Abstract
The identification of sex chromosomes is fundamental for exploring the mechanism and evolution of sex determination. Platichthys stellatus, a species exhibiting clear sexual dimorphism and homomorphic chromosome pairs, has received limited research concerning its sex determination mechanisms. Clarifying the sex chromosome of P. stellatus will enhance our understanding of sex chromosome evolution in Pleuronectiformes. This study employed whole-genome resequencing to investigate the sex chromosome and sex determination system in P. stellatus. Notably, Chr23 was identified as the sex chromosome in P. stellatus, with the sex-determining region (SDR) occupying 48.1% of the chromosome and featuring an XX/XY system. Sex chromosome turnover was observed within Pleuronectiformes, with P. stellatus, Verasper variegatus, and Hippoglossus hippoglossus sharing a common ancestral karyotype. No inversions were detected within the SDR of P. stellatus, although chromosomal rearrangements between sex chromosomes and autosomes were identified. Additionally, a sex-specific marker for P. stellatus was ascertained, enabling genetic sex identification, with significant implications for improving breeding programs and aquaculture practices.
Keywords: Sex chromosome, Platichthys stellatus, Sex determination system, Ancestral karyotype of Pleuronectiformes, Sex-specific marker
INTRODUCTION
The mechanisms underlying sex determination in fish have garnered considerable research interest due to their remarkable diversity. These mechanisms are primarily driven by genetic factors but can also be influenced by external environmental factors ( Marshall Graves, 2008). In genetic sex determination (GSD), sex is primarily determined by a specific region on the sex chromosome known as the sex-determining region (SDR) ( Bachtrog et al., 2014). This region regulates the expression of sex-related genes, triggering a cascade of molecular pathways that guide gonadal development into either testes or ovaries ( Shao & Chen, 2012).
Sex chromosomes represent the most variable component of the animal genome ( Marshall Graves, 2008). Unlike the highly conserved sex determination systems observed in mammals and birds, fish exhibit significant plasticity and turnover in both sex determination pathways and sex chromosome. In mammals, sex determination predominantly follows the XX/XY system, while birds utilize a ZZ/ZW system ( Bachtrog et al., 2014). Fish, however, display greater diversity in sex chromosome systems, although the XX/XY and ZZ/ZW systems are most prevalent. Notably, Danio rerio does not conform to these typical systems ( Bradley et al., 2011; Liew et al., 2012), with its sex determination governed by multiple genes distributed across several chromosomes, constituting a polygenic sex determination (PSD) system ( Liew & Orbán, 2014). Moreover, other unusual sex chromosome systems have been observed in other fish species, including XX/XO, XX/XY1Y2, X1X2X1X2/X1X2Y, X1X2X1X2/X1X2X1, and ZZ/ZO and ZZ/ZW1W2 ( Mei & Gui, 2014). This broad variability reflects the remarkable evolutionary plasticity in the sex determination mechanisms of fish.
Closely related fish species often exhibit variations in their sex-determining loci, resulting in the existence of multiple, distinct sex determination systems ( Hilgers & Schwarzer, 2019). Additionally, non-homologous sex chromosomes are commonly observed in many species ( Vicoso, 2019). Throughout evolution, chromosome fusions have frequently occurred, often resulting in the fixation of slightly deleterious mutations ( Zhou, 2022). The formation of new sex chromosomes can occur through the fusion of autosomes with sex chromosomes ( Gong et al., 2023), or through independent evolution of sex chromosomes ( Long et al., 2023). This independent evolution may involve sexually antagonistic polymorphisms ( Rice, 1987), which facilitate the emergence of novel sex-determining genes in regions closely linked to pre-existing or newly formed genomic regions ( Van Doorn & Kirkpatrick, 2007).
A large number of fish species lack clear heteromorphic sex chromosomes ( Eisbrenner, 2013), significantly limiting the identification of subtly different sex chromosomes using traditional cytogenetic techniques, as well as research on homomorphic sex chromosomes. However, recent advancements in genome-sequencing technologies and multi-omics analyses have opened new avenues for investigation. Genome-wide association studies (GWAS), treating sex as a binary trait, provide a powerful tool for detecting SDRs with minimal sequence differentiation or small physical size ( Palmer et al., 2019). Furthermore, calculating F ST values between males and females shows promise for identifying SDRs that exhibit lower levels of degeneration ( Vicoso, 2019).
Platichthys stellatus (starry flounder) is predominantly distributed in the North Pacific and exhibits notable sexual dimorphism. Females display growth rates two to four times faster than males ( Wang et al., 2021), while males display precocious sexual maturation. This pronounced sexual dimorphism presents challenges for aquaculture, as the differential growth and maturation rates negatively impact economic viability. Controlling sexual development in cultured fish is a key strategy for improving the economic sustainability of aquaculture.
The sex determination mechanism in P. stellatus remains unresolved, further complicated by the absence of heteromorphic chromosome pairs, making it difficult to identify the sex chromosomes ( Li et al., 2009a). In this study, we explored the sex chromosome and sex determination system in P. stellatus. Our results provide a theoretical basis for understanding the evolutionary trajectory of sex chromosomes in this species and reveal potential candidate genes for further investigation into the molecular mechanisms underlying sex determination.
MATERIALS AND METHODS
Ethics statement
All animal procedures followed the principles of the Guide for the Care and Use of Laboratory Animals at the Chinese Academy of Fishery Sciences and were approved by the Institutional Animal Care and Use Committee (IACUC) of the Yellow Sea Fisheries Research Institute (CAFS) (Qingdao, China) (Approval No.: YSFRI-2024070).
Gonadal histological examination
Histological examination was performed on the collected gonadal tissues to determine phenotypic sex. First, the tissues were fixed in 4% paraformaldehyde (PFA) at 4°C for more than 24 h, then washed in 10 mmol/L phosphate-buffered saline (PBS) (Solarbio Science, China) for 1 h. After fixation, the samples were dehydrated using graded ethanol concentrations, then embedded in paraffin blocks. Subsequently, 4 μm slices were cut from the paraffin blocks and mounted on glass slides. Hematoxylin-eosin (Solarbio Science, China) staining was applied to the slices for visualization.
