Abstract
The CRISPR-Cas13 system, an RNA-guided editing tool, has emerged as a highly efficient and stable RNA editing technique. Although the CRISPR-Cas13 system has been developed in several insect species, its application in lepidopterans has not yet been reported. In the present study, we evaluated the RNA cleavage activity of the CRISPR-Cas13 system in the silkworm ( Bombyx mori), a model lepidopteran insect, both ex vivo and in vivo. We established two stable silkworm BmE cell lines expressing PspCas13b and CasRx, respectively. Further analysis demonstrated that both PspCas13b and CasRx effectively down-regulated the transcription of exogenously-introduced target and endogenous genes in these cell lines. In addition, we generated two transgenic silkworm strains, one expressing CasRx and the other expressing RNA-guided CRISPR RNA targeting Sex combs reduced ( Scr). Further crossing experiments showed that CasRx induced a down-regulation of Scr transcription in silkworms, which impaired systemic growth of larvae. Overall, this study demonstrated that the CRISPR-Cas13 RNA editing system works efficiently in the silkworm, providing a potential alternative approach for RNA manipulation in lepidopteran insects.
Keywords: Silkworm, CRISPR, PspCas13b, CasRx, RNA editing
INTRODUCTION
Clustered regularly interspaced short palindromic repeats (CRISPR) and their associated proteins (Cas) were first identified as defense machineries in bacteria and archaea, where they function by recognizing and cleaving foreign nucleic acids. This recognition is mediated by specific CRISPR RNAs (crRNAs) that guide different Cas proteins, such as Cas9, Cas12, and Cas13, to target sequences, leading to their cleavage and degradation through endonuclease activity ( Barrangou & Marraffini, 2014; Knott & Doudna, 2018; Makarova et al., 2011a; Murugan et al., 2017; Wang & Doudna, 2023; Wang et al., 2022). Over the past decade, these systems have been rapidly adopted and optimized as highly versatile gene-editing tools, enabling precise manipulation of genes of interest across a wide range of organisms, including animals, plants, and human cells ( Knott & Doudna, 2018; Makarova et al., 2011b; Murugan et al., 2017; Wang & Doudna, 2023; Wang et al., 2022). Notably, the CRISPR-Cas9 gene-editing system, which recognizes and cleaves genomic DNA sequences marked by a protospacer adjacent motif (PAM), has garnered the most attention, with wide application in gene function analysis, molecular breeding, and therapeutic intervention ( Cong et al., 2013; Guilinger et al., 2014; Jinek et al., 2012; Madigan et al., 2023; Port et al., 2014; Wang & Doudna, 2023; Wang & Yang, 2023; Xing et al., 2014).
Unlike the CRISPR-Cas9 system targeting DNA, the programmable RNA-guided CRISPR-Cas13 editing system specifically recognizes and cleaves single-stranded RNA, enabling gene knockdown at the transcriptional level ( Abudayyeh et al., 2016; Cox et al., 2017; Mahas et al., 2019; Smargon et al., 2017). To date, four Cas13 variants have been identified, including Cas13a (formerly C2c2), Cas13b, Cas13c, and Cas13d (CasRx homolog) ( Abudayyeh et al., 2016; Huynh et al., 2020; Konermann et al., 2018; Mahas et al., 2019; Smargon et al., 2017). These enzymes contain two higher eukaryote and prokaryote nucleotide-binding (HEPN) domains, which mediate RNase activity and facilitate the precise cleavage of target transcripts associated with a protospacer flanking site (PFS) motif ( Abudayyeh et al., 2016; Cox et al., 2017; O’Connell, 2019; Shmakov et al., 2015). Generally, Cas13 enzymes are guided to recognize the PFS motif of target transcripts by a single crRNA composed of a spacer sequence that directs the system and an adjacent stem-loop scaffold with two direct repeats ( Abudayyeh et al., 2016; Konermann et al., 2018; Smargon et al., 2017). Among the Cas13 proteins, PspCas13b from Prevotella sp. P5-125, PguCas13b from Porphyromonas gulae, and CasRx from Ruminococcus flavefaciens XPD3002 have been demonstrated to present high RNA cleavage efficiency and specificity ( Cox et al., 2017; Konermann et al., 2018). The Cas13 family has also proven effective in mediating gene knockdown in vivo in various insect species such as Drosophila melanogaster, Aedes aegypti, and Sogatella furcifera ( Dalla Benetta et al., 2023; Huynh et al., 2020; Ma et al., 2023). Undoubtedly, the CRISPR-Cas13 RNA editing system has rapidly emerged as an important complement to established RNA interference (RNAi) approaches.
The domesticated silkworm ( Bombyx mori), a member of the order Lepidoptera, is not only an economically important insect for silk production but also serves as an excellent model organism for studying insect physiology and genetics ( Goldsmith et al., 2005; Ma et al., 2014). In recent years, a variety of genetic manipulation approaches have been developed to study gene functions, improve economically important traits, and produce heterologous recombinant proteins in the silkworm ( Baci et al., 2021; Chen et al., 2023a, 2023b; Jiang et al., 2021; Ma et al., 2014, 2019). These methods include the transgenic GAL4/UAS system ( Chen et al., 2023b; Imamura et al., 2003; Ma et al., 2011), zinc finger nucleases (ZFNs) ( Takasu et al., 2010), transcription activator-like effector nucleases (TALENs) ( Ma et al., 2012; Peng et al., 2018; Xu et al., 2018), CRISPR-Cas9 system ( Chen et al., 2023b; Daimon et al., 2014; Ma et al., 2014; Peng et al., 2018), and traditional double-stranded RNA (dsRNA)-mediated RNAi ( Isobe et al., 2004; Quan et al., 2002; Uhlirova et al., 2003). However, RNAi in Lepidoptera, particularly during the larval stages, often suffers from low efficiency and specificity due to poorly understood mechanisms ( Daimon et al., 2014; Kolliopoulou & Swevers, 2014; Subbaiah et al., 2013; Terenius et al., 2011). Furthermore, transgenic CRISPR-Cas9-mediated gene knockout frequently results in chimerism ( Qian et al., 2023a, 2023b), largely limiting their application in the genetic engineering in lepidopteran insects, including the silkworm. Although the CRISPR-Cas13 system has been successfully utilized in several insect species ( Dalla Benetta et al., 2023; Huynh et al., 2020; Ma et al., 2023), its use in lepidopteran insects has yet to be reported.
In the present study, we aimed to establish a more stable and robust RNA-editing tool in the silkworm using the CRISPR-Cas13 system. Employing the widely-used piggyBac transposon vector ( Handler, 2002; Tamura et al., 2000; Yusa, 2015), we engineered CRISPR-Cas13 constructs to assess the RNA cleavage activity of PspCas13b and CasRx in silkworm embryo-derived BmE cells and larvae. Both ex vivo and in vivo experiments showed that the CRISPR-Cas13 system effectively mediated specific and robust knockdown of target transcripts in the silkworm, presenting a compelling alternative to existing RNA manipulation approaches.
MATERIALS AND METHODS
Animals and cell lines
The non-diapaused silkworm strain D9L was utilized in this study. Silkworms were reared on fresh mulberry leaves at 25°C in an incubator under a 12 h light/12 h dark cycle. The embryo-derived BmE cell line was established in our laboratory ( Pan et al., 2007), and was maintained in Grace’s Insect Medium (Gibco, USA) containing 10% fetal bovine serum (Gibco, USA), streptomycin, and penicillin at 27℃.
Prediction of target sites and design of specific crRNAs
According to the working principles of the CRISPR-Cas13 editing system ( Cox et al., 2017; Smargon et al., 2017), we predicted Cas13 target sites within the mRNA sequences of four selected genes, including enhanced green fluorescent protein ( EGFP), Fizzy-related ( Fzr), which encodes a WD40 repeat protein, Myc oncogene, which encodes a basic helix-loop-helix transcription factor, and Sex combs reduced ( Scr), which belongs to the Hox family. Specific crRNAs were then designed for each gene (Supplementary Figure S1). We have previously shown that dysregulation of Fzr and Myc in the silkworm silk gland affects DNA replication in gland cells ( Qian et al., 2021, 2023b). Given that the secondary structure of target mRNA must be considered when predicting target sites ( Huynh et al., 2020; Wessels et al., 2020), we evaluated the secondary structures using two web tools: RNA-fold (http://rna.tbi.univie.ac.at/cgi-bin/RNAWebSuite/RNAfold.cgi) and RNA structure (https://rna.urmc.rochester.edu/RNAstructureWeb/Servers/Predict1/Predict1.html). Accessible regions of the target transcripts were predicted, as described previously ( Gruber et al., 2008; Reuter & Mathews, 2010). Finally, high-ranking crRNAs for guiding Cas13 variants to recognize specific target sites were designed and selected using the CRISPR-RT tool ( Huynh et al., 2020; Wang et al., 2019; Zhu et al., 2018). The target site sequences for the selected genes are provided in Supplementary Figure S1.
