Abstract
Background
The coastal habitats in the southern Gulf of Mexico face multiple threats, such as rising water temperatures, acidification, increased turbidity, invasive species and pollutants. This imperils the biodiversity of beaches, wetlands and coral reefs. To address this, there is a need for comprehensive baseline information on marine biodiversity. Several reefs in the Gulf of Mexico have been extensively studied, yet smaller reefs on the Yucatan continental shelf lack thorough exploration despite their ecological significance. These reefs serve as crucial biodiversity hotspots influenced by environmental characteristics, receiving diverse taxa from the Gulf of Mexico.
The macroalgae study at the Bank of Campeche dates back to the 1950s, but comprehensive investigations have been sporadic. The recent study aims to fill this gap, contributing to the taxonomic inventory of the area's benthic macroflora. Methodologically, extensive sampling across eight reefs was conducted, collecting and preserving macroalgae samples for identification in the laboratory.
The study documented 90 infrageneric taxa across the reefs, with Cayo Arenas exhibiting the highest species count. Additionally, three new distribution reports for Mexico were identified in the region. The distribution of species varied amongst locations, with few species in common even amongst geographically proximate reefs. The diversity found in these reefs slightly trails behind other studied regions, but surpasses previous reports for the Campeche Bank.
It is important to emphasise that the significance of this study lies in its focus on remote reefs with complicated and costly access logistics. Additionally, it is one of the first publicly available datasets published for this region.
The study aligns with existing literature on prevalent families in reef environments and highlights differences in species distribution, based on depth variations amongst reefs. The findings of new distribution records in the region and the distinctiveness of localities despite their proximity underscore the unique ecological dynamics of these reefs.
New information
These reefs are located in remote and difficult-to-access areas, highlighting the importance of the data obtained on their biodiversity and conservation status. This work presents for the first time a list of macroalgae for the reefs of Banco Nuevo, Banco Obispo, Banco Pera and Serpientes Reef. Three new distribution reports for Yucatan were identified at the Banco Obispo reef: Botryocladiashanksii E. Y. Dawson, Ceratodictyonscoparium (Montagne & Millardet) R. E. Norris and Asteromeniapeltata (W. R. Taylor) Huisman & Millar and a new report for the Mexican Atlantic, Herposiphoniaparca Setchell at the Triángulo Oeste reef. This results in distinct algal communities compared to other reefs in the region, emphasising their ecological significance and the need for continued research and conservation efforts. To our knowledge, this is one of the first interoperable datasets being published on the marine algae of the southern Gulf of Mexico reef ecosystems.
Keywords: biodiversity, seaweeds, reefs
Introduction
The habitats of coastal areas are subjected to a variety of anthropogenic and environmental pressures, which endanger the biological diversity that inhabits them. Amongst the most exposed ecosystems are beaches, coastal wetlands and coral reefs, for which the expected increase in water temperature, acidification due to the effects of global climate change, increased turbidity due to sediments and the presence of invasive species could have negative and high-impact effects in the medium and long term (Cooley et al. 2022). This scenario makes it imperative to compile reference information to establish the current conditions of marine biodiversity and to understand the current state of these ecosystems in the southern Gulf of Mexico. Knowledge of the diversity, abundance and distribution of species is the foundation for the development of conservation and natural resource utilisation projects aimed at preserving their ecological, economic and cultural importance.
The Gulf of Mexico platform has many important reef systems that have been extensively studied (Chávez-Hidalgo A et al. 2008, Horta-Puga et al. 2015). However, there is a lack of information about many smaller reefs located on the continental shelf of Yucatan, despite being important centres of biodiversity and fisheries resources (Zarco-Perelló et al. 2013).
The reefs of the Campeche Bank in the Gulf of Mexico, such as Alacranes Reef, Cayo Arenas, Cayo Arcas and Triangulos, have been the subject of various studies (Jordán-Dahlgren 2002, Tunnell et al. 2007, Zarco-Perelló et al. 2013). Nevertheless, the study of macroalgae in these environments has been scarce and discontinuous.
The first studies of macroalgae on the Campeche Bank were conducted in the 1950s, Huerta-Múzquiz collecting samples at Cayo Arenas and Alacranes Reef and identifying 19 and 21 infrageneric taxa, respectively (Huerta 1958). Subsequently, in 1960, in the eulittoral zone around Isla Pérez, 77 infrageneric taxa were identified (Huerta 1961). There are also records of collections by H. J. Humm in the Campeche Bank (Humm 1952) and reports of 17 algal species in the work of Kornicker et al. (1959). Some of the more extensive studies are those of Kim (1964), who characterised the phycoflora of Alacranes Reef with 198 infrageneric taxa and Huerta-Múzquiz et al. (1987), who compiled advances in the study of marine algae of the Yucatan Peninsula, listing 412 infrageneric taxa (140 Chlorophyta, 56 Phaeophyceae, 199 Rhodophyta and 17 Cyanobacteria) and reported algal records in Alacranes Reef, Cayo Arenas and Triangulo Oeste. For the Madascar reefs at Sisal, Yucatan, there is only the work of Ortegon-Aznar et al. (2008), who report 55 taxa of macroalgae. The most recent study reported 22 species for Alacranes, six species for Cayo Arcas and 16 species for Bajo Obispo (González-Solis et al. 2018).
Therefore, this study aims to contribute to the knowledge of macroalgae in the reef system of the Campeche Bank in the Gulf of Mexico by providing information on the composition and richness of benthic macroalgae communities.
Project description
Title
Benthic macroflora of the reef system of the Campeche Bank
Personnel
This study is part of a megaproject with the objective of generating a taxonomic inventory of the fauna and benthic macroflora of the reef system of the Campeche Bank. It aims to create a georeferenced dataset of marine biota presence/absence that follows the DarwinCore data standard (DwC) uploaded at the Caribbean OBIS Node. Additionally, it seeks to contribute to strengthening the information on biodiversity species for the Gulf of Mexico at the National Biodiversity Information System (SNIB) of CONABIO, as well as national and regional collections, updating, completing and identifying information gaps in floristic inventories and taxonomic authority catalogues.
