-R. [DOI] [PubMed] [Google Scholar]
- Bao WD, Pang P, Zhou XT, et al Loss of ferroportin induces memory impairment by promoting ferroptosis in Alzheimer's disease. Cell Death & Differentiation. 2021;28(5):1548–1562. doi: 10.1038/s41418-020-00685-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao WD, Zhou XT, Zhou LT, et al Targeting miR-124/Ferroportin signaling ameliorated neuronal cell death through inhibiting apoptosis and ferroptosis in aged intracerebral hemorrhage murine model. Aging Cell. 2020;19(11):e13235. doi: 10.1111/acel.13235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayır H, Anthonymuthu TS, Tyurina YY, et al Achieving life through death: redox biology of lipid peroxidation in ferroptosis. Cell Chemical Biology. 2020;27(4):387–408. doi: 10.1016/j.chembiol.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermúdez MA, Balboa MA, Balsinde J Lipid droplets, phospholipase A 2, arachidonic acid, and atherosclerosis . Biomedicines. 2021;9(12):1891. doi: 10.3390/biomedicines9121891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt C, Alborzinia H, Amen VS, et al Ferroptosis in health and disease. Redox Biology. 2024;75:103211. doi: 10.1016/j.redox.2024.103211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock FJ, Tait SWG Mitochondria as multifaceted regulators of cell death. Nature Reviews Molecular Cell Biology. 2020;21(2):85–100. doi: 10.1038/s41580-019-0173-8. [DOI] [PubMed] [Google Scholar]
- Buchan AM, Xue D, Slivka A A new model of temporary focal neocortical ischemia in the rat. Stroke. 1992;23(2):273–279. doi: 10.1161/01.STR.23.2.273. [DOI] [PubMed] [Google Scholar]
- Buczynski MW, Dumlao DS, Dennis EA Thematic review series: proteomics. An integrated omics analysis of eicosanoid biology. Journal of Lipid Research. 2009;50(6):1015–1038. doi: 10.1194/jlr.R900004-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell BCV, De Silva DA, Macleod MR, et al Ischaemic stroke. Nature Reviews Disease Primers. 2019;5(1):70. doi: 10.1038/s41572-019-0118-8. [DOI] [PubMed] [Google Scholar]
- Campbell BCV, Khatri P Stroke. Lancet. 2020;396(10244):129–142. doi: 10.1016/S0140-6736(20)31179-X. [DOI] [PubMed] [Google Scholar]
- Candelario-Jalil E, Paul S Impact of aging and comorbidities on ischemic stroke outcomes in preclinical animal models: a translational perspective. Experimental Neurology. 2021;335:113494. doi: 10.1016/j.expneurol.2020.113494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonell T, Rama R Iron, oxidative stress and early neurological deterioration in ischemic stroke. Current Medicinal Chemistry. 2007;14(8):857–874. doi: 10.2174/092986707780363014. [DOI] [PubMed] [Google Scholar]
- Chen B, Friedman B, Whitney MA, et al Thrombin activity associated with neuronal damage during acute focal ischemia. Journal of Neuroscience. 2012;32(22):7622–7631. doi: 10.1523/JNEUROSCI.0369-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DL, Chu B, Yang X, et al iPLA2β-mediated lipid detoxification controls p53-driven ferroptosis independent of GPX4. Nature Communications. 2021a;12(1):3644. doi: 10.1038/s41467-021-23902-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JM, Yang L, Geng LX, et al Inhibition of acyl-CoA synthetase long-chain family member 4 facilitates neurological recovery after stroke by regulation ferroptosis. Frontiers in Cellular Neuroscience. 2021b;15:632354. doi: 10.3389/fncel.2021.632354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Tang F, Du B, et al Leucine-rich repeat kinase 2 (LRRK2) inhibition upregulates microtubule-associated protein 1B to ameliorate lysosomal dysfunction and parkinsonism. MedComm. 2023;4(6):e429. doi: 10.1002/mco2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng GS, Zhao W, Xin YJ, et al Effects of ML351 and tissue plasminogen activator combination therapy in a rat model of focal embolic stroke. Journal of Neurochemistry. 2021;157(3):586–598. doi: 10.1111/jnc.15308. [DOI] [PubMed] [Google Scholar]
- Chi SI, Wang CK, Chen JJ, et al Differential regulation of H- and L-ferritin messenger RNA subunits, ferritin protein and iron following focal cerebral ischemia-reperfusion. Neuroscience. 2000;100(3):475–484. doi: 10.1016/S0306-4522(00)00317-1. [DOI] [PubMed] [Google Scholar]
- Cui LL, Zhang XJ, Yang R, et al Neuroprotection of early and short-time applying atorvastatin in the acute phase of cerebral ischemia: down-regulated 12/15-LOX, p38MAPK and cPLA2 expression, ameliorated BBB permeability. Brain Research. 2010;1325:164–173. doi: 10.1016/j.brainres.2010.02.036. [DOI] [PubMed] [Google Scholar]
- Cui Y, Zhang Y, Zhao XL, et al ACSL4 exacerbates ischemic stroke by promoting ferroptosis-induced brain injury and neuroinflammation. Brain, Behavior, and Immunity. 2021;93:312–321. doi: 10.1016/j.bbi.2021.01.003. [DOI] [PubMed] [Google Scholar]
