Abstract
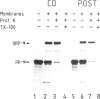
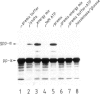
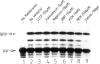
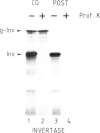
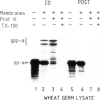
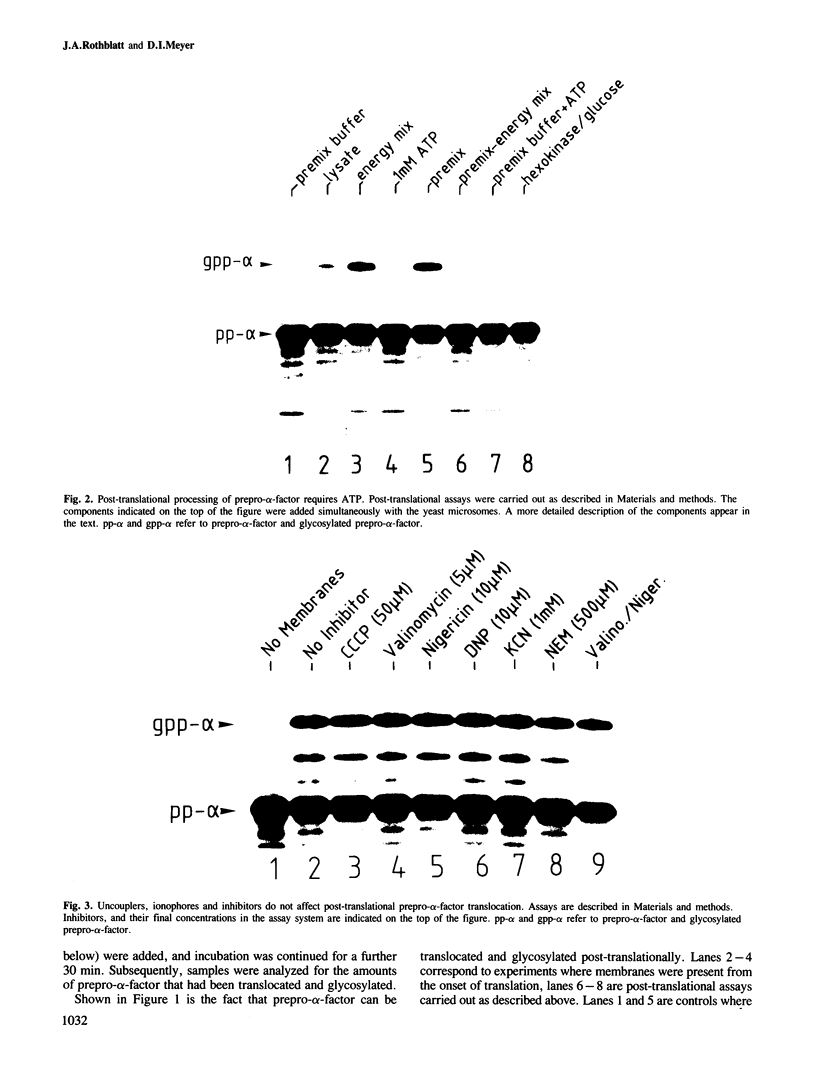
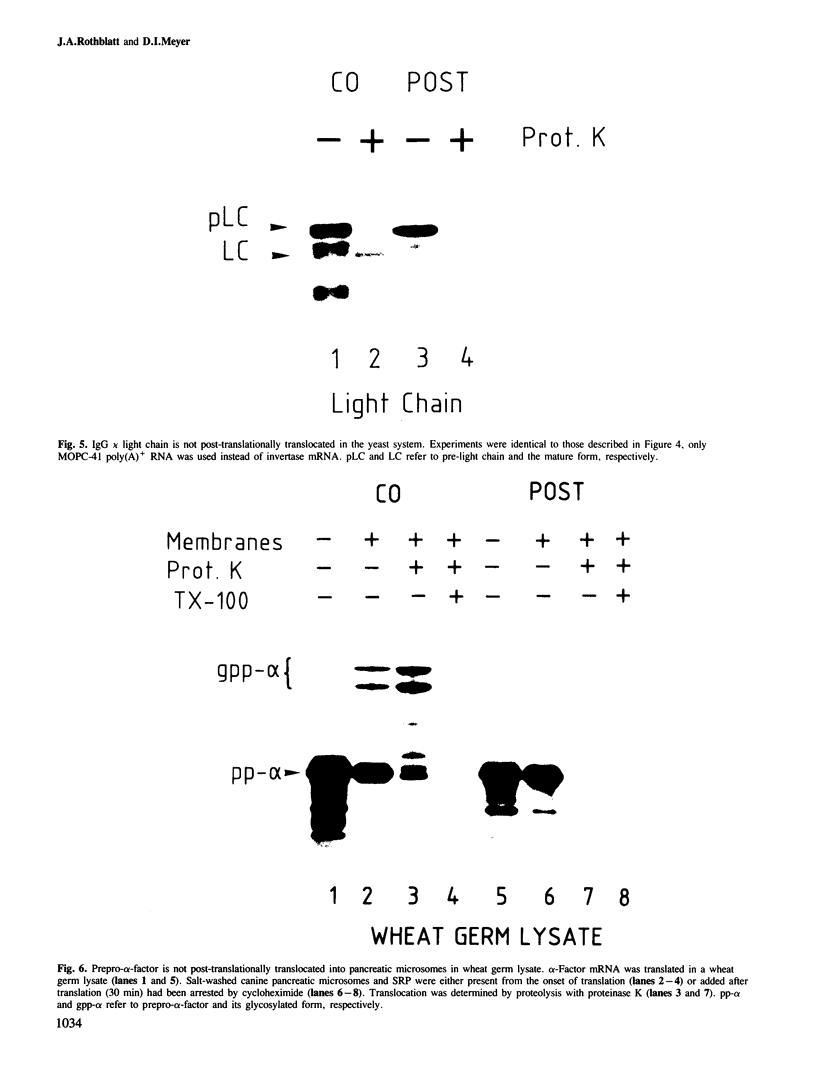
In an in vitro system comprising a yeast cell-free translation system, yeast microsomes and mRNA encoding prepro-α-factor, the translocation of this protein across the membrane of the microsomal vesicle and its glycosylation could be uncoupled from its translation. Such post-translational processing is dependent upon the presence of ATP in the system. It is not, however, affected by a variety of uncouplers, ionophores or inhibitors, including carbonyl cyanide m-chlorophenyl hydrazone (CCCP), valinomycin, nigericin, dinitrophenol (DNP), potassium cyanide (KCN) or N-ethyl maleimide (NEM). This mechanism of translocation is significant as it indicates that a protein of 18 000 daltons is capable of crossing an endoplasmic reticulum-derived membrane post-translationally. For the moment, this phenomenon seems to be restricted to prepro-α-factor in the yeast in vitro system. Neither invertase nor IgG ϰ light chain could be translocated post-translationally in yeast, nor was such processing observed for prepro-α-factor in a wheat germ system supplemented with canine pancreatic microsomes.
Keywords: secretion, protein translocation, endoplasmic reticulum, membrane biogenesis, Saccharomyces cerevisiae
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel G. Intracellular protein topogenesis. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1496–1500. doi: 10.1073/pnas.77.3.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Date T., Zwizinski C., Ludmerer S., Wickner W. Mechanisms of membrane assembly: effects of energy poisons on the conversion of soluble M13 coliphage procoat to membrane-bound coat protein. Proc Natl Acad Sci U S A. 1980 Feb;77(2):827–831. doi: 10.1073/pnas.77.2.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferro-Novick S., Novick P., Field C., Schekman R. Yeast secretory mutants that block the formation of active cell surface enzymes. J Cell Biol. 1984 Jan;98(1):35–43. doi: 10.1083/jcb.98.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser S. M., Daum G., Schatz G. Import of proteins into mitochondria. Energy-dependent uptake of precursors by isolated mitochondria. J Biol Chem. 1982 Nov 10;257(21):13034–13041. [PubMed] [Google Scholar]
