Abstract
Objectives: This study aimed to quantitatively evaluate peripheral nerve injury (PNI) after varicose vein (VV) surgery using endovenous laser ablation (EVLA).
Methods: Overall, 25 cases were analyzed. All patients underwent EVLA of the great saphenous vein (GSV) with or without resection of the varix of the GSV tributaries in stab and avulsion fashion (microphlebectomy). For evaluation of PNI, the current perception threshold (CPT) was measured preoperatively at 1 week, 1 month, 3 months, and 6 months postoperatively. In each leg, CPT was measured at 6 points. PNI was defined as >40% elevation from preoperative data.
Results: A significant elevation in CPT was observed at 2 points (knee joint level [P = 0.01] and upper portion of the lower leg [P = 0.008]) 1 week postoperatively. CPT decreased after 1 month and recovered to the same level after 6 months. PNI occurred in 52% and 36% of patients at the knee joint level and upper portion of the lower leg, respectively. Microphlebectomy was indicated as a factor associated with PNI (P <0.01).
Conclusions: Although VV surgery using EVLA is less invasive, the occurrence of transient PNI in the early postoperative period should be noted when concomitant microphlebectomy is performed.
Keywords: varicose vein, peripheral nerve injury, endovenous laser ablation, microphlebectomy
Introduction
Since endovenous thermal ablation (ETA) of the insufficient saphenous vein has been utilized, the number of varicose vein (VV) surgeries using ETA has increased annually, making it the standard therapy for VV. Great advances in ETA have led to less invasive surgery for VVs, and its safety is well established.1–4) However, peripheral nerve injury (PNI) remains a complication of ETA.5) Although Akagi et al.6) previously analyzed PNI after stripping of great saphenous vein (GSV), there are few reports on the extent of sensory disturbance and its cure after VV surgery using ETA. Endovenous laser ablation (EVLA) and radiofrequency ablation (RFA) are currently used for ETA. This study aimed to investigate perioperative and postoperative PNI in VV surgery using EVLA by evaluating quantitative sensation disturbances using a current perception threshold (CPT) measurement device.
Materials and Methods
Patients
Overall, 25 cases were enrolled in this study. From 2021 to 2023, consecutively, these cases underwent EVLA of the GSV with or without resection of the varix or its tributaries. The indications of the surgery met all the criteria as follows. The first was significant regurgitation at the sapheno-femoral junction and GSV. It was defined as the detection of regurgitation over 0.5 seconds by the M-mode of ultrasonography. All patients were symptomatic. Symptoms included fatigue, pain, muscle clamping, and leg edema. The research related to human participants complied with the institutional policies, followed the tenets of the Helsinki Declaration, and was approved by the author’s institutional review board (No. 581-2-46).
Surgical procedures
The operative procedure was as follows. All the procedures were performed by a single surgeon. The surgery was performed with the patient in the supine position. The patients were lightly sedated using a low dose of propofol or dexmedetomidine. The GSV under the knee was punctured using a 17-gauge needle under ultrasonographic guidance, and the guidewire was inserted into the GSV through the needle. Subsequently, a 6-French, 11-cm sheath was inserted into the GSV through the guidewire. A laser ablation fiber (ELVeS Radial 2 ring fiber; Biolitec, Bonn, Germany) was inserted through the sheath, and the location of the fiber tip was adjusted to within 10–15 mm of the sapheno-femoral junction. The laser system was a 1470-nm diode laser (LEONARD; Biolitec). Tumescent local anesthesia (TLA) was then administered along the GSV using an ultrasonography guide with a 20-gauge, 70-mm-long needle. The solution used for TLA was composed of 500 ml of saline, a 40 ml solution containing 400 mg xylocaine and 0.4 mg epinephrine, and 8 ml of 8.4 % sodium bicarbonate solution. The GSV was then ablated using a 10-W continuous mode. After ablation of the GSV, microphlebectomy was performed if necessary. TLA was administered around the varix, a 1-2 mm skin incision was made using a scalpel, and the varix was resected using a Varady or Crocht-type vein hook. The patient characteristics and operative data are shown in Table 1.