- Dávalos A, Castillo J, Marrugat J, et al Body iron stores and early neurologic deterioration in acute cerebral infarction. Neurology. 2000;54(8):1568–1574. doi: 10.1212/WNL.54.8.1568. [DOI] [PubMed] [Google Scholar]
- Degterev A, Ofengeim D, Yuan JY Targeting RIPK1 for the treatment of human diseases. Proceedings of the National Academy of Sciences of the United States of America. 2019;116(20):9714–9722. doi: 10.1073/pnas.1901179116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis EA Diversity of group types, regulation, and function of phospholipase A 2 . The Journal of Biological Chemistry. 1994;269(18):13057–13060. doi: 10.1016/S0021-9258(17)36794-7. [DOI] [PubMed] [Google Scholar]
- Denorme F, Wyseure T, Peeters M, et al Inhibition of thrombin-activatable fibrinolysis inhibitor and plasminogen activator inhibitor-1 reduces ischemic brain damage in mice. Stroke. 2016;47(9):2419–2422. doi: 10.1161/STROKEAHA.116.014091. [DOI] [PubMed] [Google Scholar]
- Dietrich RB, Bradley WG Jr Iron accumulation in the basal ganglia following severe ischemic-anoxic insults in children. Radiology. 1988;168(1):203–206. doi: 10.1148/radiology.168.1.3380958. [DOI] [PubMed] [Google Scholar]
- Ding H, Yan CZ, Shi HL, et al Hepcidin is involved in iron regulation in the ischemic brain. PLoS One. 2011;6(9):e25324. doi: 10.1371/journal.pone.0025324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding XL, Cao SQ, Wang Q, et al DNALI1 promotes neurodegeneration after traumatic brain injury via inhibition of autophagosome-lysosome fusion. Advanced Science. 2024;11(15):2306399. doi: 10.1002/advs.202306399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding XL, Zhang ST, Jiang LJ, et al Ultrasensitive assays for detection of plasma tau and phosphorylated tau 181 in Alzheimer's disease: a systematic review and meta-analysis. Translational Neurodegeneration. 2021;10(1):10. doi: 10.1186/s40035-021-00234-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon SJ, Lemberg KM, Lamprecht MR, et al Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon SJ, Pratt DA Ferroptosis: a flexible constellation of related biochemical mechanisms. Molecular Cell. 2023;83(7):1030–1042. doi: 10.1016/j.molcel.2023.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon SJ, Winter GE, Musavi LS, et al Human haploid cell genetics reveals roles for lipid metabolism genes in nonapoptotic cell death. ACS Chemical Biology. 2015;10(7):1604–1609. doi: 10.1021/acschembio.5b00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll S, Proneth B, Tyurina YY, et al ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nature Chemical Biology. 2017;13(1):91–98. doi: 10.1038/nchembio.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du B, Deng ZJ, Chen K, et al Iron promotes both ferroptosis and necroptosis in the early stage of reperfusion in ischemic stroke. Genes & Diseases. 2024;11(6):101262. doi: 10.1016/j.gendis.2024.101262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duce JA, Tsatsanis A, Cater MA, et al Iron-export ferroxidase activity of β-amyloid precursor protein is inhibited by zinc in Alzheimer's disease. Cell. 2010;142(6):857–867. doi: 10.1016/j.cell.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel O, Kolodziej S, Dirnagl U, et al Modeling stroke in mice - middle cerebral artery occlusion with the filament model. Journal of Visualized Experiments. 2011;(47):e2423. doi: 10.3791/2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang YY, Chen XC, Tan QY, et al Inhibiting Ferroptosis through Disrupting the NCOA4-FTH1 Interaction: A New Mechanism of Action. ACS Central Science. 2021;7(6):980–989. doi: 10.1021/acscentsci.0c01592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearnhead HO, Vandenabeele P, Vanden Berghe T How do we fit ferroptosis in the family of regulated cell death. Cell Death and Differentiation. 2017;24(12):1991–1998. doi: 10.1038/cdd.2017.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluri F, Schuhmann MK, Kleinschnitz C Animal models of ischemic stroke and their application in clinical research. Drug Design, Development and Therapy. 2015;9:3445–3454. doi: 10.2147/DDDT.S56071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonteh AN, Chilton FH Rapid remodeling of arachidonate from phosphatidylcholine to phosphatidylethanolamine pools during mast cell activation. Journal of Immunology. 1992;148(6):1784–1791. doi: 10.4049/jimmunol.148.6.1784. [DOI] [PubMed] [Google Scholar]
- Friedmann Angeli JP, Schneider M, Proneth B, et al Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nature Cell Biology. 2014;16(12):1180–1191. doi: 10.1038/ncb3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii J, Homma T, Kobayashi S Ferroptosis caused by cysteine insufficiency and oxidative insult. Free Radical Research. 2020;54(11-12):969–980. doi: 10.1080/10715762.2019.1666983. [DOI] [PubMed] [Google Scholar]
