Abstract
Background and objectives
Friedreich’s Ataxia (FRDA) is a genetic disease that affects a variety of different tissues. The disease is caused by a mutation in the frataxin gene (FXN) which is important for the synthesis of iron-sulfur clusters. The primary pathologies of FRDA are loss of motor control and cardiomyopathy. These occur due to the accumulation of reactive oxygen species (ROS) in the brain and the heart due to their high metabolic rates. Our research aims to understand how developmental processes and the kidney are impacted by a deficiency of FXN.
Methods
We utilized an antisense oligomer, or morpholino, to knockdown the frataxin gene (fxn) in zebrafish embryos. Knockdown was confirmed via RT-PCR, gel electrophoresis, and Sanger sequencing. To investigate phenotypes, we utilized several staining techniques including whole mount in situ hybridization, Alcian blue, and acridine orange, as well as dextran-FITC clearance assays.
Results
fxn deficient animals displayed otolith malformations, edema, and reduced survival. Alcian blue staining revealed craniofacial defects in fxn deficient animals, and gene expression studies showed that the pronephros, or embryonic kidney, had several morphological defects. We investigated the function of the pronephros through clearance assays and found that the renal function is disrupted in fxn deficient animals in addition to proximal tubule endocytosis. Utilizing acridine orange staining, we found that cell death is a partial contributor to these phenotypes.
Discussion and conclusion
This work provides new insights about how fxn deficiency impacts development and kidney morphogenesis. Additionally, this work establishes an additional model system to study FRDA.
Keywords: frataxin, kidney, nephron, metabolism, development, zebrafish
1 Introduction
Friedreich’s Ataxia (FRDA, OMIM #229300) is an autosomal recessive neurodegenerative disease and the most common inheritable ataxia (Anheim et al., 2010; Anheim et al., 2012). FRDA is caused by genetic mutations that reduce expression of the protein Frataxin (FXN). The vast majority (96%–98%) of patients have variable numbers of trinucleotide GAA repeats in the first intron of the FXN gene, though genetic lesions encoding other mutations have also been identified (Reetz et al., 2018; Gottesfeld, 2019). Patients typically begin losing motor function between 5 and 15 years of age (Delatycki et al., 1999; Dürr et al., 1996; Filla et al., 1990; Parkinson et al., 2013; Schöls et al., 1997), which is thought to be due to cerebellar and dorsal root ganglia degeneration (Koeppen et al., 2011; Koeppen and Mazurkiewicz, 2013). Other neurological symptoms include dysarthria, oculomotor abnormalities and hearing loss (Fahey et al., 2008; Schöls et al., 1997). While the previously mentioned symptoms are primarily neurological, there are many FRDA symptoms that are not. Scoliosis, foot deformities, and cardiomyopathy are the most common, non-ataxia symptoms of FRDA (Reetz et al., 2018). Interestingly, each of these phenotypes are positively correlated in severity and occurrence with the number of GAA repeat expansions in intron 1 of FXN (Filla et al., 1996; Reetz et al., 2018).
FXN is a mitochondrial protein involved in the biosynthesis of iron-sulfur clusters (ISC) (Adinolfi et al., 2002; Adinolfi et al., 2009; Colin et al., 2013; Huynen et al., 2001; Mühlenhoff et al., 2002; Tsai and Barondeau, 2010; Ventura et al., 2006). ISCs are used to make proteins for the electron transport chain, citric acid cycle, fatty acid biosynthesis, and glycolysis (Maio and Rouault, 2022). For example, ISCs are critical cofactors for aconitase and electron transport chain complexes I, II, and III. Decreased FXN levels lessen antioxidant defenses and the accrual of unused iron generates free radicals. Together, these changes cause cells to accumulate oxidative damage (Pallardó et al., 2021; Sivakumar and Cherqui, 2022; Kelekçi et al., 2022). Cell death is thought to transpire from this damage and insufficient energy (ATP) production (Pallardó et al., 2021; Sivakumar and Cherqui, 2022; Kelekçi et al., 2022). Tissues with high metabolic demands that are rich in mitochondria, like neurons and myocardium, are disproportionately affected over time (Koutnikova et al., 1997). However, other organs with high metabolic demands, like the kidney (Elia, 1992), are seemingly unaffected in FRDA. This is quite surprising, as related ataxias and mitochondrial disorders are very frequently characterized by renal diseases, such as the nephrotic syndrome (Martín-Hernández et al., 2005; Diomedi-Camassei et al., 2007; Quinzii and Hirano, 2010; Emma et al., 2012; Ashraf et al., 2013). As such, much still remains to be learned about how FXN deficiency affects many tissues, organs, and systems across the body.
Researchers have utilized several different model organisms to study the biological functions of FXN. Bacteria, yeast, plants, worms, and flies have allowed for important discoveries about its roles (Busi et al., 2006; Kelekçi et al., 2022; Mühlenhoff et al., 2002), as well as knockdown mouse models, knockdown mouse fibroblast cell cultures, and FRDA patient cell cultures (Kelekçi et al., 2022; Perdomini et al., 2013). In particular, murine models include a useful array of various partial or conditional Fxn knockdown strategies, the latter including tissue-specific models which enable analysis of the effects of FXN-deficiency on particular cell types. Complete knockout of Fxn is embryonic lethal in mice (Cossée et al., 2000), which has precluded assessment of the early developmental roles for FXN in mammals. Mouse fibroblast Fxn deletions also lead to cell death (Calmels et al., 2009). These studies coincide with the observation that no FRDA patients have been reported to be homozygous with deleterious point mutations which would result in a complete absence of FXN (Perdomini et al., 2013).
In the present study, we utilized the zebrafish, Danio rerio, to study the consequence of severe fxn loss of function during ontogeny, as this animal model provides unique opportunities to examine the events of early embryogenesis. Zebrafish develop externally in clear chorions which allows for direct observation of vertebrate development beginning at fertilization. Further, the zebrafish genome is highly conserved with humans (Howe et al., 2013) and shares many of the genetic regulatory mechanisms that govern development, as well as many physiological and cellular processes.
Here, we designed a knockdown zebrafish model to specifically target and reduce zygotic fxn expression while preserving the expression of maternal fxn transcripts, thus enabling development in the context of attenuated Fxn levels. In this model, we found that fxn-deficient zebrafish exhibited poor growth and failure to thrive, with compromised survival. Additionally, fxn-deficient zebrafish embryos exhibited morphological defects across numerous tissues including the central nervous system, otoliths, craniofacial structures, and the pronephros, or embryonic kidney. Within the kidney, multiple lineages were reduced, which was associated with elevated cell death and correlated with compromised ability to clear fluid and maintain water homeostasis, leading to edema. These findings illustrate that fxn plays roles in the formation of the kidney, and this new loss of function model provides an opportunity to further delineate the roles of fxn during vertebrate development.
2 Results
2.1 Molecular and computational validation of Fxn deficiency
A complete Fxn knockout in mice is embryonic lethal (Cossée et al., 2000). As a result, several conditional Fxn knockdown mouse models have been developed (Kelekçi et al., 2022). Studies using these conditional mouse models have suggested that Fxn may have roles in development (Chandran et al., 2017; Jiralerspong et al., 1997; Koeppen et al., 2017; Santos et al., 2001). These mouse models as well as work in systems ranging from bacterial to FRDA patient derived cell lines have contributed greatly to our understanding of FRDA pathology, but they have a limited ability to answer the question of how FXN deficiency impacts early aspects of embryonic development such as organogenesis.
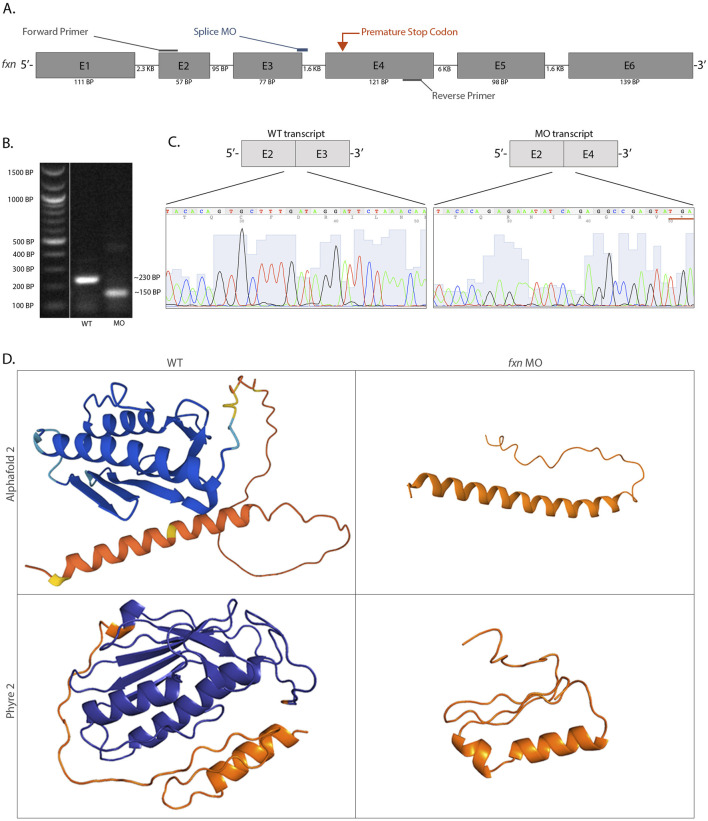
To address this question, we developed a fxn knockdown zebrafish model using an antisense oligomer morpholino strategy to specifically target zygotic fxn expression, thereby preserving expression from maternal fxn transcripts. This model allows us to model Fxn deficiency in the zebrafish, rather than a complete Fxn absence, similar to what is seen in human patients that experience a reduction of FXN (Filla et al., 1996; Gellera et al., 2007). Our morpholino (MO) targets the exon 3 splice donor site, thus was predicted to interfere with proper connection between the exon 3 and exon 4 coding sequence (Figure 1A). Wild-type zebrafish were microinjected with fxn MO at the 1 cell stage, and at the 28 somite stage (ss) we employed reverse transcriptase polymerase chain reaction (RT-PCR) followed by gel electrophoresis (Figure 1B) and Sanger sequencing (Figure 1C) to scrutinize the effect on fxn transcripts. While wild-type controls exhibited normal splicing, embryos injected with the fxn exon 3 splice donor MO exhibited exon skipping. Morphant transcripts had a complete deletion of exon 3 and exon 2 was spliced onto exon 4 (Figure 1C). This aberrant splicing event resulted in a predicted frameshift of the open reading frame and the presence of an in-frame premature stop codon near the beginning of exon 4 (Figure 1C).
