Abstract
Myasthenia gravis (MG) is an autoimmune neuromuscular junction disorder that rarely coexists with infectious thoracoabdominal aortic aneurysms (TAAA) requiring open repair. A 57-year-old patient with MG underwent elective thoracoabdominal aortic replacement. He was diagnosed with MG (Osserman classification II A). Extent IV thoracoabdominal aortic repair was performed under general anesthesia and maintained by total intravenous anesthesia. The patient was withdrawn from the ventilator on postoperative day 5 without spinal cord ischemia and myasthenic crisis. The management of infectious TAAA with myasthenia gravis warrants not only the prevention of complications associated with the crisis but also multidisciplinary treatments for infection control.
Keywords: myasthenia gravis, infectious thoracoabdominal aortic aneurysm, perioperative management
Introduction
Myasthenia gravis (MG) is an autoimmune disease caused by autoantibodies targeting the acetylcholine receptor (AchR) at the neuromuscular junction or molecules functionally associated with acetylcholine present in the postsynaptic membrane; it is characterized by fatigue that improves with rest.1) Surgical treatment is considered a factor in the onset of a crisis, with a reported occurrence frequency of approximately 10%–20%.2)
To the best of our knowledge, there have been no reports of surgery and perioperative management of infectious thoracoabdominal aortic aneurysm (TAAA) complicated MG.
This report describes the multidisciplinary management of infectious TAAA with MG.
Case Report
A 57-year-old male patient presented with lumbar traction and was examined at another hospital. However, because aortic disease could not be ruled out, he was transported to our hospital by ambulance. He was diagnosed with MG (Osserman classification II A) 2 years prior to general malaise from ptosis, thymoma incomitant, and anti-AchR antibody positivity. MG was managed with prednisolone at 5 mg/day and tacrolimus at 3.5 mg/day. One year prior, he had undergone percutaneous coronary angioplasty with a diagnosis of angina pectoris and was taking prasugrel hydrochloride at a dose of 3.75 mg/day.
He had a height of 167 cm, weight of 70 kg, and body mass index of 25. His blood pressure was 179/92 mmHg, pulse rate was 73 beats/min, and body temperature was 36.2°C. Laboratory findings revealed a white blood cell count of 17.800/mm3, hemoglobin level of 10.8 g/dL, and C-reactive protein level of 12.79 mg/dL. All the other laboratory findings were normal.
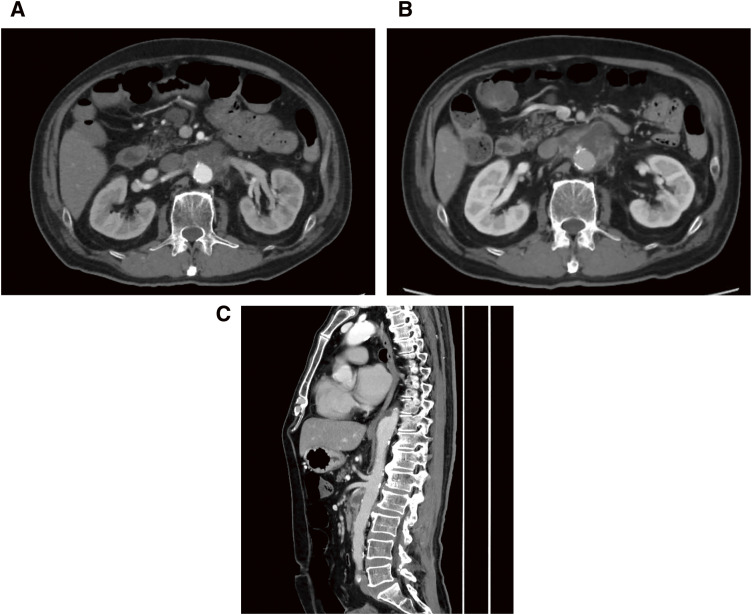
Thoracoabdominal aortic computed tomography (CT) revealed an increase in fat concentration around the superior mesenteric artery and an aneurysm-like structure (45 × 34 mm) with irregular walls (Fig. 1A). Methicillin-susceptible Staphylococcus aureus (MSSA) was detected in the blood cultures obtained at the time of admission. Based on these findings, an infectious TAAA was diagnosed, and elective surgery was planned. If the symptoms and aneurysm diameter remained stable during the course of hospitalization, a 2-week withdrawal period was provided considering oral administration of the immunosuppressant tacrolimus (3.5 mg/day) and the antiplatelet agent prasugrel hydrochloride (3.75 mg/day). Immunoglobulin therapy (0.4 g/kg/day) was administered for 5 days leading up to the day of surgery to prevent a myasthenic crisis associated with surgical stress. Contrast-enhanced CT performed immediately before surgery showed rapid expansion of the aneurysm diameter to 54 × 37 mm (Figs. 1B and 1C). The critical intercostal artery supplying the Adamkiewicz artery (AKA) was located at the level of the 7th intercostal space.
