Abstract
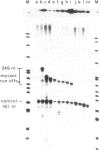
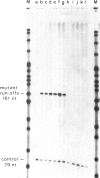
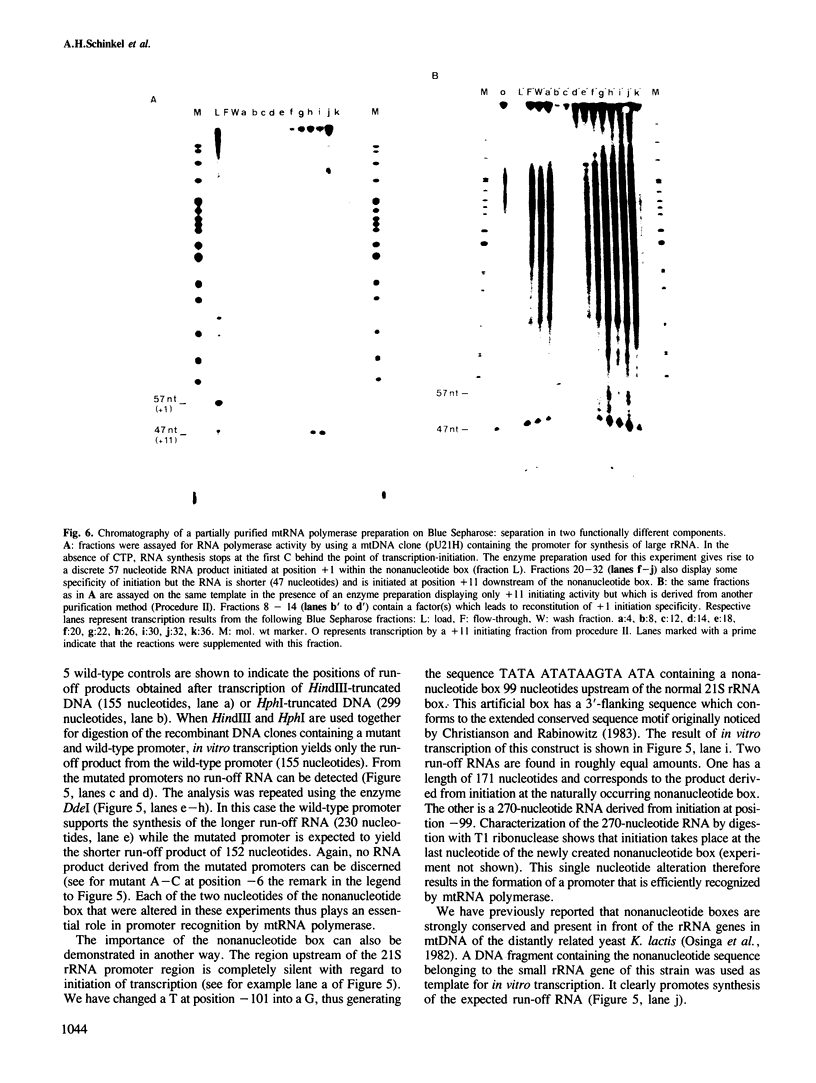
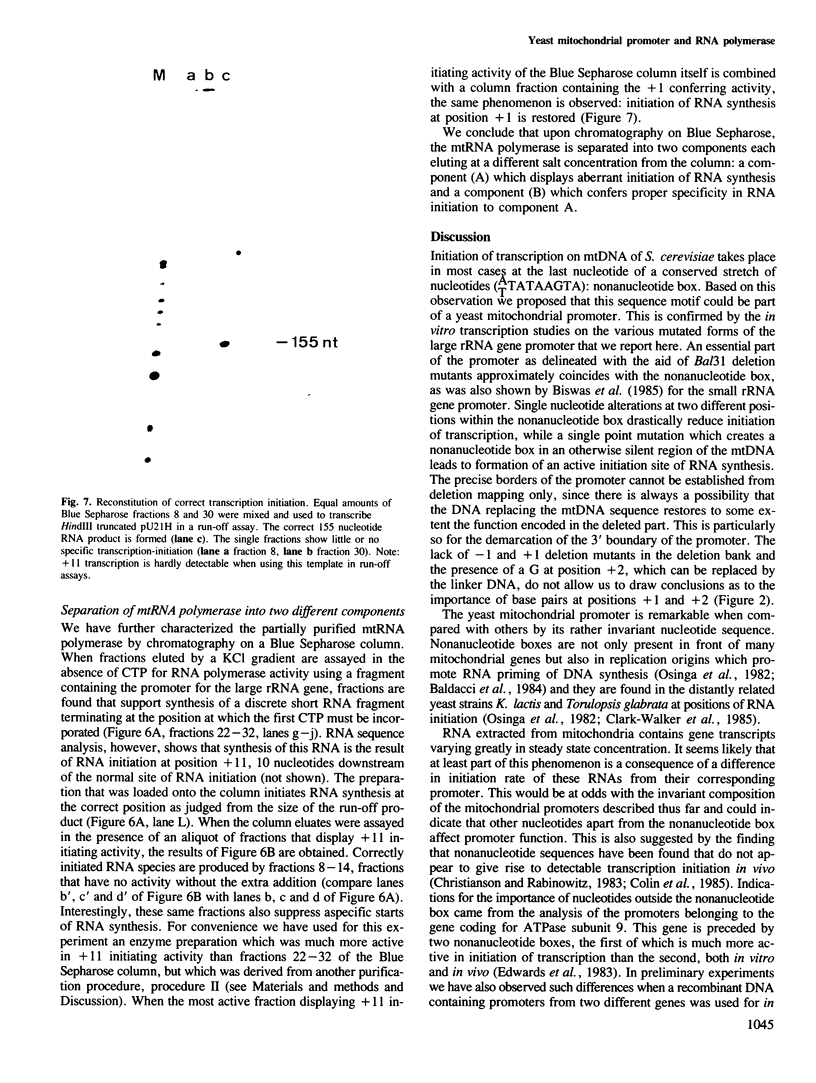
We have characterized a DNA sequence that functions in recognition of the promoter of the mitochondrial large rRNA gene by the yeast mtRNA polymerase. Promoter-containing DNA fragments were mutagenized and used as templates to study initiation of transcription in vitro with a partially purified mtRNA polymerase preparation. Deletion mutants, in which increasing stretches of DNA were removed from regions flanking the promoter, define a short area essential for correct initiation of transcription. It virtually coincides with a highly conserved stretch of nine nucleotides that is found immediately upstream of all transcriptional start sites described thus far. Two different point mutations within this nonanucleotide sequence drastically reduce promoter function. Conversely a single point mutation that results in the formation of a nonanucleotide sequence 99 nucleotides upstream of the large rRNA gene leads to a new, efficient transcription initiation site. MtRNA polymerase can be resolved into two different components by chromatography on Blue Sepharose: one retaining the capacity to synthesize RNA, the other conferring the correct specificity of initiation to the catalytic component.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldacci G., Chérif-Zahar B., Bernardi G. The initiation of DNA replication in the mitochondrial genome of yeast. EMBO J. 1984 Sep;3(9):2115–2120. doi: 10.1002/j.1460-2075.1984.tb02099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas T. K., Edwards J. C., Rabinowitz M., Getz G. S. Characterization of a yeast mitochondrial promoter by deletion mutagenesis. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1954–1958. doi: 10.1073/pnas.82.7.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson T., Edwards J. C., Mueller D. M., Rabinowitz M. Identification of a single transcriptional initiation site for the glutamic tRNA and COB genes in yeast mitochondria. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5564–5568. doi: 10.1073/pnas.80.18.5564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson T., Edwards J., Levens D., Locker J., Rabinowitz M. Transcriptional initiation and processing of the small ribosomal RNA of yeast mitochondria. J Biol Chem. 1982 Jun 10;257(11):6494–6500. [PubMed] [Google Scholar]
