Abstract
Objectives: Although direct oral anticoagulants (DOAC) have become widely used, little is known about the efficacy of DOAC for isolated distal deep vein thrombosis (DVT).
Methods: In-hospitalized orthopedic patients with isolated distal DVT who were diagnosed from 2016 to 2018 were enrolled and were followed for 1 year. Embolic events included symptomatic pulmonary embolism (PE) and DVT extension above the knee. Bleeding events were determined in the presence of bleeding academic research consortium (BARC) 2, 3 or 5 bleeding.
Results: Of 196 orthopedic patients, 84% of patients (n = 164) received DOAC (DOAC+ group), whereas 16% of patients (n = 32) did not (DOAC– group). Cumulative incidence of embolic events was observed in 1.5% of the DOAC+ group and none of the DOAC– group (p = 0.443). Cumulative incidence of bleeding events was observed in 5.1% of the DOAC+ group and none of the DOAC– group (p = 0.157). The majority of bleeding events (80%) occurred in patients with HAS-BLED scores of 3 or greater.
Conclusions: There were no significant differences in embolic events and bleeding events in retrospective data. Balancing thrombotic risk and bleeding risk remains to be key for isolated distal DVT.
Keywords: isolated distal deep vein thrombosis, anticoagulant therapy, direct oral anticoagulants, orthopedic patients
Introduction
Anticoagulant therapy for isolated distal deep vein thrombosis (DVT) has not been frequently recommended owing to its potential to increase bleeding events in patients who rarely deteriorate.1) However, isolated distal DVT could be a potential embolic source that causes significant pulmonary embolism (PE),2) and anticoagulant therapy has also been reported to be effective in DVT exacerbation and recurrence.3)
A previous randomized, double-blind, placebo-controlled trial (CACTUS) enrolled low-risk outpatients without cancer or previous venous thromboembolic disease with a first acute symptomatic DVT in the calf (n = 259) and assigned (1:1) patients to receive either the low-molecular-weight heparin nadroparin or placebo for 6 weeks. In their study, PE or extension of calf DVT developed in 3% in the nadroparin group and 5% in the placebo group, respectively (p = 0.54), whereas bleeding occurred in 4% in the nadroparin group and no patients in the placebo group, respectively (p = 0.03).1) Therefore, direct oral anticoagulants (DOAC) for calf DVT is not recommended recently. Moreover, distal DVT has a lower relapse rate than central DVT.4)
The emergence of DOAC has markedly changed our daily practice, replacing warfarin, which had been the golden standard for most oral anticoagulation therapies.5) All clinical studies comparing DOAC and warfarin for proximal DVT have shown the equivalent efficacy for embolic events but significantly fewer bleeding events in DOAC.6–10) Furthermore, food control and close monitoring are not necessary in DOAC, which is beneficial for both patients and physicians.11) Data from an atrial fibrillation (AF) study showed that the rate of patients receiving any type of oral anticoagulant (OAC) significantly increased from 53% to 70% in the last decade.5) Therefore, it is quite natural that some physicians are beginning to use DOAC even for isolated DVT. However, to date, clear evidence showing the significance of DOAC for distal DVT is not available.
The American College of Chest Physicians (ACCP) guidelines recommend that patients with low-risk distal DVT are not encouraged to take anticoagulant therapy.12) Only central extension cases or high-risk patients are recommended for anticoagulant therapy, with follow-up ultrasonography at 7–14 days.12) Japanese guidelines basically follow this strategy. (Japanese Guideline for Prevention of Venous Thromboembolism [revised edition in 2017]). However, an Asian paradox states that patients from Asia tend to experience more bleeding risk rather than embolic risk.13) Accordingly, real-world Japanese data in this category are lacking and needed.
This study aims to evaluate the use of DOAC in daily practice and to assess the efficacy and bleeding risk of DOAC in Japanese orthopedic patients with isolated distal DVT.