Sampling methods
Study extent
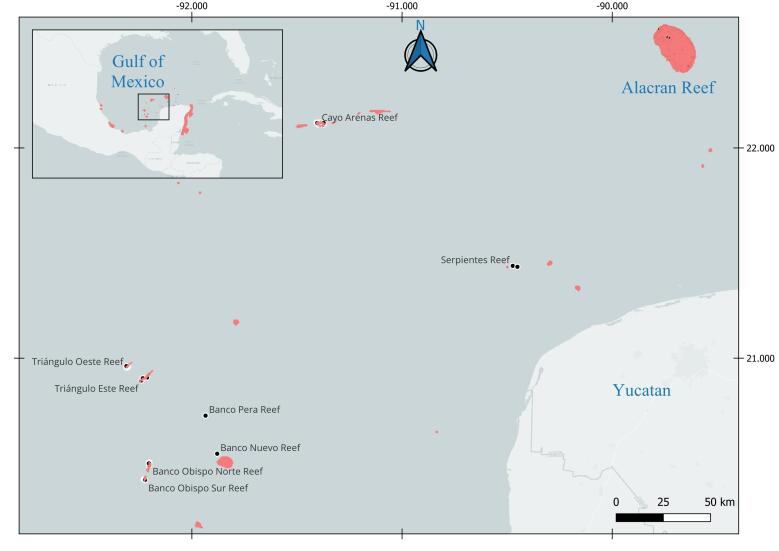
Sampling was conducted during three excursions in 2017 to eight reefs of the Campeche Bank: Arrecife Banco Nuevo (BN), Arrecife Banco Obispo Norte (BON), Arrecife Banco Obispo Este (BOE), Arrecife Banco Pera (BP), Cayo Arenas Reef West Triangle Reef (TO), East Triangle Reef (TE) and Serpientes Reef (Ser) (Fig. 1). The first excursion took place at Cayo Arenas (20 May to 28 May 2017), the second at the submerged Serpientes Reef (29 July 2017) and the last one at the submerged cays in the West (8-16 September 2017). At each site, the geographic coordinates of each sampling site were physically located using a Garmin© GPS (Global Positioning System) with an accuracy of ±4 m (Table 2). Sampling was conducted using self-contained underwater breathing apparatus (SCUBA) diving, free diving and general intertidal collection methods. Macroalgae were collected and, in the case of specimens adhering to rocks, a spatula or knife was used. The organisms were placed in sealed bags with seawater at each sampling site and station. Each sample is associated with collection information recorded in the field in the form of a label containing the following information: identification of the material at the highest possible taxonomic level in the field, location (with geographic coordinates), depth, type of substrate or environment, date and time of collection, collector's name, collection code, photograph code and collection method used.
Figure 1.
Map of the studied Reef Systems in the Gulf of Mexico. The black dots represent the sampled locations and the red colour shows the distribution of existing reefs in the Gulf of Mexico.
Table 2.
Coordinates by locality, sampling point and depth. About the sampling points, letters indicate locality initials and numbers indicate stations within each locality. Banco Obispo (BO) (S or N South or North), Triángulos Oeste (TW) Triangulos Este (TE), Arrecife Serpientes (Ser), Ext (Extra point) Arrecife Banco Pera (BP), BN: Banco Nuevo.
| Locality | Sampling point | Decimal Latitude | Decimal Longitude | Depth In Metres |
| Arrecife Banco Obispo Norte | 16_BON | 20.50072 | -92.20306 | 11 |
| Arrecife Banco Obispo Norte | 1_BON | 20.50205 | -92.20464 | 21 |
| Arrecife Banco Obispo Norte | 8_BON | 20.50371 | -92.20200 | 21 |
| Arrecife Banco Obispo Norte | 9_BON_Nocturn | 20.49049 | -92.20332 | 20 |
| Arrecife Banco Obispo Norte | 10_BON | 20.49943 | -92.20429 | 9 |
| Arrecife Banco Obispo Sur | 4 _BOS | 20.42059 | -92.22333 | 13 |
| Arrecife Banco Obispo Sur | 2_BOS | 20.42529 | -92.22865 | 25 |
| Arrecife Banco Obispo Sur | 1_BOS | 20.42343 | -92.22603 | 18 |
| Arrecife Banco Obispo Sur | 3_BOS | 20.42133 | -92.22256 | 13 |
| Arrecife Banco Pera | BP | 20.72669 | -91.93481 | 24 |
| Arrecife Cayo Arenas | Ext 001 | 22.11670 | -91.39809 | 1.5 |
| Arrecife Cayo Arenas | Point 02 | 22.12064 | -91.40636 | 9.3 |
| Arrecife Cayo Arenas | point 01 | 22.12092 | -91.38831 | 9 |
| Arrecife Cayo Arenas | point 03 | 22.11557 | -91.40161 | 6.8 |
| Arrecife Cayo Arenas | Point 06 | 22.11668 | -91.39764 | 7.5 |
| Arrecife Cayo Arenas | Ext002 | 22.11558 | -91.40008 | 1 |
| Arrecife Cayo Arenas | Point 10 | 22.11181 | -91.39808 | 13 |
| Arrecife Cayo Arenas | Point 32 | 22.11086 | -91.37644 | 11.9 |
| Arrecife Cayo Arenas | Point 09 | 22.11352 | -91.38397 | 12 |
| Arrecife Cayo Arenas | Point 34 | 22.11353 | -91.37839 | 18.5 |
| Arrecife Cayo Arenas | Point 31 | 22.11236 | -91.37344 | 15.5 |
| Arrecife Cayo Arenas | Point 30 | 22.12114 | -91.39925 | 20.6 |
| Arrecife Cayo Arenas | Point 28 | 22.11894 | -91.37289 | 6.6 |
| Arrecife Cayo Arenas | Ext 07 | 22.11868 | -91.40374 | 8 |
| Arrecife Serpientes | SER-01 | 21.43936 | -90.47292 | 9 |
| Arrecife Serpientes | SER-02 | 21.43478 | -90.45086 | 9.9 |
| Arrecife Triángulo Este | 15_TE | 20.89435 | -92.23953 | 15 |
| Arrecife Triángulo Este | 3_TE | 20.90456 | -92.23322 | 11 |
| Arrecife Triángulo Este | 15_TE | 20.90833 | -92.21313 | 28 |
| Arrecife Triángulo Oeste | 12_TW | 20.95528 | -92.31086 | 18 |
| Arrecife Triángulo Oeste | 7_TW | 20.95816 | -92.30516 | 18 |
| Arrecife Triángulo Oeste | 8_TW | 20.95856 | -92.30441 | 15 |
| Arrecife Triángulo Oeste | 1_TW | 20.95887 | -92.30717 | 0 |
| Arrecife Triángulo Oeste | 11_TW | 20.96036 | -92.30776 | 11 |
| Arrecife Triángulo Oeste | 2_TW | 20.96221 | -92.31128 | 15 |
| Banco Nuevo | BN | 20.54561 | -91.87944 | 24 |
Sampling description
For Cayo Arenas, 31 samplings were conducted, including 30 daytime samplings and one night-time dive. Organism collections were made on the coral reefs around the sandy cay and adjacent reef areas in benthic ecosystems considered as shallow waters (i.e. depths between 0 and 20 m). A total of 55% of organism collections were made using SCUBA, 29% were conducted in the intertidal zone through beach samplings and 16% were collected through free diving. During the campaign, 92 algal samples were collected from various types of environments and environmental data were recorded for each sampling point, including salinity and temperature data.
For the submerged cays sampling, a total of 79 samplings were conducted by four different working groups across six different reef areas, comprising 74 daytime samplings and five night-time dives. Organism collections were carried out on the coral reefs, specifically on TW and TE reefs, around sandy cays, in shallow benthic ecosystems considered shallow waters (i.e. depths between 0 and 30 m) and on the submerged ship found at point 11_TW. Approximately 208 algal specimens were collected during 79 dives, one snorkelling excursion and one intertidal collection, covering 61 different sites within six reefs located to the west of the Campeche Bank. Only one sampling was conducted in the intertidal zone using free diving (at Triángulos Este Reef), while the rest of the collections were made using SCUBA.