Fig. 1 (A) CT showed an increase in fat concentration around the superior mesenteric artery and an aneurysm-like form (45 × 34 mm) with irregular walls. (B and C) CT showed rapid expansion of the aneurysm with a diameter of 54 × 37 mm. CT: computed tomography.
During the surgery, propofol and fentanyl were administered as total intravenous anesthesia from induction to maintenance. Transcranial motor-evoked potential (MEP: MEE1208 Neuromaster, Nihon Koden Corporation, Tokyo, Japan) was used to monitor intraoperative spinal cord ischemia. Partial extracorporeal circulation was established via the right femoral artery and vein. An 8th intercostal thoracotomy and a retroperitoneal approach were undertaken. The aorta was clamped at Th10, and the terminal aorta was below the bifurcation of the inferior mesenteric artery. The 4-branched 20 mm thoracoabdominal graft (J graft; Japan Lifeline, Tokyo, Japan) with rifampicin immersion and a felt strip was used for all anastomoses. Upon opening the aneurysm, white pus gushed out. Owing to the effects of the infection, the adventitia of the abdominal branch adhered to the surrounding tissues and had become fragile. Consequently, detaching even the structurally sound blood vessels required additional time. Perfusion of the vessels is managed using an 8 Fr perfusion catheter to achieve a separated perfusion blood pressure of 100–150 mmHg (150–200 mL/min as irrigation rate); the visceral arteries were separately reconstructed. The intraoperative body temperature was controlled at around 34°C at rectal temperature. An omentum was not wrapped around the 4-branched graft due to insufficient length for proper securing. (Operation time, 10 h 21 min; pump time, 236 min; aortic clamp time, 191 min; bleeding 1117 mL. Transfusion: red cell products 20 units; Plasma derivatives 20 units; and platelet products 20 units.)
The same MSSA as in the blood culture was detected in the removed aortic wall. From the next day of surgery until oral intake was possible, prednisolone at 10 mg/day was administered intravenously. The patient was withdrawn from the ventilator on postoperative day 5 without spinal cord ischemia and myasthenic crisis. Postoperative contrast-enhanced CT confirmed no problems with the reconstructed blood vessels, including the anastomosis (Fig. 2).
Fig. 2 No abnormalities were observed around the artificial blood vessel and the anastomosis.
Prednisolone at 5 mg/day was administered orally from postoperative day 6, but the subsequent course was complicated by surgical site infection (SSI) in the right groin and urinary tract infection; therefore, tacrolimus was not resumed for infection control reasons.
It is not clear whether this was the effect, but due to muscle weakness of the trunk and the time required to get out of bed, so a large amount of immunoglobulin was injected intravenously again. SSI in the right groin was completely cured in 2 weeks using vacuum-assisted closure (VAC: KCI Licensing, Inc., San Antonio, TX, USA) therapy before transfers. The patient was transferred to a hospital for rehabilitation. Currently, he has resumed tacrolimus therapy and has been followed up at the outpatient clinic for 1.5 years without infection recurrence.
