Abstract
Maternal sleep deprivation (MSD) has emerged as a significant public health concern, yet its effects on offspring metabolism remain poorly understood. This study investigated the metabolomic implications of MSD on offspring cognitive development, with a particular focus on alterations in glutamate metabolism. Pregnant rats were subjected to sleep deprivation during late gestation. Plasma and brain samples from their offspring were collected at different postnatal days (P1, P7, P14, and P56) and analyzed using untargeted metabolomics with liquid chromatography-mass spectrometry. Metabolomic analysis revealed significant differences in various amino acids, including L-glutamate, L-phenylalanine, L-tyrosine, and L-tryptophan, which are crucial for cognitive function. Subsequent differential analysis and partial least squares discriminant analysis (sPLS-DA) demonstrated a gradual reduction in these metabolic differences in the brain as the offspring underwent growth and development. KEGG pathway analysis revealed differential regulation of several pathways, including alanine, aspartate, and glutamate metabolism, glutathione metabolism, arginine biosynthesis, aminoacyl-tRNA biosynthesis, histidine metabolism, and taurine and hypotaurine metabolism, at different developmental stages. Mantel and Spearman analyses indicated that the observed changes in metabolites in MSD progeny may be related to various gut microbes, Ruminococcus_1, Ruminococcaceae_UCG-005, and Eubacterium_coprostanoligenes_group. Biochemical assays further demonstrated developmental changes in the L-glutamate metabolic pathway. Collectively, these findings suggest that MSD not only affects maternal well-being but also has enduring metabolic consequences for offspring, particularly impacting pathways linked to cognitive function. This highlights the importance of addressing maternal sleep health to mitigate potential long-term consequences for offspring.
Keywords: Maternal sleep deprivation, Glutamate metabolism, Metabolomics, Cognitive development, Offspring
INTRODUCTION
Maternal sleep deprivation (MSD) has become an increasingly prevalent concern, with a substantial proportion of expectant mothers experiencing inadequate rest. According to the National Sleep Foundation, between 46% and 78% of pregnant women suffer from sleep disorders ( Smyka et al., 2020). The implications of sleep deprivation during pregnancy extend beyond maternal discomfort, influencing both maternal and fetal health. MSD during pregnancy can trigger a series of changes in neuroendocrine and immune responses, potentially disrupting uteroplacental circulation and impairing fetal brain development. These physiological changes can increase the susceptibility of offspring to emotional disorders in adulthood, such as anxiety and depression ( Pires et al., 2010). Furthermore, MSD during pregnancy can alter the developmental trajectory of offspring, manifesting as disruptions in the sleep-wake cycle, increased risk of hypertension, dysregulation of the hypothalamic-pituitary-adrenal axis, compromised renal function, and changes in sex hormone levels ( Alvarenga et al., 2013; Aswathy et al., 2018; Lima et al., 2014).
A bidirectional relationship exists between sleep and metabolism, whereby sleep disturbances can impact metabolic function, and metabolic imbalances can affect sleep patterns ( Davies et al., 2014). Sleep deprivation and related disorders have been closely linked to alterations in glucose metabolism, insulin resistance, dysregulated appetite hormones, and increased risk of obesity and type 2 diabetes ( Shigiyama et al., 2018). Adequate sleep is essential for maintaining the balance of neurotransmitters in the brain, including that of dopamine, serotonin, and norepinephrine. Disruptions in sleep can lead to neurotransmitter disruption, affecting mood, cognition, and appetite regulation ( Omond et al., 2022). Long-term sleep deprivation can have numerous deleterious effects on the body, including psychological disorders (such as low mood, depression, and anxiety), decreased attention and memory, and impaired learning ability ( Banks & Dinges, 2007; Rasch & Born, 2013). Furthermore, sleep disturbances during pregnancy can lead to a variety of metabolic disorders, including disruptions in sugar metabolism and hormone levels ( O'Keeffe & St-Onge, 2013; Parry et al., 2006)
Although extensive research has demonstrated the impact of sleep on maternal health during pregnancy, the relationship between maternal sleep disturbances and fetal health remains inadequately explored. Preliminary studies suggest that conditions such as obstructive sleep apnea, sleep disturbances, and maternal sleep position may adversely impact fetal development, potentially leading to changes in growth patterns, variations in gestational length, and even fetal death ( Warland et al., 2018). In rodent models, MSD has been shown to impair cognitive development in offspring and elevate the risk of depression ( Peng et al., 2016; Wu et al., 2014). Additionally, postnatal interventions, such as prolonged exposure to enriched environments, have been found to ameliorate the cognitive deficits in offspring resulting from MSD ( Wei et al., 2024; Zhang et al., 2023). However, the precise mechanisms by which MSD affects cognitive development in offspring remain unclear.
In a recent study, we found that MSD during pregnancy significantly impairs hippocampal CA1 synaptic transmission and long-term potentiation (LTP) ( Peng et al., 2016). We also demonstrated that MSD reduces GluA2-containing α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors at hippocampal synapses in offspring, leading to emotional and cognitive dysfunction ( Yu et al., 2018). To further explore the mechanisms by which MSD affects cognitive development, we conducted comprehensive metabolomic analyses of brain and blood samples from offspring at different developmental stages. This study aims to provide a foundation for future diagnostic and therapeutic strategies to mitigate the adverse effects of MSD on offspring, thereby improving long-term health outcomes.
MATERIALS AND METHODS
Animal and MSD model
Sprague-Dawley rats were obtained from the Animal Care Center of Chongqing Medical University and mated at the Children’s Hospital of Chongqing Medical University. Pregnant rats were individually housed in ventilated cages with unrestricted access to food and water, maintained at a controlled temperature (23–25°C) under a 12/12 h light/dark cycle (0730h–1930h). The rats were acclimated to these conditions by gentle handling for 6 days (5 min/cage/day), starting on day 15 of pregnancy ( Vecsey et al., 2013). Subsequently, the rats were randomly assigned into an MSD group and a control group. The MSD model was established during late pregnancy (gestation days 15–21) through gentle handling for 6 h per day (1200h–1800h) ( Peng et al., 2016; Radhakrishnan et al., 2015), which effectively prevented the pregnant rats from sleeping. Gentle physical stimuli, such as tapping the cage or lightly touching the rats, were used to keep the animals awake. This MSD model was established to minimize stress on the pregnant rats. All animal experiments were performed in accordance with the Chongqing Science and Technology Commission guidelines and approved by the Animal Ethics Committee of the Children’s Hospital of Chongqing Medical University (approval number CHCMU-IACUC20210114017). Every effort was made to alleviate animal suffering and reduce the number of animals used.
Sample collection and processing
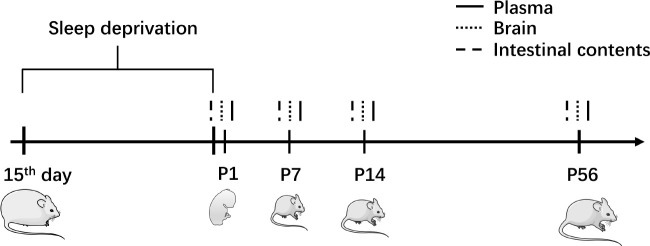
As shown in Figure 1, plasma and brain samples were collected from offspring at four developmental stages: postnatal day 1 (P1) for control and MSD groups (Ctrl-P1: n=10; MSD-P1: n=8), postnatal day 7 (P7) for control and MSD groups (Ctrl-P7: n=12; MSD-P7: n=8), postnatal day 14 (P14) for control and MSD groups (Ctrl-P14: n=11; MSD-P14: n=8), and postnatal day 56 (P56) for control and MSD groups (Ctrl-P56: n=11; MSD-P56: n=8). Plasma was obtained by collecting arterial blood from decapitated pups into anticoagulant EP tubes. The samples were centrifuged at 1 000 rpm for 10 min at room temperature, with the resulting supernatant aspirated and stored at −80°C.