Construction of plasmids for expressing Cas13 variants and crRNAs
Original plasmids containing PspCas13b, CasRx, and crRNA backbone were obtained from Addgene: pC0046-EF1a-PspCas13b-NES-HIV (#103862), pC0043-PspCas13b crRNA backbone (#103854), pXR001: EF1-CasRx-2A-EGFP (#109049), and pXR003: CasRx gRNA cloning backbone (#109053) ( Cox et al., 2017; Konermann et al., 2018). For the expression of Cas13 variants in silkworm BmE cells and living individuals, we used the transgenic piggyBac plasmid pBac[3×P3-EGFP], which directs EGFP expression specifically in insect eyes via the 3×P3 promoter. As described previously ( Qian et al., 2023b), several DNA fragments, including the ubiquitous baculovirus immediate early 1 ( IE1) promoter, Cas13 variants, nuclear localization signal (NLS), HA tag, EGFP, self-cleaving 2A peptide (2A), puromycin, and SV40 terminator, were selectively subcloned into the Asc1 site of the pBac[3×P3-EGFP] plasmid using pEASY ®-Basic Seamless Cloning and Assembly Kit (TransGen, China), generating a plasmid of approximately 9 000 bp in length. For the expression of specific crRNAs in silkworm BmE cells and living individuals, we used the transgenic plasmid pBac[3×P3-DsRed] ( Qian et al., 2023b), which drives the expression of red fluorescent protein DsRed in the eyes under the control of the 3×P3 promoter. Two crRNA backbone plasmids were constructed, containing crRNA scaffolds for PspCas13b and CasRx upstream and downstream of a customized fragment with two AarI restriction enzyme sites, respectively. The crRNA scaffolds, linked to the AarI recognition sequence, were synthesized. The synthesized spacers without or with different mutations were separately inserted into the AarI recognition site, forming different plasmids expressing full wild-type (WT) or mutated crRNAs. The related primers are listed in Supplementary Table S1.
Cell culture, plasmid transfection, and stable cell line establishment
To establish cell lines stably expressing Cas13 variants, normally cultured silkworm BmE cells were seeded into 25 cm 2 flasks approximately 24 h before transfection. When the cells reached 70%–80% confluence, two piggyBac plasmids overexpressing EGFP-fused PspCas13b or CasRx were separately co-transfected with a transgenic helper plasmid. This helper plasmid expresses a transposase under the control of the ubiquitous silkworm actin A3 promoter within the piggyBac vector and can facilitate the insertion of exogenous genes into the genome. The transfection was performed at a ratio of 1:1 (5 μg/5 μg) using X-tremeGENE HP DNA Transfection Reagent (Roche, Switzerland). One week post-transfection, the BmE cells were subjected to puromycin resistance selection using the puromycin selection reagent (200 μg/ml, BBI Life Sciences, China) for two months. Cells that survived the selection process were assumed to have stably integrated the Cas13 overexpression plasmids into their genome. To confirm successful establishment of stable cell lines, we performed flow cytometry using a MoFlo XDP flow cytometer (Beckman Coulter, USA) and microscopy analysis (Thermo Scientific, USA) to detect the number of cells expressing EGFP. Additionally, western blotting analysis was conducted to detect the expression of HA-tagged Cas13 proteins. Light and fluorescence microscopy images of the BmE cells were captured using an EVOS FL Auto microscope (Thermo Scientific, USA).
RNA cleavage activity of the CRISPR-Cas13 system in silkworm BmE cells
To evaluate the RNA cleavage activity and specificity of PspCas13b and CasRx in silkworm BmE cells, two BmE cell lines stably expressing Cas13 variants were separately seeded into 24-well plates approximately 24 h before transfection. Once the cells reached 70%–80% confluence, they were transfected with 1 μg of different crRNAs, including WT crRNAs and mutated crRNAs with mismatches, using X-tremeGENE HP DNA Transfection Reagent (Roche, Switzerland). Four days post-transfection, the cells were divided into three groups; two groups were used to detect changes in EGFP protein expression via microscopy analysis of green fluorescence (Thermo Scientific, USA) and western blotting, while the third group was analyzed for changes in EGFP transcription using reverse transcription-quantitative real-time polymerase chain reaction (RT-qPCR).
RNA sequencing (RNA-seq)
RNA-seq was conducted to investigate potential off-target effects of the CRISPR-CasRx system, following previously described protocols ( Peng et al., 2018). In brief, EGFP-crRNA was transiently transfected into BmE cells stably expressing CasRx, CasRx stably-expressed BmE cells without EGFP-crRNA transfection was used as the control. Four days post-transfection, total RNA was extracted from both the control and EGFP-crRNA-transfected cells, with three biological replicates for each condition. The RNA samples were then sequenced using the HiSeq 2500 platform (Novogene, China). Gene expression levels were quantified using FPKM (fragments per kilobase of transcript per million mapped reads). Differential expression analysis was performed using the DESeq2 package, with statistical significance determined with an adjusted P-value<0.05 and fold-change>1.2.
Embryonic injection and generation of transgenic silkworms
To generate transgenic silkworms, 2 μL of piggyBac helper plasmid (600 ng/μL) was mixed with 2 μL of piggyBac plasmids expressing either CasRx or Scr crRNA (600 ng/μL). These mixtures were then separately microinjected into 200 non-diapaused fertilized eggs within 2 h post-oviposition using a microinjector system consisting of a TransferMan NK2 micromanipulator (Eppendorf, Germany), Femto Jet 5247 microinjector (Eppendorf, Germany), and SZX16 microscope (Olympus, Japan). The microinjected eggs were cultured at 25°C with 95%–100% humidity until hatching. Offspring expressing selectable marker genes in the eyes were screened using a fluorescence microscope (Leica, Germany). EGFP and DsRed were used as markers for positive individuals expressing CasRx and Scr crRNA, respectively. Subsequently, the positive strain expressing CasRx was crossed with the positive strain expressing Scr crRNA, and the offspring co-expressing both EGFP and DsRed were selected to analyze knockdown effects of Scr. Changes in the size of the silk gland, fat body cells, and wing disc (developing into adult forewing) in male larvae following Scr knockdown were measured.
RT-qPCR examination
Silkworm BmE cells, 30 embryos on the sixth day post-oviposition, and four tissues from silkworm male larvae on the third day of the fifth larval instar (L5D3), including the silk gland from three larvae as well as the fat body, brain, and wing disc (developing into adult forewing) from six larvae, were separately collected. Total RNA (2.0 μg) was extracted from these samples using Trizol reagent (Invitrogen, USA) and subsequently treated with DNase I to eliminate any genomic DNA contamination, as described previously ( Peng et al., 2018). The cDNA was synthesized using a PrimeScript RT Master Mix Perfect Real Time Kit (Takara, Japan). RT-qPCR analysis of mRNA expression was conducted according to the instructions of the SYBR1 Premix Ex Taq TM II Kit (Takara, Japan) and following previously described protocols ( Bustin et al., 2009). Relative mRNA expression levels were calculated using the 2 -ΔΔCT method ( Livak & Schmittgen, 2001). The ribosomal protein L3 ( RpL3) gene and Actin gene were used as internal controls. Each experiment was independently repeated three times. Primer pairs for RT-qPCR analysis are listed in Supplementary Table S1, and their efficiencies were validated as described previously ( Bustin et al., 2009).
Western blotting
Total proteins were isolated from the BmE cells and silkworm embryos on the sixth day post-oviposition, then quantified using the Bradford assay (Sigma, USA), with absorbance determined at 562 nm using a microplate reader (BioTek, USA). Equal amounts of total protein were subjected to western blotting. The primary antibodies used included rabbit anti-HA (1:1 000, Cell Signaling Technology, USA), mouse anti-Tubulin (1:10 000, Beyotime, China), and rabbit anti-EGFP (1:1 000, Zoonbio Biotechnology, China). The secondary antibodies included horseradish peroxidase (HRP)-conjugated goat anti-rabbit (1:10 000; Beyotime, China) and goat anti-mouse (1:10 000; Beyotime, China).