Materials and Methods
We retrospectively enrolled consecutive orthopedic patients with isolated distal DVT from January 2016 to December 2018. We excluded patients younger than 20 years of age, hemodialysis patients, patients with active cancer, distal DVT associated with a popliteal or more proximal DVT, or clinically suspected PE. We also excluded patients on heparin only and on warfarin. The diagnosis of DVT was established using ultrasonography or enhanced computed tomography (CT). D-dimer was routinely checked in these patients by an orthopedic surgeon. Even if they were asymptomatic, echo or CT was performed aggressively in patients with elevated D-dimer before or after surgery or those who require long-term rest. Not only symptomatic DVT but also non-symptomatic DVT was included. Each orthopedic physician decided on the indication of DOAC and implementation of follow-up. We defined as DOAC+ group which received DOAC, and the DOAC– group which did not receive DOAC.
Hypertension was defined as blood pressure ≥140/90 mmHg or the use of anti-hypertensive drugs. Dyslipidemia was defined as a low-density lipoprotein cholesterol level of ≥140 mg/dl, high-density cholesterol level of ≤40 mg/dl, triglyceride level of ≥150 mg/dl, or the use of lipid-lowering medications. Diabetes was defined as fasting plasma glucose level of 126 mg/dl, casual glucose level of ≥200 mg/dl, 2-hour level of ≥200 mg/dl, hemoglobinA1c ≥6.5%, or the use of medications for diabetes. Chronic heart failure (CHF) was defined as a history of heart failure or diuretic medication use. Ischemic heart disease (IHD) was defined as receiving percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG).
HAS-BLED was defined as the sum of 1 point for hypertension (uncontrolled, systolic blood pressure >160 mmHg), 1 point for the presence of renal or liver impairment (maximum of 2 points), 1 point for stroke (previous history, particularly lacunar), 1 point for bleeding history or predisposition (anemia), 1 point for labile international normalized ratio (INR) (e.g., therapeutic time in range <60%), 1 point for elderly (>65 years), 1 point for drug (e.g., antiplatelet agents and nonsteroidal anti-inflammatory drugs), and 1 point for alcohol excess.14)
Embolic events were defined as symptomatic PE or symptomatic DVT extension above the knee. The diagnosis was established using ultrasonography or CT. Bleeding events were defined as bleeding academic research consortium (BARC) 2 (an actionable sign of hemorrhage that requires nonsurgical, medical intervention by a healthcare professional, leading to hospitalization or increased level of care, or prompting evaluation), or BARC 3 (any transfusion with overt bleeding, bleeding requiring surgical intervention for control, bleeding requiring intravenous vasoactive agents, or intracranial hemorrhage), or BARC 5 (fatal bleeding).15) These data were followed for 1 year.
The normality of data was evaluated using the Shapiro–Wilk test. Continuous variables were expressed as the means ± standard deviations or the median value (25th–75th percentiles) as appropriate unless specified. Categorical variables were expressed as numbers (percentages). Comparisons of continuous variables with normal distribution were evaluated using Student’s t-test. Comparisons of continuous variables with non-normal distribution were examined using the Wilcoxon test. Categorical variables were analyzed using the chi-squared test or Fisher’s exact test, as appropriate. Statistical analyses were performed using JMP 16 (SAS Institute, Cary, NC, USA). P-values of <0.05 were considered statistically significant. The Institutional Review Board of Showa University Fujigaoka Hospital approved this study (F2017C28).
Results
A total of 196 patients including 164 patients with DOAC and 32 patients without DOAC were analyzed. The baseline clinical characteristics of the participants are presented in Table 1. The mean age of the participants was 75 years, and 85.2% were females. There were no significant differences in lifestyle-related diseases including hypertension, dyslipidemia, and diabetes. No significant differences were found in the history of venous thromboembolism or heart disease. There were no significant differences in creatinine, hemoglobin (Hb), or D-dimer levels. The use of oral steroid therapy and antiplatelet drugs did not differ between the groups. The majority of distal DVT patients, except a few of them (4.1%), did not have DVT-related symptoms. In terms of DOAC selection, edoxaban was the most frequently selected DOAC (90.2%, 148 of 164 patients) followed by apixaban (7.3%, 12 of 164 patients) and rivaroxaban (2.4%, 4 of 164 patients). The loading dose for DVT was not used in this study population. For patients prescribed edoxaban, a reduced dose (30 mg) was frequently used (67.4%). For patients prescribed apixaban and rivaroxaban, standard maintenance dose for Japanese (10mg for apixaban and 15mg for rivaroxaban) was mostly used (83.3% for apixaban and 100% for rivaroxaban).