For the Serpientes Reef sampling, two samplings were conducted, one dive at each site on the reef peaks at depths of 8 to 13 m. A total of 29 batches with 41 algal organisms were collected.
In the laboratory, the samples were separated and preserved in formalin (4%). In some cases, pressed vouchers were created and deposited in the Algae section of the Herbarium of the Faculty of Sciences of UNAM (FCME). The pressed vouchers were consecutively assigned numbers: GM-925 to GM-1204 and they can be consulted in the dataset previously uploaded to the Ocean Biodiversity Information System (OBIS). Subsequently, identification was performed at the best possible taxonomic level by observing complete thalli and making microscopic slides using a stereoscope and an optical microscope. The main literature used for identification included the identification guides by Littler and Littler (2000) and Dawes and Mathieson (2008). Taxonomic nomenclature was verified using the Algaebase database (Guiry and Guiry 2024).
Geographic coverage
Description
Western submerged cays (Banco Obispo Triángulos Oeste and Este, Banco Pera and Banco Nuevo) Arrecife Serpientes, and Cayo Arenas at the Campeche Bank.
Coordinates
−90.45086 and −92.31128 Latitude; 22.12092 and 20.42059 Longitude.
Taxonomic coverage
Description
In this study, 90 infrageneric taxa were identified at the species level, which are located in two kingdoms: Plantae and Cromista and belong to the three phyla: Chlorophyta, Rhodophyta and Heterokontophyta, which contain five classes, 16 orders, 37 families and 62 genera of macroalgae (Table 1).
Table 1.
Infrageneric Taxa by Localities. Abbreviations: Car = cayo Arenas, BON = Banco Obispo norte, BOS = Banco Obispo Sur, S = Serpientes, TE = Triángulos Este, TN = Triángulos Norte, BN: Bajo Nuevo, BP: Banco Pera. * New distribution report for Mexican Atlantic, ** New distribution reports for Yucatan.
| Infrageneric Taxa: | Car | BON | BOS | S | TE | TN | BN | BP | |
| Num Sp | CHLOROPHYTA | ||||||||
| 1 | Anadyomenesaldanhae Joly & Oliveira | 1 | 1 | 1 | |||||
| 2 | Anadyomenestellata (Wulfen) C. Agardh | 1 | 1 | ||||||
| 3 | Bryopsispennata J.V. Lamouroux | 1 | 1 | ||||||
| 4 | Bryopsispennatavar.secunda (Harvey) Collins & Hervey | 1 | |||||||
| 5 | Bryopsisplumosa (Hudson) C. Agardh | 1 | |||||||
| 6 | Caulerpaambigua Okamura | 1 | 1 | 1 | |||||
| 7 | Caulerpachemnitzia (Esper) J.V.Lamouroux | 1 | |||||||
| 8 | Caulerpamicrophysa (Weber van Bosse) Feldmann | 1 | 1 | 1 | 1 | ||||
| 9 | Caulerparacemosa (Forsskål) J. Agardh | 1 | 1 | ||||||
| 10 | Caulerparacemosavar.macrophysa (Kützing) W. R. Taylor | 1 | |||||||
| 11 | Caulerpasertularioidesf.brevipes (J.Agardh) Svedelius | 1 | |||||||
| 12 | Caulerpaverticillata J. Agardh | 1 | 1 | 1 | 1 | 1 | |||
| 13 | Caulerpaverticillataf.charoides Weber van Bosse | 1 | 1 | ||||||
| 14 | Caulerpawebbiana Montagne | 1 | 1 | ||||||
| 15 | Cladophoraliniformis Kützing | 1 | |||||||
| 16 | Cladophoropsismacromeres W. R. Taylor | 1 | |||||||
| 17 | Cladophoropsismembranacea (Bang ex C.Agardh) Børgesen | 1 | |||||||
| 18 | Dictyosphaeriacavernosa (Forsskal) Børgesen | 1 | |||||||
| 19 | Halimedadiscoidea Decaisne | 1 | 1 | ||||||
| 20 | Halimedaincrassata (J. Ellis) J. V. Lamouroux | 1 | 1 | ||||||
| 21 | Halimedaopuntia (Linnaeus) J.V. Lamouroux | 1 | 1 | ||||||
| 22 | Halimedatuna (J.Ellis & Solander) J.V.Lamouroux | 1 | |||||||
| 23 | Penicilluscapitatus Lamarck | 1 | |||||||
| 24 | Penicillusdumetosus (J.V. Lamouroux) Blainville | 1 | |||||||
| 25 | Phyllodictyonanastomosans (Harvey) Kraft & M. J. Wynne | 1 | |||||||
| 26 | Rhipocephalusphoenixf.longifolius A.Gepp & E.Gepp | 1 | |||||||
| 27 | Siphonocladustropicus (P. Crouan & H. Crouan) J.Agardh | 1 | |||||||
| 28 | Udoteacaribaea D. S. Littler & Littler | 1 | |||||||
| 29 | Udoteacyathiformis Decaisne | 1 | |||||||
| 30 | Udoteadixonii D. S. Littler & Littler | 1 | |||||||
| 31 | Valoniamacrophysa Kützing | 1 | |||||||
| 32 | Valoniaventricosa J.Agardh | 1 | 1 | 1 | |||||
| PHAEOPHYCEAE | |||||||||
| 1 | Canistrocarpuscrispatus (J.V.Lamouroux) De Paula & De Clerck | 1 | |||||||
| 2 | Dictyopterisdelicatula J.V. Lamouroux | 1 | 1 | ||||||
| 3 | Dictyotabartayresiana J.V. Lamouroux | 1 | 1 | 1 | |||||
| 4 | Dictyotacaribaea Hörnig & Schnetter | 1 | |||||||
| 5 | Dictyota ciliolata Sonder ex Kützing | 1 | 1 | ||||||
| 6 | Dictyotahumifusa Hörnig, Schnetter & Coppejans | 1 | |||||||
| 7 | Dictyotajamaicensis W. R. Taylor | 1 | 1 | ||||||
| 8 | Dictyota pinnatifida Kützing | 1 | |||||||
| 9 | Dictyotapulchella Hörnig & Schnetter | 1 | 1 | 1 | 1 | ||||
| 10 | Feldmanniaindica (Sonder) Womersley & A. Bailey | 1 | |||||||
| 11 | Lobophoracanariensis (Sauvageau) C.W. Vieira, De Clerck & Payri | 1 | |||||||
| 12 | Lobophoradeclerckii N.E. Schultz, C.W. Schneider & L. Le Gall | 1 | |||||||
| 13 | Lobophoravariegata (J.V. Lamouroux) Womersley ex Oliveira | 1 | |||||||
| 14 | Padinahaitiensis Thivy | 1 | |||||||
| 15 | Padinaperindusiata Thivy | 1 | |||||||
| 16 | Padinasanctae-crucis Børgesen | 1 | |||||||