CCK-8 assay
The effects of CasRx on cell proliferation in BmE cells were analyzed using the CCK-8 assay with the Cell Counting Kit-8 (Beyotime, China), as described previously ( Wang et al., 2020). Briefly, on the first, third, and fifth days after seeding the BmE cells without (WT) and with CasRx expression in 96-well plates, the CCK-8 solution (10 μL per well) was added to each well. After incubating the plates for 3 h at 37°C, equal amounts of the supernatants from each well were transferred into fresh wells for absorbance measurement at 450 nm using a microplate reader (BioTek, USA). Three independent biological replicates were conducted.
Viability/cytotoxicity assay
The effects of CasRx on BmE cell viability/cytotoxicity were analyzed using a LIVE/DEAD® viability/cytotoxicity kit (Invitrogen, USA), as described previously ( Dupont et al., 2010; Wang et al., 2020). Briefly, on the third day after seeding the BmE cells without (WT) and with CasRx expression in 96-well plates, the cells were stained with Calcein-AM dye (495 nm; indicating live cells) and EthD-1 dye (528 nm; indicating dead cells), respectively. Fluorescence signals were then captured using an inverted fluorescent microscope (Olympus, Japan).
Statistical analysis
Data are presented as the mean ± standard error (SE) from three independent biological replicates. Statistical differences between two groups were determined using a two-tailed Student’s t-test ( *: P<0.05; **: P<0.01; ***: P<0.001) or, for multiple comparisons, using one-way analysis of variance (ANOVA), followed by the Tukey test (threshold set to P<0.05), in SPSS v.22.
RESULTS
Design of CRISPR-Cas13 constructs for ex vivo and in vivo analyses in the silkworm
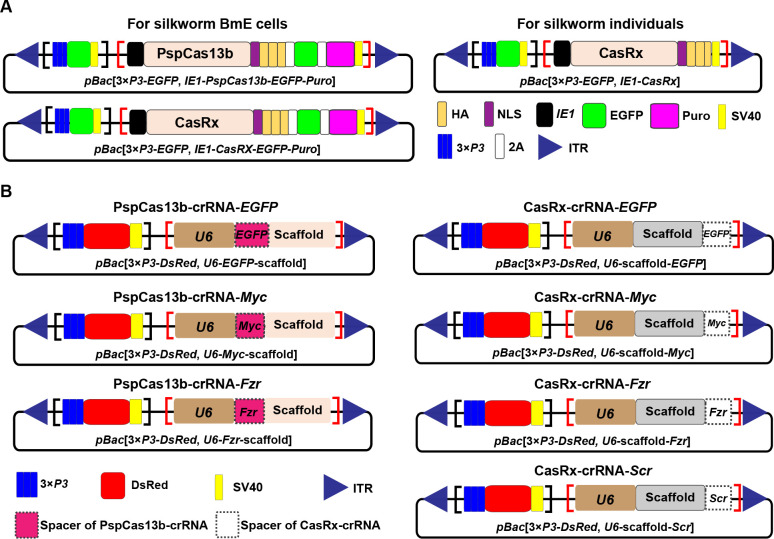
Based on previous studies examining the RNA cleavage activity and knockdown efficiency of different Cas13 enzymes in mammalian cells and Drosophila individuals ( Cox et al., 2017; Huynh et al., 2020; Konermann et al., 2018), we analyzed the potential application of two Cas13 enzymes, PspCas13b and CasRx, in the silkworm. The piggyBac vector, which presents high transposition rates and is widely used in genetic manipulation in the silkworm and other lepidopteran insects ( Handler, 2002; Tamura et al., 2000; Yusa, 2015), was used to construct plasmids expressing Cas13 proteins or specific crRNAs in silkworm embryo-derived BmE cells and living individuals. A previously established pBac[3×P3-EGFP] plasmid, which drives the expression of EGFP in the eyes under the control of the 3×P3 promoter, was used as a basic scaffold plasmid. For the expression of PspCas13b and CasRx proteins in BmE cells, pBac[3×P3-EGFP, IE1-PspCas13b-EGFP-Puro] and pBac[3×P3-EGFP, IE1-CasRx -EGFP-Puro] plasmid s were constructed ( Figure 1A). Within these plasmids, the open reading frames (ORFs) of PspCas13b and CasRx were separately fused with three repeats of HA tag (3×HA) for detection, NLS for subcellular localization, EGFP protein for stable cell line establishment and targeting analysis, and puromycin resistance expression cassette for survival selection of stable cell lines ( Figure 1A). In addition, a pBac[3×P3-EGFP, IE1-CasRx] plasmid was constructed to express the CasRx protein in silkworm individuals, in which the CasRx ORF was fused to the 3×HA tag and NLS ( Figure 1A). EGFP-fused Cas13 proteins in the above constructs were driven by a ubiquitous IE1 promoter ( Figure 1A).
Figure 1.
Design of CRISPR-Cas13 constructs for RNA manipulation in the silkworm
A: Schematic of piggyBac-based plasmids for ectopic expression of PspCas13b and CasRx in silkworm BmE cells and living individuals. B: Schematic of piggyBac-based plasmids expressing crRNAs for targeting Cas13 variants to different genes. pBac, piggyBac; ITR, inverted terminal repeats of piggyBac; EGFP, enhanced green fluorescent protein; Puro, puromycin resistance selection marker; 3×P3, eye-specific artificial promoter; IE1, ubiquitous baculovirus immediate early 1 gene promoter; U6, polymerase III U6 promoter; SV40, SV40 poly(A) terminator; 2A, 2A peptide; NLS, nuclear localization signal; HA, HA tag.
Furthermore, following the same principle, we used the previously established pBac[3×P3- DsRed] plasmid, which drives DsRed expression in the eyes under the control of the 3×P3 promoter, to construct plasmids for expressing high-ranking specific crRNAs that guide different Cas13 proteins to target different genes. As a result, we constructed several plasmids, including those expressing WT or mutated crRNAs for guiding PspCas13b or CasRx to target the exogenously-introduced EGFP gene or endogenous Myc and Fzr genes in BmE cells, as well as those expressing a crRNA for guiding CasRx to target an endogenous homeobox gene Scr in silkworm individuals ( Figure 1B; Supplementary Figure S1). The expression of all crRNAs was driven by the ubiquitous polymerase III U6 promoter ( Figure 1B).
Establishment of silkworm BmE cell lines stably expressing Cas13 variants
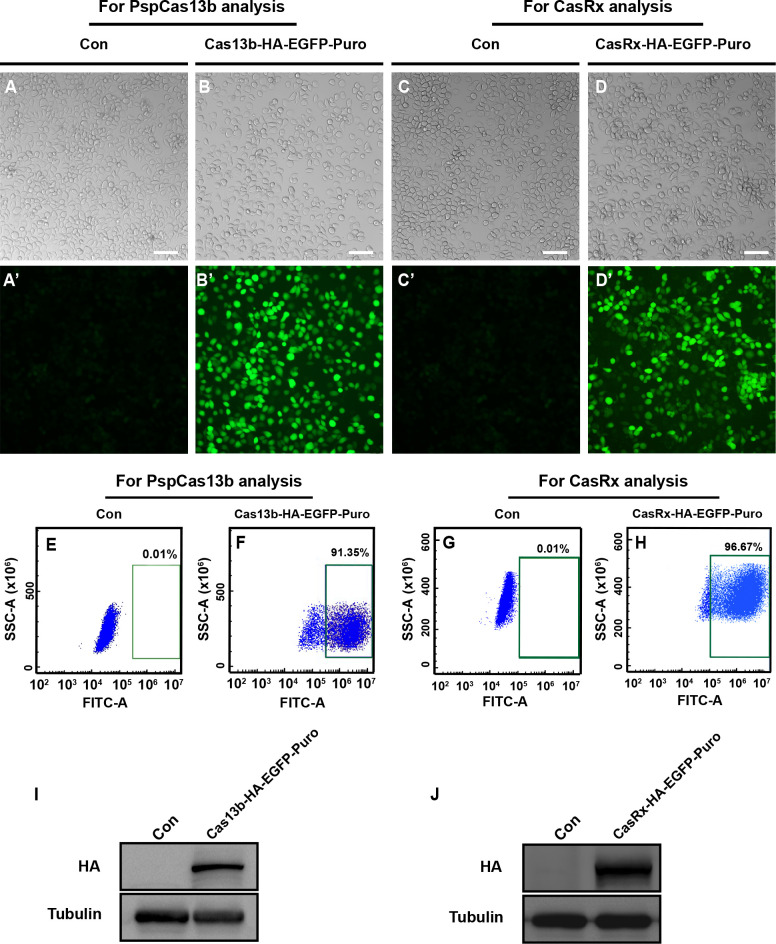
To evaluate the RNA cleavage activity of PspCas13b and CasRx in silkworm BmE cells, we first established stable BmE cell lines expressing each Cas13 protein. Following the proposed procedures (Supplementary Figure S2A), plasmids expressing EGFP-fused PspCas13b or CasRx were separately co-transfected into BmE cells with the plasmid expressing piggyBac transposase under the control of the A3 promoter (A3-Helper). These cells were cultured for one week, then subjected to puromycin selection for two months. The results indicated that compared to control BmE cells transfected with the basic pBac[3×P3-EGFP] plasmid, the surviving BmE cells transfected the plasmids expressing EGFP-fused Cas13 proteins after puromycin resistance selection exhibited clear green fluorescence ( Figure 2A–D’). Flow cytometry further confirmed that over 90% of the BmE cells successfully expressed EGFP-fused Cas13 proteins ( Figure 2E–H). Furthermore, western blotting analysis using an anti-HA antibody demonstrated that PspCas13b and CasRx were expressed in the transfected BmE cells ( Figure 2I, J). While the CCK-8 assay revealed that CasRx caused moderate inhibition of BmE cell proliferation (Supplementary Figure S3A), Live/Dead staining showed no significant impact on cell viability (Supplementary Figure S3B). Collectively, these results confirmed the successful establishment of two BmE cell lines stably expressing PspCas13b and CasRx, respectively.