Table 1 Baseline clinical characteristics.
| Overall (N = 196) | DOAC+ (N = 164) | DOAC– (N = 32) | P value | |
|---|---|---|---|---|
| Age, years | 75 (69–82) | 74 (73–76) | 68 (56–80) | 0.591 |
| Female sex, n (%) | 167 (85.2%) | 145 (88.4%) | 22 (68.8%) | 0.008 |
| Body mass index | 23.7 (21.5–26.3) | 24.4 (23.6–25.1) | 24.4 (21.4–27.3) | 0.697 |
| Hypertension, n (%) | 112 (57.1%) | 94 (57.3%) | 18 (56.3%) | 0.531 |
| Hyperlipidemia, n (%) | 59 (30.1%) | 48 (29.3%) | 11 (34.4%) | 0.674 |
| Diabetes, n (%) | 32 (16.3%) | 25 (15.2%) | 7 (21.9%) | 0.431 |
| Stroke, n (%) | 13 (6.6%) | 8 (4.9%) | 5 (15.6%) | 0.042 |
| Previous history of DVT, n (%) | 5 (2.6%) | 5 (3.1%) | 0 (0%) | 0.406 |
| Chronic heart failure, n (%) | 21 (10.7%) | 19 (11.6%) | 2 (6.3%) | 0.537 |
| Ischemic heart disease, n (%) | 15 (7.7%) | 11 (6.7%) | 4 (12.5%) | 0.275 |
| Atrial fibrillation, n (%) | 14 (7.1%) | 9 (7.9%) | 5 (7.7%) | 0.675 |
| Hb (g/dl) | 11.0 (9.4–12.1) | 10.2 (9.9–10.5) | 11.2 (9.9–12.6) | 0.055 |
| Plt (×104/mm3) | 22.3 (18.5–28.4) | 23.4 (22.2–24.6) | 26.1 (23.1–29.1) | 0.058 |
| D-dimmer (μg/ml) | 5.4 (2.8–10.0) | 7.6 (6.5–8.7) | 6.3 (4.5–7.9) | 0.917 |
| Cre (mg/dl) | 0.59 (0.50–0.71) | 0.58 (0.55–0.61) | 0.84 (0.42–1.25) | 0.106 |
| Use of oral antiplatelet drug, n (%) | 31 (15.8%) | 24 (14.6%) | 7 (21.9%) | 0.298 |
| Use of oral steroid therapy, n (%) | 15 (7.7%) | 12 (7.3%) | 3 (9.4%) | 0.716 |
| DVT-related symptom, n (%) | 8 (4.1%) | 7 (4.3%) | 1 (3.1%) | 0.767 |
| HAS-BLED score | 1.7 (1.6–1.9) | 1.8 (0.1–1.6) | 1.5 (0.2–1.1) | 0.227 |
DOAC: direct oral anticoagulant; DVT: distal deep vein thrombosis; Hb: hemoglobin; Plt: platelet; Cre: creatinine
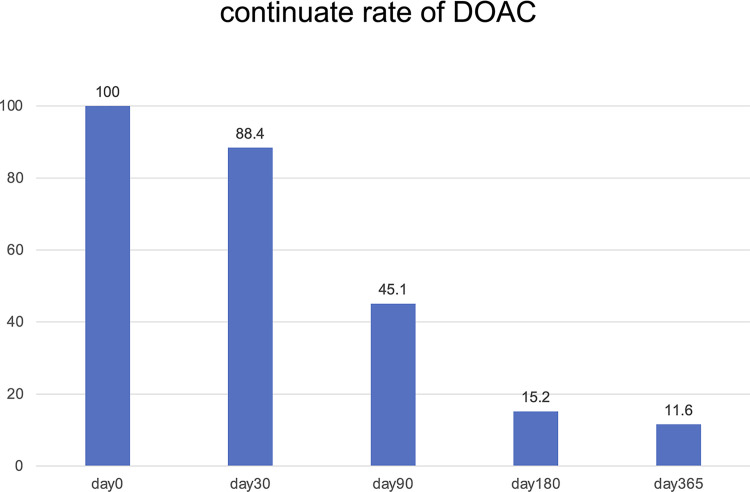
The underlying orthopedic diseases are shown in Table 2. All the DVT with orthopedic surgery occurred after the operations. DVT after orthopedic surgery was observed in 64.8% of the study population, whereas DVT without surgery was observed in 35.2% of the study population. The majority of the patients who underwent orthopedics extremity surgery received DOAC (89.0%, 113 of 127 patients). On the other hand, patients with fractures who did not undergo surgery did not receive DOAC (62.5%, 10 of 16 patients). Each orthopedic physician tended to discontinue DOAC when patients began to be physically active (Fig. 1).