Sampling and sequencing
A total of 72 P. stellatus individuals were collected from three farms located in Shandong and Jiangsu provinces, China. The gonads of each individual were used to determine physiological sex (Supplementary Figure S1). Muscle tissue samples of 37 females and 35 males had been excised and subsequently immersed in absolute ethanol. The tissues were subsequently processed for sequencing with the MGISEQ series sequencer (BGI, China).
Variant detection
Raw sequencing data were filtered for low-quality reads using SOAPnuke (v.2.1.9) ( Chen et al., 2018b), yielding high-quality clean data. These clean reads were mapped to the reference genome (GCA_016801935.1) using BWA-mem (v.0.7.18) ( Li & Durbin, 2009), with default parameters. Alignments were sorted with SAMtools (v.1.17) ( Li et al., 2009b), and variant calling was performed using GATK (v.4.0) ( Van der Auwera et al., 2013). Variant detection for each sample was carried out using the HaplotypeCaller tool in GATK. The obtained gvcf files were then merged using CombineGVCFs, followed by joint genotyping using GenotypeGVCFs to generate vcf files. Variants were filtered based on the criteria: “QD<2.0 || FS>60.0 || MQRankSum<−12.5 || ReadPosRankSum<−8.0 || SOR>3.0 || MQ<40.0 || QUAL<30.0”.
Identification of sex chromosomes and SDR
For subsequent analysis, only biallelic single nucleotide polymorphisms (SNPs) with a minor allele frequency (MAF) between 0.05 and 0.95 (--max-maf 0.95 --maf 0.05) were retained. Sites with deletion rates exceeding 20% were excluded using VCFtools (v.0.1.13) ( Danecek et al., 2011). SNP-based GWAS was performed using linear mixed models, employing the Wald test, with sex as the phenotype. Phased genotypes were processed with PLINK (v.1.90) ( Purcell et al., 2007) and used as input for GEMMA (v.0.98.5) ( Zhou & Stephens, 2012). The P-value threshold for significance was set to 6.16e-9, determined using Bonferroni correction (0.05 divided by the number of SNPs on the chromosome, 8 116 687). A 10 kb sliding window was used to calculate the F ST values between the male and female populations using VCFtools (v.0.1.13).
Change-point analysis of F ST values was conducted using the R-package “changepoint” ( Killick & Eckley, 2014), applying the binary segmentation method ( Bai, 1997), with a maximum of two changepoints. The changepoints were based on both mean and variance shifts. The boundaries between SDR and adjacent pseudo-autosomal regions (PARs) were determined through the integration of significant P-values from GWAS results and changepoint analysis of F ST values in 10 kb sliding windows, highlighting the notable variations between neighboring regions.
Recombination rates were estimated using FastEPRR ( Gao et al., 2016) after excluding SNP sites with >20% missing data and those located within repetitive regions with the parameter winLength=50 000. Recombination rates within candidate regions were evaluated to refine the identification of the SDR.
To identify the sex chromosome type in P. stellatus, bcftools (v.1.4) ( Danecek et al., 2011) was used to count genotypes for the top 100 SNPs.
Transcriptomic analysis
Transcriptome data of P. stellatus was retrieved from the NCBI database (BioProjectID: PRJNA556158) and fastp (v.0.20.0) ( Chen et al., 2018a) was employed to filter low-quality reads. Subsequent quantitative analysis was performed using salmon (v.0.14.1) ( Patro et al., 2017), and differentially expressed genes (DEGs) were identified using DESeq2 ( Love et al., 2014), applying a threshold of log 2|FoldChange|>1 and P-adjust<0.05. StringTie ( Shumate et al., 2022) was utilized for reference-guided transcriptome assembly and TransDecoder (https://github.com/TransDecoder/transdecoder.github.io/) was employed to predict alternative splicing events and open reading frames (ORFs).
Ancestral karyotype reconstruction and synteny analysis of Pleuronectiformes
The ancestral karyotype of Pleuronectiformes was reconstructed using the genomes of Platichthys stellatus (reference genome), Cynoglossus semilaevis, Hippoglossus hippoglossus, Hippoglossus stenolepis, Paralichthys olivaceus, Platichthys flesus, Pleuronectes platessa, Reinhardtius hippoglossoides, Scophthalmus maximus, Solea senegalensis, Verasper variegatus, and Oryzias latipes (outgroup). Genomes were aligned using LASTZ with parameters “T=2 C=2 H=2000 Y=3400 L=6000 K=2200 --format=axt.” ( Liu et al., 2021). Subsequently, axtChain, chainMergeSort, chainPreNet, and chainNet were used to generate “chain” and “net” files as input for DESCHRAMBLER ( Kim et al., 2017). A phylogenetic tree was constructed using OrthoFinder with homologous genes ( Emms & Kelly, 2019), and divergence times were estimated using a Bayesian relaxed molecular clock approach in MCMCTree from the PAML package ( Yang, 2007). Fossil records obtained from TIMETREE (http://www.timetree.org) provided calibration points for the estimated divergence times. Synteny analysis between P. stellatus and V. variegatus and H. hippoglossus was performed using the MCScan toolkit ( Tang et al., 2008).
Sex-specific marker identification and validation
To assess the depth of coverage for females and males in the SDR, BamDeal statistics Coverage (https://github.com/BGI-shenzhen/BamDeal) was employed. Primers were designed flanking the Y-specific sequence to amplify this region via polymerase chain reaction (PCR) in male and female P. stellatus individuals (forward: 5' AGTGAATGACCTCAGCAGCC 3' reverse: 5' GCATCAAGGACGCAGTAAAT 3'). The PCR amplification program utilized the following parameters: 3 min at 95°C, 34 cycles of 15 s at 95°C, 15 s at 55°C, 30 s at 72°C per cycle, 5 min at 72°C, held at 12°C. The PCR products were detected by 1% agarose gel electrophoresis.
RESULTS
Identification of sex chromosome and sex determination system in P. stellatus
A total of 72 P. stellatus individuals (35 males and 37 females) from three different populations were sequenced using the MGISEQ platform. A total of 6 462.95 million clean reads were obtained, with an average mapping rate of 94.8% and average coverage depth of 20.45× (Supplementary Table S1). After mapping the reads to the reference genome, 13 541 398 SNPs were retained after filtering.