To perform multidisciplinary treatments in consultation with neurologists and anesthesiologists, it was possible to treat infectious TAAA with MG.
Discussion
Reports on cardiovascular surgery associated with MG are extremely rare. Surgical treatment is considered a factor in the occurrence of crises, and the frequency of occurrence has been reported to be approximately 10%–20%.2) Since there is no high-level evidence for perioperative management, sufficient cooperation between the neurology and anesthesiology departments at each facility is required.
This patient had MG with non-thymoma and was treated with immunosuppressive therapy, including prednisolone and tacrolimus. His condition, including neurological symptoms, was stable. However, tacrolimus was withdrawn during hospitalization, considering the surgical treatment of the infectious TAAA and perioperative infection control. However, there is no solid evidence that immunosuppressive therapy is a risk factor for perioperative infections, and there are concerns that discontinuation may worsen the disease.3)
The blood concentration of tacrolimus was not measured because the drug was discontinued, and it is possible that its blood concentration decreased after surgery. Therefore, it cannot be ruled out that it may have been a factor in postoperative muscle weakness of the trunk. The rapid immunomodulating treatments were planned to prevent the exacerbation of MG symptoms associated with immunosuppressive drug withdrawal and to prevent the development of myasthenic crises associated with surgical stress.4)
According to a report by Huang et al., the effect of preoperative intravenous administration of large doses of immunoglobulin appeared on average within 3 days (1–9 days) and peaked at an average of 7 days (3–19 days) in patients scheduled for thymectomy, and the course after thymectomy was better when it was administered.5) In consultation with the neurologist, we performed an intravenous infusion of 0.4 g/kg/day of immunoglobulin for 5 days before and after surgery to prevent a crisis.6)
The use of a non-depolarizing muscle relaxant (rocuronium) is the focus of anesthesia management for MG. The efficacy of the muscle relaxant antagonist, sugammadex, in cardiac anesthesia has also been reported.7) However, as reported by Uehara et al.,8) during surgery for TAAA, where monitoring spinal cord ischemia with MEP is crucial, it is important to perform surgery with complete intravenous anesthesia without using muscle relaxants. In this case, extent IV thoracoabdominal aortic repair was performed with partial extracorporeal under complete intravenous anesthesia without using muscle relaxants.
In the past, we have experienced cases in which AKA did not need reconstruction and was not used for MEP, and operation was performed, resulting in paraplegia. Since then, MEP has been measured in almost all cases (except for cases of circulatory arrest), regardless of the reconstruction of AKA.
The stimulation conditions are 5 train for constant voltage stimulation and 500 V for strong stimulation in MEP. MEP is evaluated as a significant change of 25% or less from the initial potential of the latent waveform and is judged to be negative if the latent potential improves with the reconstruction of the intercostal artery and reperfusion of the collateral circulation tract, including the internal iliac artery and lumber artery. In this case, the latent potential decreased after we incised an aneurysm after the completion of the proximal anastomosis, but the MEP improved as a result of increasing the perfusion pressure of the internal iliac artery and lumbar artery by preceding the distal anastomosis before reconstruction of the ventral branch.
In the patients with MG, the postoperative prolonged mechanical ventilation can be predicted by several parameters such as the vital capacity <2.9 L, history of myasthenic crisis, presence of any lung disease, the duration of the disease >6 years, and the requirement of the dosage of pyridostigmine >750 mg/day. Hence, a preoperative lung function assessment is of utmost importance.4) This patient did not have any of the factors leading to respiratory failure.