| 17 | Sargassumbuxifolium (Chauvin) M.J.Wynne | 1 | |||||||
| 18 | Sargassumfurcatum Kützing | 1 | |||||||
| 19 | Stypopodiumzonale (J.V. Lamouroux) Papenfuss | 1 | 1 | 1 | 1 | ||||
| RHODOPHYTA | |||||||||
| 1 | Acanthophoraspicifera (Vahl) Børgesen | 1 | |||||||
| 2 | Amphiroabeauvoisii J.V. Lamouroux | 1 | |||||||
| 3 | Amphiroarigida J.V. Lamouroux | 1 | |||||||
| 4 | Amphiroafragilissima (Linnaeus) J.V. Lamouroux | 1 | |||||||
| 5 | Amphiroatribulus (J. Ellis & Solander) J.V. Lamouroux | 1 | 1 | 1 | |||||
| 6 | Antithamnionellabreviramosa (E.Y.Dawson) Wollaston | 1 | |||||||
| 7 | Asparagopsistaxiformis (Delile) Trevisan | 1 | 1 | ||||||
| 8 | Asteromeniapeltata(W. R. Taylor) Huisman & A. J. K. Millar ** | 1 | 1 | ||||||
| 9 | Botryocladiashanksii E. Y. Dawson ** | 1 | 1 | ||||||
| 10 | Botryocladiaspinulifera W.R. Taylor & I.A. Abbott | 1 | |||||||
| 11 | Caloglossaleprieurii (Montagne) G.Martens | 1 | |||||||
| 12 | Ceramiumnitens (C. Agardh) J. Agardh | 1 | 1 | ||||||
| 13 | Ceramiumvirgatum Roth | 1 | |||||||
| 14 | Ceratodictyonintricatum (C. Agardh) R.E. Norris | 1 | 1 | ||||||
| 15 | Ceratodictyonscoparium (Montagne & Millardet) R.E. Norris ** | 1 | 1 | 1 | 1 | ||||
| 16 | Ceratodictyon variabile (J.Agardh) R.E.Norris | 1 | |||||||
| 17 | Dichotomariaobtusatavar.major M. J. Wynne | 1 | 1 | ||||||
| 18 | Dictyurusoccidentalis J.Agardh | 1 | |||||||
| 19 | Erythrotrichiacarnea (Dillwyn) J. Agardh | 1 | |||||||
| 20 | Flahaultiategetiformans W.R. Taylor | 1 | |||||||
| 21 | Galaxaurarugosa (J. Ellis & Solander) J.V. Lamouroux | 1 | |||||||
| 22 | Gayliellaflaccida (Harvey ex Kützing) T. O. Cho & L. J. McIvor | 1 | |||||||
| 23 | Gelidiellaacerosa (Forsskål) J. Feldmann & Hamel | 1 | 1 | ||||||
| 24 | Gelidiumamericanum (W.R. Taylor) Santelices | 1 | |||||||
| 25 | Griffithsiaglobulifera Harvey ex Kützing | 1 | |||||||
| 26 | Herposiphoniaparca Setchell* | 1 | |||||||
| 27 | Hydrolithonfarinosum (J. V. Lamouroux) Penrose & Y. M. Chamberlain | 1 | |||||||
| 28 | Hypneaspinella (C. Agardh) Kützing | 1 | 1 | 1 | |||||
| 29 | Hypoglossumhypoglossoides (Stackhouse) Collins & Hervey | 1 | |||||||
| 30 | Jania adhaerens J.V.Lamouroux | 1 | |||||||
| 31 | Janiacapillacea Harvey | 1 | |||||||
| 32 | Janiapumila J.V.Lamouroux | 1 | |||||||
| 33 | Liagoraceranoides J.V.Lamouroux | 1 | |||||||
| 34 | Nitophyllumpunctatum (Stackhouse) Greville | 1 | |||||||
| 35 | Parviphycussetaceus ((Feldmann) J.Afonso-Carrillo, M.Sanson, C.Sangil & T.Diaz-Villa | 1 | 1 | ||||||
| 36 | Polysiphoniahavanensis Montagne | 1 | |||||||
| 37 | Pterocladiellacapillacea (S. G. Gmelin) Santelices & Hommersand | 1 | |||||||
| 38 | Stylonemaalsidii (Zanardini) K.M. Drew | 1 | |||||||
| 39 | Wrangeliaargus (Montagne) Montagne | 1 |
Taxa included
| Rank | Scientific Name | Common Name |
|---|---|---|
| kingdom | Plantae | Plant |
| phylum | Chlorophyta | Green Algae |
| class | Ulvophyceae | |
| order | Cladophorales | |
| order | Bryopsidales | |
| order | Siphonocladales | |
| family | Anadyomenaceae | |
| family | Bryopsidaceae | |
| family | Caulerpaceae | |
| family | Boodleaceae | |
| family | Siphonocladaceae | |
| family | Halimedaceae | |
| family | Udoteaceae | |
| family | Valoniaceae | |
| phylum | Rhodophyta | Red Algae |
| class | Florideophyceae | |
| class | Stylonematophyceae | |
| class | Compsopogonophyceae | |
| order | Ceramiales | |
| order | Corallinales | |
| order | Bonnemaisoniales | |
| order | Rhodymeniales | |
| order | Nemaliales | |
| order | Erythropeltales | |
| order | Gigartinales | |
| order | Stylonematales | |
| family | Ceramiaceae | |
| family | Lomentariaceae | |
| family | Galaxauraceae | |
| family | Dasyaceae | |
| family | Erythrotrichiaceae | |
| family | Solieriaceae | |
| family | Gelidiellaceae | |
| family | Gelidiaceae | |
| family | Wrangeliaceae | |
| family | Rhodomelaceae | |
| family | Corallinaceae | |
| family | Cystocloniaceae | |
| family | Delesseriaceae | |
| family | Liagoraceae | |
| family | Pterocladiaceae | |
| family | Stylonemataceae | |
| kingdom | Chromista | |
| phylum | Heterokontophyta | |
| class | Phaeophyceae | Brown Algae |
| order | Dictyotales | |
| order | Ectocarpales | |
| order | Fucales | |
| family | Dictyotaceae | |
| family | Acinetosporaceae | |
| family | Sargassaceae |
Temporal coverage
Formation period: 20-5-2017; 16-9-2017.
Notes
Data were collected from 20-5-2017 to 16-9-2017, at Cayo Arenas from 20 - 28 May 2017. Data were collected at Serpientes Reef on 29 July 2017. Data were collected at Triángulo Oeste, Triángulo Este, Banco Obispo Norte, Banco Obispo Sur, Banco Nuevo and Banco Pera from 8 - 16 September 2017.
Collection data
Collection name
Within theHerbarium of the Faculty of Sciences of UNAM (FCME), the pressed vouchers were consecutively assigned numbers: GM-925 to GM-1204 and they can be consulted in the dataset previously uploaded to the Ocean Biodiversity Information System (OBIS).
Collection identifier
the pressed vouchers were consecutively assigned numbers: GM-925 to GM-1204.