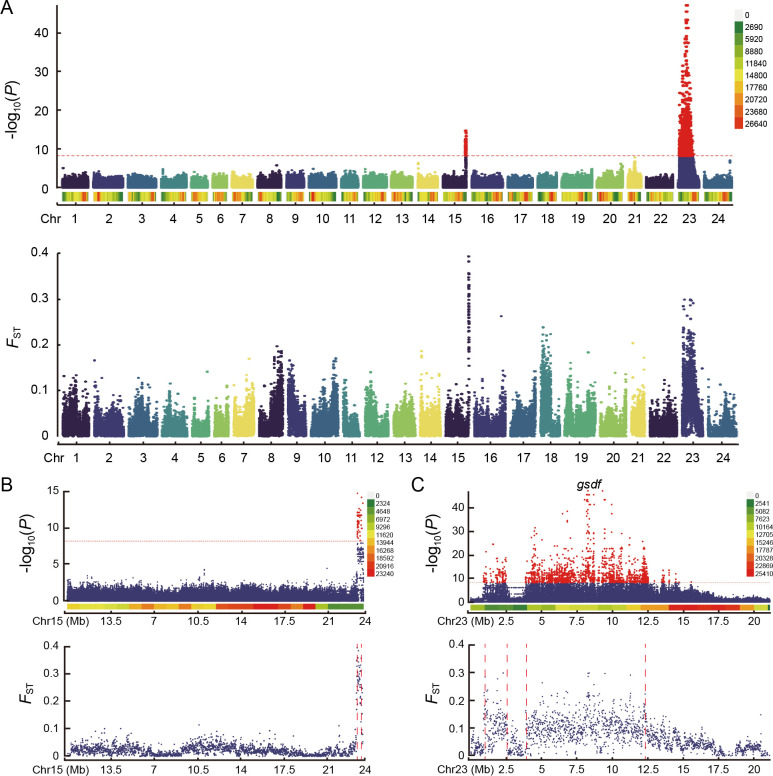
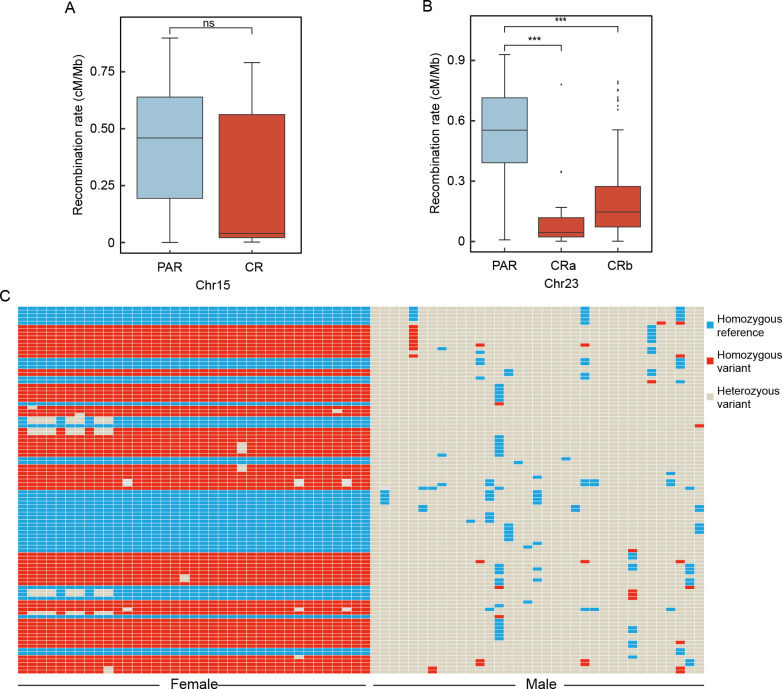
F ST estimates between the sexes and SNP-based GWAS were performed to identify sex-associated variants and potentially related genes. These analyses revealed two significant peaks located on Chr15 and Chr23 ( Figure 1A). In the distal region of Chr15, the candidate region spanned approximately 456 kb ( Figure 1B). In contrast, the candidate region on Chr23 was markedly larger, divided into two segments measuring 1.59 Mb and 8.57 Mb, respectively ( Figure 1C). Recombination rate analysis demonstrated significant inhibition of recombination in both candidate regions on Chr23, while inhibition of the candidate region on Chr15 was less pronounced compared to other regions ( Figure 2A, B).
Figure 1.
Manhattan plot for GWAS and F ST results associated with sex traits
A–C: First track shows sex-associated SNPs identified by GWAS, second track shows weighted F ST values between males and females in 10 kb windows in all chromosomes, Chr15, and Chr23, respectively. Legend on right indicates SNP density, red dashed line marks candidate region boundary.
Figure 2.
Recombination landscapes and genotypes
A, B: Recombination rates between candidate regions (CR) (red) and pseudoautosomal regions (PAR) (blue) in Chr15 and Chr23. CRa refers to first candidate region in Chr23; CRb refers to second candidate region in Chr23. ns: Not significant; ***: P<0.001. C: Genotypes of top 100 strongest sex-associated SNPs based on GWAS analysis of Chr23.
Our findings strongly support the role of Chr23 as the sex chromosome in P. stellatus, with two distinct candidate SDRs. In contrast, the relatively small candidate region on Chr15 likely represents an autosomal region associated with sex differentiation. A total of 368 genes were identified within the SDRs, 132 of which exhibited significant differences in expression between male and female gonads. Specifically, 62 genes, including gsdf, hnrnpd, herc1, nipbl, and kdm2b, showed higher expression in testes (Supplementary Table S2), while 70 genes, including fbp1, tmem120b, phyhd1, and arhgap24, showed significantly higher expression in ovaries (Supplementary Table S2). Furthermore, genes previously linked to sexual dimorphism in P. stellatus, such as eno, pgam, klf4, and sema3 ( Wang et al., 2021), were also located within the SDRs (Supplementary Table S2).
To determine the sex chromosome system of P. stellatus, the top 100 most strongly sex-associated SNPs from Chr23 were selectively sampled from the GWAS results. The genotypes indicated that females were predominantly homozygous, while males were predominantly heterozygous at these SNP loci ( Figure 2C; Supplementary Table S3), confirming an XX/XY sex chromosome system.