FIGURE 1.
An antisense morpholino oligomer targeting the exon 3 and exon 4 splice region is sufficient to completely knockdown fxn expression in zebrafish. (A) A schematic of the fxn gene with annotations showing the morpholino and primer locations. (B) After cDNA synthesis using RT-PCR, analysis of the products through gel electrophoresis revealed that morphant animals produced a shorter fxn transcript and a wild-type product was absent. (C) Sanger sequencing data revealed that the fxn morphant transcript had a complete deletion of exon 3, which encoded an in-frame premature stop codon (underlined). (D) Three-dimensional protein folding predictions suggested a highly disrupted Fxn protein structure based on the altered mRNA sequence caused by splice interference due to the fxn morpholino. The endogenous Fxn consists of three alpha helices and five beta sheets, and the altered Fxn consists of an alpha helix and an interdomain loop. The orange refers to the mitochondrial trafficking sequence, an area of the protein that has low confidence for the folding prediction because of the lack of conservation between species in this specific domain. These folding predictions strongly suggest that significant regions of the Fxn protein are abrogated in the morphants.
Next, using the fxn transcript sequences expressed by wild-type and fxn morphants, we employed the protein folding prediction algorithms Alphafold 2 and Phyre 2 to predict the three-dimensional structure of the Fxn protein (Figure 1D; Kelley et al., 2015; Jumper et al., 2021). This approach was utilized because at this time there is currently no x-ray crystallography data for zebrafish Fxn, and we wanted to visualize how the fxn transcript alteration would disrupt the Fxn protein structure. The two algorithms predicted similar structures for both the endogenous Fxn protein and the product of our zygotic Fxn knockdown, but with critical differences (Figure 1D). The endogenous immature Fxn protein predictions consist of three alpha helices and five beta-sheets (Figure 1D). This is consistent with the x-ray crystallography data of mature human Fxn which has two alpha helices and five beta-sheets (Dhe-Paganon et al., 2000). In contrast, the zygotic Fxn protein knockdown was predicted by the algorithms to have only one alpha helix (Figure 1D). Therefore, the algorithm predictions suggest that the morpholino would not only disrupt the three-dimensional structure of zebrafish Fxn, but indeed abrogate the active site due to the location of the premature stop codon.
2.2 Distinct FRDA phenotypes are present in Fxn-deficient zebrafish
In human patients with FRDA, multiple cell types are affected. Over time, there is considerable neuronal degeneration in the central and peripheral nervous systems and many patients experience severe hypertrophic cardiomyopathy, which is the most common cause of death (Koeppen and Mazurkiewicz, 2013; Koeppen et al., 2015). These neural and cardiac phenotypes have been recapitulated in Fxn-deficient mouse lines as well (Chandran et al., 2017).
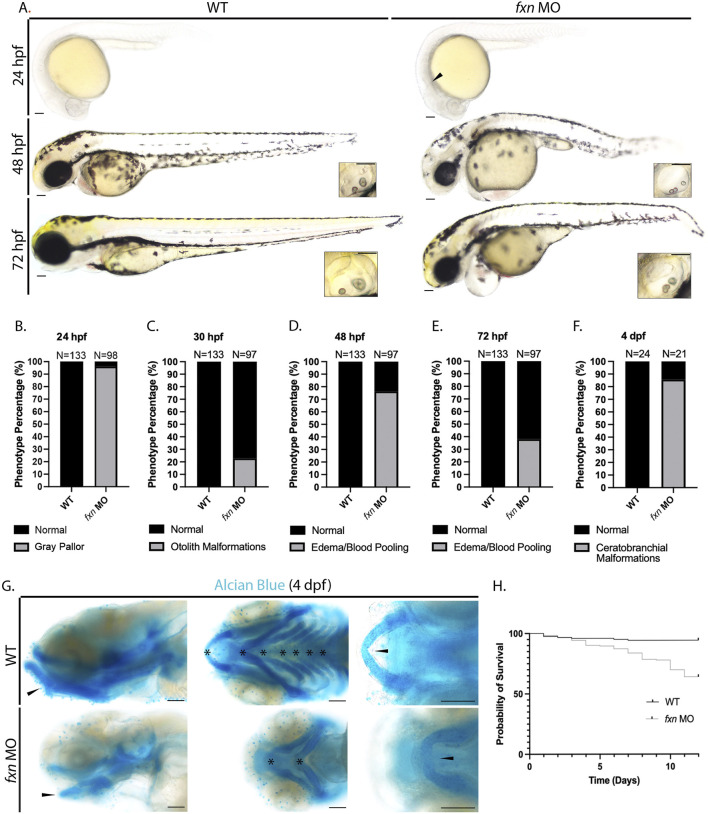
To explore and characterize development in fxn-deficient zebrafish, we first examined their live morphology at 24, 48, and 72 h post fertilization (hpf) (Figure 2A). We observed several striking phenotypes in fxn morphants compared to wild-type embryos. At 24 hpf, 96.3% of fxn-deficient embryos had a gray head pallor, a hallmark of cell death (Figures 2A,B). Also, 22.9% of the fxn-deficient embryos displayed hallmarks of malformed ear development, with three otoliths at 30 hpf compared to wild-type controls who formed otic vesicles with two otoliths (Whitfield, 2019) (Figures 2A,C). At 48 hpf, 76.4% of the fxn-deficient embryos had blood pooling in or near the pericardium which was often present with pericardial edema, the latter of which is one hallmark of renal dysfunction (Figures 2A,D). At 72 hpf, pericardial edema/blood pooling was present in 38.1% of the morphant animals (Figures 2A,D). Further, the majority of fxn-deficient embryos exhibited overt jaw malformations, where jaws were significantly smaller in size (Figure 2A). To better examine facial structure and jaw development, we performed Alcian blue staining to reveal the underlying cartilaginous cranial structures at 4 days post fertilization (dpf) (Figures 2F,G). We found that 85.7% of the morphants failed to develop ceratobranchials 1-5 by 4 dpf (Figures 2F,G). The mandibular prominence in these animals also had a convex curve when visualized laterally, rather than a concave curve that follows the profile of the maxillary prominence. Additionally, the Meckel’s cartilage also failed to fuse medially (Figure 2G). These data strongly suggest that fxn expression is required for the proper development of the pharyngeal arches and ceratobranchials.
FIGURE 2.
Morphological analysis of fxn-deficient zebrafish embryos reveal phenotypes indicative of several developmental malformations. (A) Brightfield microscopy revealed a diffuse gray pallor in the cranium of 24 hpf fxn morphants as well as blood pooling and pericardial edema at the 48 and 72 hpf stages. Insets show otolith malformations in the fxn morphants. Scale bars = 50 um. (B–F) Penetrance of observed phenotypes. (B) Gray pallor at 24 hpf. (C) Otolith malformations at 30 hpf. Edema/blood pooling at (D) 48 hpf and (E) 72 hpf. (F) Ceratobranchial malformations at 4 dpf. (G) Alcian blue staining revealed ceratobranchials 1-5 were missing in fxn morphants. Additionally, Meckel’s cartilage failed to fuse and the mandibular prominence did not curve ventrally as it does in wild-type animals. Scale bars = 50 um. (H) Kaplan-Meier survival curve documenting survival in wild-type embryos compared to fxn morphants. Fxn morphant animals displayed a 64% chance of survival compared to the 94% chance of their wild-type siblings during the first 12 days of life.
Finally, we noted that as fxn-deficient zebrafish developed between 24 and 72 hpf, they displayed a shorter tip to tail length compared to wild-type embryos, suggestive that their growth was being compromised wherein they displayed a general failure to thrive (Figure 2A). Given this observation, we examined whether fxn deficiency would impact survival. Over the course of 12 days, fxn-deficient embryos exhibited a 64% chance of survival as opposed to wild-type controls that by comparison had a 94% chance (Figure 2H). These data strongly suggest that fxn deficiency disrupts the proper development of zebrafish embryos and their transition to a healthy larval stage of ontogeny.
2.3 Fxn is required for proper pronephros morphogenesis
The nervous system, heart, and kidneys have high metabolic rates. This would cause one to hypothesize that all of these tissues would be affected in FRDA patients as well as FXN-deficient mammalian models. Further, FXN is abundantly expressed in the embryonic and adult mouse kidney (Yue et al., 2014). However, kidney function has not been reported to be affected in the lifespan of FRDA patients (Reetz et al., 2018; Shinnick et al., 2016; Watters et al., 1981) and has similarly not been observed in animal models. This paradox, even more puzzling given the compromised fluid balance with severe edema observed in our fxn-deficient zebrafish embryos which are hallmarks of compromised renal physiology (Drummond et al., 1998; Wingert and Davidson, 2008; Gerlach and Wingert, 2013; Desgrange and Cereghini, 2015; Poureetezadi and Wingert, 2016; Fatma et al., 2021), led us to investigate next the formation and function of the zebrafish pronephros.