Parent collection identifier
can be consulted in the dataset previously uploaded to the Ocean Biodiversity Information System (OBIS).
Specimen preservation method
collection is in formalin 4%.
Curatorial unit
GM-925 to GM-1204,
Usage licence
Usage licence
Other
IP rights notes
CC BY-NC 4.0
Data resources
Data package title
Macroalgae from eight reefs in the Campeche Bank, collected by the National Autonomous University of Mexico (UNAM)
Resource link
Alternative identifiers
https://obis.org/dataset/1de8b1fe-8847-4e4f-9140-cfa6dab91e4c
Number of data sets
1
Data set 1.
Data set name
Macroalgae from eight reefs in the Campeche Bank, collected by the National Autonomous University of Mexico (UNAM).
Data format
CSV
Download URL
https://ipt.iobis.org/caribbeanobis/archive.do?r=macroalgae_of_the_campeche_bank
Description
The dataset represents the macroalgal composition of eight reefs in the Campeche Bank: Banco Nuevo Reef (BN), Banco Obispo Norte Reef (BON), Banco Obispo Sur Reef (BOS), Banco Pera Reef (BP), Cayo Arenas Reef, West Triangle Reef (TO), East Triangle Reef (TE) and Serpientes Reef (Ser). The dataset contains a total of 395 records, which are taxonomically classified as follows: two at the phylum level, four at the subclass level, four at the order level, three at the family level, 185 at the genus level and 197 records at the species level. In this work, as we only put the different infrageneric taxa identified, there is a differentiation with the dataset because it contains the total number of specimens that were identified at the species level, per specimen; therefore, 195 taxa identified at the species level are reported and 185 were identified at the genus level.
Data set 1.
| Column label | Column description |
|---|---|
| occurrenceID | Biological record ID. |
| basisOfRecord | Origin or specific evidence from which the organism/sample is derived. |
| type | Type of evidence that gives rise to the record. |
| institutionCode | The full name of the institution holding the specimen or record information. |
| collectionCode | The name, acronym, alphanumeric code or initials that identify the collection or dataset from which the organism comes. |
| catalogNumber | An identifier assigned to the specimen, sample or lot in the biological collection. |
| datasetName | The name of the dataset from which the biological record is derived. |
| language | The language of the dataset. |
| recordedBy | The collector or main observer. |
| recordedByID | ID of the people (observers or collectors), groups or organisations responsible for carrying out the registration (ORCID). |
| occurrenceStatus | State that accounts for the presence or absence of a taxon at a location. |
| preparations | Preparations and methods of conservation of a specimen or a sample of the specimen. |
| disposition | The current status of a specimen in relation to the collection identified in collectionCode or collectionID. |
| samplingProtocol | The name, description or reference of the sampling method or protocol used to perform the sampling. |
| eventDate | Sampling or observation date. |
| year | Year of sampling and observation. |
| month | Month of sampling or observation. |
| day | Sampling or observation day. |
| habitat | Description of the habitat in which the event occurred. |
| fieldNumber | An indicator about the existence of or reference to field notes. |
| continent | The name of the continent on which the location takes place. |
| waterBody | The name and type of the body of water in which the location takes place. |
| country | The country name of the location. |
| countryCode | The standard code for the country of the location. |
| locality | The most location-specific geographic information. |
| maximumDepthInMetres | The greatest depth of a depth range below the local surface. |
| minimumDepthInMetres | The lowest depth of a depth range below the local surface. |
| decimalLatitude | The geographic latitude (in decimal degrees, using the spatial reference system provided in geodeticDatum). |
| decimalLongitude | The geographic longitude (in decimal degrees, using the spatial reference system provided in geodeticDatum). |
| geodeticDatum | The ellipsoid, geodetic datum or spatial reference system (SRS) on which geographic coordinates are based. |
| coordinateUncertaintyInMetres | The horizontal distance (in metres) of the decimalLatitude and decimalLongitude provided describing the smallest circle containing the entire location. |
| identifiedBy | Names of the people responsible for identifying the organism. |
| identifiedByID | ID of the persons responsible for identifying the organism (ORCID). |
| dateIdentified | The date on which the observation, collection or sample was taxonomically identified. |
| identificationRemarks | Comments or notes on identification. |
| identificationQualifier | The degree of uncertainty of the identification can be indicated by adding various terms, such as aff. and cf. to the scientific name. |
| scientificName | The canonical scientific name with the authorship corresponding to the taxonomic category to which the determination of the observed or collected organism was achieved. |
| scientificNameID | An identifier of nomenclature details (non-taxonomic) according to the scientific name documented in the scientificName element. |
| kingdom | The name of the kingdom to which the taxon belongs. |
| phylum | The name of the phylum or division to which the taxon belongs. |
| class | The name of the class or division to which the taxon belongs. |
| order | The name of the order to which the taxon belongs. |
| family | The name of the family to which the taxon belongs, |
| genus | The name of the genus to which the taxon belongs. |
| taxonRank | the taxonomic category of the most specific name present in the scientificName. |
Additional information
To complement and make this study clearer, two subsections are attached as additional information, one for results and another for discussion and conclusions.
Results
A total of 395 algae samples were gathered across eight reefs of the Campeche Bank around the sandy cays. Arrecife Banco Nuevo (BN) had 10 species from 17 samples, Arrecife Banco Obispo Norte (BON) nine species from 24 samples, Arrecife Banco Obispo Este (BOE) 20 species and Arrecife Banco Pera (BP) 24 species. Furthermore, Cayo Arenas Reef contributed a total of 28 species from 94 samples, West Triangle Reef (TO) 23 species, East Triangle Reef (TE) 15 species and Serpientes Reef (Ser) 18 species. In total, 90 species, 66 genera, 36 families, 16 orders, five classes and three phyla were identified (Table 1). Amongst these, 32 species belonged to Chlorophyta, 39 to Rhodophyta and 19 to Phaeophyceae. Cayo Arenas had the highest species count, featuring 28 species – 11 Chlorophyta, eight Rhodophyta and nine Phaeophyceae. In contrast, Banco Obispo Norte Reef had the lowest species with nine species: four Chlorophyta, four Rhodophyta and one Phaeophyceae (Fig. 3).
Figure 3.
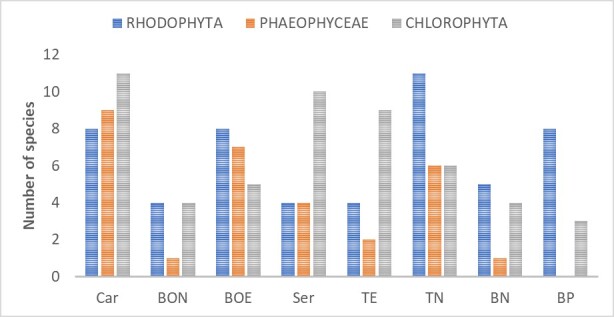
Species richness by phylum and locations (Car = cayo Arenas, BON = Banco Obispo norte, BOE = Banco Obispo Este, Ser = Serpientes, TE = Triangulos Este, TN = Triangulos Norte, BN: Bajo Nuevo, BP: Banco Pera).