The most significant SNPs based on GWAS analysis were located near the gsdf gene on Chr23, which is associated with testicular development ( Figure 1C). A total of 51 SNPs within gsdf exceeded the established significance threshold, showing strong linkage disequilibrium (Supplementary Figure S2). Sex-specific SNPs were enriched around the gsdf region (Supplementary Figure S3), and gsdf expression was highly elevated in the testes (Supplementary Figure S4), with multiple alternative splicing events identified (Supplementary Table S4).
Comparative analysis of sex chromosomes in Pleuronectiformes
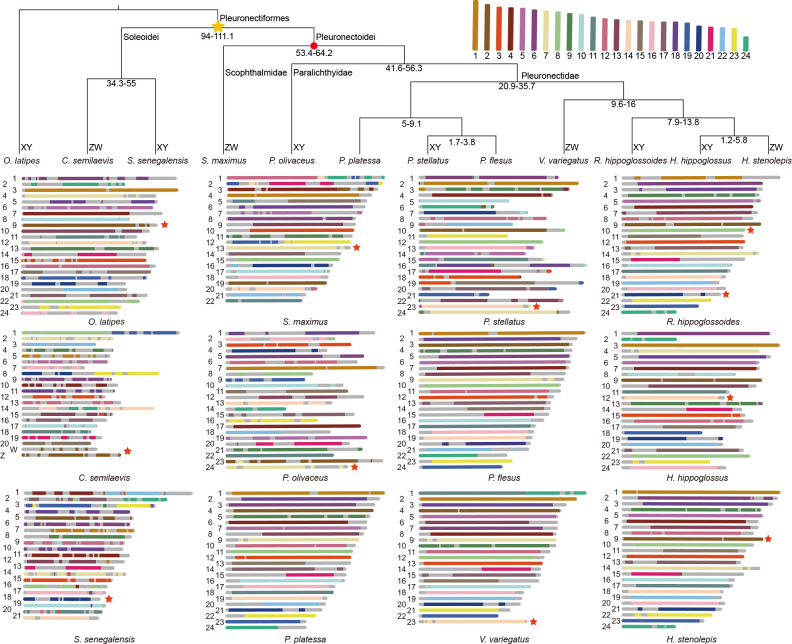
There is a notable diversity in chromosome numbers across different species within Pleuronectiformes, such as 2 n=42 in C. semilaevis ( Chen et al., 2014) and S. senegalensis ( Guerrero-Cózar et al., 2021), 2 n=44 in S. maximus ( Martínez et al., 2021), 2 n=46 in V. variegatus ( Xu et al., 2023), and 2 n=48 in P. olivaceus ( Hattori et al., 2022). To elucidate the chromosomal evolution in Pleuronectiformes, the genomes of 11 species in Pleuronectiformes were analyzed, using O. latipes as the outgroup, to reconstruct the ancestral karyotype. Results indicated that the putative ancestral karyotype consisted of 24 chromosomes and the ancestral genome was approximately 381 Mb in length (Supplementary Table S5). Chromosomal diversity in these species was shaped by ancestral chromosomal breakage and fusion events during evolutionary history. Species with 21 chromosomes underwent three ancestral karyotype fusion events, those with 22 chromosomes underwent two fusion events, and those with 23 chromosomes experienced a single fusion event. The sex chromosome karyotypes across species with known sex chromosomes in Pleuronectiformes are primarily derived from five ancestral karyotypes (alg2 in C. semilaevis ( Chen et al., 2014) and H. stenolepis ( Jasonowicz et al., 2022); alg7 in S. maximus ( Martínez et al., 2021) and P. olivaceus ( Hattori et al., 2022); alg8 in R. hippoglossoides ( Ferchaud et al., 2022); alg14 in P. stellatus, V. variegatus ( Xu et al., 2023), and H. hippoglossus ( Einfeldt et al., 2021); and alg20 in S. senegalensis ( Guerrero-Cózar et al., 2021; Rodríguez et al., 2019) and R. hippoglossoides) ( Figure 3; Supplementary Table S6). In P. stellatus, the small part of Chr15 and the majority of Chr23 were derived from alg14 ( Figure 3). Chromosomal rearrangements between the sex chromosomes and autosomes were observed, contributing to the structural diversity of these chromosomes ( Figure 4).
Figure 3.
Ancestral karyotype of Pleuronectiformes
Phylogenetic tree construction based on homologous genes and reconstruction of ancestral karyotype with O. latipes ( Matsuda et al., 2002) as an outgroup. Each node contains a number representing divergence time (million years ago, Ma) among species, with red circles highlighting fossil record used for calibration at that node. Red pentagons indicate sex chromosomes. Yellow pentagons indicate nodes representing ancestral chromosomes.
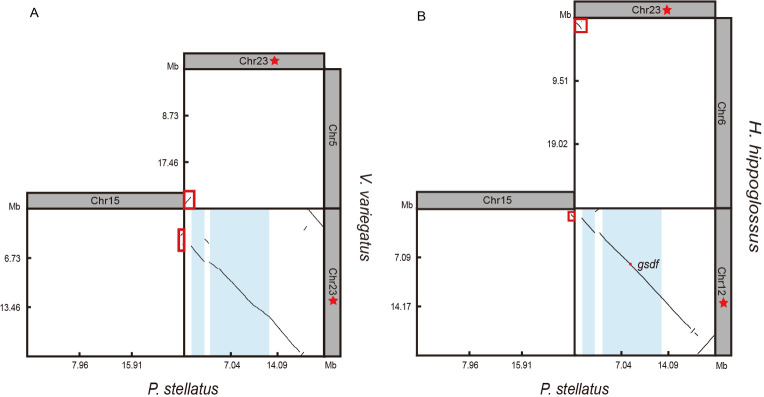
Figure 4.
Synteny analysis of sex chromosomes between Platichthys stellatus and Verasper variegatus and Hippoglossus hippoglossus
A: Synteny analysis between P. stellatus and V. variegatus. B: Synteny analysis between P. stellatus and H. hippoglossus. Red pentagons indicate sex chromosomes. Light blue represents SDRs of P. stellatus. Red box corresponds to autosomes.