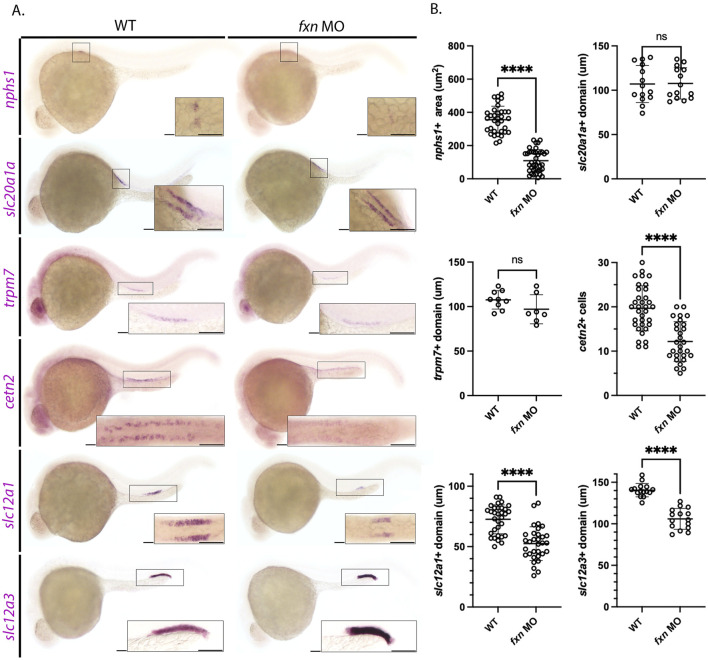
To do this, we utilized whole mount in situ hybridization (WISH) to visualize the different domains of the zebrafish pronephros at the 28 ss with previously established specific markers of each individual cell type (Figure 3). First, we used the nphs1 probe to mark the podocytes, which are essential to form the blood filtration unit used by the embryonic nephrons (Kramer-Zucker et al., 2005). The area occupied by nphs1 positive cells was decreased in fxn-deficient embryos compared to wild-type embryos (Figures 3A,B). Next, cells in the proximal convoluted tubule were marked with the probe for slc20a1a (Wingert et al., 2007). There was no statistically significant difference in the slc20a1a domain between the two groups (Figures 3A,B). Additionally, there was not a statistically significant difference in the proximal straight tubule domain, as marked with trpm7 (Figures 3A,B) (Wingert et al., 2007). Interestingly, while there was not a statistically significant difference in the domain of trpm7 positive cells, the fxn-deficient embryos appeared to be expressing less of this transcript, as the stain was fainter. Next, multiciliated cells (MCC) were marked with cetn2 (Ma and Jiang, 2007). The number of cetn2 positive cells was significantly decreased in fxn-deficient embryos compared to wild-types, suggesting that were fewer MCCs developed in the pronephros. Lastly, we examined the distal tubule, which is comprised of distal early cells that express slc12a1 and distal late cells that express slc12a3 (Wingert et al., 2007). Both distal segments were significantly shorter in length in the fxn-deficient embryos compared to wild-type controls (Figures 3A,B).
FIGURE 3.
Pronephros segment development is significantly disrupted in fxn-deficient zebrafish embryos. (A) WISH experiments reveal that fxn is required for the proper formation of the podocytes, multiciliated cells (MCCs), distal early, and distal late tubule. The previously mentioned domains were visualized via the nphs1,cetn4, slc12a1, and slc12a3 probes, respectively. Formation of the proximal convoluted and straight tubules, as assessed by expression of slc20a1a and trpm7 revealed no significant differences between wild-type embryos and fxn morphants. Scale bars = 50 um. Each boxed lateral region in the embryos corresponds to the inset, which shows a dorsal view of that corresponding area for nphs1, slc20a1a, cetn2, and slc12a1 or a lateral view of the corresponding area for trpm7 and slc12a3. (B) Unpaired t-tests of the phenotypes revealed by WISH. ****p < 0.0001.
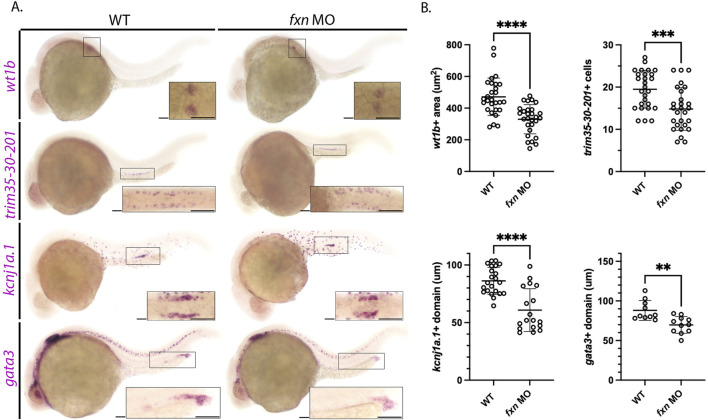
To further assess each of the renal populations that were significantly altered in the fxn-deficient embryos, WISH was performed using a panel of independent molecular markers for the affected cell type (Figure 4). Specifically, we used wt1b, trim35-30-201, kcnj1a.1, and gata3 to examine the podocytes, MCCs, distal early, and distal late, respectively (Figure 4). In each case, fxn-deficient embryos displayed significant reductions in the domain occupied by these pronephros populations compared to wild-type embryos, confirming our previous conclusions about the changes to each cell type based on our initial characterization of unique markers for each lineage (Figure 3).
FIGURE 4.
Further analysis of pronephros segment development in fxn-deficient zebrafish embryos confirms reductions in several cell populations. (A) WISH using the probes wt1b, trim35-30-201, kcnj1a.1, and gata3 which mark the podocytes, multiciliated cells (MCCs), distal early and distal late domains respectively. Fxn morphants displayed a significant reduction in podocytes, reduction in MCC number, and reduced distal domains results with this set of markers, independently recapitulating the results shown in Figure 3. Scale bars = 50 um. Each boxed lateral region in the embryos corresponds to the inset, which shows a dorsal view of that corresponding area, with the exception of the gata3 panels which show lateral views in the corresponding inset. (B) Unpaired t-tests demonstrating significantly affected pronephric domains in the fxn morphants. **p < 0.005, ***p < 0.0005, ****p < 0.0001.
Next, we utilized WISH to examine the emergence of the podocyte, distal early and distal late lineages at 22 ss and 26 ss, timepoints which precede the completion of nephron segmentation (Wingert et al., 2007; Wingert and Davidson, 2011). Additionally, these lineages were examined at 32 hpf to examine if the appearance of each population was developmentally delayed. The podocyte lineage was significantly reduced in fxn-deficient embryos compared to wild-types at the 22 ss, 26 ss and 32 hpf (Supplemental Figure S1). Likewise, the distal early lineage as marked by slc12a1 and distal late lineage as marked by slc12a3 was significantly reduced in fxn-deficient embryos compared to wild-type controls (Supplemental Figures S2, S3). Taken together, these data suggest that fxn is critical for the proper emergence of multiple lineages within the zebrafish pronephros.
To further explore this notion, we performed several control studies. First, we examined whether the fxn morpholino might have off target effects, which can be associated with edema and grey cranial pallor. The p53 morpholino is well known to attenuate common off target effects of morpholinos (Robu et al., 2007). We observed that co-injection of the p53 morpholino with the fxn morpholino led to similar phenotypes as fxn morpholino alone (Supplemental Figure S4), namely gray pallor in the cranium at 24 h post fertilization and edema between 48 and 72 hpf. In light of these results, we conclude that off target effects are not a significant component of the fxn-deficient morphant phenotypes. Additionally, wild-type zebrafish were microinjected with a standard control morpholino, raised to 24 hpf, and fixed for nephron segment analysis. WISH revealed that formation of the podocytes and tubule segments were not altered due to the standard morpholino control (Supplemental Figure S5). Taken together, these studies lead us to conclude that fxn deficiency causes specific defects in development including pronephros formation.
2.4 MCCs are significantly decreased at several different time points
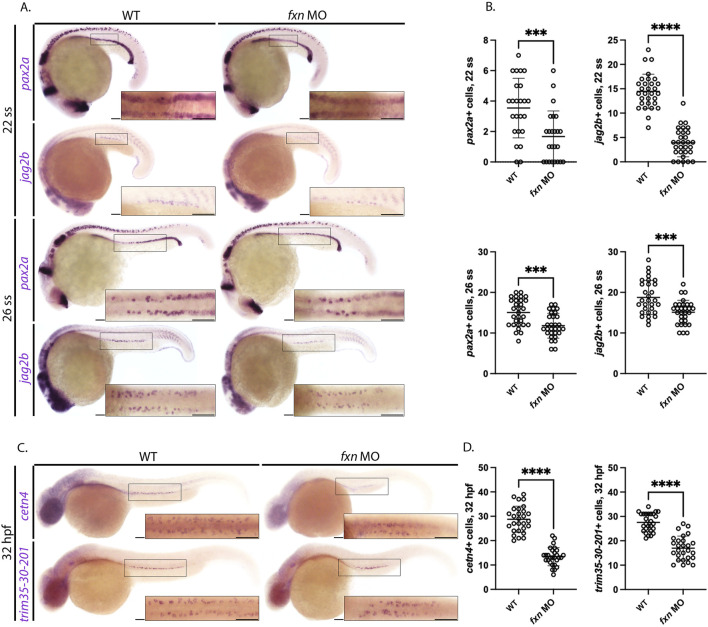
Defects in MCC development have been linked to embryonic fluid imbalance such as pericardial edema (Marra and Wingert, 2016; Marra et al., 2019; Chambers et al., 2020b). Therefore, we next sought to assess MCC specification in fxn-deficient embryos and examined whether the MCC populace was diminished at later timepoints. To characterize MCC progenitors, we examined the expression of pax2a and jag2b at the 22 ss using WISH and quantified these populations (Marra et al., 2019). MCC progenitor numbers in fxn-deficient animals were significantly decreased as compared to wild-type controls (Figures 5A,B). To examine whether the MCC populations might be diminished due to developmental delay, WISH was also performed several hours subsequent to this time point to survey the number of pax2a and jag2b expressing MCC progenitors. Fxn-deficient embryos at the 26 ss had significantly decreased pax2a+ and jag2b + MCCs compared to wild-type controls (Figures 5A,B). These data further support the conclusion that MCCs are not specified correctly in the absence of Fxn function.