Table 2 The underlying orthopedic disease.
| Overall (N = 196) | DOAC+ (N = 164) | DOAC– (N = 32) | P value | |
|---|---|---|---|---|
| With orthopedic surgery, n (%) | 127 (64.8%) | 114 (69.5%) | 13 (40.6%) | 0.002 |
| Fracture, n (%) | 8 (4.1%) | 7 (4.3%) | 1 (3.1%) | 0.767 |
| Arthritis, n (%) | 119 (60.7%) | 107 (65.2%) | 12 (37.5%) | 0.005 |
| Without orthopedic surgery, n (%) | 69 (35.2%) | 50 (30.5%) | 19 (59.4%) | 0.002 |
| Fracture, n (%) | 16 (8.2%) | 6 (3.7%) | 10 (31.3%) | <0.001 |
| Arthritis, n (%) | 53 (27.0%) | 44 (26.8%) | 9 (28.1%) | 0.666 |
DOAC: direct oral anticoagulant
Fig. 1 Continue rates of DOAC. The continued rates of DOAC were 88.4%, 45.1%, 15.2%, and 11.6% in days 30, 90, 180, and 365, respectively. Approximately half of the patients discontinued DOACs within 90 days. DOAC: direct oral anticoagulant.
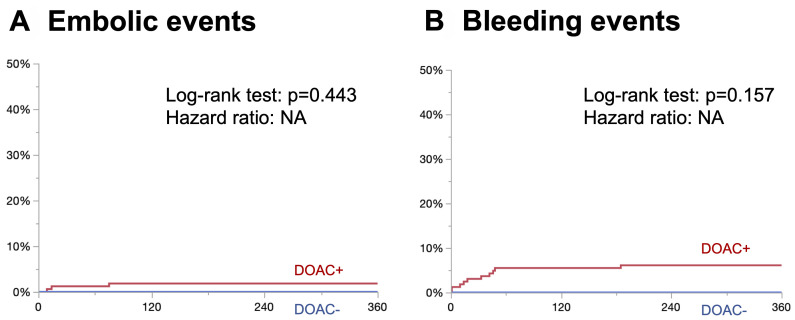
Cumulative incidence of embolic events and bleeding events are shown in Figs. 2A and 2B. A tiny proportion of patients developed embolic events in the DOAC+ group, whereas no patients developed embolic events in the DOAC– group (log-rank test, p = 0.443; hazard ratio, NA) (Fig. 2A). Similarly, a tiny proportion of patients developed bleeding events in the DOAC+ group, whereas no patients developed bleeding events in the DOAC– group (log-rank test, p = 0.157; hazard ratio, NA) (Fig. 2B). All embolic events are listed in Supplementary Table 1, which were all DVT extension above the knee and occurred while taking DOAC. Similarly, all bleeding events are listed in Supplementary Table 2, which were mostly primary disease and procedure-related and occurred while taking DOAC. Patient characteristics and HAS-BLED scores were compared between patients with bleeding and those without in the DOAC+ group (Table 3). In patients with bleeding events, body mass index (BMI) and hemoglobin levels were significantly lower, whereas D-dimer and HAS-BLED scores were significantly greater. Patients with bleeding events mostly had >2 HAS-BLED scores and had significantly high-risk bleeding events (positive predictive value [PPV], 1/4 [85.7%]; area under the curve [AUC], 0.843).