- Christianson T., Rabinowitz M. Identification of multiple transcriptional initiation sites on the yeast mitochondrial genome by in vitro capping with guanylyltransferase. J Biol Chem. 1983 Nov 25;258(22):14025–14033. [PubMed] [Google Scholar]
- Clark-Walker G. D., McArthur C. R., Sriprakash K. S. Location of transcriptional control signals and transfer RNA sequences in Torulopsis glabrata mitochondrial DNA. EMBO J. 1985 Feb;4(2):465–473. doi: 10.1002/j.1460-2075.1985.tb03652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colin Y., Baldacci G., Bernardi G. A new putative gene in the mitochondrial genome of Saccharomyces cerevisiae. Gene. 1985;36(1-2):1–13. doi: 10.1016/0378-1119(85)90064-2. [DOI] [PubMed] [Google Scholar]
- Deters D., Müller U., Homberger H. Breakage of yeast cells: large scale isolation of yeast mitochondria with a continuous-flow disintegrator. Anal Biochem. 1976 Jan;70(1):263–267. doi: 10.1016/s0003-2697(76)80067-x. [DOI] [PubMed] [Google Scholar]
- Edwards J. C., Levens D., Rabinowitz M. Analysis of transcriptional initiation of yeast mitochondrial DNA in a homologous in vitro transcription system. Cell. 1982 Dec;31(2 Pt 1):337–346. doi: 10.1016/0092-8674(82)90127-1. [DOI] [PubMed] [Google Scholar]
- Edwards J. C., Osinga K. A., Christianson T., Hensgens L. A., Janssens P. M., Rabinowitz M., Tabak H. F. Initiation of transcription of the yeast mitochondrial gene coding for ATPase subunit 9. Nucleic Acids Res. 1983 Dec 10;11(23):8269–8282. doi: 10.1093/nar/11.23.8269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levens D., Howley P. M. Novel method for identifying sequence-specific DNA-binding proteins. Mol Cell Biol. 1985 Sep;5(9):2307–2315. doi: 10.1128/mcb.5.9.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levens D., Lustig A., Rabinowitz M. Purification of mitochondrial RNA polymerase from Saccharomyces cerevisiae. J Biol Chem. 1981 Feb 10;256(3):1474–1481. [PubMed] [Google Scholar]