Figure 2.
Establishment of stable silkworm BmE cell lines expressing Cas13 variants
A–D’: Microscopy analysis of BmE cells expressing EGFP-fused Cas13 proteins. White light microscopy (A–D) and fluorescence microscopy (A’–D’) were conducted. Scale bar: 50 μm. E–H: Flow cytometry analysis of BmE cells expressing EGFP-fused Cas13 proteins. Control, original BmE cell without transfection. I, J: Western blotting validation of expressions of HA-tagged Cas13 proteins in BmE cells. Anti-HA and anti-Tubulin antibodies were used. EGFP, enhanced green fluorescent protein; Puro, puromycin selection marker gene; HA, HA tag.
RNA cleavage activity of Cas13 variants in silkworm BmE cells
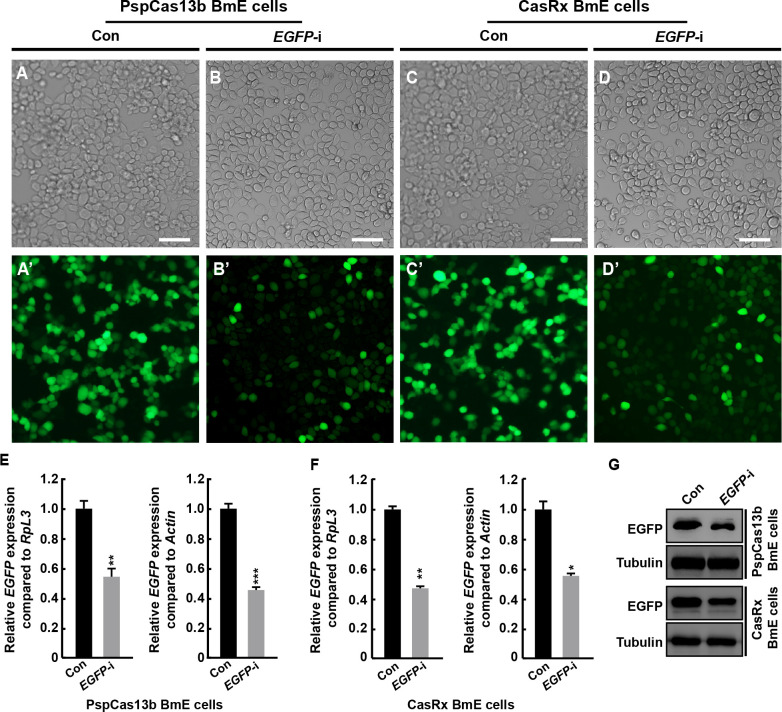
Next, we selected the exogenously-introduced EGFP gene as a target to examine the RNA cleavage activity of PspCas13b and CasRx in silkworm BmE cells. Two constructs expressing different crRNAs designed to guide Cas13 variants to target EGFP were transiently transfected into cultured BmE cells stably expressing Cas13 variants (Supplementary Figure S2A). Two additional constructs expressing crRNAs targeting the DsRed gene were used as controls. On the fourth day post-transfection, the fluorescence signal in BmE cells expressing EGFP-fused Cas13 variants was markedly decreased following transfection of the plasmid carrying specific crRNAs compared to the control ( Figure 3A–D’). In addition, RT-qPCR analysis showed a significant reduction in EGFP transcript levels following crRNA expression compared with the control ( Figure 3E, F). Western blotting analysis also confirmed a corresponding decrease in EGFP protein levels following crRNA expression ( Figure 3G). Notably, RNA-seq analysis revealed that only one endogenous gene was down-regulated following EGFP knockdown compared to the control (Supplementary Figure S4; EGFP was not annotated as it is not included in the silkworm reference genome). Collectively, these results indicate that the CRISPR-CasRx system exhibits very high target specificity in silkworm BmE cells.
Figure 3.
Target RNA cleavage of Cas13 variants in silkworm BmE cells
A–D’: Microscopy analysis of changes in green fluorescence in EGFP-Cas13-expressed BmE cells following transfection of crRNAs targeting EGFP. White light microscopy (A–D) and fluorescence microscopy (A’–D’) were conducted. Scale bar: 50 μm. E, F: RT-qPCR analysis of EGFP transcripts in EGFP-Cas13-expressed BmE cells following transfection of EGFP-targeting crRNAs. RpL3 and Actin genes were used as an internal control. Significant differences were determined using Student’s t-test. *: P<0.05; **: P<0.01; ***: P<0.001 versus control. G, H: Western blotting validation of EGFP protein expression changes in EGFP-Cas13-expressed BmE cells following transfection with EGFP-targeting crRNAs. Anti-EGFP and anti-Tubulin antibodies were used. Control, transfection of crRNA targeting DsRed transcript; EGFP-i, RNA cleavage following transfection of EGFP-targeting crRNA.
In addition, we examined the RNA cleavage activity of PspCas13b and CasRx on two endogenous genes, Myc and Fzr. The results demonstrated a significant reduction in the mRNA levels of both genes following crRNA expression (Supplementary Figure S5A–D). Taken together, these findings confirm the successful establishment of two BmE cell lines stably expressing PspCas13b and CasRx.
RNA cleavage fidelity of Cas13 variants in silkworm BmE cells
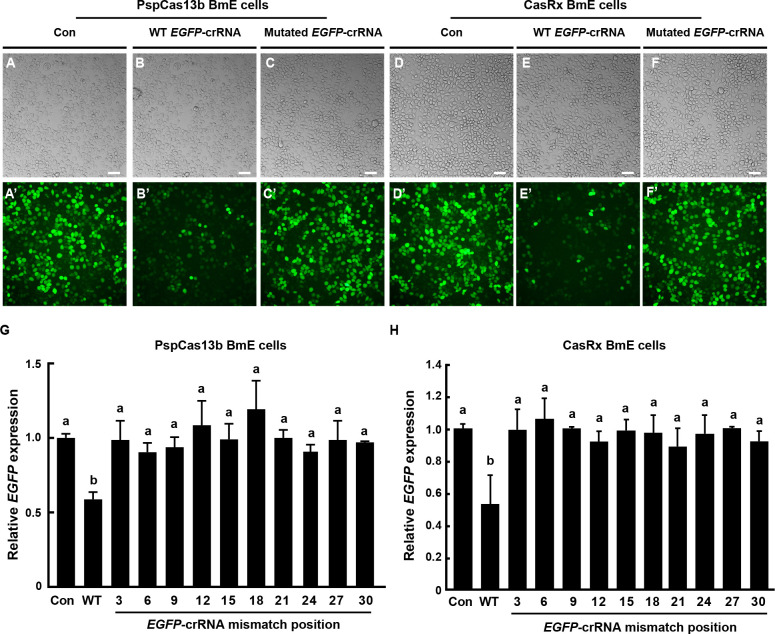
The RNA cleavage activity of Cas13 proteins can be disrupted by a single mismatch in the core region of the spacer or two mismatches in the crRNA ( Huynh et al., 2020). Therefore, to assess the fidelity of PspCas13b and CasRx in the silkworm BmE cells, we analyzed the impact of mismatches between EGFP-crRNAs and their target EGFP RNAs on RNA cleavage. As described previously ( Cox et al., 2017; Huynh et al., 2020; Molina Vargas et al., 2024; Smargon et al., 2017), we generated a series of mutated EGFP-crRNAs, each containing a single mismatch of three consecutive nucleotides within the crRNA spacer. These mismatches were denoted numerically, with “3” indicating a mutation in nucleotides #1–3, closest to the scaffold, “6” denoting nucleotides #4–6, and so on ( Figures 1, 4). Subsequent analysis in BmE cells overexpressing EGFP and different Cas13 variants demonstrated that a single mismatch in nucleotides #1–3 within the EGFP-crRNA spacer did not down-regulate EGFP expression compared to the WT EGFP-crRNA ( Figure 4A–F’). RT-qPCR analyses further revealed that all mismatches at different positions within the WT EGFP-crRNA spacer abolished the cleavage activity of Cas13 variants toward EGFP RNA ( Figure 4G, H). These results confirmed the high fidelity of Cas13 variants, which maintain robust on-target RNA cleavage activity in silkworm BmE cells.