Figure 1.
Animal model and sampling
Pregnant rats were exposed to MSD for 6 h daily during gestation days 15–21, with prior acclimation to gentle handling procedures. Plasma, brain tissue, and intestinal content samples were collected from offspring on postnatal days 1, 7, 14, and 56 (P1, P7, P14, and P56, respectively).
Each brain was bisected; the left hemisphere was placed in a centrifuge tube and immediately immersed in liquid nitrogen, while the right hemisphere was dissected into hippocampal and cortical regions, each placed into separate centrifuge tubes and immersed in liquid nitrogen. All samples were subsequently transferred to a −80°C freezer for preservation.
Metabolite extraction
Plasma samples (50 μL) were centrifuged at 14 000 × g for 10 min at 4–8°C, with the resulting supernatant transferred to a new 1.5 mL microcentrifuge tube. Methanol cooled to −80°C was added to the supernatant to achieve a final 80% (v/v) methanol solution. The mixture was vortexed and left at −20°C for 60 min, then centrifuged at 14 000 × g for 20 min at 4°C. The final supernatant was transferred to a new 1.5 mL microcentrifuge tube and freeze-dried to form a precipitate. Whole-brain tissue samples were weighed and ground in liquid nitrogen, then mixed with methanol and water (v/v 4:1). Subsequent procedures were identical to those used for the plasma samples.
Liquid chromatography-mass spectrometry (LC-MS)
Mass spectrometry was performed using the Sciex TripleTOF 6600 system (AB SCIEX, USA) equipped with an electrospray ionization (ESI) source in both positive and negative ionization modes. The ESI source conditions were as follows: nebulizer (Gas 1), 50 psi; heater (Gas 2), 45 psi; curtain gas flow, 30 psi; source temperature, 550°C; ion spray voltage floating, +5 500 V (+) and −4 500 V (−).
The mass spectrometer was coupled with a Shimadzu high-performance liquid chromatograph (HPLC) (ExionLC™ AD, USA). Separations were performed using an ACQUITY UPLC HSS T3 column (100 mm × 2.1 mm, 1.8 μm, Waters, USA) and ACQUITY UPLC BEH amide column (100 mm × 2.1 mm, 1.7 μm, Waters, USA). The T3 column was operated at a flow rate of 0.3 mL/min with water containing 0.1% formic acid (A) and acetonitrile (B) as mobile phases. The amide column was operated at a flow rate of 0.2 mL/min with a gradient elution of 15 mmol/L ammonium formate (A) and acetonitrile (B). A 5 μL sample volume was injected for each analysis. To minimize the potential impact of fluctuations in instrument detection signals, samples were kept in an automatic injector at 4°C and analyzed in random order. Quality control (QC) samples were inserted at regular intervals throughout the analysis to monitor and assess system stability and data reliability.
Biochemical assays
Concentrations of glutamate, glutathione, and cysteine in blood, hippocampal, and cortical samples were detected using a Glutamate measurement kit (Sangon, China), Reduced glutathione (GSH) assay kit (Jiancheng, China), and Cysteine (Cys) content test kit (Jiancheng, China), respectively.
Metabolite data processing and annotation
The MS-derived raw data were subjected to data preprocessing using Compound Discoverer (v.3.0) (Thermo Fisher Scientific, USA), including peak extraction, peak alignment, peak calibration, and normalization. Metabolite structural identification was performed using accurate mass matching (<25 ppm) and tandem mass spectrometry (MS/MS) spectral matching. Annotation of LC-MS-based untargeted metabolomic data was performed using MetDNA2 (http://metdna.zhulab.cn/) ( Shen et al., 2019).
Statistical analysis
Supervised partial least squares discriminant analysis (sPLS-DA) was performed using R (v.4.2.2) and the mixOmics (v.6.22.0) and MASS (v.7.3.60) packages. The sPLS-DA model was implemented with variable importance in projection (VIP)>1 and P<0.05. Volcano plot analysis was conducted with a significance threshold of P<0.05 using the ggrepel (v.0.9.4) and ggplot2 (v.3.4.4) packages, while heatmap visualization was performed using the pheatmap (v.1.0.12) package in R.
Spearman rank correlation coefficients were determined using the Mantel test in ggcorrplot (v.0.1.4.1) to investigate the correlations between gut microbiota and blood/brain metabolites. Gut microbiota sequencing data were obtained from our previous research ( Yao et al., 2022). Data analysis was carried out using GraphPad Prism (v.8.0), applying Student t-tests to assess significance, which was determined at P<0.05.
Receiver operating characteristic (ROC) analysis was utilized to predict biomarkers in MSD offspring. Average ROC curves were constructed using outcomes from bootstrap resampling, and the area under the curve (AUC) was determined for mean ROC curves. Metabolites with an AUC value greater than 0.5 were considered potential biomarkers for MSD offspring.
Functional enrichment analysis
Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) enrichment analyses were performed to determine changes in biological pathways and functions caused by differential metabolites. Enrichment analyses were performed using the Genomes (KEGG) database and MetaboAnalyst (v.3.0) (http://www.metaboanalyst.ca/MetaboAnalyst/).
RESULTS
Alterations in plasma metabolites in MSD offspring
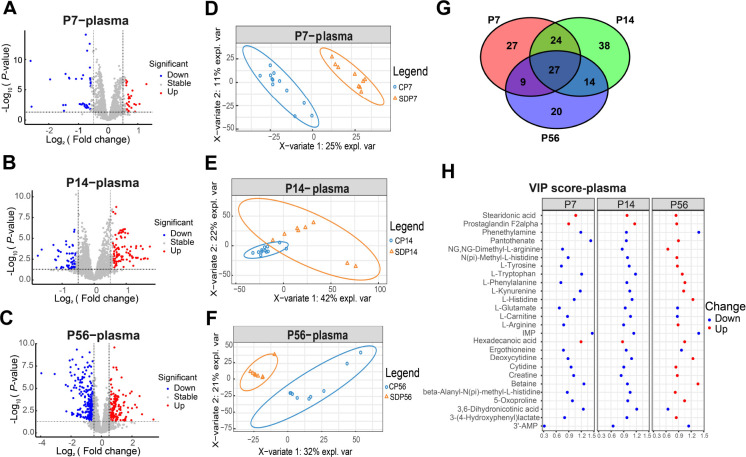
Untargeted metabolomic analysis of plasma samples from offspring at different developmental stages (P7, P14, P56) in both the MSD and control groups revealed significant alterations ( Figure 2A–I). Data from P1 were excluded from Figure 2 and Supplementary Table S1 due to varying degrees of hemolysis during plasma collection. LC-MS profiling identified a total of 7 533 peaks. At P7, 58 metabolites were significantly reduced and 64 were significantly elevated. At P14, 110 metabolites were significantly decreased and 169 were markedly increased. At P56, 294 metabolites were significantly reduced and 128 were markedly elevated ( Figure 2A–C). The top 50 differentially expressed metabolites at each developmental stage were visualized through hierarchical cluster heatmaps (Supplementary Figure S1). The sPLS-DA results are depicted in Figure 2D–F.