Comparative synteny analysis was performed between P. stellatus and V. variegatus and H. hippoglossus ( Figure 4), revealing all three species shared a conserved ancestral sex chromosome karyotype. In P. stellatus, the sex chromosome (Chr23) was linearly related to Chr5 and Chr23 of V. variegatus ( Figure 4A) and Chr6 and Chr12 of H. hippoglossus ( Figure 4B). Additionally, the sex chromosomes of V. variegatus (Chr23) and H. hippoglossus (Chr12) exhibited a linear relationship with Chr15 and Chr23 of P. stellatus ( Figure 4). These findings suggest that chromosomal crossovers between autosomes and sex chromosomes contributed to the formation of the sex chromosomes. Further analysis identified chromosomal rearrangements within the sex chromosomes, although no structural variations were detected within the SDRs of Chr23 ( Figure 4).
Application of sex-specific markers
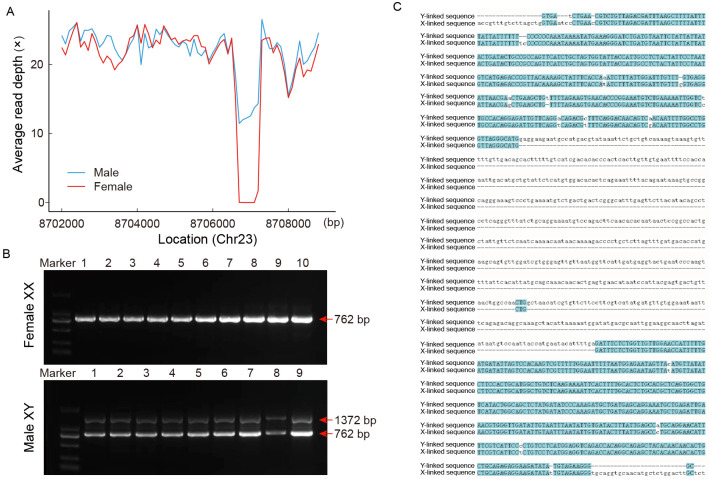
The top 100 SNPs identified through GWAS were predominantly enriched around the gsdf locus (Chr23: 8250000–8400000) and an additional genomic region on Chr23: 8700000–8750000 (Supplementary Table S7). Detailed analysis of this region between male and female P. stellatus individuals revealed a significant difference in the Chr23: 8706600–8707300 segment. In female individuals, sequencing depths in this region approached zero, whereas in males, depths were approximately half that of other regions ( Figure 5A), suggesting the presence of a Y-chromosome specific sequence. PCR primers flanking this sequence were used to amplify the region, with gel electrophoresis clearly distinguishing two bands in males and one band in females ( Figure 5B). The upper band in males corresponded to the product from the Y chromosome, while the lower band was from the X chromosome. The Y chromosome fragment was 1 327 bp in length and the X chromosome fragment was 762 bp. Alignment of these sequences revealed a 620 bp insertion unique to the Y chromosome ( Figure 5C).
Figure 5.
Application of sex-specific markers and sex identification
A: Average read depth of female (red) and male (blue) individuals. B: Sex identification by sex-specific marker. One band for females, two bands for males. C: Alignment of primer amplification sequences from X and Y chromosomes.
DISCUSSION
The remarkable plasticity of fish gonads and the frequent turnover of sex determination systems and sex chromosomes present substantial challenges for evolutionary biology ( Martínez et al., 2021). Fish exhibit a wide diversity in sex determination mechanisms, with sex chromosomes playing an important role in understanding these processes. In P. stellatus, the absence of heteromorphic sex chromosome pairs ( Li et al., 2009a) complicates the identification of its sex chromosomes and thus the study of its sex determination mechanisms. Advances in sequencing technologies and the ability to perform large-scale genomic analyses have made it possible to accurately identify sex chromosomes, facilitating the exploration of sex determination mechanisms and sex chromosome evolution in fish. Based on sex-specific differences in SNPs, we explored the genetic disparities between males and females at the genomic level. Integrating GWAS, F ST analysis, and recombination rate studies, Chr23 was identified as the sex chromosome in P. stellatus. The SDR on Chr23 was approximately 10.16 Mb, constituting 48.1% of the chromosome. Both the size and proportion of SDRs in sex chromosomes can vary significantly among fish species. For instance, in yellow catfish, the SDR is approximately 0.3 Mb, accounting for only 0.7% of the sex chromosome ( Gong et al., 2023). In contrast, the SDR constitutes 63.9% and 95.1% of the sex chromosome in seahorse species Hippocampus abdominalis and Hippocampus erectus, respectively ( Long et al., 2023).
The study of SDRs is crucial for understanding the early evolutionary processes of emerging sex chromosomes ( Bachtrog et al., 2014). In P. stellatus, the SDRs not only included genes related to gonadal development but also genes associated with sexual dimorphism. A notable feature of the SDRs on Chr23 was their significantly reduced recombination rate compared to other regions in the same chromosome, suggesting that these regions were subjected to recombination inhibition ( Figure 2B). Several factors may contribute to this inhibition of recombination, such as sexually antagonistic polymorphisms ( Rice, 1987) or chromosomal inversions ( Lahn & Page, 1999). For example, in frogs, recombination inhibition occurs between sexually antagonistic genes and sex-determining genes ( Perrin, 2021). In humans ( Lemaitre et al., 2009), papaya ( Wang et al., 2012), and three-spined sticklebacks ( Peichel et al., 2020), recombination inhibition primarily occurs due to inversions. Recombination inhibition can also be generated through recombination modifiers, which regulate crossover events at specific locations in avian genomes ( Chen et al., 2014). In the case of P. stellatus, the absence of observed inversions or translocations in the SDRs ( Figure 4) suggests that recombination inhibition may be attributed to sexually antagonistic polymorphism ( Rice, 1987) or the influence of recombination modifiers. However, further investigation is needed to elucidate the precise mechanisms underlying recombination inhibition in these regions.
Platichthys stellatus is a male-heterogametic species, characterized by recombination inhibition within its SDRs between the X and Y chromosomes, while maintaining homomorphic sex chromosomes. This retention of homomorphy may be explained by the origin of sex chromosomes in Pleuronectiformes, derived from five ancestral karyotypes ( Figure 3). A possible hypothesis for this phenomenon is that the turnover of sex chromosomes in these species resulted in minimal degradation, allowing the sex chromosomes to remain in an evolutionary “youthful” state, similar to that observed in willows ( Hu et al., 2023) and reptiles ( Zhu et al., 2022).