- Funk CD Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294(5548):1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- Ganz T Hepcidin and iron regulation, 10 years later. Blood. 2011;117(17):4425–4433. doi: 10.1182/blood-2011-01-258467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner HW Oxygen radical chemistry of polyunsaturated fatty acids. Free Radical Biology and Medicine. 1989;7(1):65–86. doi: 10.1016/0891-5849(89)90102-0. [DOI] [PubMed] [Google Scholar]
- Gaschler MM, Stockwell BR Lipid peroxidation in cell death. Biochemical and Biophysical Research Communications. 2017;482(3):419–425. doi: 10.1016/j.bbrc.2016.10.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2019 Stroke Collaborators Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the global burden of disease study 2019. The Lancet-Neurology. 2021;20(10):795–820. doi: 10.1016/S1474-4422(21)00252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluck N, Schwob O, Krimsky M, et al Activation of cytosolic phospholipase A 2 and fatty acid transacylase is essential but not sufficient for thrombin-induced smooth muscle cell proliferation . American Journal of Physiology-Cell Physiology. 2008;294(6):C1597–C1603. doi: 10.1152/ajpcell.00206.2007. [DOI] [PubMed] [Google Scholar]
- Guan XY, Li XL, Yang XJ, et al The neuroprotective effects of carvacrol on ischemia/reperfusion-induced hippocampal neuronal impairment by ferroptosis mitigation. Life Sciences. 2019;235:116795. doi: 10.1016/j.lfs.2019.116795. [DOI] [PubMed] [Google Scholar]
- Guo J, Tuo QZ, Lei P Iron, ferroptosis, and ischemic stroke. Journal of Neurochemistry. 2023;165(4):487–520. doi: 10.1111/jnc.15807. [DOI] [PubMed] [Google Scholar]
- Gupta S, Sharma SS Neuroprotective effects of trolox in global cerebral ischemia in gerbils. Biological & Pharmaceutical Bulletin. 2006;29(5):957–961. doi: 10.1248/bpb.29.957. [DOI] [PubMed] [Google Scholar]
- Hacke W, Kaste M, Bluhmki E, et al Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. The New England Journal of Medicine. 2008;359(13):1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- Han J, Sun L, Xu YW, et al Activation of PPARγ by 12/15-lipoxygenase during cerebral ischemia-reperfusion injury. International Journal of Molecular Medicine. 2015;35(1):195–201. doi: 10.3892/ijmm.2014.1998. [DOI] [PubMed] [Google Scholar]
- Han LY, Zhong CK, Bu XQ, et al Prognostic value of lipoprotein-associated phospholipase A 2 mass for all-cause mortality and vascular events within one year after acute ischemic stroke . Atherosclerosis. 2017;266:1–7. doi: 10.1016/j.atherosclerosis.2017.09.013. [DOI] [PubMed] [Google Scholar]
- Huang X, Yang X, Zhang MX, et al SELENOI functions as a key modulator of ferroptosis pathway in colitis and colorectal cancer . Advanced Science. 2024;11(28):2404073. doi: 10.1002/advs.202404073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingold I, Berndt C, Schmitt S, et al. 2018. Selenium utilization by GPX4 is required to prevent hydroperoxide-induced ferroptosis. Cell, 172(3): 409–422. e21.
- Jia CL, Gou YJ, Gao YH, et al Rosmarinic acid liposomes suppress ferroptosis in ischemic brain via inhibition of TfR1 in BMECs. Phytomedicine. 2024;132:155835. doi: 10.1016/j.phymed.2024.155835. [DOI] [PubMed] [Google Scholar]
- Jiang XJ, Stockwell BR, Conrad M Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22(4):266–282. doi: 10.1038/s41580-020-00324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao LL, Li XL, Luo YX, et al Iron metabolism mediates microglia susceptibility in ferroptosis. Frontiers in Cellular Neuroscience. 2022;16:995084. doi: 10.3389/fncel.2022.995084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin R, Zhu XL, Li GH Embolic middle cerebral artery occlusion (MCAO) for ischemic stroke with homologous blood clots in rats. Journal of Visualized Experiments. 2014;(91):51956. doi: 10.3791/51956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung JE, Karatas H, Liu Y, et al STAT-dependent upregulation of 12/15-lipoxygenase contributes to neuronal injury after stroke. Journal of Cerebral Blood Flow & Metabolism. 2015;35(12):2043–2051. doi: 10.1038/jcbfm.2015.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan VE, Mao GW, Qu F, et al Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nature Chemical Biology. 2017;13(1):81–90. doi: 10.1038/nchembio.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SA, Ilies MA The phospholipase A2 superfamily: structure, isozymes, catalysis, physiologic and pathologic roles. International Journal of Molecular Sciences. 2023;24(2):1353. doi: 10.3390/ijms24021353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kil J, Lobarinas E, Spankovich C, et al Safety and efficacy of ebselen for the prevention of noise-induced hearing loss: a randomised, double-blind, placebo-controlled, phase 2 trial. The Lancet. 2017;390(10098):969–979. doi: 10.1016/S0140-6736(17)31791-9. [DOI] [PubMed] [Google Scholar]