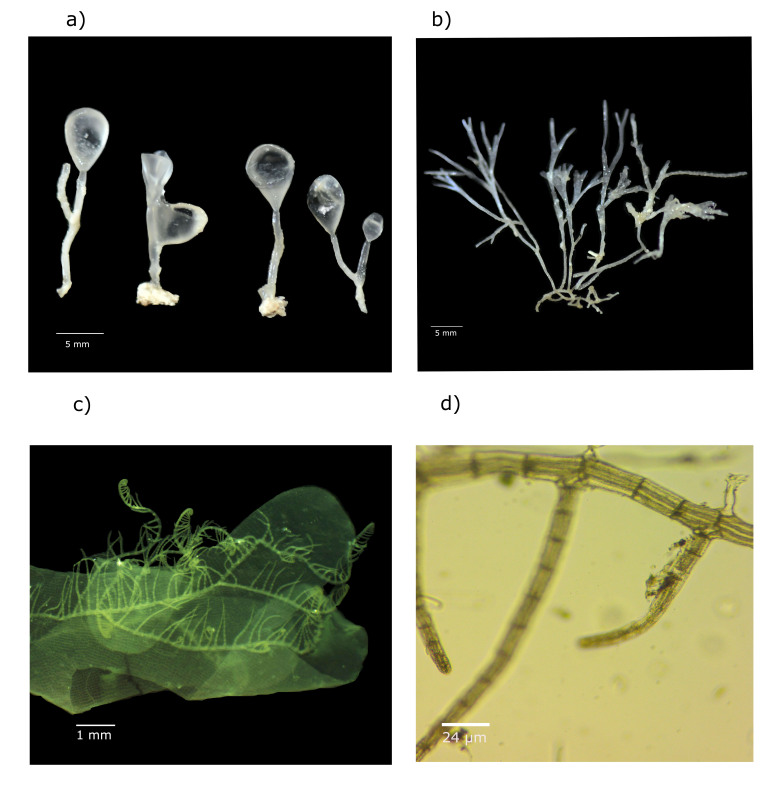
Four species were newly reported in this region: Asteromeniapeltata (W. R. Taylor) Huisman & A. J. K. Millar, Botryocladiashanksii E. Y. Dawson, 1962, Ceratodictyonscoparium (Montagne & Millardet) R. E. Norris, 1987 and Herposiphoniaparca Setchell, 1926 (Fig. 2).
Figure 2.
New records: a Botryocladiashanksii E. Y. Dawson, 1962 (GM-1082) Banco Obispo Sur Reef; b Ceratodictyonscoparium (Montagne & Millardet) R.E.Norris, 1987 (GM-1004) Triángulo Oeste Reef; c-d Herposiphoniaparca Setchell, 1926 (GM-1186) Triángulo Oeste Reef.
Asteromeniapeltata: This algae species has a thalli adhering to the substrate by small attachment discs. Plants with stipitate stems and peltate or lobed dorsiventral laminae.The laminae widen to reach 4 cm in width and can be simple or irregularly lobed circles; often the laminae anastomose with each other at the margins. In their natural environment, plants are iridescent with colours ranging from yellow to reddish-brown (Mendoza-González and Mateo-Cid 2007). Its distribution spans Mexican Caribbean, Caribbean Islands such as Bahamas and Cuba and parts of the Western Atlantic and South America including Colombia and Venezuela (Guiry and Guiry 2024).
Botryocladiashanksii: Is characterised by its red hue, grows epilithically, forming large clumps up to 26 mm tall. These plants are attached by a discoid holdfast, producing one or two solid terete axes (around 0.5 mm in diameter). These axes are dichotomously branched 1–2 times, bearing up to ten lateral vesicles confined to their distal parts (Afonso Carrillo and Sobrino 2004). Their distribution spans various regions, including Atlantic Islands like the Canary Islands, Central American areas like Belize and Costa Rica, Caribbean Islands like Cuba and parts of the Western Atlantic and South America, including Colombia (Guiry and Guiry 2024) (Fig. 2a).
Ceratodictyonscoparium: This species exhibits a tufted, dark red thallus that is notably tough, hard and wiry. It features creeping and erect axes, with the lower sections primarily terete. However, the erect axes, particularly distally, are distinctly flattened and irregularly subdichotomously branched or palmate. The axes possess a cartilaginous nature. Tetrasporangia are found within inflated nemathecia covering branch apices, typically divided cruciately, decussately or frequently in a tetrahedral manner. It grows epilithically in the subtidal zones. Its distribution spans Central America, including Panama; Caribbean Islands such as Cuba; and South America, notably Brazil (Huisman 2018) (Fig. 2B).
Herposiphoniaparca: This algae species has prostrated primary axes that produce erect, epiphytic, ecocutate branches, measuring 1–2 mm in height. These branches adhere to the substrate via unicellular rhizoids terminating in haptera, lacking an open connection to pericentral cells. The erect branches are sparsely branched, typically with 8–12 segments, each consisting of six to eight pericentral cells. The diameter of prostrate shafts ranges from 76–78 μm, while the diameter of erect shafts in the middle portion measures 58–67 μm. Apices feature vegetative trichoblasts. Tetrahedral tetrasporangia are formed in the distal portions of the determinate erect branches, arranged either in straight or spiral series. Terminal cistocars and spiral spermatotial branches are formed on the apices of the determinate branches (Garcia et al. 2008). Its distribution includes North America, particularly Florida; Central America, encompassing Belize; Caribbean Islands like Cuba; and regions across the Western Atlantic and South America, including Brazil, Colombia and Venezuela (Guiry and Guiry 2024) (Fig. 2C and D).
Each location exhibited its own set of specific species and, while some species were shared amongst them, the most prevalent species (comprising 55% of the findings) across the localities was Caulerpaverticillata. This was followed by Caulerpamicrophysa (Weber van Bosse) Feldmann, Dictyotapulchella Hörnig & Schnetter, Stypopodiumzonale (J.V. Lamouroux) Papenfuss and Ceratodictyonscoparium, which collectively constituted 44% of the species in all the localities. Additionally, species like Amphiroatribulus (J. Ellis & Solander) J. V. Lamouroux, Hypneaspinella (C. Agardh) Kützing, Dictyotabartayresiana J. V. Lamouroux, and Anadyomenesaldanhae Joly & Oliveira, accounted for 33% of the observed species.
Discussion and conclusion
The diversity found within these reef banks, totalling 90 infrageneric taxa, slightly trails behind the findings in Sisal reefs, which recorded 123 species (Vilchis et al. 2024). Nevertheless, this count surpasses the previous report on five Campeche Bank reefs, where the highest species count was 21 at Bajo Obispo (González-Solis et al. 2018). This discrepancy is likely attributed to variations in sampling techniques and sampling effort. Interestingly, C.verticillata, the species with the highest relative frequency across five out of eight localities (Table 2), was not documented in the last floristic study of the Bank of Campeche (González-Solis et al. 2018). However, previous studies have indicated its abundance in Sisal reefs, along with H.spinella, both of which are macroalgae commonly found in reef ecosystems (Littler and Littler 2000).