Despite the significant diversity in sex chromosome structures across Pleuronectiformes, there is also a notable preference for the five ancestral karyotypes. With the exception of C. semilaevis, these sex chromosomes exhibit minimal signs of degeneration, suggesting that sex chromosome turnover can occur without the accumulation of a large number of deleterious mutations. This process is facilitated by an ancestral pattern of reduced recombination, which promotes the turnover of sex chromosomes ( Ferchaud et al., 2022; Long et al., 2023). Additionally, sexually antagonistic polymorphisms play a crucial role in driving sex chromosome turnover by enabling the formation of new sex-determining genes or novel SDRs through polymorphic variation ( Van Doorn & Kirkpatrick, 2007). These mechanisms have contributed to the diversity and independent evolution of sex chromosomes in Pleuronectiformes.
Moreover, the small terminal region of Chr15 and the sex chromosomes share a common ancestral karyotype ( Figure 3). It is mainly attributed to chromosomal rearrangement and other potential possiblities. Chromosomal rearrangements between sex chromosomes and autosomes during evolutionary processes frequently occur in Pleuronectiformes (Figures 3, 4) ( Cheng et al., 2020; Ferchaud et al., 2022). These rearrangements not only connect previously separate chromosomes but also reduce recombination rates along each chromosome arm, thereby preventing recombination between fused and unfused homologous chromosomes in polymorphic populations ( Dumas & Britton-Davidian, 2002). This suppression of recombination may represent a key factor in the evolution of sex chromosome differentiation ( Ferchaud et al., 2022).
The diversity of sex determination in fish, coupled with the primordial nature of sex chromosomes, underscores the ongoing uncertainty surrounding the identification of sex-determining genes ( Shao & Chen, 2012). Various genes linked to male sex determination, such as gsdf, sox3, amhr2, sdy, and dmrt1, have been identified, alongside genes related to female sex differentiation, such as foxl2, cyp19a1, s f1, and wnt4 ( Mei & Gui, 2014). In our analysis, sex-associated SNPs were distributed within and around the gsdf gene ( Figure 1C; Supplementary Figure S3), which also exhibited exclusive high expression in the testes (Supplementary Figure S4). Previous studies of the Nile tilapia have shown that knockout of gsdf results in ovarian development in XY individuals, indicating that gsdf functions downstream of dmrt1 in the male sex determination pathway ( Jiang et al., 2016). Similarly, in H. hippoglossus, which shares homologous sex chromosomes with P. stellatus ( Figure 3; Supplementary Table S6), gsdf serves as the sex-determining gene ( Edvardsen et al., 2022; Einfeldt et al., 2021). In addition to gene expression, alternative splicing events ( Lu et al., 2022) and competing endogenous RNA (ceRNA) crosstalk mediated by non-coding RNAs (ncRNAs) ( Tang et al., 2022) have been shown to influence the regulatory mechanisms governing gonadal development. The prevalence of alternative splicing and its role in modulating gsdf expression may also be related to gonadal development. Given these findings, we speculate that gsdf may be a potential candidate gene for sex determination in P. stellatus. However, further experimental research is required to determine its specific function in P. stellatus.
The sex-specific marker identified in this study accurately provides an effective tool for determining the genetic sex of P. stellatus, offering a robust foundation for exploring sex determination mechanisms and optimizing juvenile breeding strategies. While P. stellatus adults display clear sexual dimorphism, with females significantly larger than males, such differences are not apparent during the juvenile stage. The ability to accurately identify sex at an early developmental stage using sex-specific markers is highly valuable for enhancing the efficiency of aquaculture. Additionally, these markers hold potential for use in screening and breeding sex-reversed individuals, further contributing to advances in aquaculture practices.
In conclusion, this study identified Chr23 as the sex chromosome in P. stellatus, confirming an XX/XY system, with the SDR accounting for 48.1% of the sex chromosome. This research addresses the gap in the study of homomorphic sex chromosomes, particularly in cases with subtle differentiation between the sexes ( Long et al., 2023). Results also showed that the sex chromosomes of P. stellatus, V. variegatus, and H. hippoglossus were derived from the same ancestral karyotype, with rearrangements detected between the sex chromosomes and autosomes. These findings provide a theoretical basis for understanding the evolutionary mechanisms of sex chromosomes in Pleuronectiformes. Additionally, the identification of potential candidate sex-determining genes and the application of sex-specific markers offer significant opportunities for advancing research on sex determination and facilitating sex control breeding strategies in aquaculture.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
Acknowledgments
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
C.W.S. designed the research. W.J.L. analyzed the data and wrote the manuscript. Y.M.Z., H.J.H., and Y.Y.L. performed histological sectioning and validation experiments. Y.Y.L. and C.L. collected samples. S.L.H. and Z.L.S. performed ancillary analyses. B.H.L. performed experiments in the manuscript review response. S.L., K.Q.L., L.B.D., Q.W., and H.Y.W. revised the manuscript. All authors read and approved the final version of the manuscript.
Funding Statement
This work was supported by the National Key Research and Development Program of China (2022YFD2400100), National Natural Science Foundation of Shandong Province of China (ZR2023QC006), and National Natural Science Foundation of China (32403053)
DATA AVAILABILITY
The raw resequencing data can be obtained from the NCBI (BioProjectID PRJNA1178810), GSA (CRA015152) and Science Data Bank (doi: 10.57760/sciencedb.j00139.00087).
References
- Bachtrog D, Mank JE, Peichel CL, et al Sex determination: why so many ways of doing it? PLoS Biology. 2014;12(7):e1001899. doi: 10.1371/journal.pbio.1001899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai JS Estimating multiple breaks one at a time. Econometric Theory. 1997;13(3):315–352. doi: 10.1017/S0266466600005831. [DOI] [Google Scholar]
- Bradley KM, Breyer JP, Melville DB, et al An SNP-Based Linkage Map for zebrafish reveals sex determination loci. G3 (Bethesda) 2011;1(1):3–9. doi: 10.1534/g3.111.000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SF, Zhou YQ, Chen YR, et al. 2018a. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics (Oxford, England), 34(17): i884–i890.