Figure 2.
Untargeted metabolomic analysis of plasma samples from offspring at different developmental stages
A–C: Volcano plot of differential metabolites between MSD and control offspring at P7 (A), P14 (B), and P56 (C). D–F: sPLS-DA of metabolomes between MSD and control offspring at P7 (D), P14 (E), and P56 (F). G: Venn diagram of overlapping differential metabolites between MSD and control offspring at different time points in plasma. H: VIP scores of 27 common differential metabolites identified in the Venn diagram.
Differential analysis between the MSD and control groups identified significant changes in 27 metabolites across all three developmental time points (P7, P14, and P56) ( Figure 2G; Supplementary Table S1). The VIP scores for these metabolites are shown in Figure 2H. Most of these metabolites were amino acids and their derivatives, including L-tryptophan, L-histidine, L-glutamate, L-carnitine, and L-phenethylamine, which are closely associated with brain function. L-tryptophan and L-histidine, essential for neurotransmitter synthesis ( Wurtman, 2011), showed lower concentrations during early development in MSD-exposed offspring, but higher blood concentrations in adulthood. L-glutamate, a critical excitatory neurotransmitter, showed a decreasing, though not statistically significant (VIP<1), trend, as did its precursor, glutamine. Previous untargeted LC-MS analyses have demonstrated that sleep deprivation is associated with elevated blood levels of L-glutamate ( Hu et al., 2021). Betaine, which plays a key role in methylation ( Zhao et al., 2018), and 5-oxoproline, known for its antioxidant properties ( Pederzolli et al., 2007), are both associated with cognitive function. Pantothenate (vitamin B5) is crucial for energy metabolism and serves as a precursor for coenzyme A (CoA), which is involved in multiple metabolic pathways. L-carnitine supports energy metabolism via the transportation of fatty acids ( Madsen et al., 2018). Stearidonic acid, an omega-3 polyunsaturated fatty acid, exhibits potential neuroprotective benefits. Studies on acute sleep deprivation have also reported significant increases in tryptophan, biogenic amines, serotonin, and taurine ( Davies et al., 2014), which may directly impact offspring development during pregnancy.
Alterations in brain metabolites in MSD offspring
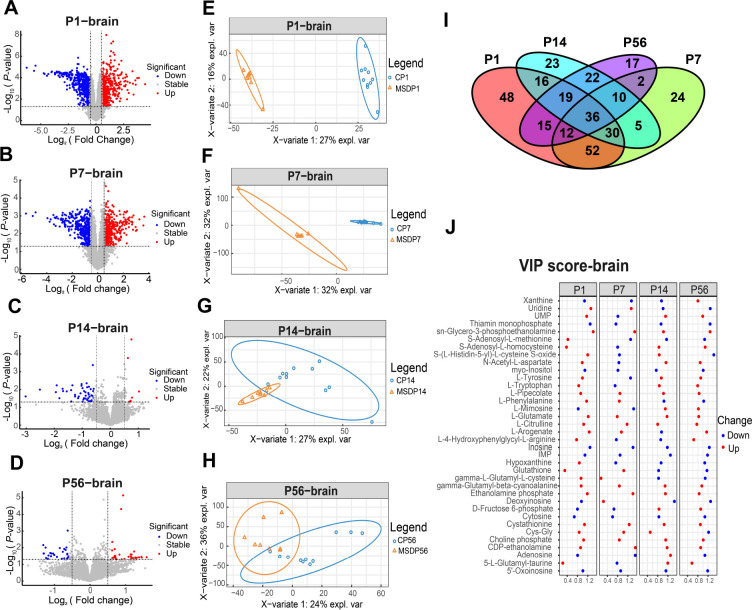
Untargeted metabolomics analysis was conducted on brain samples from MSD-exposed offspring at different developmental stages (P1, P7, P14, and P56). This included variance analysis with volcano plots ( Figure 3A–D), sPLS-DA analysis ( Figure 3E–H), and hierarchical clustering analysis of the top 50 differential metabolites at each time point visualized using heatmaps (Supplementary Figure S2). LC-MS analysis identified a total of 7 533 peaks. As shown in Figure 3A–D, distinct metabolic differences were observed in the brain samples of MSD-exposed offspring at each developmental stage. At P1, 600 metabolites were elevated, while 559 were reduced. At P7, 665 metabolites were increased, with 619 decreased. At P14, 150 metabolites were increased, with 146 decreased. At P56, 37 metabolites were elevated and 16 were reduced. These findings demonstrate a progressive decline in the number of differentially abundant metabolites between MSD and control offspring with age, with the fewest changes observed at P56. The sPLS-DA results ( Figure 3E–H) corroborated this trend, showing a reduction in metabolic profile divergence between MSD and control offspring as they aged. These findings suggest that MSD profoundly impacts brain development in offspring, with metabolic alterations normalizing over time. Our previous research demonstrated cognitive impairments in adult MSD-impacted offspring ( Peng et al., 2016), indicating that these deficits may originate during development, thus highlighting the importance of early-life interventions and potential for restoration in the adult brain.
Figure 3.
Untargeted metabolomic analysis of brain samples from offspring at different developmental stages
A–D: Volcano plot of differential metabolites between MSD and control offspring at P1 (A), P7 (B), P14 (C), and P56 (D). E–H: sPLS-DA of metabolomes between MSD and control offspring at P1 (E), P7 (F), P14 (G), and P56 (H). I: Venn diagram of differential metabolites between MSD and control groups at different time points in the brain. J: VIP scores of 36 common differential metabolites identified in the Venn diagram.
Metabolites in brain samples from MSD and control offspring were analyzed at four developmental stages (P1, P7, P14, and P56), with a total of 36 metabolites found to exhibit significant changes across all stages ( Figure 3I; Supplementary Table S2). The VIP scores of these metabolites are shown in Figure 3J. In contrast to the plasma results, L-glutamate levels were elevated in the brains of MSD offspring. Glutathione levels increased during the neonatal and early childhood stages but were lower than those of the control group in adolescence and adulthood. Reduced glutathione biosynthesis can lead to increased cytoplasmic glutamate and miniature excitatory postsynaptic current (mEPSC) frequency, suggesting that glutathione may serve as a physiological reservoir for glutamate neurotransmission ( Sedlak et al., 2019). Phospholipid derivatives, such as sn-glycero-3-phosphoethanolamine, choline phosphate, ethanolamine phosphate, and inositol, are essential for cell membrane structure and function ( Feng et al., 2020; Hadinoto et al., 2013; Kim et al., 2013; White et al., 2021). Abnormalities in membrane function can disrupt neurotransmitter release and reuptake, impact neuronal development and connectivity, and influence synaptic plasticity ( Wheal et al., 1998). Our previous study showed that MSD increases AMPA receptor internalization ( Yu et al., 2018), decreases LTP, and reduces neuronal generation ( Peng et al., 2016), all of which impair learning and cognitive functions in offspring. These findings support the notion that disturbances in cell membrane function can profoundly affect synaptic transmission and cognitive development. Additionally, metabolites involved in antioxidant and redox processes, including gamma-L-glutamyl-L-cysteine, 5-L-glutamyl-taurine, cystathionine, and glutathione, displayed significant changes, indicating perturbation of antioxidant defense mechanisms in offspring ( Wurtman, 2008).