FIGURE 5.
Fxn-deficient zebrafish embryos form reduced multiciliated cell (MCC) progenitors. (A) WISH analysis at the 22 and 26 ss utilizing the probes pax2a and jag2b revealed that the population of MCC progenitors was significantly reduced in fxn morphants compared to wild-type control embryos. Scale bars = 50 um. (B) Unpaired t-test of the corresponding WISH experiments in panel (A). (C) WISH analysis at the 32 hpf time point utilized the probes trim35-30-201 and cetn4, which are expressed by differentiating MCCs, and revealed that MCC numbers were still significantly reduced at this later timepoint. Scale bars = 50 um. Each boxed lateral region in the embryos corresponds to the inset, which shows a dorsal view of that corresponding area. (D) Unpaired t-test of the corresponding WISH experiments in panel (C). ***p < 0.0005, ****p < 0.0001.
To examine the progression of MCC development in the context of fxn deficiency, we conducted WISH analysis on 32 hpf animals using the trim35-30-201 and cetn2 riboprobes, which mark differentiating MCCs (Ma and Jiang, 2007; Liu et al., 2007; Marra and Wingert, 2016). While both the wild-types and fxn-deficient embryos formed MCCs that expressed these more mature markers, consistent with the ability of this lineage to differentiate, the number of trim35-30-201+ and cent2+ MCCs were significantly reduced in number in fxn-deficient embryos compared to wild-types (Figures 5C,D). In sum, these findings suggest that fewer MCCs are patterned in the fxn-deficient pronephros.
2.5 The pronephros in Fxn-deficient zebrafish is dysfunctional
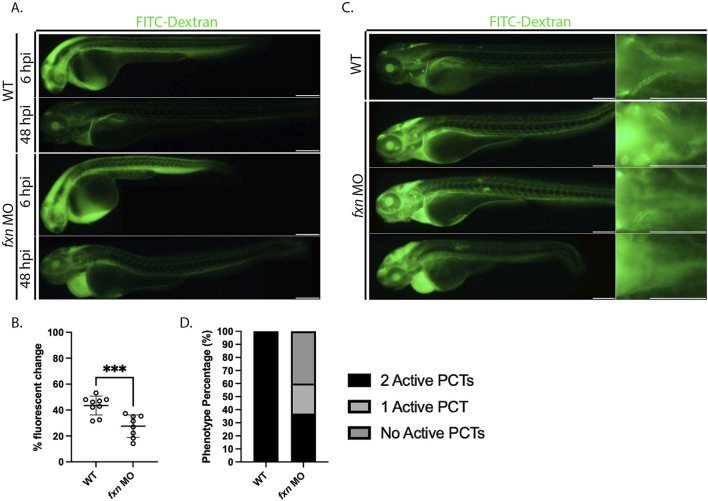
Because we observed a variety of renal cell populations being affected by fxn-deficiency, we hypothesized that pronephros dysfunction may indeed underlie the edema exhibited by fxn-deficient embryos. To test this hypothesis, we injected 36 hpf wild-type controls and fxn-deficient embryos and with 40 kD FITC-Dextran, as this compound can be utilized as a proxy to visualize and measure fluid clearance within live animals in real time (Weaver et al., 2022) (Figure 6). After introduction of this tracer, embryos were imaged at 6 h post injection (hpi) and 48 hpi to assess fluorescent intensity (Figure 6A). As observed previously, fxn-deficient embryos developed pronounced pericardial edema over this time period (Figure 6A). Upon analysis of the percentage fluorescent change between 6 and 48 hpi, fxn-deficient embryos displayed edema a significantly decreased ability to clear the FITC-dextran compared to wild-types (Figure 6B).
FIGURE 6.
Fxn-deficient zebrafish embryos display renal clearance defects and some proximal reabsorption defects. (A) fxn morphants failed to excrete the FITC-Dextran at the same rate as their wild-type siblings. Images of the same wild-type and fxn-deficient animals 6 hours and 48 h after injection with the FITC-dextran. FITC-Dextran (40 kDa) was injected around 36 h post-fertilization. (B) An unpaired t-test of the percent fluorescent change of each animal. Percent fluorescent change was calculated using the 6 h post injection (hpi) and 48 hpi fluorescent intensity of the same animal. (C) Morphants exhibit decreased endocytosis activity in the proximal convoluted tubule (PCT). Among the morphants, there were three primary phenotypes. Morphant animals either had endocytosis activity in two PCTs, one PCT, or neither. These findings suggest that dysfunction within the PCT segments is one contributor to the fluid imbalance observed in fxn-deficient animals. (D) The proportions of proximal convoluted tubule phenotypes in the wild-type and fxn morphant groups. Scale bars are 200 um. ***p < 0.0005.
We also used FITC-Dextran to visualize proximal convoluted tubule (PCT) endocytosis activity. (Figure 6C). Receptor mediated endocytosis is utilized extensively by the proximal tubule to reabsorb materials in the renal filtrate (Anzenberger et al., 2006). While wild-type embryos exhibited robust endocytosis in the PCT of both nephrons, the fxn morphants displayed a range of reduced PCT function. Approximately 35% of fxn morphants had PCT endocytosis activity in both nephrons. However, about 25% of fxn morphants had PCT endocytosis activity in nephron only and about 40% of the fxn morphants had no PCT endocytosis in either nephron (Figure 6D). From this, we conclude that nephron function is compromised, and thus concluded that Fxn function is necessary to achieve proper pronephros function during development.
2.6 Cell death is amplified in Fxn-deficient zebrafish
Decreased expression of FXN leading to ROS and consequent cell death is hypothesized to be a major underlying pathogenic component of FRDA, commonly affecting the nervous system (Abeti et al., 2016; Koeppen et al., 2011; Koeppen and Mazurkiewicz, 2013). In our morphological studies, we had observed gray pallor in the developing central nervous system of Fxn-deficient embryos, which is a harbinger of cell death (Cheng et al., 2015). Thus, next we sought to characterize cell survival in fxn-deficient zebrafish to test the hypothesis that Fxn loss of function was indeed causing an increase in cell death across tissues during embryogenesis.
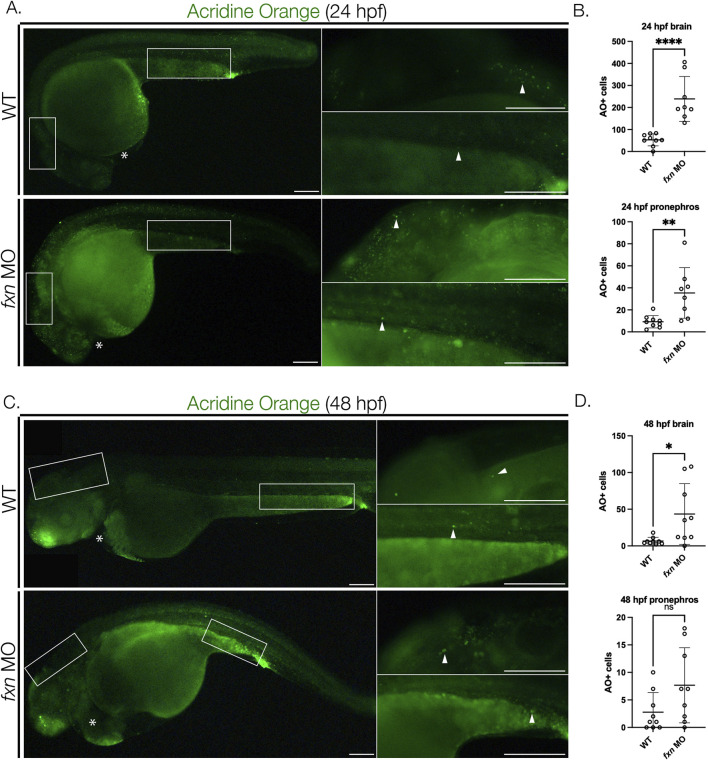
To test this, we used 24 and 48 hpf Fxn-deficient embryos to perform acridine orange (AO) staining (Tucker and Lardelli, 2007), which is a DNA intercalating fluorescent dye that rapidly and robustly labels apoptotic cells and readily penetrates deep tissues in whole mount embryos (van Ham et al., 2010). Next, we quantified cell death in several regions, focusing on the brain as well as the pronephros and the heart. Fxn-deficient zebrafish had significantly more AO + cells in the brain at both 24 and 48 hpf compared to wild-types (Figures 7A–D). Fxn-deficient embryos also had significantly more AO + cells in the pronephros at 24 hpf than wild-type controls (Figures 7A,B). At the 48 hpf time point, however, there was not a significant difference in AO + cell number between wild-types and Fxn-deficient embryos (Figures 7C,D). Interestingly, we did not observe AO + cells within the heart in either wild-type or fxn-deficient embryos at 24 or 48 hpf (Figure 7). These observations suggest that cell death is a component of the developmental defects which transpire during both pronephros and central nervous system formation, but not the heart, in fxn-deficient zebrafish.
FIGURE 7.