Fig. 2 Kaplan–Meier curve. (A) Embolic events. No significant differences in embolic events were noted between the 2 groups (log-rank test, p = 0.443; hazard ratio, NA). (B) Bleeding events (BARC >2). No significant differences in bleeding events were observed between the 2 groups (log-rank test, p = 0.157; hazard ratio, NA). DOAC: direct oral anticoagulant; BARC: bleeding academic research consortium.
Table 3 Predictors of bleeding events (only in the DOAC+ group).
| Overall (N = 164) | Bleeding event+ (N = 10) | Bleeding event– (N = 154) | P value | |
|---|---|---|---|---|
| Age, years | 74 (73–76) | 76.8 (71.2–82.4) | 74.8 (73.4–76.2) | 0.522 |
| Female sex, n (%) | 145 (88.4%) | 9 (90.0%) | 136 (88.3%) | 0.719 |
| Body mass index | 24.4 (23.6–25.1) | 22.0 (20.5–23.6) | 24.6 (24.0–25.3) | 0.018 |
| Hypertension, n (%) | 94 (57.3%) | 7 (70.0%) | 87 (56.5%) | 0.519 |
| Hyperlipidemia, n (%) | 48 (29.3%) | 2 (20.0%) | 46 (29.9%) | 0.725 |
| Diabetes, n (%) | 25 (15.2%) | 4 (40.0%) | 21 (13.6%) | 0.047 |
| Stroke, n (%) | 8 (4.9%) | 1 (10.0%) | 7 (4.6%) | 0.923 |
| Previous history of DVT, n (%) | 5 (3.1%) | 0 (0.0%) | 5 (3.3%) | 0.727 |
| Chronic heart failure, n (%) | 19 (11.6%) | 0 (0.0%) | 19 (12.3%) | 0.607 |
| Ischemic heart disease, n (%) | 11 (6.7%) | 0 (0.0%) | 11 (7.1%) | 0.489 |
| Atrial fibrillation, n (%) | 9 (7.9%) | 0 (0.0%) | 9 (5.8%) | 0.427 |
| Hb (g/dl) | 10.2 (9.9–10.5) | 9.6 (8.2–11.0) | 10.8 (10.5–11.1) | 0.036 |
| Plt (×104/mm3) | 23.4 (22.2–24.6) | 20.2 (16.3–24.1) | 23.6 (22.4–24.8) | 0.221 |
| D-dimmer (μg/ml) | 7.6 (6.5–8.7) | 12.8 (6.3–19.2) | 7.2 (6.0–8.5) | 0.007 |
| Cre (mg/dl) | 0.58 (0.55–0.61) | 0.64 (0.50–0.79) | 0.62 (0.59–0.66) | 0.783 |
| Use of oral antiplatelet drug, n (%) | 24 (14.6%) | 1 (10.0%) | 23 (14.9%) | 0.554 |
| Use of oral steroid therapy, n (%) | 12 (7.3%) | 0 (0.0%) | 12 (7.8%) | 0.457 |
| DVT-related symptom, n (%) | 7 (4.3%) | 1 (10.0%) | 6 (3.9%) | 0.362 |
| HAS-BLED score | 1.8 (0.1–1.6) | 3 (2.3–3.7) | 1.7 (1.6–1.8) | <0.001 |
In patients with bleeding events, body mass index and hemoglobin were significantly lower, whereas D-dimer and HAS-BLED scores were significantly greater.
DOAC: direct oral anticoagulant; DVT: distal deep vein thrombosis; Hb: hemoglobin; Plt: platelet; Cre: creatinine
Discussion
The main findings of this study can be summarized as follows. DOAC was prescribed in most of the patients, accounting for 84% of our study population. No symptomatic PE events were observed in either group. Few cases of DVT extension above the knee were observed in the DOAC+ group. Bleeding events were observed only in the DOAC+ group; however, these events were mostly primarily disease-related or procedure-related in patients with relatively high bleeding risk. A statistically significant difference was not observed between the groups in terms of embolic events and bleeding events.