Table 1 Patients’ characteristics and operative data.
| Age | 65 |
| Gender (men:women) | 9:16 |
| CEAP classification | C1:0 C2: 20 C3: 5 C4: 0 C5: 0 C6: 0 |
| LEED | 84.6 ± 8.1 |
| Length of treated vein | 40.4 ± 4.2 |
| Varicosectomy | 18 |
| PNI at points 1, 2, 5, and 6 | 0 |
| PNI at point 3 | 13 (52%) |
| PNI at point 4 | 9 (36%) |
CEAP: Clinical-Etiology-Anatomy-Pathophysiology classification; LEED: linear endovenous energy density; PNI: peripheral nerve injury
Data
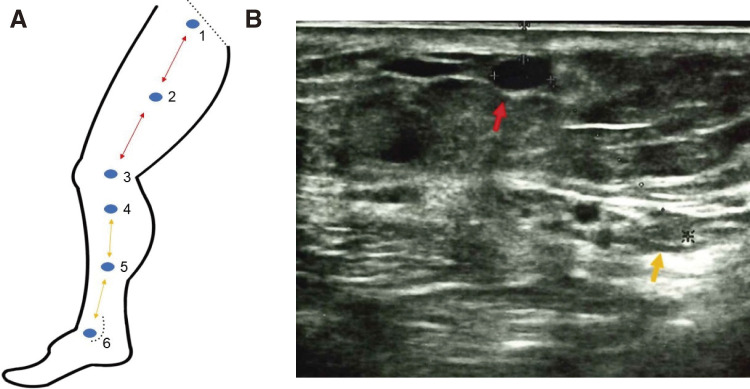
Painvision (Nipro, Osaka, Japan) was used for the perioperative and postoperative quantitative evaluation of PNI. Painvision is a tool that can easily measure the CPT of the skin. It provides electronic stimulation through an electrode attached to a target point. The stimulation is gradually strengthened from 0 to 256 μA, then the patient is instructed to press the button that is connected to the machine when the patient perceives any stimulation at the point. The intensity of stimulation at each instance was recorded. The time taken until the stimulation reaches 256 μA from 0 μA is set in 3 different patterns (60, 80, and 100 seconds). The average of the 3 records was regarded as the sensory threshold at the skin point. The sensory threshold was measured at 6 points on 1 leg (Fig. 1A). The detailed definitions of these 6 points are described in Fig. 1A legend. In point 1, the sapheno-femoral junction was depicted just beneath the linear echography probe. In point 4, the saphenous nerve and the GSV could be seen on the ultrasonography monitor simultaneously (Fig. 1B). At all points, the GSV was shown in the middle of the echography monitor, and the distances between 2 adjacent points (1–2, 2–3, 3–4, 4–5, and 5–6) were recorded. As point 2 is in the middle between points 1 and 3, the distances between points 1 and 2 and between points 2 and 3 were equal. Similarly, the distances between 4 and 5, and 5 and 6 were equal. Since the ablated vein remained as a scar even in the postoperative period, it was possible that the 6 points were reproducibly marked both preoperatively and postoperatively by depicting the GSV in the middle of the echography monitor at each point. At point 4, the distance between the GSV and the saphenous nerve was measured. The thresholds at each point were recorded preoperatively and 1 week, 1 month, and 6 months postoperatively. None of the patients required medications for PNI, such as pregabalin or mecobalamin.
Fig. 1 (A) Scheme and the definition of the 6 points. Point 1: The sapheno-femoral junction was depicted. Point 2: The midpoint between points 1 and 3. Point 3: The knee joint level. Point 4: The saphenous nerve and the GSV could be seen on the ultrasonography monitor simultaneously below the knee (B). Point 5 was the midpoint between points 4 and 6. Point 6: The level of the medial malleolus. Straight dotted line: inguinal ligament, curved dotted line: medial malleolus. The distances between 1 and 2, 2 and 3 are equal (red arrowed line). The distances between 4 and 5, 5 and 6 are equal (yellow arrowed line). (B) The GSV and the saphenous nerve at the point 4. Red arrow: GSV; yellow arrow: saphenous nerve. These 2 components are depicted simultaneously. In this case, the distance between them is 14 mm. GSV: great saphenous vein.