- Hortsch M., Meyer D. I. Transfer of secretory proteins through the membrane of the endoplasmic reticulum. Int Rev Cytol. 1986;102:215–242. doi: 10.1016/s0074-7696(08)61276-0. [DOI] [PubMed] [Google Scholar]
- Julius D., Schekman R., Thorner J. Glycosylation and processing of prepro-alpha-factor through the yeast secretory pathway. Cell. 1984 Feb;36(2):309–318. doi: 10.1016/0092-8674(84)90224-1. [DOI] [PubMed] [Google Scholar]
- Kolansky D. M., Conboy J. G., Fenton W. A., Rosenberg L. E. Energy-dependent translocation of the precursor of ornithine transcarbamylase by isolated rat liver mitochondria. J Biol Chem. 1982 Jul 25;257(14):8467–8471. [PubMed] [Google Scholar]
- Kreil G. Transfer of proteins across membranes. Annu Rev Biochem. 1981;50:317–348. doi: 10.1146/annurev.bi.50.070181.001533. [DOI] [PubMed] [Google Scholar]
- Randall L. L. Translocation of domains of nascent periplasmic proteins across the cytoplasmic membrane is independent of elongation. Cell. 1983 May;33(1):231–240. doi: 10.1016/0092-8674(83)90352-5. [DOI] [PubMed] [Google Scholar]
- Rees-Jones R., Al-Awqati Q. Proton-translocating adenosinetriphosphatase in rough and smooth microsomes from rat liver. Biochemistry. 1984 May 8;23(10):2236–2240. doi: 10.1021/bi00305a022. [DOI] [PubMed] [Google Scholar]
- Rothblatt J. A., Meyer D. I. Secretion in yeast: reconstitution of the translocation and glycosylation of alpha-factor and invertase in a homologous cell-free system. Cell. 1986 Feb 28;44(4):619–628. doi: 10.1016/0092-8674(86)90271-0. [DOI] [PubMed] [Google Scholar]
- Schleyer M., Schmidt B., Neupert W. Requirement of a membrane potential for the posttranslational transfer of proteins into mitochondria. Eur J Biochem. 1982 Jun 15;125(1):109–116. doi: 10.1111/j.1432-1033.1982.tb06657.x. [DOI] [PubMed] [Google Scholar]
- Watts C., Wickner W., Zimmermann R. M13 procoat and a pre-immunoglobulin share processing specificity but use different membrane receptor mechanisms. Proc Natl Acad Sci U S A. 1983 May;80(10):2809–2813. doi: 10.1073/pnas.80.10.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W. T., Lodish H. F. Multiple mechanisms of protein insertion into and across membranes. Science. 1985 Oct 25;230(4724):400–407. doi: 10.1126/science.4048938. [DOI] [PubMed] [Google Scholar]