- Kim H, Urquhart R, Pontarelli F, et al Protocol for establishing a global ischemia model using a 4-vessel occlusion in rats. STAR Protocols. 2023;4(4):102630. doi: 10.1016/j.xpro.2023.102630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo Y, Asanuma M, Nishibayashi S, et al Late-onset lipid peroxidation and neuronal cell death following transient forebrain ischemia in rat brain. Brain Research. 1997;772(1-2):37–44. doi: 10.1016/S0006-8993(97)00836-6. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Ogawa N, Asanuma M, et al Regional differences in late-onset iron deposition, ferritin, transferrin, astrocyte proliferation, and microglial activation after transient forebrain ischemia in rat brain. Journal of Cerebral Blood Flow & Metabolism. 1995;15(2):216–226. doi: 10.1038/jcbfm.1995.27. [DOI] [PubMed] [Google Scholar]
- Kramer RM, Roberts EF, Manetta JV, et al Thrombin-induced phosphorylation and activation of Ca 2+-sensitive cytosolic phospholipase A 2 in human platelets . The Journal of Biological Chemistry. 1993;268(35):26796–26804. doi: 10.1016/S0021-9258(19)74383-X. [DOI] [PubMed] [Google Scholar]
- Kramer RM, Roberts EF, Um SL, et al p38 mitogen-activated protein kinase phosphorylates cytosolic phospholipase A 2 (cPLA 2) in thrombin-stimulated platelets. Evidence that proline-directed phosphorylation is not required for mobilization of arachidonic acid by cPLA 2 . The Journal of Biological Chemistry. 1996;271(44):27723–27729. doi: 10.1074/jbc.271.44.27723. [DOI] [PubMed] [Google Scholar]
- Krishnaswamy S. 2013. The transition of prothrombin to thrombin. Journal of Thrombosis and Haemostasis, 11 Suppl 1(0 1): 265–276.
- Kuriakose D, Xiao ZC Pathophysiology and treatment of stroke: present status and future perspectives. International Journal of Molecular Sciences. 2020;21(20):7609. doi: 10.3390/ijms21207609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapong WR, Tang F, Liu P, et al Choriocapillaris reduction accurately discriminates against early-onset Alzheimer's disease. Alzheimer's & Dementia. 2024;20(6):4185–4198. doi: 10.1002/alz.13871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labat-Gest V, Tomasi S Photothrombotic ischemia: a minimally invasive and reproducible photochemical cortical lesion model for mouse stroke studies. Journal of Visualized Experiments. 2013;(76):e50370. doi: 10.3791/50370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan B, Ge JW, Cheng SW, et al Extract of Naotaifang, a compound Chinese herbal medicine, protects neuron ferroptosis induced by acute cerebral ischemia in rats. Journal of Integrative Medicine. 2020;18(4):344–350. doi: 10.1016/j.joim.2020.01.008. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Zivin JA Ebselen, a seleno-organic antioxidant, is neuroprotective after embolic strokes in rabbits: synergism with low-dose tissue plasminogen activator. Stroke. 2003;34(8):2013–2018. doi: 10.1161/01.STR.0000081223.74129.04. [DOI] [PubMed] [Google Scholar]
- Lee JM, Grabb MC, Zipfel GJ, et al Brain tissue responses to ischemia. The Journal of Clinical Investigation. 2000;106(6):723–731. doi: 10.1172/JCI11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TK, Kim H, Song M, et al Time-course pattern of neuronal loss and gliosis in gerbil hippocampi following mild, severe, or lethal transient global cerebral ischemia. Neural Regeneration Research. 2019;14(8):1394–1403. doi: 10.4103/1673-5374.253524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei P, Ayton S TRIMming the tangles. Science Bulletin. 2023;68(21):2507–2509. doi: 10.1016/j.scib.2023.09.019. [DOI] [PubMed] [Google Scholar]
- Lei P, Ayton S, Finkelstein DI, et al Tau deficiency induces parkinsonism with dementia by impairing APP-mediated iron export. Nature Medicine. 2012;18(2):291–295. doi: 10.1038/nm.2613. [DOI] [PubMed] [Google Scholar]
- Li C, Sun GC, Chen BL, et al Nuclear receptor coactivator 4-mediated ferritinophagy contributes to cerebral ischemia-induced ferroptosis in ischemic stroke. Pharmacological Research. 2021;174:105933. doi: 10.1016/j.phrs.2021.105933. [DOI] [PubMed] [Google Scholar]
- Li HW, Wang CY, Yu XC, et al Measurement of cerebral oxygen extraction fraction using quantitative BOLD approach: a review. Phenomics. 2023;3(1):101–118. doi: 10.1007/s43657-022-00081-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wei G, Song ZM, et al SIRT5 regulates ferroptosis through the Nrf2/HO-1 signaling axis to participate in ischemia-reperfusion injury in ischemic stroke. Neurochemical Research. 2024;49(4):998–1007. doi: 10.1007/s11064-023-04095-4. [DOI] [PubMed] [Google Scholar]
- Li L, Li YW, Zhao JY, et al Quantitative analysis of iron concentration and expression of ferroportin 1 in the cortex and hippocampus of rats induced by cerebral ischemia. Journal of Clinical Neuroscience. 2009;16(11):1466–1472. doi: 10.1016/j.jocn.2009.01.020. [DOI] [PubMed] [Google Scholar]