Dysregulation of glutamate and cognitive-related protein metabolism
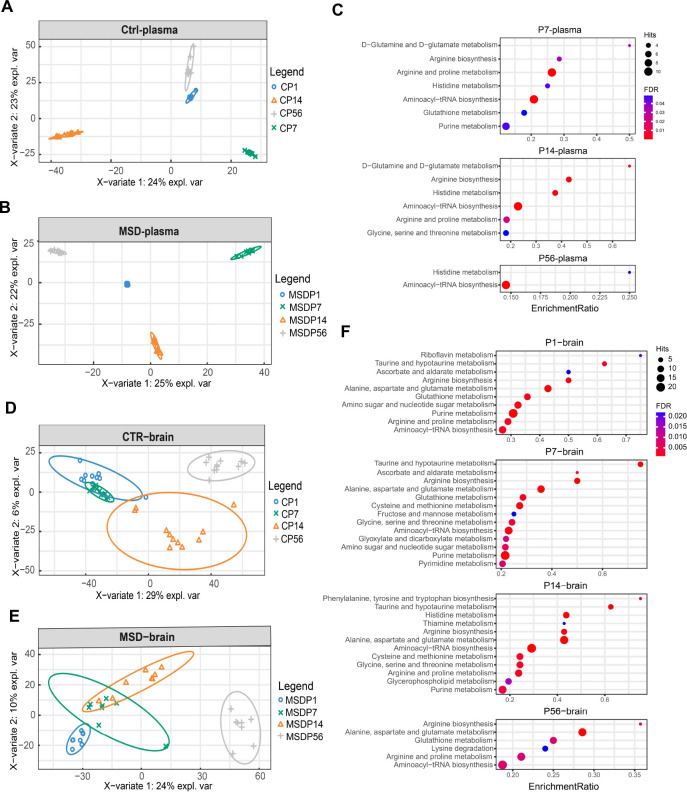
To investigate the impact of MSD on metabolite profiles and developmental outcomes in offspring, sPLS-DA ( Figure 4A, B) and pathway analysis ( Figure 4C) were conducted on blood samples collected at various developmental stages. The results identified significant changes in pathways involved in amino acid metabolism and RNA transcription (false discovery rate (FDR)<0.1, Supplementary Table S3). Over time, MSD offspring exhibited notable abnormalities in several metabolic pathways, including D-glutamine and D-glutamate metabolism, arginine biosynthesis, histidine metabolism, and phenylalanine metabolism (FDR<0.1, Supplementary Table S3). These pathways are closely associated with neurotransmission and cognitive function ( He et al., 2024; Petroff, 2002; Sutanto et al., 2022), indicating that disruptions in these pathways may contribute to the cognitive developmental consequences observed in MSD-exposed offspring.
Figure 4.
sPLS-DA and pathway analysis of MSD and control offspring
A–B: sPLS-DA plots of metabolomes in control (A) and MSD plasma (B) across all developmental stages. C: Graphical representation of pathway analysis of plasma samples (FDR<0.05). D, E: sPLS-DA plots of metabolomes in control (D) and MSD brain (E) across all developmental stages. F: Graphical representation of pathway analysis of brain samples (FDR<0.05).
The sPLS-DA results of brain samples ( Figure 4D, E) revealed that metabolic profiles in P14 MSD-exposed offspring were more closely aligned with those of earlier developmental stages (P1 and P7) than with control counterparts. Further examination of brain metabolite pathways demonstrated that MSD exerts a profound and enduring impact on brain metabolism in offspring. Key metabolic pathways affected included alanine, aspartate, and glutamate metabolism, glutathione metabolism, arginine biosynthesis, and aminoacyl-tRNA biosynthesis ( Figure 4F; Supplementary Table S4). Consistent with the plasma results, MSD significantly disrupted the metabolism of amino acids essential for cognitive function in offspring, corroborating previous research on the metabolic effects of sleep deprivation ( Yoon et al., 2019). Changes in taurine and hypotaurine metabolism were observed at P1, P7, and P14. Acute sleep deprivation has been shown to markedly increase taurine levels, potentially contributing to its antidepressant effects ( Davies et al., 2014). This suggests that metabolic changes associated with insufficient sleep during pregnancy may be transmitted to the fetus and persist during growth and development.
Metabolite biomarker and correlation analyses of gut microbiota
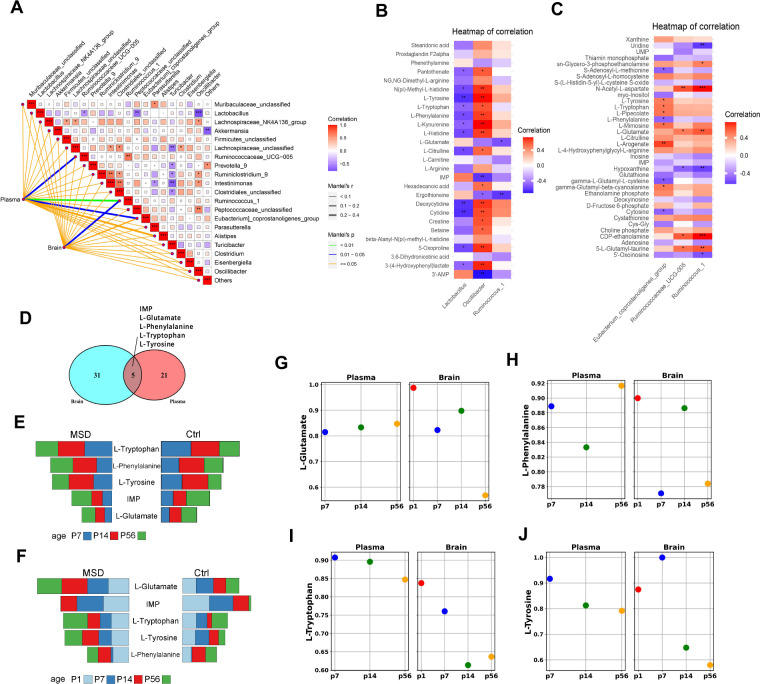
To further explore the connection between glutamate metabolism and gut microbiota, Mantel tests were conducted to assess correlations between gut microbiota and plasma (27) and brain (36) metabolites showing long-term changes ( Figure 5A). Significant correlations were detected between plasma metabolites and gut microbes Ruminococcus_1, Ruminococcaceae_UCG-005, and Eubacterium_coprostanoligenes_group, as well as between brain metabolites and Ruminococcus_1 (Mantel P<0.05, Figure 5A). Both Ruminococcus_1 and Ruminococcaceae_UCG-005 belong to the family Ruminococcaceae, which is involved in the production of short-chain fatty acids, particularly butyrate ( Liu et al., 2019). Ruminococcaceae abundance decreases with age ( Biagi et al., 2016), although Ruminococcaceae_UCG-005 taxa can be enhanced through physical activity ( Tabone et al., 2021). Eubacterium_coprostanoligenes_group is known to mediate the effects of a high-fat diet on dyslipidemia through cholic acid metabolism ( Wei et al., 2021), with oral administration shown to significantly reduce cholesterol levels ( Li et al., 1995).