Cell death is elevated in fxn-deficient zebrafish embryos. (A) Images of AO-stained animals at 24 hpf. Fxn morphants animals have significantly increased cell death in the brain and pronephros at 24 hpf and increased cell death in the brain at 48 hpf. White boxes denote the locations for the panels on the right top (brain) and right bottom (pronephros). Scale bars = 50 um. (B) Unpaired t-tests of cell death in the brain and pronephros at 24 hpf. (C) Images of AO-stained animals at 48 hpf. White boxes denote the locations for the panels on the right top (brain) and right bottom (pronephros). Within the right top brain panel, each white box indicates a single positive cell. In fxn morphants, areas of the brain commonly had multiple cells as indicated by the white box. Scale bars = 50 um. (D) Unpaired t-tests of cell death in the brain and pronephros at 48 hpf. *p < 0.05, **p < 0.005, ****p < 0.0001.
2.7 Pronephros segment formation is differentially compromised in fxn-deficient embryos
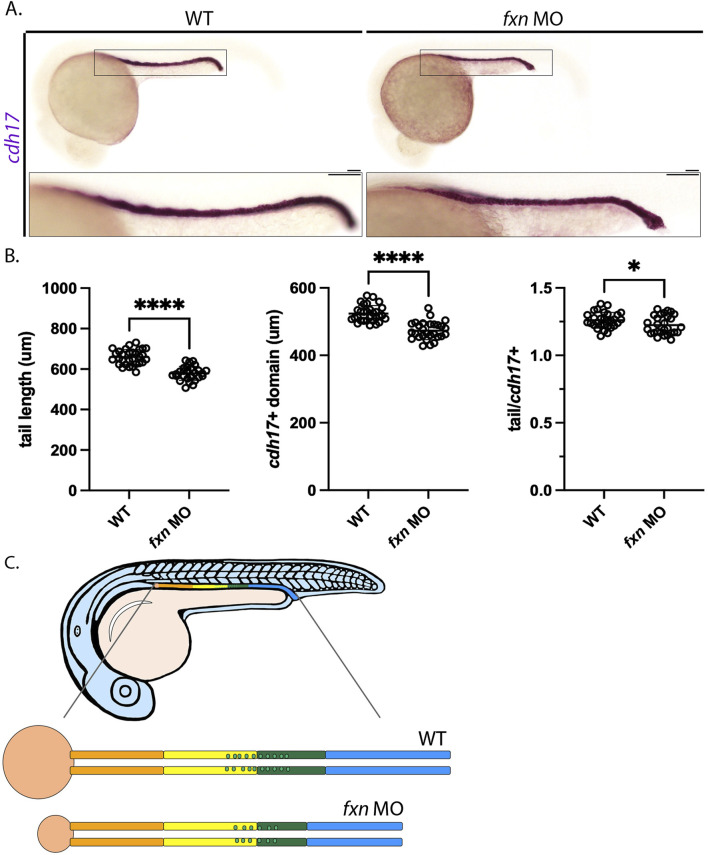
As noted previously, we found that fxn-deficient embryos were typically smaller in tip to tail length than wild-types of a comparable developmental age, suggesting compromised growth over time (Figure 2). Thus, we wondered whether the decreases seen in the several pronephros populations like the distal tubules and MCCs were simply due to the animal being smaller. To examine this possibility, we conducted WISH using cdh17, which is a pronephros specific marker (Figure 8). cdh17 stains the length of the nephron tubules (Wingert et al., 2007), thus allowing us to compare the absolute length of the pronephros versus the embryo tail length from tip to tail (Figure 8A). By measuring and comparing these lengths in 24 hpf wild-types and fxn-deficient embryos, we found that the pronephros and the tail were both significantly shorter than in fxn-deficient embryos compared to wild-type control embryos at this age (Figure 8B). When we calculated the ratio of tail/cdh17 length, fxn-deficient embryos were found to have a reduction in pronephros size that was slightly less severe than the decrease in tail length (Figure 8B). This suggests that the pronephros undergoes a decrease in size that is very similar to the overall embryonic size decrease in fxn-deficient embryos. We also examined performed tail analysis to explore whether the decreases in distal tubule length were proportionate to the smaller pronephros size. Examination of two distal early markers (slc12a1, kcnj1a.1) and two distal late markers (slc12a3, ptgs1) revealed that the decreased lengths of these segments were approximately 25% while tail lengths were reduced by approximately 10%–12% (Supplemental Figure S6). This analysis reveals that the distal segments of fxn-deficient embryos are disproportionately reduced compared to the tail or the nephron overall (Figure 8C). Future studies are needed to ascertain whether changes in regions such as the cloaca, collecting duct or Corpuscles of Stannius, endocrine glands associated with the pronephros lineage, are affected in fxn-deficient embryos and how changes in these cell types relate to the distal segment reductions.
FIGURE 8.
Tail/pronephros tubule ratio comparisons indicate that the embryonic kidney that develops within fxn-deficient zebrafish embryos was decreased in length proportionally to the decreased in tail length. (A) WISH analysis of pronephros tubule formation as marked by cdh17 revealed that this structure was shorter in fxn morphants than it is in their wild-type siblings (scale bars = 50 um). Each boxed lateral region in the embryos corresponds to the inset, which shows an enlarged lateral view of that corresponding area. (B) Unpaired t-tests of tail length and pronephros length as marked by cdh17 revealed that the length of both structures was significantly shorter in the morphants than in their wild-type siblings. An unpaired t-test of the ratios of tail and pronephros length also showed a statistically significant difference. *p < 0.05, p ****p < 0.0001. (C) fxn deficiency leads to formation of a pronephros which is decreased in size in porportion to the reduction in embryo size, however the distal segments are disporportionally reduced suggesting these lineages are uniquely affected by the loss of Fxn activity.
3 Discussion
The roles of FXN in mammalian embryogenesis remain poorly understood. Here, we have created a new loss of function model for Fxn zygotic deficiency in the zebrafish, and utilized this paradigm to begin exploring the effects of diminished Fxn expression during vertebrate organogenesis. These studies have revealed that Fxn is required for the proper development of otolith sensory structures, pharyngeal arches, ceratobranchials, and several renal lineages that comprise the nephron functional units within the pronephros. We found that cell death is significantly increased during Fxn deficiency in both the central nervous system and pronephros—an observation which correlates with the finding that pronephros formation is differentially compromised in Fxn-deficient embryos. Lastly, we found that Fxn-deficient zebrafish embryos had an impaired ability to clear fluids. In part, fluid imbalance in Fxn-deficient zebrafish correlates to alterations in PCT reabsorption, which was compromised in ∼60% of Fxn-deficient embryos. Collectively, these studies have documented a number of significant phenotypic consequences for the loss of Fxn expression during early embryonic stages of zebrafish development.
Interestingly, several of the tissues that are affected by Fxn deficiency during zebrafish development are also affected in animal models of FRDA and human patients as well. For example, FRDA mouse models and patients experience neuron attrition or increased cell death during neuronal differentiation (Koeppen, 2011; Santos et al., 2001; Simon et al., 2004), and in the present study we found that neuron survival was reduced in Fxn-deficient zebrafish embryos at 24 and 48 hpf. Although the cause of death is unknown, Fxn-deficient zebrafish embryos had a lower chance of survival than their wild-type siblings, which parallels shorter life expectancies in mouse models (Perdomini et al., 2014) and humans (Andermann et al., 1976).
Contrary to our findings in this report, problems stemming from craniofacial malformations, otoliths, pharyngeal arches, and ceratobranchial malformations have not been reported in other model organisms or FRDA patient studies. However, one study demonstrated that zebrafish otoliths, pharyngeal arches, and ceratobranchials are all derived from the neural crest (Kague et al., 2012). This may correlate with the study which demonstrated that apoptosis is increased in differentiating neuroectoderm (Santos et al., 2001).
In addition, alterations in kidney form and function have not been previously associated with FXN deficiency in other models or humans. Thus, our findings suggest for the first time that Fxn has roles in supporting embryonic kidney development. Future studies are necessary to explore whether Fxn has direct or indirect roles in the emergence, differentiation and survival of the intermediate mesoderm in higher vertebrates. Our work implicates Fxn as requisite for proper survival of renal populations. More research is needed to further understand the dynamics of cell loss, such as the mechanisms of cell death, and whether cell proliferation is compromised. The latter may be relevant given the early population changes in the podocyte, MCC and distal segments, and our observations that pronephros growth is differentially compromised in the context of Fxn deficiency.
In conclusion, we have created a zebrafish model of zygotic Fxn deficiency that can be utilized moving forward to expand our fundamental understanding about the developmental roles of fxn. This model is particularly well suited to examine the effects of Fxn during early ontogeny and organogenesis. Future studies are needed to create complementary genetic models of fxn loss of function using tools such as CRISPR/Cas9, and to investigate molecular changes associated with reduced Fxn levels. For example, Fxn dysfunction is closely associated with mitochondrial pathologies, which were not examined in the present work. Overall, our hope is that the fxn deficiency model described here will prove useful to other FRDA researchers in the collective effort to understand the effects of decreased FXN expression in humans, and to assist with efforts to identify new therapeutics to treat this devastating condition.
4 Materials and methods
4.1 Zebrafish husbandry
Zebrafish were cared for by the Freimann Life Sciences animal facility. All studies were approved by the University of Notre Dame Institutional Animal Care and Use Committee (IACUC), under protocol numbers 19–06-5,412 and 22–07-7,335. The Tübingen strain was used for all our zebrafish studies. Embryos were incubated at 28°C in E3 embryo media. Once the animals reached the relevant developmental stage (Westerfield, 1993), they were used for live imaging or anesthetized with 0.02% tricaine and then fixed with 4% paraformaldehyde/1x phosphate buffered saline for gene expression studies (Kimmel et al., 1995).