For postoperative management, prednisolone (10 mg/day) was administered intravenously until it could be administered orally, in consideration of surgical stress. The patient was weaned from the mechanical ventilator support on postoperative day 5 without complications of crisis. However, since the amount of 1-time ventilation decreased and the concentration of carbon dioxide in the blood remained at 45–50 mmHg after extubation, non-invasive positive pressure ventilation (NIPPV) was used. Respiratory muscle fatigue caused by MG is often reversible, and the effectiveness of NIPPV for MG has been reported.9)
On the other hand, it has been reported that the risk of postoperative complications, including infections such as pneumonia, sepsis, and postoperative hemorrhage, is increased in surgical procedures in patients with MG.10)
In the case of an infectious aneurysm, the artificial vessel and felt strip are immersed in 60 mL of rifampicin solution for 5–10 minutes. Since the J graft is not gelatin-coated on the outside of the artificial vessels, there is a possibility that the effect of rifampicin may be insufficient, so in the case of SSI, continuous irrigation and VAC treatment should be performed, and if the respiratory condition allows, the use of muscle flap using musculus latissimus dorsi was considered. In addition, we need to pay attention to the management of perioperative antibiotics because aminoglycosides, polypeptides, quinolones, and macrolides worsen the symptoms of MG.1) At initial admission to the hospital, this patient was administered 4.5 g tazobactam/piperacillin infused every 8 h, along with vancomycin at a dose of 2 g every 12 h and cefazolin at a dose of 2 g every 8 h after MSSA was confirmed. After 5 weeks of intravenous administration, cefaclor was switched to oral administration to control the infection.
Conclusion
In the management of infectious TAAA with MG, it is essential not only to prevent complications of crisis but also to perform multidisciplinary treatments that consider infection control.
Declarations
Informed consent
Written informed consent was obtained from the patient for the publication of the case details.
Disclosure statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author contributions
Study conception: KC
Data collection: KC
Writing: KC
Critical review and revision: all authors
Final approval of the article: all authors
Accountability for all aspects of the work: all authors.
References
- 1).Gilhus NE. Myasthenia gravis. N Engl J Med 2016; 375: 2570–81. [DOI] [PubMed] [Google Scholar]
- 2).Dresser L, Wlodarski R, Rezania K, et al. Myasthenia gravis: epidemiology, pathophysiology and clinical manifestations. J Clin Med 2021; 10: 2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Global guidelines for the prevention of surgical site infection. Geneva: World Health Organization, 2016; p. 1–186. [PubMed] [Google Scholar]
- 4).Ramesh B. Myasthenia gravis: a challenge. Indian J Anaesth 2015; 59: 197–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Huang CS, Hsu HS, Kao KP, et al. Intravenous immunoglobulin in the preparation of thymectomy for myasthenia gravis. Acta Neurol Scand 2003; 108: 136–8. [DOI] [PubMed] [Google Scholar]
- 6).Elovaara I, Apostolski S, van Doorn P, et al. EFNS guidelines for the use of intravenous immunoglobulin in treatment of neurological diseases: EFNS task force on the use of intravenous immunoglobulin in treatment of neurological diseases. Eur J Neurol 2008; 15: 893–908. [DOI] [PubMed] [Google Scholar]
- 7).Collins S, Roberts H, Hewer I. Anesthesia and perioperative considerations for patients with myasthenia gravis. AANA J 2020; 88: 485–91. [PubMed] [Google Scholar]
- 8).Uehara K, Fujiwara Y, Morishima M, et al. Open thoracoabdominal aortic aneurysm repair in a patient with myasthenia gravis. Interact Cardiovasc Thorac Surg 2022; 34: 510–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Seneviratne J, Mandrekar J, Wijdicks EF, et al. Noninvasive ventilation in myasthenic crisis. Arch Neurol 2008; 65: 54–8. [DOI] [PubMed] [Google Scholar]
- 10).Chang YW, Chou YC, Yeh CC, et al. Outcomes after major surgery in patients with myasthenia gravis: a nationwide matched cohort study. PLoS One 2017; 12: e0180433. [DOI] [PMC free article] [PubMed] [Google Scholar]