Figure 5.
Analysis of relationship between metabolites and gut microbiota
A: Mantel analysis of correlation between common differential metabolites in plasma and brain with gut microbiota. B, C: Spearman analysis of correlation between identified gut microbiota from Mantel test and common differential metabolites in plasma (B) and brain samples (C). *: P<0.05; **: P<0.001; ***: P<0.0001. D: Venn diagram showcasing common differential metabolites in both brain and plasma. E, F: Comparative analysis of concentrations of five common differential metabolites in plasma (E) and brain samples (F) between MSD and control groups. G–J: Dots, with colors corresponding to AUCs depicted in Supplementary Figures S3, S4, signify areas under the ROC curves for metabolites exhibiting biomarker potential for MSD-exposed offspring. Ctrl: Control group. MSD: Maternal sleep deprivation group.
Spearman correlation analysis indicated that Lactobacillus and Oscillibacter were significantly correlated with various plasma metabolites, particularly those involved in amino acid metabolism, such as L-tyrosine, L-citrulline, L-tryptophan, L-kynurenine, L-phenylalanine, and L-histidine ( Figure 5B, C). Oscillibacter, a member of the Ruminococcaceae family, has been reported to reduce blood triglyceride levels and marginally impact body mass index and waist-to-hip ratio ( Liu et al., 2022). Similarly, Lactobacillus is directly involved in tryptophan metabolism, producing a wide range of tryptophan-derived metabolites ( Montgomery et al., 2022), and its abundance decreases when tryptophan intake is strictly limited ( Zapata et al., 2018). In conditions like chronic intermittent hypoxia, as observed in obstructive sleep apnea, an increase in Lactobacillus species occurs alongside a decrease in tryptophan levels ( Wang et al., 2022). In brain metabolites, positive correlations were observed between Ruminococcus_1, Ruminococcaceae_UCG-005, and L-glutamate, a crucial neurotransmitter in the central nervous system ( Figure 4C). The Ruminococcaceae family has been implicated in the synthesis of both glutamate and gamma aminobutyric acid (GABA) ( Radjabzadeh et al., 2022). Extensive correlations were identified between various members of the Ruminococcaceae family, including Ruminococcus_1, Ruminococcaceae_UCG-005, and Oscillibacter, and different metabolites. Previous studies have indicated that Ruminococcus_1 and Ruminococcaceae_UCG-005 show an increasing trend in sleep-deprived mothers and their offspring, correlating positively with neuroinflammatory factors IL-1β and TNF-α ( Yao et al., 2022). These findings suggest that the Ruminococcaceae family may be a promising target for future research aimed at ameliorating the impact of sleep deprivation on offspring.
The shared differential metabolites identified in both brain and plasma across different growth stages included L-glutamate, L-phenylalanine, L-tryptophan, L-tyrosine, and inosine monophosphate ( Figure 5D). Changes in the levels of these four amino acid metabolites between the MSD and control groups at different periods are shown in Figure 5E, F. ROC analysis and the corresponding AUC values are shown in Supplementary Figures S3, S4 and Figure 5G–J. The AUC values for L-glutamate in the brain were 0.99, 0.82, 0.90, and 0.57, suggesting that L-glutamate is a highly sensitive biomarker for detecting the effects of MSD in the brain ( Figure 5G; Supplementary Figure S4).
Disruption of glutamate metabolism in MSD offspring
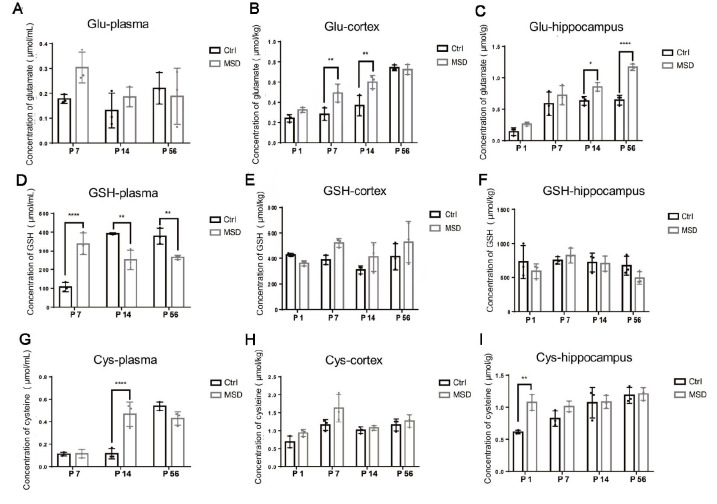
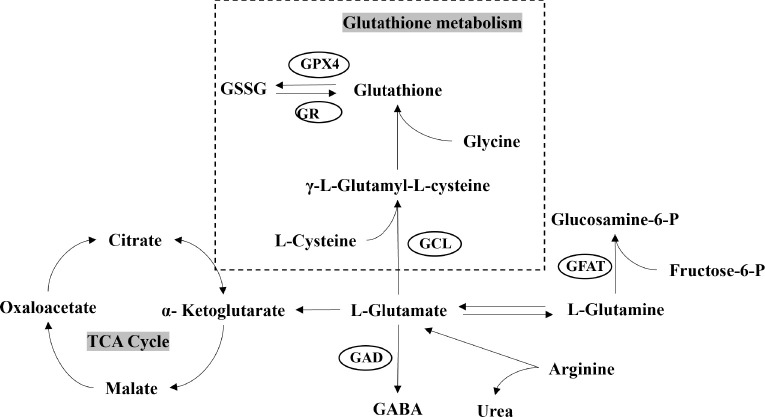
Biochemical assays revealed a significant elevation in glutamate levels at various developmental stages in the hippocampus and cortex of MSD offspring ( Figure 6B, C), while no notable differences were detected in the plasma ( Figure 6A). In contrast, plasma glutathione levels showed an initial rise during the early stage (P7), followed by a marked decline in the later stages (P14, P56) ( Figure 6D), with no significant differences observed in the cortex ( Figure 6E) or hippocampus ( Figure 6F). Cysteine levels also showed a consistent upward trend during development ( Figure 6G–I). Glutamate combines with cysteine and glycine under the catalytic action of glutamate-cysteine ligase (GCL) to form γ-glutamylcysteine, which is further modified by glutathione synthetase, incorporating an additional glutamate molecule to produce glutathione ( Wu et al., 2004) ( Figure 7). Consequently, the observed increases in glutamate and cysteine levels during early development led to a rise in glutathione production, while the subsequent reduction in glutathione levels during later stages may have resulted from a negative feedback regulation mechanism.
Figure 6.
Biochemical assessment of glutamate, glutathione, and cysteine levels
A–C: Glutamate concentrations in plasma (A), cortex (B), and hippocampus (C). D–F: Glutathione concentrations in plasma (D), cortex (E), and hippocampus (F). G–I: Cysteine concentrations in plasma (G), cortex (H), and hippocampus (I). Glu: Glutamate; GSH: Glutathione; Cys: Cysteine. Ctrl: Control group. MSD: Maternal sleep deprivation group. *: P<0.05; **: P<0.001.
Figure 7.
Overview of glutamate and glutathione metabolism
GSSG: Glutathione disulfide; GABA: Gamma-aminobutyric acid; GPX4: Glutathione peroxidase 4; GR: Glutathione reductase; GCL: γ-glutamylcysteine ligase; GFAT: Glutamine fructose-6-phosphate amidotransferase; GAD: Glutamate decarboxylase; TCA: Tricarboxylic acid cycle.