Statistical analysis
Continuous variables were presented as the mean and standard deviation. Categorical variables were presented as numbers and percentages. An increase in the threshold of >40% was defined as PNI. All cases were classified into 2 groups according to the absence of PNI and presence of PNI at least at 1 point, and clinical factors, including age, sex, linear endovenous energy density (LEED), length of treated vein, varicosectomy, the distance between the GSV and the saphenous nerve at point 4, were compared between the 2 groups. Continuous and categorical variables were analyzed using an unpaired T-test and the Chi-square test, respectively. The thresholds over time (pre-operation, 1 week after the operation, 1 month after the operation, and 6 months after the operation) at each point were compared with the repeated analysis of the covariance model with 1 factor (analysis of variance [ANOVA]). If the ANOVA was significant, pairwise comparisons were conducted post hoc with the Bonferroni method. Eta squared (η2) was used to assess effect size. Statistical significance was set at P <0.05. All analyses were performed using the JMP statistical software (JMP Statistical Discovery LLC, Cary, NC, USA).
Results
Clinical results
There were no major complications such as severe endovenous heat-induced thrombosis or major bleeding.
Six months postoperatively, ultrasonography revealed complete obliteration of the GSV in all cases. None of the patients complained of symptoms indicating nerve injury such as burning pain, sensory loss, or extreme sensitivity to touch.
Changes in the threshold
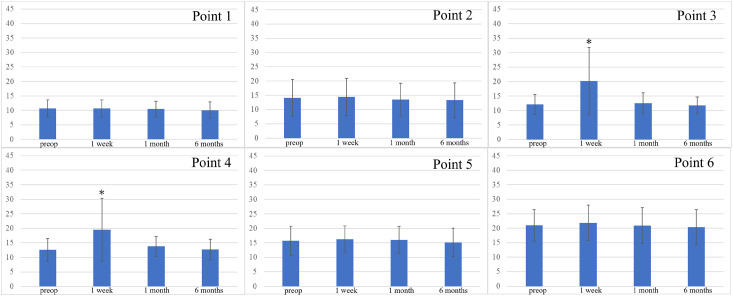
At points 1, 2, 5, and 6, no significant changes were observed in the thresholds throughout the observation period (Fig. 2). The thresholds 1 week after the operation were significantly higher at points 3 (P = 0.008, η2 = 0.35) and 4 (P = 0.02, η2 = 0.29). However, they gradually improved, and 6 months after the operation, there was no significant difference from the preoperative data.
Fig. 2 Change in CPT during the observation period. At points 3 and 4, CPT was significantly higher at 1 week postoperatively (*P = 0.008 at point 3, 0.02 at point 4). Preop: preoperative; CPT: current perception threshold.
Although the threshold was significantly higher at points 3 and 4, no patient complained of numbness in the lower leg.
Factors affecting PNI
Examination of CPT elevation at each measurement site according to the aforementioned definition of positive PNI showed that nerve injury occurred in 52% and 36% of patients at point 3 and point 4, respectively (Table 2). PNI was observed at least at 1 point in 18 cases (72%). Analysis of the factors associated with PNI showed that varicosectomy was the predominant factor (P <0.01) (Table 2).
Table 2 Results of the comparison between the PNI-positive and PNI-negative groups.
| PNI (+) N:18 | PNI (–) N:7 | P | |
|---|---|---|---|
| Sex (female) | 9 (50%) | 6 (85%) | 0.17 |
| Age | 64.6 ± 15.8 | 71 ± 14.2 | 0.42 |
| LEED | 85.1 ± 2.0 | 85 ± 3.2 | 0.98 |
| Length of treated vein | 40.8 ± 1.0 | 39.4 ± 1.63 | 0.49 |
| Distance to saphenous nerve at point 4 | 13.0 ± 1.02 | 13.4 ± 1.64 | 0.86 |
| Varicosectomy | 16 (88%) | 2 (28%) | <0.01 |
| Anterior | 11 | 1 | 0.03 |
| Posterior | 6 | 1 | 0.34 |
| Number of stab and avulsion wound | 3.6 ± 2.0 | 1.5 ± 0.7 | 0.26 |
Varicosectomy was a factor that induced PNI.