Figure 4.
RNA cleavage fidelity of Cas13 variants in silkworm BmE cells
A–F’: Microscopy analysis showing effect of EGFP-targeting crRNA mutations with a single mismatch in nucleotides #1–3 toward the 3’ end of the spacer on EGFP protein expression in BmE cells expressing EGFP and Cas13 variants. White light microscopy (A–F) and fluorescence microscopy (A’–F’) were conducted. Scale bar: 50 μm. G, H: RT-qPCR analysis showing effect of EGFP-targeting crRNA mutations with different mismatches in the spacer on EGFP transcript levels in BmE cells expressing EGFP and Cas13 variants. Values are mean±SE (error bars) from three independent biological replicates. Different letters indicate significant differences at P<0.05, as determined by one-way ANOVA followed by Tukey test. Each number represents a single mismatch with three consecutive nucleotides toward the 3’ end of the crRNA spacer, namely “3” for mutation of nucleotides #1–3, “6” for nucleotides #4–6, and so on. Control, transfection of crRNA targeting DsRed transcript; WT, wild-type.
Characterization of Cas13-mediated RNA cleavage in silkworm individuals
We next investigated the RNA cleavage activity of the Cas13 variant CasRx in silkworm individuals in vivo (Supplementary Figure S2B). Through embryonic microinjection of the above constructed plasmids and screening for eye-specific fluorescent protein expression, we generated two transgenic silkworm strains: one ubiquitously expressing CasRx and the other expressing a specific crRNA targeting the homeobox gene Scr ( Figure 1; Supplementary Figures S2B, S6A–I). Western blotting analysis of total proteins extracted from silkworm embryos on the sixth day post-oviposition confirmed the expression of CasRx in the transgenic strain (Supplementary Figure S6M). The transgenic CasRx strain was crossed with the transgenic crRNA -Scr strain, inducing interference of Scr transcription ( Scr-i; Supplementary Figure S6J–L).
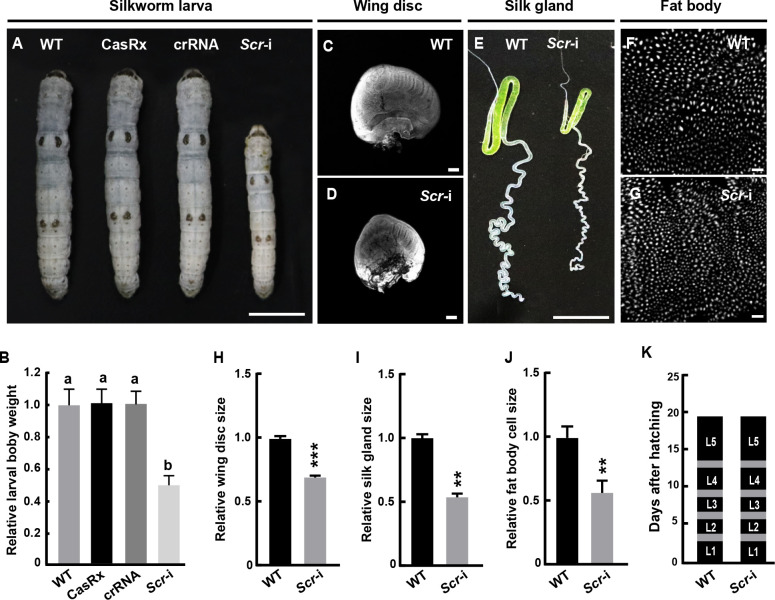
Notably, compared to the three controls, including the WT, CasRx line, and crRNA-Scr line, silkworm larvae with CRISPR-CasRx-mediated Scr transcript editing ( Scr-i) exhibited a marked reduction in overall body size and weight ( Figure 5A, B). In addition, the wing disc, silk gland, and fat body cells were significantly reduced in Scr-i larvae compared to WT larvae ( Figure 5C–J). However, there was no significant change in the duration of larval development ( Figure 5K). Importantly, RT-qPCR analysis confirmed a significant decrease in Scr transcription in the whole embryos of Scr-i larvae compared to WT (Supplementary Figure S7A). Further analysis revealed that Scr-i led to a significant decrease in Scr transcription in several larval tissues, including the silk gland, fat body, brain, and wing disc (Supplementary Figure S7B–E). Collectively, these data indicate that the CRISPR-Cas13 system functions efficiently in vivo in the silkworm and that Scr regulates systemic growth in silkworm larvae.
Figure 5.
CRISPR-CasRx-mediated Scr knockdown in silkworm larvae
A, B: CRISPR-CasRx-mediated editing of the Scr RNA resulted in a decrease in the size and weight of larval body. Scale bar: 1 cm. Values are mean±SE (error bars) from three independent biological replicates. Different letters indicate significant differences at P<0.05, determined by one-way ANOVA followed by Tukey test. C–J: Scr RNA editing decreased the size of larval wing disc (C, D, H), silk gland (E, I), fat body cells (F, G, J). Scale bar: 1 cm for silk gland; 50 μm for fat body cells; 200 μm for wing disc. K: Scr RNA editing had no effect on the duration of larval development. Gray areas denote larval molting. L, larval instars; L1 to L5 represent first to fifth larval instars. Values are mean±SE (error bars) from three independent biological replicates. Significant differences were determined using Student’s t-test, **: P<0.01 and ***: P<0.001 versus WT as the control. WT, wild-type.
DISCUSSION
The RNA-guided RNA-targeting CRISPR-Cas13 system has recently been developed and employed for RNA editing in mammalian cells, plants, and several insects, such as Drosophila ( Huynh et al., 2020; Konermann et al., 2018; Mahas et al., 2019). To date, four Cas13 variants, including Cas13a, Cas13b, Cas13c, and CasRx/Cas13d, have been characterized as single-effector RNases capable of precisely recognizing and cleaving target RNA ( Abudayyeh et al., 2016; Huynh et al., 2020; Konermann et al., 2018; Mahas et al., 2019; Smargon et al., 2017). In the present study, we focused on two highly efficient Cas13 variants, PspCas13b and CasRx, to establish CRISPR-Cas13 tools and assess their RNA cleavage activity in the silkworm, both ex vivo and in vivo. This work broadens the applicability of RNA manipulation in lepidopteran insects such as the silkworm and opens new possibilities for utilizing the CRISPR-Cas13 system in the development of novel biological control strategies for managing lepidopteran pests.
RNAi was first established as an RNA-editing technique in Caenorhabditis elegans to silence endogenous mRNA via the mediation of exogenous dsRNA. Since its inception, RNAi has been applied in multiple organisms, including insects ( Bellés, 2010; Fire et al., 1998; Meister & Tuschl, 2004; Perrimon et al., 2010; Terenius et al., 2011). In the silkworm, in vivo RNAi has been utilized to analyze gene function and enhance antiviral capacity ( Dai et al., 2008; Isobe et al., 2004; Jiang et al., 2013, 2021; Kanginakudru et al., 2007; Masumoto et al., 2009; Wu et al., 2016), by direct injection of in vitro-synthesized dsRNA into embryos or larvae ( Dai et al., 2008; Deng et al., 2012; Meng et al., 2015a; Nakao, 2012; Wu et al., 2016) or through transgenic expression of RNA hairpins ( Isobe et al., 2004; Jiang et al., 2013; Kanginakudru et al., 2007; Subbaiah et al., 2013). Despite its widespread use, RNAi-mediated knockdown often suffers from low efficiency, likely due to a lack of robust RNAi machinery in some species. This inefficiency has been observed for certain genes, such as the bilin-binding protein gene and pheromone-binding protein gene, or in specific tissues, such as larval epidermis and pupal wings ( Kobayashi et al., 2012; Terenius et al., 2011). Our study confirmed high and stable knockdown efficiency of the CRISPR-Cas13 RNA-editing system in the silkworm, both ex vivo and in vivo. These results, combined with previous observations regarding high targeting specificity of CRISPR-Cas13 in Drosophila, mammalian cells, and plants ( Huynh et al., 2020; Mahas et al., 2019; Smargon et al., 2017), highlight this RNA-editing system as a promising alternative for RNA engineering in the silkworm and potentially other lepidopteran insects.