DISCUSSION
Untargeted metabolomic analysis of blood and brain samples from MSD-exposed offspring revealed significant differences compared to the control group. The disparity in brain metabolites between MSD-exposed offspring and controls gradually diminished over time, suggesting a possible normalization or compensatory mechanism as the brain adjusts its metabolic processes during development. Specifically, 27 blood metabolites and 36 brain metabolites displayed significant differences across various stages (Supplementary Tables S1, S2). Plasma samples from P1 were excluded due to hemolysis, likely caused by the susceptibility of neonatal vasculature and mechanical stresses of the collection procedure, which exacerbated red blood cell fragility. Pathway analysis identified key alterations in several metabolic pathways critical to development, including alanine, aspartate, and glutamate metabolism, glutathione metabolism, arginine biosynthesis, aminoacyl-tRNA biosynthesis and histidine metabolism. These pathways, which have been previously associated with the impact of sleep deprivation on metabolism ( Davies et al., 2014), highlight the extensive effects of MSD. These findings suggest that MSD not only affects the mother but also has cascading effects on the offspring, impacting their physiological and cognitive functions throughout growth.
L-glutamate, a critical amino acid and neurotransmitter in the central nervous system, is essential for physiological processes such as cognition, learning, and memory ( Chakraborty et al., 2023; Möller, 2023). Prior studies have shown that MSD elevates microglial activation in offspring, creating an inflammatory environment that triggers significant glutamate release ( Piani et al., 1992; Zhao et al., 2014). This release is facilitated through gap junction hemichannels composed of connexin proteins, which are up-regulated in response to brain injury and inflammation ( Yawata et al., 2008). Glutamate plays a pivotal role in multiple metabolic pathways and is closely associated with various differentially expressed metabolites identified in MSD-exposed offspring. It is synthesized via transamination, where an amino group is transferred to α-ketoglutarate, a process catalyzed by glutamate dehydrogenase. This enzyme also facilitates the reversible conversion of glutamate to α-ketoglutarate, a key intermediate of the tricarboxylic acid (TCA) cycle that participates in various key metabolic pathways ( Figure 7) ( Xiao et al., 2016) and is involved in ammonia and NAD(P)H interconversion ( Tapiero et al., 2002; Xiao et al., 2016). Sleep deprivation has been shown to increase glutamate levels while reducing glutamine ( Hu et al., 2021), thereby disrupting the highly active glutamate-glutamine cycle, which is essential for neurotransmitter balance, nitrogen regulation, and energy metabolism in the brain ( Ramadan et al., 2013). Moreover, glutamate serves as a precursor for the synthesis of GABA, an inhibitory neurotransmitter in the central nervous system. The balance between glutamate and GABA neurotransmission is essential for proper brain function and sleep regulation ( Petroff, 2002).
Our previous research showed that AMPA glutamate receptors undergo endocytosis, and inhibiting this process can improve cognition in MSD offspring ( Yu et al., 2018). Given this, we focused on changes in glutamate, proposing that sustained elevation of glutamate levels may induce a negative feedback mechanism, leading to the down-regulation of AMPA receptors on the postsynaptic membrane. This hypothesis is supported by evidence showing that prolonged exposure to high glutamate concentrations can diminish glutamate receptor functionality, potentially due to compensatory mechanisms aimed at preventing excitotoxicity and preserving synaptic stability. Persistently high levels of glutamate have been linked to various neurological conditions, including drug addiction, neurodegenerative disorders, and brain injury ( Kogan & Aghajanian, 1995; Yadav et al., 2023). The compensatory reduction in AMPA receptor expression in response to sustained high glutamate levels may serve as a protective adaptation to mitigate the risk of excitotoxic damage.
Emerging research has suggested that prenatal sleep deprivation can markedly impair hippocampal neurogenesis and cognitive development in offspring ( Zhao et al., 2008, 2014). Notably, studies have shown that intermittent sleep deprivation during pregnancy can increase proinflammatory markers while decreasing anti-inflammatory markers in the hippocampus of young animals, leading to reduced hippocampal neurogenesis, impaired spatial learning, compromised memory function, and diminished pleasure sensation ( Zhao et al., 2014). One potential explanation for these observations is the dysregulation of microglial activation, which plays a crucial role in mediating inflammatory responses ( Zhao et al., 2015). Activated microglia are known to release glutamate and adenosine triphosphate (ATP) ( Illes et al., 2020), which may account for the elevated glutamate levels observed in MSD-exposed offspring in the present study. Therefore, a key area of study should focus on determining whether MSD disrupts glutamate metabolism by activating microglial cells in offspring, thereby contributing to cognitive impairment. Future studies should also investigate whether modulating glutamate metabolism can alleviate synaptic and cognitive deficits in MSD-impacted offspring.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
Acknowledgments
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
W.T.H. and Z.F.D. conceived the study and wrote the manuscript. W.T.H. and Z.Y.Y. conducted MSD experiments and sampling. W.T.H., J.H.F., and Y.P.C. analyzed the data. W.T.H. and D.X.L. performed the biochemical assays. All authors read and approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank all other members of the Dong lab for helpful discussions and technical support.
Funding Statement
This work was supported by the National Natural Science Foundation of China (32371030, 82071395), Natural Science Foundation of Chongqing (CSTB2024NSCQ-LZX0008), Science and Technology Research Program of Chongqing Municipal Education Commission (KJQN202300424), and CQMU Program for Youth Innovation in Future Medicine (W0044)
DATA AVAILABILITY
The metabolomic MS data are available in the Open Archive for Miscellaneous Data under accession number OMIX004781 (https://ngdc.cncb.ac.cn/omix/).