- Chen SL, Zhang GJ, Shao CW, et al Whole-genome sequence of a flatfish provides insights into ZW sex chromosome evolution and adaptation to a benthic lifestyle. Nature Genetics. 2014;46(3):253–260. doi: 10.1038/ng.2890. [DOI] [PubMed] [Google Scholar]
- Chen YX, Chen YS, Shi CM, et al SOAPnuke: a MapReduce acceleration-supported software for integrated quality control and preprocessing of high-throughput sequencing data. Gigascience. 2018b;7(1):gix120. doi: 10.1093/gigascience/gix120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng YB, Shang DT, Luo MJ, et al Whole genome-wide chromosome fusion and new gene birth in the Monopterus albus genome . Cell & Bioscience. 2020;10(1):67. doi: 10.1186/s13578-020-00432-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danecek P, Auton A, Abecasis G, et al The variant call format and VCFtools. Bioinformatics. 2011;27(15):2156–2158. doi: 10.1093/bioinformatics/btr330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas D & Britton-Davidian J Chromosomal rearrangements and evolution of recombination: comparison of chiasma distribution patterns in Standard and Robertsonian populations of the house mouse. Genetics. 2002;162(3):1355–1366. doi: 10.1093/genetics/162.3.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvardsen RB, Wallerman O, Furmanek T, et al Heterochiasmy and the establishment of gsdf as a novel sex determining gene in Atlantic halibut . PLoS Genetics. 2022;18(2):e1010011. doi: 10.1371/journal.pgen.1010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einfeldt AL, Kess T, Messmer A, et al Chromosome level reference of Atlantic halibut Hippoglossus hippoglossus provides insight into the evolution of sexual determination systems . Molecular Ecology Resources. 2021;21(5):1686–1696. doi: 10.1111/1755-0998.13369. [DOI] [PubMed] [Google Scholar]
- Eisbrenner WD. 2013. Sex Determination in Tasmanian Atlantic Salmon. Master thesis, Simon Fraser University, Vancouver.
- Emms DM, Kelly S OrthoFinder: phylogenetic orthology inference for comparative genomics. Genome Biology. 2019;20(1):238. doi: 10.1186/s13059-019-1832-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferchaud AL, Merot C, Normandeau E, et al Chromosome-level assembly reveals a putative Y-autosomal fusion in the sex determination system of the Greenland Halibut ( Reinhardtius hippoglossoides) . G3 (Bethesda) 2022;12(1):jkab376. doi: 10.1093/g3journal/jkab376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Ming C, Hu W, et al New software for the fast estimation of population recombination rates (FastEPRR) in the genomic era. G3 Genes| Genomes| Genetics. 2016;6(6):1563–1571. doi: 10.1534/g3.116.028233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong GR, Xiong Y, Xiao SJ, et al Origin and chromatin remodeling of young X/Y sex chromosomes in catfish with sexual plasticity. National Science Review. 2023;10(2):nwac239. doi: 10.1093/nsr/nwac239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Cózar I, Gomez-Garrido J, Berbel C, et al Chromosome anchoring in Senegalese sole ( Solea senegalensis) reveals sex-associated markers and genome rearrangements in flatfish . Scientific Reports. 2021;11(1):13460. doi: 10.1038/s41598-021-92601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori RS, Kumazawa K, Nakamoto M, et al Y-specific amh allele, amhy, is the master sex-determining gene in Japanese flounder Paralichthys olivaceus . Frontiers in Genetics. 2022;13:1007548. doi: 10.3389/fgene.2022.1007548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgers L, Schwarzer J The untapped potential of medaka and its wild relatives. Elife. 2019;8:e46994. doi: 10.7554/eLife.46994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu N, Sanderson BJ, Guo MH, et al Evolution of a ZW sex chromosome system in willows. Nature Communications. 2023;14(1):7144. doi: 10.1038/s41467-023-42880-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasonowicz AJ, Simeon A, Zahm M, et al Generation of a chromosome-level genome assembly for Pacific halibut ( Hippoglossus stenolepis) and characterization of its sex-determining genomic region . Molecular Ecology Resources. 2022;22(7):2685–2700. doi: 10.1111/1755-0998.13641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang DN, Yang HH, Li MH, et al gsdf is a downstream gene of dmrt1 that functions in the male sex determination pathway of the Nile tilapia . Molecular Reproduction and Development. 2016;83(6):497–508. doi: 10.1002/mrd.22642. [DOI] [PubMed] [Google Scholar]
- Killick R, Eckley IA Changepoint: an R package for changepoint analysis. Journal of Statistical Software. 2014;58(3):1–19. [Google Scholar]
- Kim J, Farré M, Auvil L, et al Reconstruction and evolutionary history of eutherian chromosomes. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(27):E5379–E5388. doi: 10.1073/pnas.1702012114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahn BT & Page DC Four evolutionary strata on the human X chromosome. Science. 1999;286(5441):964–967. doi: 10.1126/science.286.5441.964. [DOI] [PubMed] [Google Scholar]
- Lemaitre C, Braga MDV, Gautier C, et al Footprints of inversions at present and past pseudoautosomal boundaries in human sex chromosomes. Genome Biology and Evolution. 2009;1:56–66. doi: 10.1093/gbe/evp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Chen SQ, Liu ZH, et al Karyotype of Platichthys stellatus pallas . Progress in Fishery Sciences. 2009a;30(2):20–25. [Google Scholar]
- Li H, Durbin R Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25(14):1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, et al The sequence alignment map format and SAMtools. Bioinformatics. 2009b;25(16):2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew WC, Bartfai R, Lim Z, et al Polygenic sex determination system in zebrafish. PLoS One. 2012;7(4):e34397. doi: 10.1371/journal.pone.0034397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew WC, Orbán L Zebrafish sex: a complicated affair. Briefings in Functional Genomics. 2014;13(2):172–187. doi: 10.1093/bfgp/elt041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Gao JB, Cui XX, et al A towering genome: experimentally validated adaptations to high blood pressure and extreme stature in the giraffe. Science Advances. 2021;7(12):eabe9459. doi: 10.1126/sciadv.abe9459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long X, Charlesworth D, Qi JF, et al Independent evolution of sex chromosomes and male pregnancy-related genes in two seahorse species. Molecular Biology and Evolution. 2023;40(1):msac279. doi: 10.1093/molbev/msac279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biology. 2014;15(12):550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y F, Liu Q, Liu K Q, et al Identification of global alternative splicing and sex-specific splicing via comparative transcriptome analysis of gonads of Chinese tongue sole ( Cynoglossus semilaevis) . Zoological Research. 2022;43(3):319–330. doi: 10.24272/j.issn.2095-8137.2021.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall Graves JA Weird animal genomes and the evolution of vertebrate sex and sex chromosomes. Annual Review of Genetics. 2008;42:565–586. doi: 10.1146/annurev.genet.42.110807.091714. [DOI] [PubMed] [Google Scholar]