4.2 Morpholino knockdown
Morpholinos were obtained from Gene Tools, LLC. The morpholino sequence targeting the zebrafish fxn Exon 3/Exon4 splice site is 5′-CTAAATTTACCTGAGGTGATGTGCC-3’. The morpholino was injected into zebrafish embryos at the 1 cell stage and uninjected wild-type siblings were raised as controls. The concentration and volume used are 3 g/L and ∼1.5 nL respectively. The standard control morpholino used was 5′-CCTCTTACCTCAGTTACAATTTATA-3’. The p53 morpholino used was 5’ –AGAATTGATTTTGCCGACCTCCTCT-3’.
4.3 RT-PCR and sanger sequencing
RNA was extracted from approximately 30 animals from wild-type and morphant groups using TRIZOL Invitrogen (15596026). Next, cDNA was generated using qScript cDNA Supermix (Quantabio). The primers used were: forward 5′- TCAATAAGTGGTGGTAGGAGTGTTT-3′, and reverse 5′-CCAGTGAAGTTTTCATCTGTGAGAT-3’. Our samples were sequenced at the University of Notre Dame Genomics and Bioinformatics Core Facility. The trace files were analyzed using 4Peaks (4Peaks by A. Griekspoor and Tom Groothuis, nucleobytes. com.)
4.4 Whole-mount in situ hybridization (WISH)
WISH was performed in the same manner as described by previous publications (Chambers B. E. et al., 2020; Chambers J. M. et al., 2020; Marra et al., 2019). The antisense RNA, digoxigenin-labeled probes that were used are cdh17, wt1b, nphs1, slc20a1a, trpm7, trim35-30-201, cetn4, pax2a, jag2b, slc12a1, kcnj1a.1, slc12a3, and gata3. These probes were generated using in vitro transcription from IMAGE clone templates as described by previous publications (Gerlach and Wingert, 2014; Wingert et al., 2007).
4.5 FITC-dextran injections
Wild-type and fxn morphant animals were incubated in 0.003% phenylthiourea (Sigma-Aldrich, P7629) starting at 8 hpf. Animals were anesthetized at 36 hpf with 0.02% tricaine and subsequently injected with 3 nL of 40 kDa FITC-Dextran (Invitrogen, D-1845) (5 mg/mL). The injection site was around somite three in muscular tissue as described in a previous publication (Kroeger et al., 2017). The animals were then imaged at 6- and 48-hours post injection. The fluorescent change of the entire animal was measured using the FIJI software. The percent change of fluorescent intensity was calculated for each animal by keeping animals separate in a 96-well plate. The change in fluorescent intensity for both groups was analyzed using an unpaired t-test.
4.6 Acridine orange (AO)
Cell death was approximated using AO (Sigma-Aldrich A6014) as described in previous publications (Kroeger et al., 2017; Weaver et al., 2022). 50 mg of AO were dissolved into 50 mL of MilliQ water as a stock solution (100x). The stock solution was stored at −20 C in a light-protected container. AO was diluted down to a 1x concentration in E3 embryo medium before being applied to 24 and 48 hpf animals which had previously been incubated in 0.003% phenylthiourea starting at 8 hpf. Animals were incubated for 30 min at room temperature in the AO/E3 solution and then washed three times over the course of 10 min in E3. Samples were then anesthetized in a tricaine/E3 solution and immediately imaged in methylcellulose.
4.7 Alcian blue (AB)
AB (Thermofisher 05500-5G) staining was conducted as described in a previous publication (Neuhauss et al., 1996; Weaver et al., 2022). Wild-type and fxn morphants were fixed at 4 dpf using 4% paraformaldehyde at 4 C for 16 h. Dehydration was done using 100% methanol at −20 C. The rehydration was done with a series of methanol washes of decreasing concentration. Animals were bleached for 2 h, rinsed in PBST, and then digested using a proteinase K (Roche 3115836001) (10 mg/mL for 15 min). Animals were then rinsed in PBST again and incubated at room temperature on a rocker for 16 h in an Alcian Blue (0.1%), ethanol (70%), and HCl (5%) solution. The animals were then destained on a rocker in an ethanol (70%) and HCL (5%) solution for 8 h. After destaining, the animals were washed in PBST, dehydrated in a series of ethanol washes that increased in concentration, and stored in glycerol prior to imaging.
4.8 Image acquisition
WISH, AB, AO, and live animal images were all taken on a Nikon Eclipse microscope with a DS-Fi2 camera. Methylcellulose with small amounts of tricaine was used to hold the animals in place for the live images and glycerol was used to hold the fixed animals.
4.9 Quantification and statistical analysis
All measurements were taken using the Nikon Elements imaging software on 10x images. Surface area measurements were taken using the auto-select tool. Length measurements were taken using the polyline tool. MCCs were counted manually using the binoculars on the microscope. All of the WISH data were entered into GraphPad Prism and analyzed using unpaired t-tests. AB data was analyzed using the FIJI software. AO images were analyzed by counting fluorescent cells manually. Live, brightfield images were analyzed by quantifying the phenotypes manually.
Acknowledgments
The authors are grateful for the support of the Freimann Zebrafish Facility at the University of Notre Dame for the care of our animal colony, and for the services provided by the Genomics and Bioinformatics Core Facility. This work is dedicated to AH. In this study, the AI tools Alphafold 2 and Phyre 2 were used to predict protein structures.
Funding Statement
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was submitted by funds to RAW from the University of Notre Dame College of Science.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, and further inquires can be directed to the corresponding authors.
Ethics statement
The animal study was approved by University of Notre Dame IACUC. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
WE: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Supervision, Writing–original draft, Writing–review and editing. AD: Data curation, Formal Analysis, Investigation, Writing–review and editing. EG: Data curation, Formal Analysis, Investigation, Writing–review and editing. MR: Data curation, Formal Analysis, Investigation, Writing–review and editing. CG: Data Curation, Formal Analysis, Investigation, Writing–review and editing. AJD: Data Curation, Formal Analysis, Investigation, Writing–review and editing. RW: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Writing–original draft, Writing–review and editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2024.1496244/full#supplementary-material
References
- Abeti R., Parkinson M. H., Hargreaves I. P., Angelova P. R., Sandi C., Pook M. A., et al. (2016). Mitochondrial energy imbalance and lipid peroxidation cause cell death in Friedreich's ataxia. Cell Death Dis. 7 (5), e2237. 10.1038/cddis.2016.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adinolfi S., Iannuzzi C., Prischi F., Pastore C., Iametti S., Martin S. R., et al. (2009). Bacterial frataxin CyaY is the gatekeeper of iron-sulfur cluster formation catalyzed by IscS. Nat. Struct. Mol. Biol. 16 (4), 390–396. 10.1038/nsmb.1579 [DOI] [PubMed] [Google Scholar]
- Adinolfi S., Trifuoggi M., Politou A. S., Martin S., Pastore A. (2002). A structural approach to understanding the iron-binding properties of phylogenetically different frataxins. Hum. Mol. Genet. 11 (16), 1865–1877. 10.1093/hmg/11.16.1865 [DOI] [PubMed] [Google Scholar]
- Andermann E., Remillard G. M., Goyer C., Blitzer L., Andermann F., Barbeau A. (1976). Genetic and family studies in Friedreich's ataxia. Can. J. Neurol. Sci. 3 (4), 287–301. 10.1017/s0317167100025476 [DOI] [PubMed] [Google Scholar]
- Anheim M., Fleury M., Monga B., Laugel V., Chaigne D., Rodier G., et al. (2010). Epidemiological, clinical, paraclinical and molecular study of a cohort of 102 patients affected with autosomal recessive progressive cerebellar ataxia from Alsace, Eastern France: implications for clinical management. Neurogenetics 11 (1), 1–12. 10.1007/s10048-009-0196-y [DOI] [PubMed] [Google Scholar]
- Anheim M., Tranchant C., Koenig M. (2012). The autosomal recessive cerebellar ataxias. N. Engl. J. Med. 366 (7), 636–646. 10.1056/NEJMra1006610 [DOI] [PubMed] [Google Scholar]
- Anzenberger U., Bit-Avragim N., Rohr S., Rudolph F., Dehmel B., Willnow T., et al. (2006). Elucidation of megalin/LRP2-dependent endocytic transport processes in the larval zebrafish pronephros. Journal of cell science 119 (Pt 10), 2127–2137. 10.1242/jcs.02954 [DOI] [PubMed] [Google Scholar]
- Ashraf S., Gee H. Y., Woerner S., Xie L. X., Vega-Warner V., Lovric S., et al. (2013). ADCK4 mutations promote steroid-resistant nephrotic syndrome through CoQ10 biosynthesis disruption. J. Clin. Invest. 123 (12), 5179–5189. 10.1172/JCI69000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busi M. V., Maliandi M. V., Valdez H., Clemente M., Zabaleta E. J., Araya A., et al. (2006). Deficiency of Arabidopsis thaliana frataxin alters activity of mitochondrial Fe-S proteins and induces oxidative stress. Plant J. 48 (6), 873–882. 10.1111/j.1365-313X.2006.02923.x [DOI] [PubMed] [Google Scholar]