Anterior: varicosectomy at the anterior site to the GSV trunk.
Posterior: varicosectomy at the posterior site to the GSV trunk.
PNI: peripheral nerve injury; LEED: linear endovenous energy density; GSV: great saphenous vein
Discussion
Since Navarro reported EVLA of the GSV using an 810-nm diode laser,7) which was approved in the United States of America in 1999, EVLA has become the main treatment modality for VVs worldwide. EVLA uses pulsed light to ablate and obliterate the GSV. It has been suggested that the low penetration of energy means that EVLA is less likely to damage the surrounding tissues. However, EVLA is associated with complications such as leg pain, subcutaneous hemorrhage, or PNI.8–11) Currently, 1470-nm diode lasers are mainly utilized for EVLA. Wavelengths >1000 nm were classified as water-specific laser wavelengths (WSLW). WSLW lasers are expected to decrease complications after EVLA because they are directly absorbed by the water contained in the vein wall.12) Complications after EVLA using a 1470-nm laser are relatively rare.13–16) However, PNI is a complication of EVLA. Hirokawa et al.17) reported that the PNI rate after EVLA using a 1470-nm laser was approximately 2.0%. Laser ablation of the below-knee segment increases the risk of saphenous nerve injury in particular.17) In the saphenous compartment of the lower leg, the saphenous nerve runs adjacent to the GSV.17) It is supposed that the nerve fibers located just near the ablated vein are damaged by the emitted heat. However, in the present study, no ablation-related factors were identified as significant causes of nerve injury. The average LEED score in the present study was relatively high. However, during surgery, a sufficient amount of the TLA solution was administered along the targeted GSV. In echography, the treated portion of the GSV was completely surrounded by the low-echo area, indicating the presence of TLA solution. This may be attributed to the avoidance of damage to the nerve near the ablated GSV.
On the other hand, the current study suggests that microphlebectomy was a significant factor for PNI. Varicosectomy in this fashion was mainly performed at the knee level or the upper portion of the lower leg. Although microphlebectomy at anterior sites to the GSV trunk was identified as a significant factor for PNI, microphlebectomy at posterior sites was not. Baba et al.18) reported occurrence of nerve injury after microphlebectomy with EVLA using a 1470-nm laser was 1.7%. In the medial area of the lower leg or knee, the branches of the saphenous nerve (medial crural cutaneous branches) are distributed on the skin as sensory nerves. The saphenous nerve is the terminal branch of the femoral nerve derived from the lumbar plexus.19,20) When microphlebectomy is performed, until the vein is caught by the hook, the hook is repeatedly inserted into the subcutaneous tissue, and the tissue is dissected bluntly and blindly. During this procedure, small subcutaneous nerves may have been damaged. The skin at the knee level was relatively thick. Therefore, relatively high damage may have been caused to the subcutaneous tissue when searching for a varix. The small nerve branches of the saphenous nerve distributed to the subcutaneous tissue may have been damaged, resulting in an elevation of the sensory threshold. In our data, the number of microphlebectomy in each patient did not affect the frequency of PNI. On the other hand, the location of the microphlebectomy was more likely to be related to PNI, especially that anterior to the GSV. According to an anatomical study of the distribution of the saphenous nerve, the saphenous nerve descends from the tibial tuberosity and distributes in 3 different patterns within the lower thigh.21) One of these patterns exhibits poor nerve distribution posterior to the GSV, while all patterns show nerve distribution anterior to the GSV, where PNI may be inevitable with varicosectomy at the same site.
In this study, the elevated sensory thresholds recovered to the preoperative level 6 months after the operation. Even after 1 month, there was no significant difference from the preoperative data. Nerve fibers are classified into Aα, Aβ, Aγ, Aδ, B, and C, according to their diameter, conduction velocity, and their function.22) Among these, Painvision is said to stimulate Aβ and Aδ nerve fibers which are related to tactile sensation. Classically, according to Seddon, the PNI is classified into 5 groups according to severity.22) Among these, classes 1 and 2 showed full recovery of nerve function after weeks to months. In the more severe groups (classes 3–5), recovery was incomplete.22) In this study, the elevation of the CPT peaked after 1 week postoperatively, began recovery afterward, and then recovered to the preoperative level 6 months postoperatively. Judging from this tendency, even if some extent of nerve damage is caused by the microphlebectomy procedure, the severity might be relatively mild. In previous studies on PNI after a stripping operation, it was reported that neurological symptoms improved in many cases over time.23–25) The results of this study are consistent with those of previous studies,23–25) although they were related to stripping and not EVLA.