- Liang TY, Qiang TT, Ren LF, et al Near-infrared fluorescent probe for hydrogen sulfide: high-fidelity ferroptosis evaluation in vivo during stroke . Chemical Science. 2022;13(10):2992–3001. doi: 10.1039/D1SC05930K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JX, Zheng HW, Cucchiara BL, et al Association of Lp-PLA 2-A and early recurrence of vascular events after TIA and minor stroke . Neurology. 2015;85(18):1585–1591. doi: 10.1212/WNL.0000000000001938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton P Ischemic cell death in brain neurons. Physiological Reviews. 1999;79(4):1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- Liu XY, Du Y, Liu J, et al Ferrostatin-1 alleviates cerebral ischemia/reperfusion injury through activation of the AKT/GSK3β signaling pathway. Brain Research Bulletin. 2023;193:146–157. doi: 10.1016/j.brainresbull.2022.12.009. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zheng Y, Karatas H, et al 12/15-lipoxygenase inhibition or knockout reduces warfarin-associated hemorrhagic transformation after experimental stroke. Stroke. 2017;48(2):445–451. doi: 10.1161/STROKEAHA.116.014790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YY, Min JW, Feng S, et al Therapeutic role of a cysteine precursor, OTC, in ischemic stroke is mediated by improved proteostasis in mice. Translational Stroke Research. 2020;11(1):147–160. doi: 10.1007/s12975-019-00707-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochhead JJ, Ronaldson PT, Davis TP. 2024. The role of oxidative stress in blood-brain barrier disruption during ischemic stroke: Antioxidants in clinical trials. Biochemical Pharmacology, 116186.
- Luoqian JY, Yang WY, Ding XL, et al Ferroptosis promotes T-cell activation-induced neurodegeneration in multiple sclerosis. Cellular & Molecular Immunology. 2022;19(8):913–924. doi: 10.1038/s41423-022-00883-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyden P, Pereira B, Chen B, et al Direct thrombin inhibitor argatroban reduces stroke damage in 2 different models. Stroke. 2014;45(3):896–899. doi: 10.1161/STROKEAHA.113.004488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiafico SP, Tuo QZ, Li XL, et al Tau suppresses microtubule-regulated pancreatic insulin secretion. Molecular Psychiatry. 2023;28(9):3982–3993. doi: 10.1038/s41380-023-02267-w. [DOI] [PubMed] [Google Scholar]
- Manni MM, Tiberti ML, Pagnotta S, et al Acyl chain asymmetry and polyunsaturation of brain phospholipids facilitate membrane vesiculation without leakage. eLife. 2018;7:e34394. doi: 10.7554/eLife.34394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao YY, Zhang M, Tuma RF, et al Deficiency of PAR4 attenuates cerebral ischemia/reperfusion injury in mice. Journal of Cerebral Blood Flow & Metabolism. 2010;30(5):1044–1052. doi: 10.1038/jcbfm.2009.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez NS, Machado JM, Pérez-Saad H, et al Global brain ischemia in Mongolian gerbils: assessing the level of anastomosis in the cerebral circle of willis. Acta Neurobiologiae Experimentalis. 2012;72(4):377–384. doi: 10.55782/ane-2012-1909. [DOI] [PubMed] [Google Scholar]
- Menzies SA, Hoff JT, Betz AL Middle cerebral artery occlusion in rats: a neurological and pathological evaluation of a reproducible model. Neurosurgery. 1992;31(1):100–107. doi: 10.1227/00006123-199207000-00014. [DOI] [PubMed] [Google Scholar]
- Millán M, Degregorio-Rocasolano N, Pérez De La Ossa N, et al Targeting pro-oxidant iron with deferoxamine as a treatment for ischemic stroke: safety and optimal dose selection in a randomized clinical trial. Antioxidants. 2021;10(8):1270. doi: 10.3390/antiox10081270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan M, Sobrino T, Castellanos M, et al Increased body iron stores are associated with poor outcome after thrombolytic treatment in acute stroke. Stroke. 2007;38(1):90–95. doi: 10.1161/01.STR.0000251798.25803.e0. [DOI] [PubMed] [Google Scholar]
- Millerot-Serrurot E, Bertrand N, Mossiat C, et al Temporal changes in free iron levels after brain ischemia: relevance to the timing of iron chelation therapy in stroke. Neurochemistry International. 2008;52(8):1442–1448. doi: 10.1016/j.neuint.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Naito MG, Xu DC, Amin P, et al Sequential activation of necroptosis and apoptosis cooperates to mediate vascular and neural pathology in stroke. Proceedings of the National Academy of Sciences of the United States of America. 2020;117(9):4959–4970. doi: 10.1073/pnas.1916427117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura A, Sakai S, Taketomi Y, et al. 2023. PLA2G2E-mediated lipid metabolism triggers brain-autonomous neural repair after ischemic stroke. Neuron, 111(19): 2995–3010. e9.