- Calmels N., Seznec H., Villa P., Reutenauer L., Hibert M., Haiech J., et al. (2009). Limitations in a frataxin knockdown cell model for Friedreich ataxia in a high-throughput drug screen. BMC Neurol. 9, 46. 10.1186/1471-2377-9-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers B. E., Clark E. G., Gatz A. E., Wingert R. A. (2020a). Kctd15 regulates nephron segment development by repressing Tfap2a activity. Development 147 (23), dev191973. 10.1242/dev.191973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers J. M., Addiego A., Flores-Mireles A. L., Wingert R. A. (2020b). Ppargc1a controls ciliated cell development by regulating prostaglandin biosynthesis. Cell Rep. 33 (6), 108370. 10.1016/j.celrep.2020.108370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandran V., Gao K., Swarup V., Versano R., Dong H., Jordan M. C., et al. (2017). Inducible and reversible phenotypes in a novel mouse model of Friedreich's Ataxia. Elife 6, e30054. 10.7554/eLife.30054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C. N., Verdun V. A., Wingert R. A. (2015). Recent advances in elucidating the genetic mechanisms of nephrogenesis using zebrafish. Cells 4 (2), 218–233. 10.3390/cells4020218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colin F., Martelli A., Clémancey M., Latour J. M., Gambarelli S., Zeppieri L., et al. (2013). Mammalian frataxin controls sulfur production and iron entry during de novo Fe4S4 cluster assembly. J. Am. Chem. Soc. 135 (2), 733–740. 10.1021/ja308736e [DOI] [PubMed] [Google Scholar]
- Cossée M., Puccio H., Gansmuller A., Koutnikova H., Dierich A., LeMeur M., et al. (2000). Inactivation of the Friedreich ataxia mouse gene leads to early embryonic lethality without iron accumulation. Hum. Mol. Genet. 9 (8), 1219–1226. 10.1093/hmg/9.8.1219 [DOI] [PubMed] [Google Scholar]
- Delatycki M. B., Paris D. B., Gardner R. J., Nicholson G. A., Nassif N., Storey E., et al. (1999). Clinical and genetic study of Friedreich ataxia in an Australian population. Am. J. Med. Genet. 87 (2), 168–174. [DOI] [PubMed] [Google Scholar]
- Desgrange A., Cereghini S. (2015). Nephron patterning: lessons from Xenopus, zebrafish, and mouse studies. Cells 4 (3), 483–499. 10.3390/cells4030483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhe-Paganon S., Shigeta R., Chi Y. I., Ristow M., Shoelson S. E. (2000). Crystal structure of human frataxin. J. Biol. Chem. 275 (40), 30753–30756. 10.1074/jbc.C000407200 [DOI] [PubMed] [Google Scholar]
- Diomedi-Camassei F., Di Giandomenico S., Santorelli F. M., Caridi G., Piemonte F., Montini G., et al. (2007). COQ2 nephropathy: a newly described inherited mitochondriopathy with primary renal involvement. J. Am. Soc. Nephrol. 18 (10), 2773–2780. 10.1681/ASN.2006080833 [DOI] [PubMed] [Google Scholar]
- Drummond I. A., Majumdar A., Hentschel H., Elger M., Solnica-Krezel L., Schier A. F., et al. (1998). Early development of the zebrafish pronephros and analysis of mutations affecting pronephric function. Development 125 (23), 4655–4667. 10.1242/dev.125.23.4655 [DOI] [PubMed] [Google Scholar]
- Dürr A., Cossee M., Agid Y., Campuzano V., Mignard C., Penet C., et al. (1996). Clinical and genetic abnormalities in patients with Friedreich's ataxia. N. Engl. J. Med. 335 (16), 1169–1175. 10.1056/NEJM199610173351601 [DOI] [PubMed] [Google Scholar]
- Elia M. (1992). “Organ and tissue contribution to metabolic rate,” in Energy metabolism: tissue determinants and cellular corollaries. Editors Kinney, J. M., Tucker H. N. (New York, NY: Raven Press, Ltd; ), 61–79. [Google Scholar]
- Emma F., Bertini E., Salviati L., Montini G. (2012). Renal involvement in mitochondrial cytopathies. Pediatr. Nephrol. 27 (4), 539–550. 10.1007/s00467-011-1926-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey M. C., Cremer P. D., Aw S. T., Millist L., Todd M. J., White O. B., et al. (2008). Vestibular, saccadic and fixation abnormalities in genetically confirmed Friedreich ataxia. Brain 131 (Pt 4), 1035–1045. 10.1093/brain/awm323 [DOI] [PubMed] [Google Scholar]
- Fatma S., Nayak U., Swain R. K. (2021). Methods to generate and evaluate zebrafish models of human kidney diseases. Int. J. Dev. Biol. 65 (7-8-9), 475–485. 10.1387/ijdb.210041rs [DOI] [PubMed] [Google Scholar]
- Filla A., DeMichele G., Caruso G., Marconi R., Campanella G. (1990). Genetic data and natural history of Friedreich's disease: a study of 80 Italian patients. J. Neurol. 237 (6), 345–351. 10.1007/BF00315657 [DOI] [PubMed] [Google Scholar]
- Filla A., De Michele G., Cavalcanti F., Pianese L., Monticelli A., Campanella G., et al. (1996). The relationship between trinucleotide (GAA) repeat length and clinical features in Friedreich ataxia. Am. J. Hum. Genet. 59 (3), 554–560. [PMC free article] [PubMed] [Google Scholar]
- Gellera C., Castellotti B., Mariotti C., Mineri R., Seveso V., Didonato S., et al. (2007). Frataxin gene point mutations in Italian Friedreich ataxia patients. Neurogenetics 8 (4), 289–299. 10.1007/s10048-007-0101-5 [DOI] [PubMed] [Google Scholar]
- Gerlach G. F., Wingert R. A. (2013). Kidney organogenesis in the zebrafish: insights into vertebrate nephrogenesis and regeneration. Wiley Interdiscip. Rev. Dev. Biol. 2 (5), 559–585. 10.1002/wdev.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach G. F., Wingert R. A. (2014). Zebrafish pronephros tubulogenesis and epithelial identity maintenance are reliant on the polarity proteins Prkc iota and zeta. Dev. Biol. 396 (2), 183–200. 10.1016/j.ydbio.2014.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesfeld J. M. (2019). Molecular mechanisms and therapeutics for the gaa·ttc expansion disease friedreich ataxia. neurotherapeutics. the journal of the American Society for Experimental NeuroTherapeutics 16 (4), 1032–1049. 10.1007/s13311-019-00764-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe K., Clark M. D., Torroja C. F., Torrance J., Berthelot C., Muffato M., et al. (2013). The zebrafish reference genome sequence and its relationship to the human genome. Nature 496 (7446), 498–503. 10.1038/nature12111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynen M. A., Snel B., Bork P., Gibson T. J. (2001). The phylogenetic distribution of frataxin indicates a role in iron-sulfur cluster protein assembly. Hum. Mol. Genet. 10 (21), 2463–2468. 10.1093/hmg/10.21.2463 [DOI] [PubMed] [Google Scholar]
- Javed F., He Q., Davidson L. E., Thornton J. C., Albu J., Boxt L., et al. (2010). Brain and high metabolic rate organ mass: contributions to resting energy expenditure beyond fat-free mass. Am. J. Clin. Nutr. 91 (4), 907–912. 10.3945/ajcn.2009.28512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiralerspong S., Liu Y., Montermini L., Stifani S., Pandolfo M. (1997). Frataxin shows developmentally regulated tissue-specific expression in the mouse embryo. Neurobiol. Dis. 4 (2), 103–113. 10.1006/nbdi.1997.0139 [DOI] [PubMed] [Google Scholar]
- Jumper J., Evans R., Pritzel A., Green T., Figurnov M., Ronneberger O., et al. (2021). Highly accurate protein structure prediction with AlphaFold. Nature 596 (7873), 583–589. 10.1038/s41586-021-03819-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kague E., Gallagher M., Burke S., Parsons M., Franz-Odendaal T., Fisher S. (2012). Skeletogenic fate of zebrafish cranial and trunk neural crest. PLoS One 7 (11), e47394. 10.1371/journal.pone.0047394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelekçi S., Yıldız A. B., Sevinç K., Çimen D. U., Önder T. (2022). Perspectives on current models of Friedreich's ataxia. Front. Cell Dev. Biol. 10, 958398. 10.3389/fcell.2022.958398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley L. A., Mezulis S., Yates C. M., Wass M. N., Sternberg M. J. (2015). The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 10 (6), 845–858. 10.1038/nprot.2015.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel C. B., Ballard W. W., Kimmel S. R., Ullmann B., Schilling T. F. (1995). Stages of embryonic development of the zebrafish. Dev. Dyn. 203 (3), 253–310. 10.1002/aja.1002030302 [DOI] [PubMed] [Google Scholar]
- Koeppen A. H. (2011). Friedreich's ataxia: pathology, pathogenesis, and molecular genetics. J. Neurol. Sci. 303 (1-2), 1–12. 10.1016/j.jns.2011.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeppen A. H., Becker A. B., Qian J., Gelman B. B., Mazurkiewicz J. E. (2017). Friedreich ataxia: developmental failure of the dorsal root entry zone. J. Neuropathol. Exp. Neurol. 76 (11), 969–977. 10.1093/jnen/nlx087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeppen A. H., Davis A. N., Morral J. A. (2011). The cerebellar component of Friedreich's ataxia. Acta Neuropathol. 122 (3), 323–330. 10.1007/s00401-011-0844-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeppen A. H., Mazurkiewicz J. E. (2013). Friedreich ataxia: neuropathology revised. Exp. Neurol. 72 (2), 78–90. 10.1097/NEN.0b013e31827e5762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeppen A. H., Ramirez R. L., Becker A. B., Bjork S. T., Levi S., Santambrogio P., et al. (2015). The pathogenesis of cardiomyopathy in Friedreich ataxia. PLoS One 10 (3), e0116396. 10.1371/journal.pone.0116396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutnikova H., Campuzano V., Foury F., Dollé P., Cazzalini O., Koenig M. (1997). Studies of human, mouse and yeast homologues indicate a mitochondrial function for frataxin. Nat. Genet. 16 (4), 345–351. 10.1038/ng0897-345 [DOI] [PubMed] [Google Scholar]