This study had some limitations. This was not a randomized controlled study. The small size of the patient group is also a limitation of this study. In addition, the definition of PNI remains controversial. In this study, PNI was defined as >40% elevation of CPT from the preoperative data. However, no definitive criteria have been established. In the report by Akagi et al., in which CPT was analyzed after GSV stripping, an increase in CPT value of more than 20% (hyposensitivity) or a decrease to below 50% (hypersensitivity) was defined as positive PNI.6) In our study, no patient showed hypersensitivity. In addition, as previously mentioned, when the CPT is measured using Painvision, patients are instructed to press a button when stimulated. Therefore, to a certain extent, this evaluation depends on attentiveness. From this perspective, this study may not be entirely objective. Further studies are necessary to clearly define and evaluate PNI using objective data.
Conclusion
Although VV surgery using EVLA is less invasive, the occurrence of transient PNI in the early postoperative period should be noted when concomitant microphlebectomy is performed.
Declarations
Statement of patient consent
Written informed consent was obtained from the patient for publishing this article.
Acknowledgments
We would like to thank Editage for editing English in our manuscript.
Author contributions
Study conception: AH, SS, and KS
Data collection: AH
Analysis: AH
Investigation: AH, SS, and KS
Funding acquisition: None
Manuscript preparation: AH and SS
Critical review and revision: all authors
Final approval of the article: all authors
Accountability for all aspects of the work: all authors.
Disclosure statement
The authors do not have any conflicts of interest to declare.
References
- 1).Fernández CF, Roizental M, Carvallo J. Combined endovenous laser therapy and microphlebectomy in the treatment of varicose veins: efficacy and complications of a large single-center experience. J Vasc Surg 2008; 48: 947–52. [DOI] [PubMed] [Google Scholar]
- 2).Theivacumar NS, Dellagrammaticas D, Mavor AI, et al. Endovenous laser ablation: does standard above-knee great saphenous vein ablation provide optimum results in patients with both above- and below-knee reflux? A randomized controlled trial. J Vasc Surg 2008; 48: 173–8. [DOI] [PubMed] [Google Scholar]
- 3).Gifford SM, Kalra M, Gloviczki P, et al. Reflux in the below-knee great saphenous vein can be safely treated with endovenous ablation. J Vasc Surg Venous Lymphat Disord 2014; 2: 397–402. [DOI] [PubMed] [Google Scholar]
- 4).Zhu HP, Zhou YL, Zhang X, et al. Combined endovenous laser therapy and pinhole high ligation in the treatment of symptomatic great saphenous varicose veins. Ann Vasc Surg 2014; 28: 301–5. [DOI] [PubMed] [Google Scholar]
- 5).Utoh J, Tsukamoto Y. Prevention of saphenous nerve injury after below-knee laser ablation of incompetent great saphenous veins: a trial of two-step ablation and an early result. Phlebology 2023; 38: 484–5. [DOI] [PubMed] [Google Scholar]
- 6).Akagi D, Arita H, Komiyama T, et al. Objective assessment of nerve injury after greater saphenous vein stripping. Eur J Vasc Endovasc Surg 2007; 33: 625–30. [DOI] [PubMed] [Google Scholar]
- 7).Navarro L, Min RJ, Boné C. Endovenous laser: a new minimally invasive method of treatment for varicose veins-preliminary observations using an 810 nm diode laser. Dermatol Surg 2001; 27: 117–22. [DOI] [PubMed] [Google Scholar]
- 8).Woźniak W, Mlosek RK, Ciostek P. Complications and failure of endovenous laser ablation and radiofrequency ablation procedures in patients with lower extremity varicose veins in a 5-year follow-up. Vasc Endovascular Surg 2016; 50: 475–83. [DOI] [PubMed] [Google Scholar]