In addition, we found that CRISPR-Cas13-mediated knockdown of the homeobox gene Scr significantly impeded the systemic growth of silkworm larvae. Scr encodes a transcription factor that regulates target gene transcription by binding directly to the DNA-binding site through its homeodomain ( Gehring et al., 1994). This gene is predominantly expressed in the head and thoracic regions during embryogenesis and plays key roles in body segmentation and organ development in insects ( Angelini & Kaufman, 2005; Chai et al., 2008; Heffer & Pick, 2013; Hughes & Kaufman, 2002; Mulhair & Holland, 2024; Riley et al., 1987). In Drosophila, for example, Scr is essential for the formation of posterior head and anterior thorax structures and contributes to the development of the central and peripheral nervous systems during the embryonic stage ( Lemotte et al., 1989; Percival-Smith et al., 1997; Struhl, 1982) . Mutations in Scr disrupt the development of the sex comb, larval salivary gland, and specific organs in the head and pre-thoracic segments of adults ( Calvo-Martín et al., 2017; Devi & Shyamala, 2013; Panzer et al., 1992). In the silkworm, Scr is involved in the development of the embryonic silk gland ( Kokubo et al., 1997), and modulates larval molting by negatively regulating ecdysone biosynthesis ( Daimon et al., 2021). In our previous study, we revealed that Scr positively regulates the transcription of enzymes involved in juvenile hormone biosynthesis and TALEN-based Scr knockout results in embryonic lethality in the silkworm ( Meng et al., 2015b). Here, we generated two transgenic silkworm strains, one expressing CasRx and the other expressing crRNA-Scr. We found that CRISPR-CasRx-mediated Scr knockdown decreased body and organ size in larvae, suggesting that Scr regulates silkworm systemic growth. However, being different from previous reports ( Daimon et al., 2021; Meng et al., 2015b), Scr knockdown did not affect silkworm embryonic viability or larval molting. We speculate that this may be due to incomplete inhibition of Scr transcription by the CRISPR-CasRx system (Supplementary Figure S7). Our findings, together with evidence that ecdysone can restrict systemic growth in Drosophila ( Delanoue et al., 2010), suggest that CRISPR-CasRx-mediated Scr knockdown may moderately increase ecdysone production, thereby indirectly impairing organ growth or directly altering organ-specific development in larvae. In this study, Scr transcript editing was achieved using a single high-ranking crRNA target site located outside the homeodomain. In future work, we will generate additional crRNA strains targeting lower-ranking sites within Scr and tissue-specific CasRx strains to further validate the CRISPR-Cas13 system and decipher the role of Scr in regulating systemic growth in the silkworm. In summary, this study provides transgenic RNA-editing lines and new clues for comprehensively characterizing the function of Scr in silkworm growth and development.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
Acknowledgments
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
Y.H.T., X.Z., Z.C.D., H.L., Y.Y., T.J.Z., D.Q.Y., and W.L.Q. performed the experiments and analyzed the data. D.J.C. and Y.H.T. designed the experiments. D.J.C. conceived and supervised the study. Y.H.T. and X.Z. wrote the manuscript. D.J.C. and W.L.Q edited the manuscript. All authors read and approved the final version of the manuscript.
Funding Statement
This work was supported by the National Natural Science Foundation of China (32070496, 32370555), Fundamental Research Funds for the Central Universities (SWU120033), and Technology Innovation and Application Development Program of Chongqing (CSTB2024TIAD-KPX0023)
Contributor Information
Wen-Liang Qian, Email: qianwl@swu.edu.cn.
Dao-Jun Cheng, Email: chengdj@swu.edu.cn.
DATA AVAILABILITY
All raw data have been deposited in the Sequence Read Archive of the National Center for Biotechnology Information (NCBI) database under accession number PRJNA1132555, Genome Sequence Archive (GSA) database under accession number CRA018506, and Science Data Bank (doi: 10.57760/sciencedb.j00139.00078).
References
- Abudayyeh OO, Gootenberg JS, Konermann S, et al C2c2 is a single-component programmable RNA-guided RNA-targeting CRISPR effector. Science. 2016;353(6299):aaf5573. doi: 10.1126/science.aaf5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelini DR, Kaufman TC Comparative developmental genetics and the evolution of arthropod body plans. Annual Review of Genetics. 2005;39:95–119. doi: 10.1146/annurev.genet.39.073003.112310. [DOI] [PubMed] [Google Scholar]
- Baci GM, Cucu AA, Giurgiu AI, et al Advances in editing silkworms ( Bombyx mori) genome by using the CRISPR-Cas system . Insects. 2021;13(1):28. doi: 10.3390/insects13010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrangou R, Marraffini LA CRISPR-Cas systems: prokaryotes upgrade to adaptive immunity. Molecular Cell. 2014;54(2):234–244. doi: 10.1016/j.molcel.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellés X Beyond Drosophila: RNAi in vivo and functional genomics in insects . Annual Review of Entomology. 2010;55:111–128. doi: 10.1146/annurev-ento-112408-085301. [DOI] [PubMed] [Google Scholar]
- Bustin SA, Benes V, Garson JA, et al The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clinical Chemistry. 2009;55(4):611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- Calvo-Martín JM, Papaceit M, Segarra C Sex combs reduced ( Scr) regulatory region of Drosophila revisited . Molecular Genetics and Genomics. 2017;292(4):773–787. doi: 10.1007/s00438-017-1309-1. [DOI] [PubMed] [Google Scholar]
- Chai CL, Zhang Z, Huang FF, et al A genomewide survey of homeobox genes and identification of novel structure of the Hox cluster in the silkworm. Insect Biochemistry and Molecular Biology. 2008;38(12):1111–1120. doi: 10.1016/j.ibmb.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Chen K, Yu Y, Zhang ZJ, et al Engineering a complex, multiple enzyme-mediated synthesis of natural plant pigments in the silkworm. Proceedings of the National Academy of Sciences of the United States of America. 2023a;120(33):e2306322120. doi: 10.1073/pnas.2306322120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Yu Y, Zhang ZJ, et al. 2023b. The morphogen Hedgehog is essential for proper adult morphogenesis in Bombyx mori. Insect Biochemistry and Molecular Biology, 153: 103906.
- Cong L, Ran FA, Cox D, et al Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339(6121):819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DBT, Gootenberg JS, Abudayyeh OO, et al RNA editing with CRISPR-Cas13. Science. 2017;358(6366):1019–1027. doi: 10.1126/science.aaq0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai HJ, Ma L, Wang J, et al Knockdown of ecdysis-triggering hormone gene with a binary UAS/GAL4 RNA interference system leads to lethal ecdysis deficiency in silkworm . Acta Biochimica et Biophysica Sinica. 2008;40(9):790–795. doi: 10.1093/abbs/40.9.790. [DOI] [PubMed] [Google Scholar]
- Daimon T, Kiuchi T, Takasu Y Recent progress in genome engineering techniques in the silkworm. Development, Growth and Differentiation. 2014;56(1):14–25. doi: 10.1111/dgd.12096. [DOI] [PubMed] [Google Scholar]
- Daimon T, Koyama T, Yamamoto G, et al. 2021. The number of larval molts is controlled by Hox in caterpillars. Current Biology, 31(4): 884–891. e3.
- Dalla Benetta E, López-Denman AJ, Li HH, et al Engineered antiviral sensor targets infected mosquitoes. The CRISPR Journal. 2023;6(6):543–556. doi: 10.1089/crispr.2023.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delanoue R, Slaidina M, Léopold P The steroid hormone ecdysone controls systemic growth by repressing dMyc function in Drosophila fat cells . Developmental Cell. 2010;18(6):1012–1021. doi: 10.1016/j.devcel.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Deng HM, Zhang JL, Li Y, et al Homeodomain POU and Abd-A proteins regulate the transcription of pupal genes during metamorphosis of the silkworm. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(31):12598–12603. doi: 10.1073/pnas.1203149109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi TR, Shyamala BV. 2013. Male- and female-specific variants of doublesex gene products have different roles to play towards regulation of Sex combs reduced expression and sex comb morphogenesis in Drosophila. Journal of Biosciences, 38(3): 455–460.