- Matsuda M, Nagahama Y, Shinomiya A, et al DMY is a Y-specific DM-domain gene required for male development in the medaka fish. Nature. 2002;417(6888):559–563. doi: 10.1038/nature751. [DOI] [PubMed] [Google Scholar]
- Martínez P, Robledo D, Taboada X, et al A genome-wide association study, supported by a new chromosome-level genome assembly, suggests sox2 as a main driver of the undifferentiatiated ZZ/ZW sex determination of turbot ( Scophthalmus maximus) . Genomics. 2021;113(4):1705–1718. doi: 10.1016/j.ygeno.2021.04.007. [DOI] [PubMed] [Google Scholar]
- Mei J, Gui JF Genetic basis and biotechnological manipulation of sexual dimorphism and sex determination in fish. Scientia Sinica Vitae. 2014;44(12):1198–1212. doi: 10.1360/zc2014-44-12-1198. [DOI] [PubMed] [Google Scholar]
- Palmer DH, Rogers TF, Dean R, et al How to identify sex chromosomes and their turnover. Molecular Ecology. 2019;28(21):4709–4724. doi: 10.1111/mec.15245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patro R, Duggal G, Love MI, et al Salmon provides fast and bias-aware quantification of transcript expression. Nature Methods. 2017;14(4):417–419. doi: 10.1038/nmeth.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peichel CL, Mccann SR, Ross JA, et al Assembly of the threespine stickleback Y chromosome reveals convergent signatures of sex chromosome evolution. Genome Biology. 2020;21(1):177. doi: 10.1186/s13059-020-02097-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin N Sex-chromosome evolution in frogs: what role for sex-antagonistic genes? Philosophical Transactions of the Royal Society B Biological Science. 2021;376(1832):20200094. doi: 10.1098/rstb.2020.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, et al PLINK: a tool set for whole-genome association and population-based linkage analyses. The American Journal of Human Genetics. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice WR The accumulation of sexually antagonistic genes as a selective agent promoting the evolution of reduced recombination between primitive sex chromosomes. Evolution. 1987;41(4):911–914. doi: 10.2307/2408899. [DOI] [PubMed] [Google Scholar]
- Rodríguez ME, Molina B, Merlo MA, et al Evolution of the Proto Sex-Chromosome in Solea senegalensis . International Journal of Molecular Sciences. 2019;20(20):511. doi: 10.3390/ijms20205111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao CW, Chen SL Sex-determining genes and its association with mechanism of sex chromosome evolution in Vertebrate. Journal of Agricultural Biotechnology. 2012;20(12):1463–1474. [Google Scholar]
- Shumate A, Wong B, Pertea G, et al Improved transcriptome assembly using a hybrid of long and short reads with StringTie. PLoS Computational Biology. 2022;18(6):e1009730. doi: 10.1371/journal.pcbi.1009730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang HB, Bowers JE, Wang XY, et al Synteny and collinearity in plant genomes. Science. 2008;320(5875):486–488. doi: 10.1126/science.1153917. [DOI] [PubMed] [Google Scholar]
- Tang LL, Huang F, You WX, et al CeRNA crosstalk mediated by ncRNAs is a novel regulatory mechanism in fish sex determination and differentiation. Genome Research. 2022;32(8):1502–1515. doi: 10.1101/gr.275962.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Auwera GA, Carneiro MO, Hartl C, et al. 2013. From FastQ data to high confidence variant calls: the Genome Analysis Toolkit best practices pipeline. Current Protocols in Bioinformatics, 43(1110): 11.10. 1–11.10. 33.
- Van Doorn GS & Kirkpatrick M Turnover of sex chromosomes induced by sexual conflict. Nature. 2007;449(7164):909–912. doi: 10.1038/nature06178. [DOI] [PubMed] [Google Scholar]
- Vicoso B Molecular and evolutionary dynamics of animal sex-chromosome turnover. Nature Ecology & Evolution. 2019;3(12):1632–1641. doi: 10.1038/s41559-019-1050-8. [DOI] [PubMed] [Google Scholar]
- Wang JP, Na JK, Yu QY, et al Sequencing papaya X and Y h chromosomes reveals molecular basis of incipient sex chromosome evolution . Proceedings of the National Academy of Sciences of the United States of America. 2012;109(34):13710–13715. doi: 10.1073/pnas.1207833109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Tian YS, Zhang JJ, et al Involvement of glycolysis activation in flatfish sexual size dimorphism: insights from transcriptomic analyses of Platichthys stellatus and Cynoglossus semilaevis . Comparative Biochemistry and Physiology Part D: Genomics and Proteomics. 2021;39:100832. doi: 10.1016/j.cbd.2021.100832. [DOI] [PubMed] [Google Scholar]
- Xu XW, Sun PC, Gao CB, et al Assembly of the poorly differentiated Verasper variegatus W chromosome by different sequencing technologies . Scientific Data. 2023;10(1):893. doi: 10.1038/s41597-023-02790-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZH PAML 4: phylogenetic analysis by maximum likelihood. Molecular Biology and Evolution. 2007;24(8):1586–1591. doi: 10.1093/molbev/msm088. [DOI] [PubMed] [Google Scholar]
- Zhou Q A chromosome minimalist view of genome regulation and evolution based on mouse chromosome engineering. Zoological research. 2022;43(6):949–951. doi: 10.24272/j.issn.2095-8137.2022.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Stephens M Genome-wide efficient mixed-model analysis for association studies. Nature Genetics. 2012;44(7):821–824. doi: 10.1038/ng.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu ZX, Matsubara K, Shams F, et al Diversity of reptile sex chromosome evolution revealed by cytogenetic and linked-read sequencing. Zoological Research. 2022;43(5):719–733. doi: 10.24272/j.issn.2095-8137.2022.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data to this article can be found online.
Data Availability Statement
The raw resequencing data can be obtained from the NCBI (BioProjectID PRJNA1178810), GSA (CRA015152) and Science Data Bank (doi: 10.57760/sciencedb.j00139.00087).