References
- Alvarenga TA, Aguiar MFP, Mazaro-Costa R, et al Effects of sleep deprivation during pregnancy on the reproductive capability of the offspring. Fertility and Sterility. 2013;100(6):1752–1757. doi: 10.1016/j.fertnstert.2013.08.014. [DOI] [PubMed] [Google Scholar]
- Aswathy BS, Kumar VM, Gulia KK The effects of rapid eye movement sleep deprivation during late pregnancy on newborns' sleep. Journal of Sleep Research. 2018;27(2):197–205. doi: 10.1111/jsr.12564. [DOI] [PubMed] [Google Scholar]
- Banks S, Dinges DF Behavioral and physiological consequences of sleep restriction. Journal of Clinical Sleep Medicine. 2007;3(5):519–528. doi: 10.5664/jcsm.26918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagi E, Franceschi C, Rampelli S, et al Gut microbiota and extreme longevity. Current Biology. 2016;26(11):1480–1485. doi: 10.1016/j.cub.2016.04.016. [DOI] [PubMed] [Google Scholar]
- Chakraborty P, Dey A, Gopalakrishnan AV, et al Glutamatergic neurotransmission: a potential pharmacotherapeutic target for the treatment of cognitive disorders. Ageing Research Reviews. 2023;85:101838. doi: 10.1016/j.arr.2022.101838. [DOI] [PubMed] [Google Scholar]
- Davies SK, Ang JE, Revell VL, et al Effect of sleep deprivation on the human metabolome. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(29):10761–10766. doi: 10.1073/pnas.1402663111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Xin QW, Zhang WL, et al Cell-membrane-targeted drug delivery system based on choline-phosphate-functionalized β-cyclodextrin. Macromolecular Bioscience. 2020;20(12):2000069. doi: 10.1002/mabi.202000069. [DOI] [PubMed] [Google Scholar]
- Hadinoto K, Sundaresan A, Cheow WS Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: a review. European Journal of Pharmaceutics and Biopharmaceutics. 2013;85(3):427–443. doi: 10.1016/j.ejpb.2013.07.002. [DOI] [PubMed] [Google Scholar]
- He JM, Hou TY, Wang QW, et al L-arginine metabolism ameliorates age-related cognitive impairment by Amuc_1100-mediated gut homeostasis maintaining. Aging Cell. 2024;23(4):e14081. doi: 10.1111/acel.14081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Li PX, Zhang R, et al Integrated metabolomics and proteomics analysis reveals energy metabolism disorders in the livers of sleep-deprived mice. Journal of Proteomics. 2021;245:104290. doi: 10.1016/j.jprot.2021.104290. [DOI] [PubMed] [Google Scholar]
- Illes P, Rubini P, Ulrich H, et al Regulation of microglial functions by purinergic mechanisms in the healthy and diseased CNS. Cells. 2020;9(5):1108. doi: 10.3390/cells9051108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Hernandez MLG, Balla T Inositol lipid regulation of lipid transfer in specialized membrane domains. Trends in Cell Biology. 2013;23(6):270–278. doi: 10.1016/j.tcb.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan JH, Aghajanian GK Long-term glutamate desensitization in locus coeruleus neurons and its role in opiate withdrawal. Brain Research. 1995;689(1):111–121. doi: 10.1016/0006-8993(95)00545-2. [DOI] [PubMed] [Google Scholar]
- Li L, Buhman KK, Hartman PA, et al Hypocholesterolemic effect of Eubacterium coprostanoligenes ATCC 51222 in rabbits. Letters in Applied Microbiology. 1995;20(3):137–140. doi: 10.1111/j.1472-765X.1995.tb00410.x. [DOI] [PubMed] [Google Scholar]
- Lima ILB, Rodrigues AFAC, Bergamaschi CT, et al Chronic sleep restriction during pregnancy-repercussion on cardiovascular and renal functioning of male offspring. PLoS One. 2014;9(11):e113075. doi: 10.1371/journal.pone.0113075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SM, Li EY, Sun ZY, et al Altered gut microbiota and short chain fatty acids in Chinese children with autism spectrum disorder. Scientific Reports. 2019;9(1):287. doi: 10.1038/s41598-018-36430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XM, Tong X, Zou YQ, et al Mendelian randomization analyses support causal relationships between blood metabolites and the gut microbiome. Nature Genetics. 2022;54(1):52–61. doi: 10.1038/s41588-021-00968-y. [DOI] [PubMed] [Google Scholar]
- Madsen KL, Preisler N, Rasmussen J, et al L-Carnitine improves skeletal muscle fat oxidation in primary carnitine deficiency. The Journal of Clinical Endocrinology & Metabolism. 2018;103(12):4580–4588. doi: 10.1210/jc.2018-00953. [DOI] [PubMed] [Google Scholar]
- Möller HE Considerations on gradual glutamate accumulation related to cognitive task performance. Journal of Cerebral Blood Flow & Metabolism. 2023;43(3):476–478. doi: 10.1177/0271678X221139550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery TL, Eckstrom K, Lile KH, et al Lactobacillus reuteri tryptophan metabolism promotes host susceptibility to CNS autoimmunity . Microbiome. 2022;10(1):198. doi: 10.1186/s40168-022-01408-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keeffe M, St-Onge MP Sleep duration and disorders in pregnancy: implications for glucose metabolism and pregnancy outcomes. International Journal of Obesity. 2013;37(6):765–770. doi: 10.1038/ijo.2012.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omond SET, Hale MW, Lesku JA Neurotransmitters of sleep and wakefulness in flatworms. Sleep. 2022;45(5):zsac053. doi: 10.1093/sleep/zsac053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry BL, Martínez LF, Maurer EL, et al Sleep, rhythms and women's mood. Part I. Menstrual cycle, pregnancy and postpartum. Sleep Medicine Reviews. 2006;10(2):129–144. doi: 10.1016/j.smrv.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Pederzolli CD, Sgaravatti ÂM, Braum CA, et al 5-Oxoproline reduces non-enzymatic antioxidant defenses in vitro in rat brain . Metabolic Brain Disease. 2007;22(1):51–65. doi: 10.1007/s11011-006-9041-2. [DOI] [PubMed] [Google Scholar]
- Peng Y, Wang W, Tan T, et al Maternal sleep deprivation at different stages of pregnancy impairs the emotional and cognitive functions, and suppresses hippocampal long-term potentiation in the offspring rats. Molecular Brain. 2016;9:17. doi: 10.1186/s13041-016-0197-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroff OAC GABA and glutamate in the human brain. The Neuroscientist. 2002;8(6):562–573. doi: 10.1177/1073858402238515. [DOI] [PubMed] [Google Scholar]
- Piani D, Spranger M, Frei K, et al Macrophage-induced cytotoxicity of N-methyl-D-aspartate receptor positive neurons involves excitatory amino acids rather than reactive oxygen intermediates and cytokines. European Journal of Immunology. 1992;22(9):2429–2436. doi: 10.1002/eji.1830220936. [DOI] [PubMed] [Google Scholar]
- Pires GN, Andersen ML, Giovenardi M, et al Sleep impairment during pregnancy: possible implications on mother-infant relationship. Medical Hypotheses. 2010;75(6):578–582. doi: 10.1016/j.mehy.2010.07.036. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan A, Aswathy BS, Kumar VM, et al Sleep deprivation during late pregnancy produces hyperactivity and increased risk-taking behavior in offspring. Brain Research. 2015;1596:88–98. doi: 10.1016/j.brainres.2014.11.021. [DOI] [PubMed] [Google Scholar]
- Radjabzadeh D, Bosch JA, Uitterlinden AG, et al Gut microbiome-wide association study of depressive symptoms. Nature Communications. 2022;13(1):7128. doi: 10.1038/s41467-022-34502-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadan S, Lin A, Stanwell P Glutamate and glutamine: a review of in vivo MRS in the human brain . NMR in Biomedicine. 2013;26(12):1630–1646. doi: 10.1002/nbm.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasch B, Born J About sleep's role in memory. Physiological Reviews. 2013;93(2):681–766. doi: 10.1152/physrev.00032.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlak TW, Paul BD, Parker GM, et al The glutathione cycle shapes synaptic glutamate activity. Proceedings of the National Academy of Sciences of the United States of America. 2019;116(7):2701–2706. doi: 10.1073/pnas.1817885116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen XT, Wang RH, Xiong X, et al Metabolic reaction network-based recursive metabolite annotation for untargeted metabolomics. Nature Communications. 2019;10(1):1516. doi: 10.1038/s41467-019-09550-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigiyama F, Kumashiro N, Tsuneoka Y, et al Mechanisms of sleep deprivation-induced hepatic steatosis and insulin resistance in mice. American Journal of Physiology - Endocrinology and Metabolism. 2018;315(5):E848–E858. doi: 10.1152/ajpendo.00072.2018. [DOI] [PubMed] [Google Scholar]