- Dupont KM, Sharma K, Stevens HY, et al Human stem cell delivery for treatment of large segmental bone defects. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(8):3305–3310. doi: 10.1073/pnas.0905444107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fire A, Xu SQ, Montgomery MK, et al. 1998. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature, 391(6669): 806–811.
- Gehring WJ, Qian YQ, Billeter M, et al Homeodomain-DNA recognition. Cell. 1994;78(2):211–223. doi: 10.1016/0092-8674(94)90292-5. [DOI] [PubMed] [Google Scholar]
- Goldsmith MR, Shimada T, Abe H The genetics and genomics of the silkworm. Annual Review of Entomology. 2005;50:71–100. doi: 10.1146/annurev.ento.50.071803.130456. [DOI] [PubMed] [Google Scholar]
- Gruber AR, Lorenz R, Bernhart SH, et al The vienna RNA websuite. Nucleic Acids Research. 2008;36(S2):W70–W74. doi: 10.1093/nar/gkn188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilinger JP, Thompson DB, Liu DR Fusion of catalytically inactive Cas9 to FokI nuclease improves the specificity of genome modification. Nature Biotechnology. 2014;32(6):577–582. doi: 10.1038/nbt.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handler AM Use of the piggyBac transposon for germ-line transformation of insects . Insect Biochemistry and Molecular Biology. 2002;32(10):1211–1220. doi: 10.1016/S0965-1748(02)00084-X. [DOI] [PubMed] [Google Scholar]
- Heffer A, Pick L Conservation and variation in Hox genes: how insect models pioneered the evo-devo field . Annual Review of Entomology. 2013;58:161–179. doi: 10.1146/annurev-ento-120811-153601. [DOI] [PubMed] [Google Scholar]
- Hughes CL, Kaufman TC Hox genes and the evolution of the arthropod body plan. Evolution & Development. 2002;4(6):459–499. doi: 10.1046/j.1525-142x.2002.02034.x. [DOI] [PubMed] [Google Scholar]
- Huynh N, Depner N, Larson R, et al. 2020. A versatile toolkit for CRISPR-Cas13-based RNA manipulation in Drosophila. Genome Biology, 21(1): 279.
- Imamura M, Nakai J, Inoue S, et al. 2003. Targeted gene expression using the GAL4/UAS system in the silkworm Bombyx mori. Genetics, 165(3): 1329–1340.
- Isobe R, Kojima K, Matsuyama T, et al Use of RNAi technology to confer enhanced resistance to BmNPV on transgenic silkworms. Archives of Virology. 2004;149(10):1931–1940. doi: 10.1007/s00705-004-0349-0. [DOI] [PubMed] [Google Scholar]
- Jiang L, Goldsmith MR, Xia QY Advances in the arms race between silkworm and baculovirus. Frontiers in Immunology. 2021;12:628151. doi: 10.3389/fimmu.2021.628151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Zhao P, Cheng TC, et al A transgenic animal with antiviral properties that might inhibit multiple stages of infection. Antiviral Research. 2013;98(2):171–173. doi: 10.1016/j.antiviral.2013.02.015. [DOI] [PubMed] [Google Scholar]
- Jinek M, Chylinski K, Fonfara I, et al A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337(6096):816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanginakudru S, Royer C, Edupalli SV, et al Targeting ie-1 gene by RNAi induces baculoviral resistance in lepidopteran cell lines and in transgenic silkworms . Insect Molecular Biology. 2007;16(5):635–644. doi: 10.1111/j.1365-2583.2007.00753.x. [DOI] [PubMed] [Google Scholar]
- Knott GJ, Doudna JA CRISPR-Cas guides the future of genetic engineering. Science. 2018;361(6405):866–869. doi: 10.1126/science.aat5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi I, Tsukioka H, Kômoto N, et al SID-1 protein of Caenorhabditis elegans mediates uptake of dsRNA into Bombyx cells . Insect Biochemistry and Molecular Biology. 2012;42(2):148–154. doi: 10.1016/j.ibmb.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Kokubo H, Ueno K, Amanai K, et al Involvement of the Bombyx Scr gene in development of the embryonic silk gland . Developmental Biology. 1997;186(1):46–57. doi: 10.1006/dbio.1997.8578. [DOI] [PubMed] [Google Scholar]
- Kolliopoulou A, Swevers L Recent progress in RNAi research in Lepidoptera: intracellular machinery, antiviral immune response and prospects for insect pest control. Current Opinion in Insect Science. 2014;6:28–34. doi: 10.1016/j.cois.2014.09.019. [DOI] [PubMed] [Google Scholar]
- Konermann S, Lotfy P, Brideau NJ, et al. 2018. Transcriptome engineering with RNA-targeting type VI-D CRISPR effectors. Cell, 173(3): 665–676. e14.
- Lemotte PK, Kuroiwa A, Fessler LI, et al The homeotic gene Sex Combs Reduced of Drosophila: gene structure and embryonic expression . The EMBO Journal. 1989;8(1):219–227. doi: 10.1002/j.1460-2075.1989.tb03367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD Analysis of relative gene expression data using real-time quantitative PCR and the 2 −ΔΔ CT method . Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Ma L, Xu HF, Zhu JQ, et al Ras1 CA overexpression in the posterior silk gland improves silk yield . Cell Research. 2011;21(6):934–943. doi: 10.1038/cr.2011.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma SY, Chang JS, Wang XG, et al. 2014. CRISPR/Cas9 mediated multiplex genome editing and heritable mutagenesis of BmKu70 in Bombyx mori. Scientific Reports, 4: 4489.
- Ma SY, Smagghe G, Xia QY Genome editing in Bombyx mori: new opportunities for silkworm functional genomics and the sericulture industry . Insect Science. 2019;26(6):964–972. doi: 10.1111/1744-7917.12609. [DOI] [PubMed] [Google Scholar]
- Ma SY, Zhang SL, Wang F, et al Highly efficient and specific genome editing in silkworm using custom TALENs. PLoS One. 2012;7(9):e45035. doi: 10.1371/journal.pone.0045035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YF, Zhang MQ, Gong LL, et al Efficient nanoparticle-based CRISPR-Cas13d induced mRNA disruption of an eye pigmentation gene in the white-backed planthopper. Sogatella furcifera. Insect Science. 2023;30(6):1552–1564. doi: 10.1111/1744-7917.13203. [DOI] [PubMed] [Google Scholar]
- Madigan V, Zhang F, Dahlman JE Drug delivery systems for CRISPR-based genome editors. Nature Reviews Drug Discovery. 2023;22(11):875–894. doi: 10.1038/s41573-023-00762-x. [DOI] [PubMed] [Google Scholar]
- Mahas A, Aman R, Mahfouz M CRISPR-Cas13d mediates robust RNA virus interference in plants. Genome Biology. 2019;20(1):263. doi: 10.1186/s13059-019-1881-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarova KS, Aravind L, Wolf YI, et al Unification of Cas protein families and a simple scenario for the origin and evolution of CRISPR-Cas systems. Biology Direct. 2011a;6:38. doi: 10.1186/1745-6150-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarova KS, Haft DH, Barrangou R, et al Evolution and classification of the CRISPR-Cas systems. Nature Reviews Microbiology. 2011b;9(6):467–477. doi: 10.1038/nrmicro2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masumoto M, Yaginuma T, Niimi T Functional analysis of Ultrabithorax in the silkworm, Bombyx mori, using RNAi . Development Genes and Evolution. 2009;219(9-10):437–444. doi: 10.1007/s00427-009-0305-9. [DOI] [PubMed] [Google Scholar]
- Meister G, Tuschl T Mechanisms of gene silencing by double-stranded RNA. Nature. 2004;431(7006):343–349. doi: 10.1038/nature02873. [DOI] [PubMed] [Google Scholar]
- Meng M, Cheng DJ, Peng J, et al The homeodomain transcription factors antennapedia and POU-M2 regulate the transcription of the steroidogenic enzyme gene Phantom in the silkworm . Journal of Biological Chemistry. 2015a;290(40):24438–24452. doi: 10.1074/jbc.M115.651810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng M, Liu C, Peng J, et al Homeodomain protein Scr regulates the transcription of genes involved in juvenile hormone biosynthesis in the silkworm. International Journal of Molecular Sciences. 2015b;16(11):26166–26185. doi: 10.3390/ijms161125945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina Vargas AM, Sinha S, Osborn R, et al New design strategies for ultra-specific CRISPR-Cas13a-based RNA detection with single-nucleotide mismatch sensitivity. Nucleic Acids Research. 2024;52(2):921–939. doi: 10.1093/nar/gkad1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulhair PO, Holland PWH. 2024. Evolution of the insect Hox gene cluster: Comparative analysis across 243 species. Seminars in Cell & Developmental Biology, 152–153: 4–15.