At the family level, the findings align with existing literature (Dreckmann 1998, Mendoza-González et al. 2016, Vilchis et al. 2018, García García et al. 2020, Pedroche and Sentíes 2020, Ortegón-Aznar and León-Tejera 2022) that highlights Caulerpaceae, Halimedaceae, Dictyotaceae and Rhodomelaceae as the most prevalent families with the highest species count in reef environments. When considering macroalgae distribution by reef, Cayo Arenas, TE and Serpientes reported more chlorophytes, whereas Bajos Obispo, B. Pera and B. Nuevo were dominated by rhodophytes. This distinction likely arises from the depth variations amongst these reefs, where green algae tend to thrive in the shallower reefs, influencing their development and distribution (Garduño-Solórzano et al. 2005).
In Table 1, these species are newly recorded for Yucatan: Asteromeniapeltata, Botryocladiashanksii, Ceratodictyonscoparium and Herposiphoniaparca Setchell. Regarding the newly-reported species in Yucatan, A.saldanhae had been previously documented in the Gulf of Mexico in Veracruz by Vázquez-Machorro et al. (2016). C.scoparium, on the other hand, had been reported in the Mexican Caribbean around Puerto Morelos and in the Gulf of Mexico by Suárez and Martínez-Daranas (2020). However, for H.parca, no records existed, marking it as a new finding for the Mexican Atlantic.
There is a few species shared between reefs indicating a low similarity despite their belonging to the same archipelago. Typically, the similarity between localities increases with proximity, as observed by Nekola and White (1999). Yet, the intricate geographical location and geological history of the Yucatan Peninsula have fostered diverse habitats, resulting in high species richness adapted to specific microenvironments. This variation leads to noticeable differences in the biological composition, even amongst nearby localities (Vilchis et al. 2018).
These reefs serve as significant biodiversity hubs, interconnected with the environmental characteristics of the carbonate shelf where they reside. They receive Gulf of Mexico waters that transport various taxa, contributing to their higher biological diversity, as observed in studies by Horta-Puga et al. 2007, Ortiz-Lozano et al. 2013 and Zarco-Perelló et al. 2013. These factors potentially result in different algal communities compared to other Campeche Bank reefs (Zarco-Perelló et al. 2013).
Acknowledgements
We are grateful for the financial support of the Harte Charitable Foundation, through the Harte Research Institute (Biodiversity of the Southern Gulf of Mexico) and the CONABIO NE018 Project (updating knowledge of the diversity of shallow-water (< 50 m) benthic marine invertebrate species in the Southern Gulf of Mexico). We also thank Dr. Patricia Guadarrama for her support and technical assistance at the Laboratorio de Ecología y Manejo de Costa y Mares, UMDI-Sisal, F Ciencias, UNAM. We thank Dr. Daniel León Álvarez and Jonathan Morales for receiving and assigning catalogue numbers to the algae in the Collection Sección de algas del Herbario (FCME) de la Facultad de Ciencias, UNAM. We are grateful to Dr. Carolina Peralta for her guidance in managing our dataset in OBIS.
Author contributions
Conceptualisation: M.A.R, A.M.S, B.M.D, R.E.C.C, C.G.S, I.O.A and N.S.; field methodology: M.A and NS.; formal analysis, I.O.A and R.E.C.C; writing original draft, I.O.A, R.E.C.C; data curation, A.M.S, B.M.D, M.A.R, C.G.S and I.O.A; writing—review and editing, I.O.A, R.E.C.C, A.M.S, B.M.D, M.A.R, C.G.S and N.S.; funding acquisition: N.S. All authors have read and agreed to the published version of the manuscript.
References
- Afonso Carrillo J., Sobrino C. Two amphi-Atlantic species of Botryocladia (Rhodymeniales, Rhodophyta) in the Canary Islands (Eastern Atlantic) Cryptogamie Algologie. 2004;25(2):147–159. [Google Scholar]
- Chávez-Hidalgo A, Cruz-Agüero G, Chávez E. A. Evidences on the connectivity of coral reefs of the Gulf of Mexico and the Mexican Caribbean Proc 11th Int Coral Reef Symp, Ft. Lauderdale. Symposium Proceedings of the 11th International Coral Reef., editor. Proc.11th Int Coral Reef Symp 2008
- Cooley S., Schoeman D., Bopp L., Boyd P., Donner S., Ghebrehiwet D. Y., Ito S. -I., Kiessling W., Martinetto P., Ojea E., Racault M. -F., Rost B., Skern-Mauritzen M. In: Climate Change 2022: Impacts, adaptation and vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Pörtner H. O., Roberts D. C., Tignor M., Poloczanska E. S., Mintenbeck K., Alegría A., Craig M., Langsdorf S., Löschke S., Möller V., Okem A., Rama B., editors. Cambridge University Press; Cambridge,UK and New York, NY, USA: 2022. Oceans and coastal ecosystems and their services.379-550. [DOI] [Google Scholar]
- Dawes C. J., Mathieson A. C. The seaweed of Florida. University Press of Florida; 2008. 541 [Google Scholar]
- Dreckmann K. M. Clasificación y nomenclatura de las macroalgas marinas bentónicas del Atlántico mexicano. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad. Cd. Mx. 1998;México:149. [Google Scholar]
- García García Annie May Ek, Cabrera Becerril Ernesto, Núñez Reséndiz María Luisa, Dreckmann Kurt M., Sentíes Abel. Actualización taxonómica de las algas rojas (Rhodophyta) marinas bentónicas del Atlántico mexicano. Acta Botanica Mexicana. 2020;127 doi: 10.21829/abm127.2020.1677. [DOI] [Google Scholar]
- Garcia Mayra, Gil Nelson, Gomez Santiago. Nuevos registros de Herposiphonia Parca y H. Arcuata (Rhodomelaceae, Rhodophyta), para la costa de Venezuela. http://ve.scielo.org/scielo.php?script=sci_arttext&pid=S0252-82742008000100004&lng=es&tlng=es. Ernstia. 2008;18(1):059–071. [Google Scholar]
- Garduño-Solórzano G., Godinez J. L., Ortega M. M. Distribución geográfica y afinidad por el sustrato de las algas verdes (Chlorophyceae) bénticas de las costas mexicanas del Golfo de México y Mar Caribe. Boletín de la Sociedad Botánica de México. 2005;76:61–78. [Google Scholar]