- Kramer-Zucker A. G., Wiessner S., Jensen A. M., Drummond I. A. (2005). Organization of the pronephric filtration apparatus in zebrafish requires Nephrin, Podocin and the FERM domain protein Mosaic eyes. Dev. Biol. 285 (2), 316–329. 10.1016/j.ydbio.2005.06.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeger P. T., Jr, Drummond B. E., Miceli R., McKernan M., Gerlach G. F., Marra A. N., et al. (2017). The zebrafish kidney mutant zeppelin reveals that brca2/fancd1 is essential for pronephros development. Dev. Biol. 428 (1), 148–163. 10.1016/j.ydbio.2017.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Pathak N., Kramer-Zucker A., Drummond I. A. (2007). Notch signaling controls the differentiation of transporting epithelia and multiciliated cells in the zebrafish pronephros. Development 134 (6), 1111–1122. 10.1242/dev.02806 [DOI] [PubMed] [Google Scholar]
- Ma M., Jiang Y. J. (2007). Jagged2a-notch signaling mediates cell fate choice in the zebrafish pronephric duct. PLoS Genet. 3 (1), e18. 10.1371/journal.pgen.0030018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maio N., Rouault T. A. (2022). Mammalian iron sulfur cluster biogenesis and human diseases. IUBMB Life 74 (7), 705–714. 10.1002/iub.2597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra A. N., Adeeb B. D., Chambers B. E., Drummond B. E., Ulrich M., Addiego A., et al. (2019). Prostaglandin signaling regulates renal multiciliated cell specification and maturation. Proc. Natl. Acad. Sci. USA. 116 (17), 8409–8418. 10.1073/pnas.1813492116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra A. N., Wingert R. A. (2016). Epithelial cell fate in the nephron tubule is mediated by the ETS transcription factors etv5a and etv4 during zebrafish kidney development. Dev. Biol. 411 (2), 231–245. 10.1016/j.ydbio.2016.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Hernández E., García-Silva M. T., Vara J., Campos Y., Cabello A., Muley R., et al. (2005). Renal pathology in children with mitochondrial diseases. Pediatr. Nephrol. 20 (9), 1299–1305. 10.1007/s00467-005-1948-z [DOI] [PubMed] [Google Scholar]
- Mühlenhoff U., Richhardt N., Ristow M., Kispal G., Lill R. (2002). The yeast frataxin homolog Yfh1p plays a specific role in the maturation of cellular Fe/S proteins. Hum. Mol. Genet. 11 (17), 2025–2036. 10.1093/hmg/11.17.2025 [DOI] [PubMed] [Google Scholar]
- Neuhauss S. C., Solnica-Krezel L., Schier A. F., Zwartkruis F., Stemple D. L., Malicki J., et al. (1996). Mutations affecting craniofacial development in zebrafish. Development 123, 357–367. 10.1242/dev.123.1.357 [DOI] [PubMed] [Google Scholar]
- Pallardó F. V., Pagano G., Rodríguez L. R., Gonzalez-Cabo P., Lyakhovich A., Trifuoggi M. (2021). Friedreich Ataxia: current state-of-the-art, and future prospects for mitochondrial-focused therapies. Transl. Res. 229, 135–141. 10.1016/j.trsl.2020.08.009 [DOI] [PubMed] [Google Scholar]
- Parkinson M. H., Boesch S., Nachbauer W., Mariotti C., Giunti P. (2013). Clinical features of Friedreich's ataxia: classical and atypical phenotypes. J. Neurochem. 126 (Suppl. 1), 103–117. 10.1111/jnc.12317 [DOI] [PubMed] [Google Scholar]
- Perdomini M., Belbellaa B., Monassier L., Reutenauer L., Messaddeq N., Cartier N., et al. (2014). Prevention and reversal of severe mitochondrial cardiomyopathy by gene therapy in a mouse model of Friedreich's ataxia. Nat. Med. 20 (5), 542–547. 10.1038/nm.3510 [DOI] [PubMed] [Google Scholar]
- Perdomini M., Hick A., Puccio H., Pook M. A. (2013). Animal and cellular models of Friedreich ataxia. J. Neurochem. 126 (Suppl. 1), 65–79. 10.1111/jnc.12219 [DOI] [PubMed] [Google Scholar]
- Poureetezadi S. J., Wingert R. A. (2016). Little fish, big catch: zebrafish as a model for kidney disease. Kidney Int. 89 (6), 1204–1210. 10.1016/j.kint.2016.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puccio H., Simon D., Cossée M., Criqui-Filipe P., Tiziano F., Melki J., et al. (2001). Mouse models for Friedreich ataxia exhibit cardiomyopathy, sensory nerve defect and Fe-S enzyme deficiency followed by intramitochondrial iron deposits. Nat. Genet. 27 (2), 181–186. 10.1038/84818 [DOI] [PubMed] [Google Scholar]
- Quinzii C. M., Hirano M. (2010). Coenzyme Q and mitochondrial disease. Dev. Disabil. Res. Rev. 16 (2), 183–188. 10.1002/ddrr.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reetz K., Dogan I., Hohenfeld C., Didszun C., Giunti P., Mariotti C., et al. (2018). Nonataxia symptoms in friedreich ataxia: report from the registry of the European friedreich's ataxia consortium for translational studies (EFACTS). Neurology 91 (10), e917–e930. 10.1212/WNL.0000000000006121 [DOI] [PubMed] [Google Scholar]
- Robu M. E., Larson J. D., Nasevicius A., Beiraghi S., Brenner C., Farber S. A., et al. (2007). p53 activation by knockdown technologies. PLoS Genet. 3 (5), e78. 10.1371/journal.pgen.0030078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos M. M., Ohshima K., Pandolfo M. (2001). Frataxin deficiency enhances apoptosis in cells differentiating into neuroectoderm. Hum. Mol. Genet. 10 (18), 1935–1944. 10.1093/hmg/10.18.1935 [DOI] [PubMed] [Google Scholar]
- Schöls L., Amoiridis G., Przuntek H., Frank G., Epplen J. T., Epplen C. (1997). Friedreich's ataxia. Revision of the phenotype according to molecular genetics. Brain 120 (Pt 12), 2131–2140. 10.1093/brain/120.12.2131 [DOI] [PubMed] [Google Scholar]
- Shinnick J. E., Isaacs C. J., Vivaldi S., Schadt K., Lynch D. R. (2016). Friedreich Ataxia and nephrotic syndrome: a series of two patients. BMC Neurol. 16, 3. 10.1186/s12883-016-0526-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon D., Seznec H., Gansmuller A., Carelle N., Weber P., Metzger D., et al. (2004). Friedreich ataxia mouse models with progressive cerebellar and sensory ataxia reveal autophagic neurodegeneration in dorsal root ganglia. J. Neurosci. 24 (8), 1987–1995. 10.1523/JNEUROSCI.4549-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivakumar A., Cherqui S. (2022). Advantages and limitations of gene therapy and gene editing for Friedreich's Ataxia. Front. Genome 4, 903139. 10.3389/fgeed.2022.903139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. L., Barondeau D. P. (2010). Human frataxin is an allosteric switch that activates the Fe-S cluster biosynthetic complex. Biochemistry 49 (43), 9132–9139. 10.1021/bi1013062 [DOI] [PubMed] [Google Scholar]
- Tucker B., Lardelli M. (2007). A rapid apoptosis assay measuring relative acridine orange fluorescence in zebrafish embryos. Zebrafish 4 (2), 113–116. 10.1089/zeb.2007.0508 [DOI] [PubMed] [Google Scholar]
- Van Ham T. J., Mapes J., Kokel D., Peterson R. T. (2010). Live imaging of apoptotic cells in zebrafish. FASEB J. 24 (11), 4336–4342. 10.1096/fj.10-161018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura N., Rea S. L., Testi R. (2006). Long-lived C. elegans mitochondrial mutants as a model for human mitochondrial-associated diseases. Exp. Gerontol. 41 (10), 974–991. 10.1016/j.exger.2006.06.060 [DOI] [PubMed] [Google Scholar]
- Watters G. V., Zlotkin S. H., Kaplan B. S., Humphreys P., Drummond K. N. (1981). Friedreich's ataxia with nephrotic syndrome and convulsive disorder: clinical and neurophysiological studies with renal and nerve biopsies and an autopsy. Can. J. Neurol. Sci. 8 (1), 55–60. 10.1017/s0317167100042852 [DOI] [PubMed] [Google Scholar]
- Weaver N. E., Healy A., Wingert R. A. (2022). Gldc is essential for renal progenitor patterning during kidney development. Biomedicines 10 (12), 3220. 10.3390/biomedicines10123220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. (1993). The zebrafish book. Eugene: University of Oregon Press. [Google Scholar]
- Whitfield T. T. (2019). Cilia in the developing zebrafish ear. Philos. Trans. R. Soc. Lond. B Biol. Sci. 375 (1792), 20190163. 10.1098/rstb.2019.0163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingert R. A., Davidson A. J. (2008). The zebrafish pronephros: a model to study nephron segmentation. Kidney Int. 73 (10), 1120–1127. 10.1038/ki.2008.37 [DOI] [PubMed] [Google Scholar]
- Wingert R. A., Davidson A. J. (2011). Zebrafish nephrogenesis involves dynamic spatiotemporal expression changes in renal progenitors and essential signals from retinoic acid and irx3b. Dev. Dyn. 240 (8), 2011–2027. 10.1002/dvdy.22691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingert R. A., Selleck R., Yu J., Song H. D., Chen Z., Song A., et al. (2007). The cdx genes and retinoic acid control the positioning and segmentation of the zebrafish pronephros. PLoS Genet. 3 (10), 1922–1938. 10.1371/journal.pgen.0030189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue F., Cheng Y., Breschi A., Vierstra J., Wu W., Ryba T., et al. (2014). A comparative encyclopedia of DNA elements in the mouse genome. Nature 515 (7527), 355–364. 10.1038/nature13992 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, and further inquires can be directed to the corresponding authors.