- Murugan K, Babu K, Sundaresan R, et al The revolution continues: Newly discovered systems expand the CRISPR-Cas toolkit. Molecular Cell. 2017;68(1):15–25. doi: 10.1016/j.molcel.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao H Anterior and posterior centers jointly regulate Bombyx embryo body segmentation . Developmental Biology. 2012;371(2):293–301. doi: 10.1016/j.ydbio.2012.08.029. [DOI] [PubMed] [Google Scholar]
- O‘Connell MR Molecular mechanisms of RNA targeting by Cas13-containing type VI CRISPR-Cas systems. Journal of Molecular Biology. 2019;431(1):66–87. doi: 10.1016/j.jmb.2018.06.029. [DOI] [PubMed] [Google Scholar]
- Pan MH, Xiao SQ, Chen M, et al. 2007. Establishment and characterization of two embryonic cell lines of Bombyx mori. In Vitro Cellular & Developmental Biology-Animal, 43(2): 101–104.
- Panzer S, Weigel D, Beckendorf SK Organogenesis in Drosophila melanogaster: embryonic salivary gland determination is controlled by homeotic and dorsoventral patterning genes . Development. 1992;114(1):49–57. doi: 10.1242/dev.114.1.49. [DOI] [PubMed] [Google Scholar]
- Peng J, Li Z, Yang Y, et al Comparative transcriptome analysis provides novel insight into morphologic and metabolic changes in the fat body during silkworm metamorphosis. International Journal of Molecular Sciences. 2018;19(11):3525. doi: 10.3390/ijms19113525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percival-Smith A, Weber J, Gilfoyle E, et al. 1997. Genetic characterization of the role of the two HOX proteins, Proboscipedia and Sex Combs Reduced, in determination of adult antennal, tarsal, maxillary palp and proboscis identities in Drosophila melanogaster. Development, 124(24): 5049–5062.
- Perrimon N, Ni JQ, Perkins L In vivo RNAi: today and tomorrow. Cold Spring Harbor Perspectives in Biology. 2010;2(8):a003640. doi: 10.1101/cshperspect.a003640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Port F, Chen HM, Lee T, et al. 2014. Optimized CRISPR/Cas tools for efficient germline and somatic genome engineering in Drosophila. Proceedings of the National Academy of Sciences of the United States of America, 111(29): E2967–E2976.
- Qian WL, Guo MG, Peng J, et al. 2023a. Decapentaplegic retards lipolysis during metamorphosis in Bombyx mori and Drosophila melanogaster. Insect Biochemistry and Molecular Biology, 155: 103928.
- Qian WL, Li H, Zhang X, et al Fzr regulates silk gland growth by promoting endoreplication and protein synthesis in the silkworm. PLoS Genetics. 2023b;19(1):e1010602. doi: 10.1371/journal.pgen.1010602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian WL, Yang Y, Li Z, et al Enhanced Myc expression in silkworm silk gland promotes DNA replication and silk production . Insects. 2021;12(4):361. doi: 10.3390/insects12040361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan GX, Kanda T, Tamura T Induction of the white egg 3 mutant phenotype by injection of the double-stranded RNA of the silkworm white gene . Insect Molecular Biology. 2002;11(3):217–222. doi: 10.1046/j.1365-2583.2002.00328.x. [DOI] [PubMed] [Google Scholar]
- Reuter JS, Mathews DH RNAstructure: software for RNA secondary structure prediction and analysis. BMC Bioinformatics. 2010;11:129. doi: 10.1186/1471-2105-11-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley PD, Carroll SB, Scott MP The expression and regulation of Sex combs reduced protein in Drosophila embryos . Genes & Development. 1987;1(7):716–730. doi: 10.1101/gad.1.7.716. [DOI] [PubMed] [Google Scholar]
- Shmakov S, Abudayyeh OO, Makarova KS, et al Discovery and functional characterization of diverse class 2 CRISPR-Cas systems. Molecular Cell. 2015;60(3):385–397. doi: 10.1016/j.molcel.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smargon AA, Cox DBT, Pyzocha NK, et al. 2017. Cas13b is a type VI-B CRISPR-associated RNA-guided RNase differentially regulated by accessory proteins Csx27 and Csx28. Molecular Cell, 65(4): 618–630. e7.
- Struhl G Genes controlling segmental specification in the Drosophila thorax . Proceedings of the National Academy of Sciences of the United States of America. 1982;79(23):7380–7384. doi: 10.1073/pnas.79.23.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbaiah EV, Royer C, Kanginakudru S, et al Engineering silkworms for resistance to baculovirus through multigene RNA interference. Genetics. 2013;193(1):63–75. doi: 10.1534/genetics.112.144402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasu Y, Kobayashi I, Beumer K, et al Targeted mutagenesis in the silkworm Bombyx mori using zinc finger nuclease mRNA injection . Insect Biochemistry and Molecular Biology. 2010;40(10):759–765. doi: 10.1016/j.ibmb.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Tamura T, Thibert C, Royer C, et al Germline transformation of the silkworm Bombyx mori L. using a piggyBac transposon-derived vector . Nature Biotechnology. 2000;18(1):81–84. doi: 10.1038/71978. [DOI] [PubMed] [Google Scholar]
- Terenius O, Papanicolaou A, Garbutt JS, et al RNA interference in Lepidoptera: an overview of successful and unsuccessful studies and implications for experimental design. Journal of Insect Physiology. 2011;57(2):231–245. doi: 10.1016/j.jinsphys.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Uhlirova M, Foy BD, Beaty BJ, et al Use of sindbis virus-mediated RNA interference to demonstrate a conserved role of Broad-Complex in insect metamorphosis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(26):15607–15612. doi: 10.1073/pnas.2136837100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang BB, Yang H Progress of CRISPR-based programmable RNA manipulation and detection. WIREs RNA. 2023;14(6):e1804. doi: 10.1002/wrna.1804. [DOI] [PubMed] [Google Scholar]
- Wang F, Hou K, Chen WJ, et al Transgenic PDGF-BB/sericin hydrogel supports for cell proliferation and osteogenic differentiation. Biomaterials Science. 2020;8(2):657–672. doi: 10.1039/C9BM01478K. [DOI] [PubMed] [Google Scholar]
- Wang JY, Doudna JA CRISPR technology: a decade of genome editing is only the beginning. Science. 2023;379(6629):eadd8643. doi: 10.1126/science.add8643. [DOI] [PubMed] [Google Scholar]
- Wang QX, Liu X, Zhou JH, et al The CRISPR-Cas13a gene-editing system induces collateral cleavage of RNA in glioma cells. Advanced Science. 2019;6(20):1901299. doi: 10.1002/advs.201901299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YY, Huang C, Zhao WQ Recent advances of the biological and biomedical applications of CRISPR/Cas systems. Molecular Biology Reports. 2022;49(7):7087–7100. doi: 10.1007/s11033-022-07519-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels HH, Méndez-Mancilla A, Guo XY, et al Massively parallel Cas13 screens reveal principles for guide RNA design. Nature Biotechnology. 2020;38(6):722–727. doi: 10.1038/s41587-020-0456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Wang PY, Zhao QL, et al Mutation of a cuticle protein gene, BmCPG10, is responsible for silkworm Non-moulting in the 2 nd instar mutant . PLoS One. 2016;11(4):e0153549. doi: 10.1371/journal.pone.0153549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing HL, Dong L, Wang ZP, et al A CRISPR/Cas9 toolkit for multiplex genome editing in plants. BMC Plant Biology. 2014;14:327. doi: 10.1186/s12870-014-0327-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Dong QL, Yu Y, et al. 2018. Mass spider silk production through targeted gene replacement in Bombyx mori. Proceedings of the National Academy of Sciences of the United States of America, 115(35): 8757–8762.
- Yusa K. 2015. piggyBac transposon. Microbiology Spectrum, 3(2): MDNA3–0028–2014.
- Zhu HX, Richmond E, Liang C CRISPR-RT: a web application for designing CRISPR-C2c2 crRNA with improved target specificity. Bioinformatics. 2018;34(1):117–119. doi: 10.1093/bioinformatics/btx580. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data to this article can be found online.
Data Availability Statement
All raw data have been deposited in the Sequence Read Archive of the National Center for Biotechnology Information (NCBI) database under accession number PRJNA1132555, Genome Sequence Archive (GSA) database under accession number CRA018506, and Science Data Bank (doi: 10.57760/sciencedb.j00139.00078).