- Nieto ML, Venable ME, Bauldry SA, et al Evidence that hydrolysis of ethanolamine plasmalogens triggers synthesis of platelet-activating factor via a transacylation reaction. The Journal of Biological Chemistry. 1991;266(28):18699–18706. doi: 10.1016/S0021-9258(18)55119-X. [DOI] [PubMed] [Google Scholar]
- Oh M, Jang SY, Lee JY, et al The lipoprotein-associated phospholipase A2 inhibitor Darapladib sensitises cancer cells to ferroptosis by remodelling lipid metabolism. Nature Communications. 2023;14(1):5728. doi: 10.1038/s41467-023-41462-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oubidar M, Boquillon M, Marie C, et al Ischemia-induced brain iron delocalization: effect of iron chelators. Free Radical Biology & Medicine. 1994;16(6):861–867. doi: 10.1016/0891-5849(94)90205-4. [DOI] [PubMed] [Google Scholar]
- Park UJ, Lee YA, Won SM, et al Blood-derived iron mediates free radical production and neuronal death in the hippocampal CA1 area following transient forebrain ischemia in rat. Acta Neuropathologica. 2011;121(4):459–473. doi: 10.1007/s00401-010-0785-8. [DOI] [PubMed] [Google Scholar]
- Pérez-Chacón G, Astudillo AM, Balgoma D, et al Control of free arachidonic acid levels by phospholipases A 2 and lysophospholipid acyltransferases . Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2009;1791(12):1103–1113. doi: 10.1016/j.bbalip.2009.08.007. [DOI] [PubMed] [Google Scholar]
- Phillis JW, O'Regan MH. 2003. The role of phospholipases, cyclooxygenases, and lipoxygenases in cerebral ischemic/traumatic injuries. Critical Reviews TM in Neurobiology, 15(1): 61–90.
- Prass K, Ruscher K, Karsch M, et al. 2002. Desferrioxamine induces delayed tolerance against cerebral ischemia in vivo and in vitro. Journal of Cerebral Blood Flow & Metabolism, 22(5): 520–525.
- Rajput PS, Lyden PD, Chen B, et al Protease activated receptor-1 mediates cytotoxicity during ischemia using in vivo and in vitro models . Neuroscience. 2014;281:229–240. doi: 10.1016/j.neuroscience.2014.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhold SL, Zimmerman GA, Prescott SM, et al Phospholipid remodeling in human neutrophils: parallel activation of a deacylation/reacylation cycle and platelet-activating factor synthesis. Journal of Biological Chemistry. 1989;264(36):21652–21659. doi: 10.1016/S0021-9258(20)88235-0. [DOI] [PubMed] [Google Scholar]
- Robinson RG, Shoemaker WJ, Schlumpf M, et al Effect of experimental cerebral infarction in rat brain on catecholamines and behaviour. Nature. 1975;255(5506):332–334. doi: 10.1038/255332a0. [DOI] [PubMed] [Google Scholar]
- Ryan F, Zarruk JG, Lößlein L, et al Ceruloplasmin plays a neuroprotective role in cerebral ischemia. Frontiers in Neuroscience. 2018;12:988. doi: 10.3389/fnins.2018.00988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz SI, Baron JC, Fisher M, et al Stroke treatment academic industry roundtable X: brain cytoprotection therapies in the reperfusion era. Stroke. 2019;50(4):1026–1031. doi: 10.1161/STROKEAHA.118.023927. [DOI] [PubMed] [Google Scholar]
- Seiler A, Schneider M, Förster H, et al Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metabolism. 2008;8(3):237–248. doi: 10.1016/j.cmet.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Sergeeva M, Strokin M, Wang H, et al Arachidonic acid and docosahexaenoic acid suppress thrombin-evoked Ca 2+ response in rat astrocytes by endogenous arachidonic acid liberation . Journal of Neurochemistry. 2002;82(5):1252–1261. doi: 10.1046/j.1471-4159.2002.01052.x. [DOI] [PubMed] [Google Scholar]
- Singh NK, Rao GN Emerging role of 12/15-Lipoxygenase (ALOX15) in human pathologies. Progress in Lipid Research. 2019;73:28–45. doi: 10.1016/j.plipres.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolowski JD, Soldozy S, Sharifi KA, et al Preclinical models of middle cerebral artery occlusion: new imaging approaches to a classic technique. Frontiers in Neurology. 2023;14:1170675. doi: 10.3389/fneur.2023.1170675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockwell BR, Friedmann Angeli JP, Bayir H, et al Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171(2):273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striggow F, Riek M, Breder J, et al The protease thrombin is an endogenous mediator of hippocampal neuroprotection against ischemia at low concentrations but causes degeneration at high concentrations. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(5):2264–2269. doi: 10.1073/pnas.040552897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun WY, Tyurin VA, Mikulska-Ruminska K, et al Phospholipase iPLA 2β averts ferroptosis by eliminating a redox lipid death signal . Nature Chemical Biology. 2021;17(4):465–476. doi: 10.1038/s41589-020-00734-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Zheng YF, Wang CX, et al Glutathione depletion induces ferroptosis, autophagy, and premature cell senescence in retinal pigment epithelial cells. Cell Death & Disease. 2018;9(7):753. doi: 10.1038/s41419-018-0794-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallima H, El Ridi R Arachidonic acid: physiological roles and potential health benefits - A review. Journal of Advanced Research. 2018;11:33–41. doi: 10.1016/j.jare.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang F, Zhou LY, Li P, et al Inhibition of ACSL4 alleviates parkinsonism phenotypes by reduction of lipid reactive oxygen species. Neurotherapeutics. 2023;20(4):1154–1166. doi: 10.1007/s13311-023-01382-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tou JS Platelet-activating factor regulates phospholipid metabolism in human neutrophils. Lipids. 1989;24(9):812–817. doi: 10.1007/BF02544589. [DOI] [PubMed] [Google Scholar]
- Traystman RJ Animal models of focal and global cerebral ischemia. ILAR Journal. 2003;44(2):85–95. doi: 10.1093/ilar.44.2.85. [DOI] [PubMed] [Google Scholar]
- Tuo QZ, Lei P, Jackman KA, et al Tau-mediated iron export prevents ferroptotic damage after ischemic stroke. Molecular Psychiatry. 2017;22(11):1520–1530. doi: 10.1038/mp.2017.171. [DOI] [PubMed] [Google Scholar]
- Tuo QZ, Liu Y, Xiang Z, et al Thrombin induces ACSL4-dependent ferroptosis during cerebral ischemia/reperfusion. Signal Transduction and Targeted Therapy. 2022a;7(1):59. doi: 10.1038/s41392-022-00917-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuo QZ, Masaldan S, Southon A, et al Characterization of selenium compounds for anti-ferroptotic activity in neuronal cells and after cerebral ischemia-reperfusion injury. Neurotherapeutics. 2021a;18(4):2682–2691. doi: 10.1007/s13311-021-01111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuo QZ, Zhang ST, Lei P Mechanisms of neuronal cell death in ischemic stroke and their therapeutic implications. Medicinal Research Reviews. 2022b;42(1):259–305. doi: 10.1002/med.21817. [DOI] [PubMed] [Google Scholar]
- Tuo QZ, Zou JJ, Lei P Rodent models of vascular cognitive impairment. Journal of Molecular Neuroscience. 2021b;71(5):1–12. doi: 10.1007/s12031-020-01733-2. [DOI] [PubMed] [Google Scholar]
- Ugidos IF, Santos-Galdiano M, Pérez-Rodríguez D, et al. 2017. Neuroprotective effect of 2-hydroxy arachidonic acid in a rat model of transient middle cerebral artery occlusion. Biochimica et Biophysica Acta (BBA) - Biomembranes, 1859(9 Pt B): 1648–1656.
- Van Den Brink-Van Der Laan E, Killian JA, De Kruijff B Nonbilayer lipids affect peripheral and integral membrane proteins via changes in the lateral pressure profile. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2004;1666(1-2):275–288. doi: 10.1016/j.bbamem.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Van Leyen K, Kim HY, Lee SR, et al Baicalein and 12/15-lipoxygenase in the ischemic brain. Stroke. 2006;37(12):3014–3018. doi: 10.1161/01.STR.0000249004.25444.a5. [DOI] [PubMed] [Google Scholar]
- Walter K What is acute ischemic stroke. JAMA. 2022;327(9):885. doi: 10.1001/jama.2022.1420. [DOI] [PubMed] [Google Scholar]
- Wang AX, Jia BX, Zhang XL, et al Efficacy and safety of butylphthalide in patients with acute ischemic stroke: a randomized clinical trial. JAMA Neurology. 2023;80(8):851–859. doi: 10.1001/jamaneurol.2023.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Liu SH, Fu YP, et al Cdk5 activation induces hippocampal CA1 cell death by directly phosphorylating NMDA receptors. Nature Neuroscience. 2003;6(10):1039–1047. doi: 10.1038/nn1119. [DOI] [PubMed] [Google Scholar]
- Wang PN, Cui YM, Ren QQ, et al Mitochondrial ferritin attenuates cerebral ischaemia/reperfusion injury by inhibiting ferroptosis. Cell Death & Disease. 2021;12(5):447. doi: 10.1038/s41419-021-03725-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang PN, Ren QQ, Shi MT, et al Overexpression of mitochondrial ferritin enhances blood-brain barrier integrity following ischemic stroke in mice by maintaining iron homeostasis in endothelial cells. Antioxidants. 2022;11(7):1257. doi: 10.3390/antiox11071257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson BD, Dietrich WD, Busto R, et al Induction of reproducible brain infarction by photochemically initiated thrombosis. Annals of Neurology. 1985;17(5):497–504. doi: 10.1002/ana.410170513. [DOI] [PubMed] [Google Scholar]