- González-Solis Alicia, Torruco Daniel, Torruco-González Ángel Daniel. Biodiversidad de macroalgas en arrecifes coralinos de la Sonda de Campeche, el Caribe Mexicano y Belice. Gayana. Botánica. 2018;75(1):501–511. doi: 10.4067/s0717-66432018000100501. [DOI] [Google Scholar]
- Guiry M. D., Guiry G. M. https://www.algaebase.org. [2024-04-10T00:32:03+00:00]. https://www.algaebase.org
- Horta-Puga G., Vargas-Hernández J. M., Carricart-Ganivet J. P. In: Coral Reefs of the Southern Gulf of Mexico. 1. Tunnell Jr J W., Withers K, Chávez E., et al., editors. Vol. 1. Texas A&M University; Corpus Christi: 2007. Reef Corals.95-101. [Google Scholar]
- Horta-Puga G., Tello-Musi J. L., Carricart-Ganivet J. P., Carriquiry J., Vilaescusa-Celaya J. In: Aportes al conocimiento del Sistema Arrecifal Veracruzano: hacia el Corredor Arrecifal del Suroeste del Golfo de México. Granados-Barba L., Ortiz-Lozano L., Salas-Monreal D., González-Gándara C., et al., editors. Universidad Autónoma de Campeche; 2015. Veracruz Reef System: a hermatypic coral community thriving in a sedimentary terrigenous environment.181-208. [Google Scholar]
- Huerta L. Contribución al conocimiento de las algas de los bajos de la Sonda de Campeche, Cozumel e Isla Mujeres. Anales de la Escuela Nacional de Ciencias Biológicas, México. 1958;9:115–123. [Google Scholar]
- Huerta L. Flora marina de los alrededores de la Isla Pérez, arrecife Alacranes, Sonda de Campeche, México. Anales de la Escuela Nacional de Ciencias Biológicas, México. 1961;10(14):11–22. [Google Scholar]
- Huerta-Múzquiz L., Mendoza-González A. C., Mateo-Cid L. E. Avance sobre un estudio de las algas marinas de la península de Yucatán. Phytologia. 1987;62:23–53. [Google Scholar]
- Huisman J. M. ABRS, Canberra; 2018. Algae of Australia: Marine benthic algae of north-western Australia, Vol 2. Red algae. 672 pp. [Google Scholar]
- Humm H. J. Marine algae from Campeche banks. Florida State University Studies. 1952;7:27. [Google Scholar]
- Jordán-Dahlgren E. Gorgonian distribution patterns in coral reef environments of the Gulf of Mexico: evidence of sporadic ecological connectivity? Coral Reefs. 2002;21(2):205–215. doi: 10.1007/s00338-002-0226-9. [DOI] [Google Scholar]
- Kim C. S. Marine algae of Alacrán reef, southern Gulf of Mexico. Ph.D. Thesis. Duke University. Durham; 1964. 213 [Google Scholar]
- Kornicker L. S., Bonet F., Cann R., Hoskin C. M. Alacran Reef, Campeche Bank, Mexico. Publications of the Institute of Marine Science, University of Texas. 1959;6:1–22. [Google Scholar]
- Littler D. S., Littler M. M. Caribbean reef plants. An identification guide to the reef plants of the Caribbean, Bahamas, Florida and Gulf of Mexico. OffShore Graphics, Inc; 2000. 542 [Google Scholar]
- Mendoza-González A. Catalina, Mateo-Cid Luz Elena. Cinco nuevos registros de algas rojas (Rhodophyta) para el caribe mexicano. Polibotánica. 2007;23:101–119. [Google Scholar]
- Mendoza-González C. A., Mateo-Cid L. E., García-López D. Y., Acosta-Calderon J. A., Vázquez-Rodríguez A., Hernández-Casas C. M., Gerardo A., Garduño-Acosta G. A. In: Marine benthos: biology, ecosystem functions and environmental impact. Riosmena-Rodríguez R., editor. Nova Science Publishers Inc; New York, EUA: 2016. Marine seaweeds of the Yucatan Peninsula: diversity, economic importance and conservation. [Google Scholar]
- Nekola J. C., White P. S. The distance decay of similarity in biogeography and ecology. Journal of Biogeography. 1999;26:867–878. doi: 10.1046/j.1365-2699.1999.00305.x. [DOI] [Google Scholar]
- Ortegon-Aznar Ileana, Leon Tejera Hilda, Gold-Morga M., Ramirez-Miss Noemi. Proceedings of the 11th International Coral Reef Symposium, pp. 1373-1376. Ft. Lauderdale, Florida; 2008. Preliminary results on marine algae of Madagascar Reef, Yucatan, México: A functional group approach. [Google Scholar]
- Ortegón-Aznar I., León-Tejera H. Diversidad de macroalgas y cianoprocariontes marinos de la costa norte de la Península de Yucatán, México. Hidrobiológica. 2022;32(3):309–317. doi: 10.24275/uam/izt/dcbs/hidro/2022v32n3/Ortegon. [DOI] [Google Scholar]
- Ortiz-Lozano Leonardo, Pérez-España Horacio, Granados-Barba Alejandro, González-Gándara Carlos, Gutiérrez-Velázquez Ana, Martos Javier. The Reef Corridor of the Southwest Gulf of Mexico: Challenges for its management and conservation. Ocean & Coastal Management. 2013;86:22–32. doi: 10.1016/j.ocecoaman.2013.10.006. [DOI] [Google Scholar]
- Pedroche F,, Sentíes A. Diversidad de macroalgas marinas en México. Una actualización florística y nomenclatural. Cymbella. 2020;6(1):4–55. [Google Scholar]
- Suárez A. M., Martínez-Daranas B. Similitud de la ficoflora marina en zonas del Atlántico Occidental Tropical y Subtropical. Caldasia. 2020;42(1):85–95. doi: 10.15446/caldasia.v42n1.73372. [DOI] [Google Scholar]
- Tunnell J W, A. Chávez E., K Withers, et al. Coral reefs of the Southern Gulf of Mexico. Harte Research Institute, Texas A&M University - Corpus Christi; 2007. 361. [Google Scholar]
- Vázquez-Machorro Angélica, Godínez-Ortega José Luis, Granados-Barba Alejandro, Ramírez-García Pedro. Estructura y composición de la macroflora dominante del pecio Ana Elena, Sistema Arrecifal Veracruzano, Golfo de México. Hidrobiológica. 2016;26(2):259–267. doi: 10.24275/uam/izt/dcbs/hidro/2016v26n2/Vazquez. [DOI] [Google Scholar]
- Vilchis Martha Isabel, Dreckmann Kurt M., García-Trejo Erick A., Hernández Oscar E., Sentíes Abel. Patrones de distribución de las grandes macroalgas en el golfo de México y el Caribe mexicano: una contribución a la biología de la conservación. Revista Mexicana de Biodiversidad. 2018;89(1) doi: 10.22201/ib.20078706e.2018.1.2226. [DOI] [Google Scholar]
- Vilchis M I, Ortegon-Aznar I, Álvarez-Rocha M, Tuz-Sulub A, Galindo-de Santiago M C, N Simões, P Pinzón J, A Duran, et al. Macroalgas marinas bentónicas de ambientes insulares en la zona de transición biótica del Atlántico mexicano. Revista Mexicana de Biodiversidad. 2024 doi: 10.22201/ib.20078706e.2024.95.5415. [DOI]
- Zarco-Perelló S., Mascaró M., Garza-Pérez R., Simoes N. Topography and coral community of the Sisal reefs, Campeche Bank, Yucatán, México. Hidrobiológica. 2013;23(1):28–41. [Google Scholar]