- Smyka M, Kosińska-Kaczyńska K, Sochacki-Wójcicka N, et al Sleep problems in pregnancy-a cross-sectional study in over 7000 pregnant women in Poland. International Journal of Environmental Research and Public Health. 2020;17(15):5306. doi: 10.3390/ijerph17155306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutanto CN, Loh WW, Kim JE The impact of tryptophan supplementation on sleep quality: a systematic review, meta-analysis, and meta-regression. Nutrition Reviews. 2022;80(2):306–316. doi: 10.1093/nutrit/nuab027. [DOI] [PubMed] [Google Scholar]
- Tabone M, Bressa C, García-Merino JA, et al The effect of acute moderate-intensity exercise on the serum and fecal metabolomes and the gut microbiota of cross-country endurance athletes. Scientific Reports. 2021;11(1):3558. doi: 10.1038/s41598-021-82947-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapiero H, Mathé G, Couvreur P, et al II. Glutamine and glutamate. Biomedicine & Pharmacotherapy. 2002;56(9):446–457. doi: 10.1016/s0753-3322(02)00285-8. [DOI] [PubMed] [Google Scholar]
- Vecsey CG, Wimmer MEJ, Havekes R, et al Daily acclimation handling does not affect hippocampal long-term potentiation or cause chronic sleep deprivation in mice. Sleep. 2013;36(4):601–607. doi: 10.5665/sleep.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Zou JJ, Xu HJ, et al Effects of chronic intermittent hypoxia and chronic sleep fragmentation on gut microbiome, serum metabolome, liver and adipose tissue morphology. Frontiers in Endocrinology. 2022;13:820939. doi: 10.3389/fendo.2022.820939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warland J, Dorrian J, Morrison JL, et al Maternal sleep during pregnancy and poor fetal outcomes: a scoping review of the literature with meta-analysis. Sleep Medicine Reviews. 2018;41:197–219. doi: 10.1016/j.smrv.2018.03.004. [DOI] [PubMed] [Google Scholar]
- Wei RM, Zhang YM, Zhang KX, et al An enriched environment ameliorates maternal sleep deprivation-induced cognitive impairment in aged mice by improving mitochondrial function via the Sirt1/PGC-1α pathway. Aging. 2024;16(2):1128–1144. doi: 10.18632/aging.205385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Jiang WB, Tian Z, et al Fecal g. Streptococcus and g. Eubacterium_coprostanoligenes_group combined with sphingosine to modulate the serum dyslipidemia in high-fat diet mice . Clinical Nutrition. 2021;40(6):4234–4245. doi: 10.1016/j.clnu.2021.01.031. [DOI] [PubMed] [Google Scholar]
- Wheal HV, Chen Y, Mitchell J, et al Molecular mechanisms that underlie structural and functional changes at the postsynaptic membrane during synaptic plasticity. Progress in Neurobiology. 1998;55(6):611–640. doi: 10.1016/S0301-0082(98)00026-4. [DOI] [PubMed] [Google Scholar]
- White CJ, Ellis JM, Wolfgang MJ The role of ethanolamine phosphate phospholyase in regulation of astrocyte lipid homeostasis. Journal of Biological Chemistry. 2021;297(1):100830. doi: 10.1016/j.jbc.2021.100830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GY, Fang YZ, Yang S, et al Glutathione metabolism and its implications for health. The Journal of Nutrition. 2004;134(3):489–492. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- Wu MF, Li XY, Feng B, et al Poor sleep quality of third-trimester pregnancy is a risk factor for postpartum depression. Medical Science Monitor. 2014;20:2740–2745. doi: 10.12659/MSM.891222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtman RJ Synapse formation and cognitive brain development: effect of docosahexaenoic acid and other dietary constituents. Metabolism. 2008;57(Suppl 2):S6–S10. doi: 10.1016/j.metabol.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtman RJ. 2011. Non-nutritional uses of nutrients. European Journal of Pharmacology, 668 Suppl 1: S10–S15.
- Xiao DF, Zeng LM, Yao K, et al The glutamine-alpha-ketoglutarate (AKG) metabolism and its nutritional implications. Amino Acids. 2016;48(9):2067–2080. doi: 10.1007/s00726-016-2254-8. [DOI] [PubMed] [Google Scholar]
- Yadav P, Podia M, Kumari SP, et al Glutamate receptor endocytosis and signaling in neurological conditions. Progress in Molecular Biology and Translational Science. 2023;196:167–207. doi: 10.1016/bs.pmbts.2022.10.001. [DOI] [PubMed] [Google Scholar]
- Yao ZY, Li XH, Zuo L, et al Maternal sleep deprivation induces gut microbial dysbiosis and neuroinflammation in offspring rats. Zoological Research. 2022;43(3):380–390. doi: 10.24272/j.issn.2095-8137.2022.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yawata I, Takeuchi H, Doi Y, et al Macrophage-induced neurotoxicity is mediated by glutamate and attenuated by glutaminase inhibitors and gap junction inhibitors. Life Sciences. 2008;82(21-22):1111–1116. doi: 10.1016/j.lfs.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Yoon SJ, Long NP, Jung KH, et al Systemic and local metabolic alterations in sleep-deprivation-induced stress: a multiplatform mass-spectrometry-based lipidomics and metabolomics approach. Journal of Proteome Research. 2019;18(9):3295–3304. doi: 10.1021/acs.jproteome.9b00234. [DOI] [PubMed] [Google Scholar]
- Yu YZ, Huang ZL, Dai CF, et al Facilitated AMPAR endocytosis causally contributes to the maternal sleep deprivation-induced impairments of synaptic plasticity and cognition in the offspring rats. Neuropharmacology. 2018;133:155–162. doi: 10.1016/j.neuropharm.2018.01.030. [DOI] [PubMed] [Google Scholar]
- Zapata RC, Singh A, Ajdari NM, et al Dietary tryptophan restriction dose-dependently modulates energy balance, gut hormones, and microbiota in obesity-prone rats. Obesity. 2018;26(4):730–739. doi: 10.1002/oby.22136. [DOI] [PubMed] [Google Scholar]
- Zhang YM, Wei RM, Li XY, et al Long-term environmental enrichment overcomes depression, learning, and memory impairment in elderly CD-1 mice with maternal sleep deprivation exposure. Frontiers in Aging Neuroscience. 2023;15:1177250. doi: 10.3389/fnagi.2023.1177250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao CM, Deng W, Gage FH Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132(4):645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Zhao GF, He F, Wu CL, et al Betaine in inflammation: mechanistic aspects and applications. Frontiers in Immunology. 2018;9:1070. doi: 10.3389/fimmu.2018.01070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao QY, Peng C, Wu XH, et al Maternal sleep deprivation inhibits hippocampal neurogenesis associated with inflammatory response in young offspring rats. Neurobiology of Disease. 2014;68:57–65. doi: 10.1016/j.nbd.2014.04.008. [DOI] [PubMed] [Google Scholar]
- Zhao QY, Xie XF, Fan YH, et al Phenotypic dysregulation of microglial activation in young offspring rats with maternal sleep deprivation-induced cognitive impairment. Scientific Reports. 2015;5:9513. doi: 10.1038/srep09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data to this article can be found online.
Data Availability Statement
The metabolomic MS data are available in the Open Archive for Miscellaneous Data under accession number OMIX004781 (https://ngdc.cncb.ac.cn/omix/).