- Wu JR, Tuo QZ, Lei P Ferroptosis, a recent defined form of critical cell death in neurological disorders. Journal of Molecular Neuroscience. 2018;66(2):197–206. doi: 10.1007/s12031-018-1155-6. [DOI] [PubMed] [Google Scholar]
- Wu MX, Chen ZY, Jiang M, et al Friend or foe: role of pathological tau in neuronal death. Molecular Psychiatry. 2023;28(6):2215–2227. doi: 10.1038/s41380-023-02024-z. [DOI] [PubMed] [Google Scholar]
- Wu Q, Wu WS, Su L, et al Mitochondrial ferritin is a hypoxia-inducible factor 1α-inducible gene that protects from hypoxia-induced cell death in brain. Antioxidants & Redox Signaling. 2019;30(2):198–212. doi: 10.1089/ars.2017.7063. [DOI] [PubMed] [Google Scholar]
- Xu J, Wang AX, Meng X, et al Edaravone dexborneol versus edaravone alone for the treatment of acute ischemic stroke: a phase III, randomized, double-blind, comparative trial. Stroke. 2021;52(3):772–780. doi: 10.1161/STROKEAHA.120.031197. [DOI] [PubMed] [Google Scholar]
- Xu S, Tuo QZ, Meng J, et al Thrombin induces ferroptosis in triple-negative breast cancer through the cPLA2α/ACSL4 signaling pathway. Translational Oncology. 2024;39:101817. doi: 10.1016/j.tranon.2023.101817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Sano K, Takakura K, et al Ebselen in acute ischemic stroke: a placebo-controlled, double-blind clinical trial. Ebselen Study Group. Stroke. 1998;29(1):12–17. doi: 10.1161/01.STR.29.1.12. [DOI] [PubMed] [Google Scholar]
- Yan HF, Tuo QZ, Lei P Cell density impacts the susceptibility to ferroptosis by modulating IRP1-mediated iron homeostasis. Journal of Neurochemistry. 2024;168(7):1359–1373. doi: 10.1111/jnc.16085. [DOI] [PubMed] [Google Scholar]
- Yan HF, Tuo QZ, Yin QZ, et al The pathological role of ferroptosis in ischemia/reperfusion-related injury. Zoological Research. 2020;41(3):220–230. doi: 10.24272/j.issn.2095-8137.2020.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HF, Zou T, Tuo QZ, et al Ferroptosis: mechanisms and links with diseases. Signal Transduction and Targeted Therapy. 2021;6(1):49. doi: 10.1038/s41392-020-00428-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Liu XL, Song CL, et al Structure-activity relationship studies of phenothiazine derivatives as a new class of ferroptosis inhibitors together with the therapeutic effect in an ischemic stroke model. European Journal of Medicinal Chemistry. 2021;209:112842. doi: 10.1016/j.ejmech.2020.112842. [DOI] [PubMed] [Google Scholar]
- Yang WS, Stockwell BR Ferroptosis: death by lipid peroxidation. Trends in Cell Biology. 2016;26(3):165–176. doi: 10.1016/j.tcb.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye FH, Garton HJL, Hua Y, et al The role of thrombin in brain injury after hemorrhagic and ischemic stroke. Translational Stroke Research. 2021;12(3):496–511. doi: 10.1007/s12975-020-00855-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yigitkanli K, Pekcec A, Karatas H, et al Inhibition of 12/15-lipoxygenase as therapeutic strategy to treat stroke. Annals of Neurology. 2013;73(1):129–135. doi: 10.1002/ana.23734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JH, Seo Y, Jo YS, et al Brain lipidomics: from functional landscape to clinical significance. Science Advances. 2022;8(37):eadc9317. doi: 10.1126/sciadv.adc9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu XL, Long YC Crosstalk between cystine and glutathione is critical for the regulation of amino acid signaling pathways and ferroptosis. Scientific Reports. 2016;6:30033. doi: 10.1038/srep30033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H, Li XM, Zhang XY, et al Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochemical and Biophysical Research Communications. 2016;478(3):1338–1343. doi: 10.1016/j.bbrc.2016.08.124. [DOI] [PubMed] [Google Scholar]
- Zhang J, Barasch N, Li RC, et al Inhibition of cytosolic phospholipase A 2 alpha protects against focal ischemic brain damage in mice . Brain Research. 2012;1471:129–137. doi: 10.1016/j.brainres.2012.06.031. [DOI] [PubMed] [Google Scholar]
- Zhou BR, Liu J, Kang R, et al Ferroptosis is a type of autophagy-dependent cell death. Seminars in Cancer Biology. 2020;66:89–100. doi: 10.1016/j.semcancer.2019.03.002. [DOI] [PubMed] [Google Scholar]