Warfarin, which used to be the gold standard for anticoagulant therapy, was occasionally avoided owing to concerns about bleeding complications and the inconvenience of PT-INR adjustment.5) DOAC made this problem easier and is currently the main drug used for anticoagulant therapy. The proportion of Japanese patients on oral anticoagulants for AF was 53% in 2011, which increased to 70% in 2022.5) Similar to the situation of AF, anticoagulant therapy using DOAC became easier to introduce even for DVT. Around 2015, anticoagulant therapy using DOAC was indicated for DVT in Japan.6–10) Although a randomized placebo-controlled trial of anticoagulant therapy using DOAC for isolated distal DVT has not been conducted, DOAC can be prescribed.
The benefits of anticoagulant therapy in isolated distal DVT have been unclear.16) In 2008, the ACCP guidelines recommended a 3-month of anticoagulant therapy; however, since 2012, it has been recommended that patients with low-risk distal DVT should not take anticoagulant therapy, and only central extension cases or high-risk patients should take anticoagulant therapy, with follow-up ultrasonography at 7–14 days.12,16–18) Distal DVT is not covered by the NICE guidelines because it does not become severe.19) The CACTUS trial, which was a double-blind randomized controlled trial (RCT), reported that nadroparin was not superior to placebo in preventing the extension of calf DVT to the proximal veins, contralateral proximal DVT, and symptomatic PE, and led to significantly increased major and clinically relevant non-major bleeding events.1) These are the rationale for the current Japanese guidelines not to uniformly administer anticoagulant therapy in patients with distal DVT. However, in our study, 84% of the orthopedic patients received DOAC. It was likely that attending physicians initiated DOAC despite the potential risk of bleeding in patients postoperatively.
Sevitt et al.20) reported from autopsy data that the most frequent site of DVT was the soleal vein. According to a study at Tottori University, distal DVT was a PE source when the isolated thrombosis of the large soleal vein was >7 mm in diameter, and the soleal vein was the most frequent and significant location of calf DVT, suggesting that these were an occasional embolic source of critical PE.2) Based on the above, to prevent PE, treating soleus DVT at its source in the first place may be imperative. Moreover, a study on anticoagulant therapy using heparin for patients with asymptomatic isolated distal DVT during hospitalization showed that the rate of DVT extension above the knee was higher in orthopedic patients, patients with malignancies, and patients with immobility, and recommended anticoagulant therapy in these patients until they are ambulatory or have a negative echo.21)
In this study, bleeding events were numerically greater in the DOAC+ group but not statistically significant. The use of anticoagulant therapy frequently comes with balancing embolic and bleeding risks.14) The HAS-BLED score assesses major bleeding events in patients with AF, and a score of >3 indicates a high bleeding risk.14) Similarly, in our study, the HAS-BLED score of patients with bleeding events was significantly >2 (Table 3). We may need to be more cautious when patients have a high HAS-BLED score >2.
This study had some limitations. First, each attending physician decided whether to take the DOAC and period. Significant selection bias existed in our study population. Depending on the subjectivity of the attending physician, administration, and duration of DOAC use were determined. It was likely that orthopedic physicians were motivated to administrate DOAC for patients with the operation. Second, isolated distal DVT developed few embolic and bleeding events, and it may be not easy to conclude from our study.
Conclusion
A substantial proportion of orthopedic patients received DOAC for isolated distal DVT. Regardless of DOAC prescription, thrombotic events were very rare in this population. Although bleeding events occurred in patients receiving DOAC, these events were mostly primarily disease related and procedure related in patients with high bleeding risk. DOAC could be an option when thrombotic and bleeding risks were carefully evaluated.
Declarations
Acknowledgments
We would like to thank the cardiology medical staff at Showa University Fujigaoka Hospital.
Disclosure statement
All authors have no conflicts of interest to disclose.
Author contributions
Study conception: AO, MS, and HM
Data collection: AO and MS
Analysis: AO and HM
Investigation: AO, MS, and HM
Manuscript preparation: AO and HM
Funding acquisition: HS
Critical review and revision: all authors
Final approval of the article: all authors
Accountability for all aspects of the work: all authors.
Supplementary information
Embolic event cases.
Bleeding event cases.