- 9).van den Bos RR, Neumann M, De Roos KP, et al. Endovenous laser ablation-induced complications: review of the literature and new cases. Dermatol Surg 2009; 35: 1206–14. [DOI] [PubMed] [Google Scholar]
- 10).Dexter D, Kabnick L, Berland T, et al. Complications of endovenous lasers. Phlebology 2012; 27 Suppl, 1: 40–5. [DOI] [PubMed] [Google Scholar]
- 11).Healy DA, Kimura S, Power D, et al. A systematic review and meta-analysis of thrombotic events following endovenous thermal ablation of the great saphenous vein. Eur J Vasc Endovasc Surg 2018; 56: 410–24. [DOI] [PubMed] [Google Scholar]
- 12).Almeida J, Mackay E, Javier J, et al. Saphenous laser ablation at 1470 nm targets the vein wall, not blood. Vasc Endovascular Surg 2009; 43: 467–72. [DOI] [PubMed] [Google Scholar]
- 13).Doganci S, Demirkilic U. Comparison of 980 nm laser and bare-tip fibre with 1470 nm laser and radial fibre in the treatment of great saphenous vein varicosities: a prospective randomised clinical trial. Eur J Vasc Endovasc Surg 2010; 40: 254–9. [DOI] [PubMed] [Google Scholar]
- 14).Pannier F, Rabe E, Maurins U. First results with a new 1470-nm diode laser for endovenous ablation of incompetent saphenous veins. Phlebology 2009; 24: 26–30. [DOI] [PubMed] [Google Scholar]
- 15).Dermody M, O’Donnell TF, Balk EM. Complications of endovenous ablation in randomized controlled trials. J Vasc Surg Venous Lymphat Disord 2013; 1: 427–36.e1. [DOI] [PubMed] [Google Scholar]
- 16).Pan Y, Zhao J, Mei J, et al. Comparison of endovenous laser ablation and high ligation and stripping for varicose vein treatment: a meta-analysis. Phlebology 2014; 29: 109–19. [DOI] [PubMed] [Google Scholar]
- 17).Hirokawa M, Kurihara N. Comparison of bare-tip and radial fiber in endovenous laser ablation with 1470 nm diode laser. Ann Vasc Dis 2014; 7: 239–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Baba T, Ohki T, Kanaoka Y, et al. Utility of the Ginza forceps for superficial phlebectomy during endovenous laser ablation of the great saphenous vein. Surg Today 2017; 47: 1384–90. [DOI] [PubMed] [Google Scholar]
- 19).Dayan V, Cura L, Cubas S, et al. Surgical anatomy of the saphenous nerve. Ann Thorac Surg 2008; 85: 896–900. [DOI] [PubMed] [Google Scholar]
- 20).Kerver AL, Leliveld MS, den Hartog D, et al. The surgical anatomy of the infrapatellar branch of the saphenous nerve in relation to incisions for anteromedial knee surgery. J Bone Joint Surg Am 2013; 95: 2119–25. [DOI] [PubMed] [Google Scholar]
- 21).Wilmot VV, Evans DJ. Categorizing the distribution of the saphenous nerve in relation to the great saphenous vein. Clin Anat 2013; 26: 531–6. [DOI] [PubMed] [Google Scholar]
- 22).Hussain G, Wang J, Rasul A, et al. Current status of therapeutic approaches against peripheral nerve injuries: a detailed story from injury to recovery. Int J Biol Sci 2020; 16: 116–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Morrison C, Dalsing MC. Signs and symptoms of saphenous nerve injury after greater saphenous vein stripping: prevalence, severity, and relevance for modern practice. J Vasc Surg 2003; 38: 886–90. [DOI] [PubMed] [Google Scholar]
- 24).Sam RC, Silverman SH, Bradbury AW. Nerve injuries and varicose vein surgery. Eur J Vasc Endovasc Surg 2004; 27: 113–20. [DOI] [PubMed] [Google Scholar]
- 25).Wood JJ, Chant H, Laugharne M, et al. A prospective study of cutaneous nerve injury following long saphenous vein surgery. Eur J Vasc Endovasc Surg 2005; 30: 654–8. [DOI] [PubMed] [Google Scholar]