- Levens D., Ticho B., Ackerman E., Rabinowitz M. Transcriptional initiation and 5' termini of yeast mitochondrial RNA. J Biol Chem. 1981 May 25;256(10):5226–5232. [PubMed] [Google Scholar]
- Osinga K. A., De Haan M., Christianson T., Tabak H. F. A nonanucleotide sequence involved in promotion of ribosomal RNA synthesis and RNA priming of DNA replication in yeast mitochondria. Nucleic Acids Res. 1982 Dec 20;10(24):7993–8006. doi: 10.1093/nar/10.24.7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osinga K. A., De Vries E., Van der Horst G., Tabak H. F. Processing of yeast mitochondrial messenger RNAs at a conserved dodecamer sequence. EMBO J. 1984 Apr;3(4):829–834. doi: 10.1002/j.1460-2075.1984.tb01892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osinga K. A., Tabak H. F. Initiation of transcription of genes for mitochondrial ribosomal RNA in yeast: comparison of the nucleotide sequence around the 5'-ends of both genes reveals a homologous stretch of 17 nucleotides. Nucleic Acids Res. 1982 Jun 25;10(12):3617–3626. doi: 10.1093/nar/10.12.3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osinga K. A., Van der Bliek A. M., Van der Horst G., Groot Koerkamp M. J., Tabak H. F., Veeneman G. H., Van Boom J. H. In vitro site-directed mutagenesis with synthetic DNA oligonucleotides yields unexpected deletions and insertions at high frequency. Nucleic Acids Res. 1983 Dec 20;11(24):8595–8608. doi: 10.1093/nar/11.24.8595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palleschi C., Francisci S., Bianchi M. M., Frontali L. Initiation of transcription of a mitochondrial tRNA gene cluster in S. cerevisiae. Nucleic Acids Res. 1984 Oct 11;12(19):7317–7326. doi: 10.1093/nar/12.19.7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkley C. S., Keller M. J., Jaehning J. A. A multicomponent mitochondrial RNA polymerase from Saccharomyces cerevisiae. J Biol Chem. 1985 Nov 15;260(26):14214–14223. [PubMed] [Google Scholar]
- Zassenhaus H. P., Martin N. C., Butow R. A. Origins of transcripts of the yeast mitochondrial var 1 gene. J Biol Chem. 1984 May 10;259(9):6019–6027. [PubMed] [Google Scholar]