References
- 1).Righini M, Galanaud J-P, Guenneguez H, et al. Anticoagulant therapy for symptomatic calf deep vein thrombosis (CACTUS): a randomised, double-blind, placebo-controlled trial. Lancet Haematol 2016; 3: e556–62. [DOI] [PubMed] [Google Scholar]
- 2).Ohgi S, Tachibana M, Ikebuchi M, et al. Pulmonary embolism in patients with isolated soleal vein thrombosis. Angiology 1998; 49: 759–64. [DOI] [PubMed] [Google Scholar]
- 3).Lee CH, Cheng CL, Lin LJ, et al. Epidemiology and predictors of short-term mortality in symptomatic venous thromboembolism. Circ J 2011; 75: 1998–2004. [DOI] [PubMed] [Google Scholar]
- 4).Galanaud JP, Sevestre MA, Genty C, et al. Incidence and predictors of venous thromboembolism recurrence after a first isolated distal deep vein thrombosis. J Thromb Haemost 2014; 12: 436–43. [DOI] [PubMed] [Google Scholar]
- 5).Akao M, Ogawa H, Masunaga N, et al. 10-year trends of antithrombotic therapy status and outcomes in Japanese atrial fibrillation patients—the Fushimi AF registry. Circ J 2022; 86: 726–36. [DOI] [PubMed] [Google Scholar]
- 6).Büller HR, Décousus H, Grosso MA, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013; 369: 1406–15. [DOI] [PubMed] [Google Scholar]
- 7).Nakamura M, Wang YQ, Wang C, et al. Efficacy and safety of edoxaban for treatment of venous thromboembolism: a subanalysis of East Asian patients in the Hokusai-VTE trial. J Thromb Haemost 2015; 13: 1606–14. [DOI] [PubMed] [Google Scholar]
- 8).Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010; 363: 2499–510. [DOI] [PubMed] [Google Scholar]
- 9).Büller HR, Prins MH, Lensin AW, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012; 366: 1287–97. [DOI] [PubMed] [Google Scholar]
- 10).Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med 2013; 369: 799–808. [DOI] [PubMed] [Google Scholar]
- 11).Wright JN, Vazquez SR, Kim K, et al. Assessing patient preferences for switching from warfarin to direct oral anticoagulants. J Thromb Thrombolysis 2019; 48: 596–602. [DOI] [PubMed] [Google Scholar]
- 12).Stevens SM, Woller SC, Baumann Kreuziger L, et al. Executive summary: antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. Chest 2021; 160: 2247–59. [DOI] [PubMed] [Google Scholar]
- 13).Ohno J, Sotomi Y, Hirata A, et al. Dose of direct oral anticoagulants and adverse outcomes in Asia. Am J Cardiol 2021; 139: 50–6. [DOI] [PubMed] [Google Scholar]
- 14).Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010; 138: 1093–100. [DOI] [PubMed] [Google Scholar]
- 15).Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation 2011; 123: 2736–47. [DOI] [PubMed] [Google Scholar]
- 16).Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 2012; 141 Suppl: e419S–96S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Kearon C, Kahn SR, Agnelli G, et al. Antithrombotic therapy for venous thromboembolic disease: American College of Chest physicians evidence-based clinical practice guidelines (8th Edition). Chest. 8th Ed. 2008; 133 Suppl: 454S–545S. [DOI] [PubMed] [Google Scholar]
- 18).Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016; 149: 315–52. [DOI] [PubMed] [Google Scholar]
- 19).Chong LY, Fenu E, Stansby G, et al. Management of venous thromboembolic diseases and the role of thrombophilia testing: summary of NICE guidance. BMJ 2012; 344 jun27 1: e3979. [DOI] [PubMed] [Google Scholar]
- 20).Sevitt S, Gallagher N. Venous thrombosis and pulmonary embolism. A clinico-pathological study in injured and burned patients. Br J Surg 1961; 48: 475–89. [DOI] [PubMed] [Google Scholar]
- 21).Singh K, Yakoub D, Giangola P, et al. Early follow-up and treatment recommendations for isolated calf deep venous thrombosis. J Vasc Surg 2012; 55: 136–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Embolic event cases.
Bleeding event cases.