Abstract
Introduction:
Two phase 3 randomized controlled studies (ADJUNCT ONE (Clinicaltrials.gov: NCT01836523), ADJUNCT TWO (Clinicaltrials.gov: NCT02098395)) evaluated liraglutide (1.8, 1.2 or 0.6 mg) vs placebo in participants with type 1 diabetes (T1D) as an adjunct to insulin therapy. This paper aims to improve our understanding of the potential mechanisms leading to premature discontinuation of this treatment regimen.
Methods:
Post hoc comparisons were conducted on baseline characteristics and adverse event (AE) rates of participants completing and not completing the ADJUNCT studies due to AEs/lack of tolerance using summary tables and variance analysis.
Results:
Non-completers (liraglutide and placebo combined) had lower baseline body mass index (BMI) (ADJUNCT ONE: 27.8 kg/m2 vs 29.8 kg/m2, P < .0001; ADJUNCT TWO: 26.3 kg/m2 vs 29.2 kg/m2, P < .0001), longer duration of T1D (25.8 years vs 21.0 years, P < .0001; 24.1 years vs 21.0 years, P = .04), lower daily insulin doses by continuous infusion (46.4 U vs 57.3 U, P = .01; 40.9 U vs 57.4 U, P = .12) or multiple injections (58.4 U vs 68.5 U, P = .006; 56.0 U vs 65.8 U, P =.03) and a higher proportion of participants with undetectable C-peptide (91.5% vs 81.3%; 87.0% vs 84.9%) compared to completers. When analyzed by treatment group, only duration of T1D and C-peptide differed between completers and non-completers among liraglutide (and not placebo) participants. The AE rates were higher for non-completers.
Conclusion:
Individuals with longer-standing T1D and low levels of C-peptide at baseline were more likely to discontinue adjunctive liraglutide treatment due to AEs/lack of tolerance than individuals with residual insulin production. Lower BMI predicted a greater likelihood of non-completion for the included participants, regardless of treatment. These new findings may be relevant for clinical practice.
Keywords: GLP-1 receptor agonist, discontinuation, adjunctive therapy, liraglutide, type 1 diabetes, baseline data
Introduction
In 2022, there were 8.75 million individuals worldwide living with type 1 diabetes (T1D). 1 Despite significant advancements in insulin therapies and technologies, two-thirds of individuals with T1D fail to achieve the recommended target for glycated hemoglobin (HbA1C) of less than 7%.2,3 Further, the prevalence of overweight and obesity in individuals with T1D is increasing 4 with more than 60% of the adult T1D population in the United States classified as overweight or obese. 5 Individuals with T1D who have obesity may develop insulin resistance, visceral fat accumulation, dyslipidemia, microvascular complications, fatty liver disease, diabetic kidney disease and other characteristics of metabolic syndrome, increasing their susceptibility to cardiovascular disease (CVD).6-13 Many epidemiological studies have reported significantly increased risk of CVD complications in T1D 14 with a mortality three times that of individuals without diabetes.10,15,16
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) have become standard of care in managing type 2 diabetes and obesity and studies have shown improved CVD outcomes with these agents.17-19 Several comprehensive metadata studies have demonstrated GLP-1 RAs as effective in improving glucose control, decreasing insulin requirements, facilitating weight loss, and improving cardiovascular disease risk factors without increasing the risk of severe hypoglycemia in individuals with T1D.13,20-23 However, these agents are currently not approved by the regulators and are being used off-label for the management of obesity and CV risk in people with T1D.13,24-27
Liraglutide is the only GLP-1 RA that has been evaluated in phase 3 studies in T1D, i.e., ADJUNCT ONE 28 and ADJUNCT TWO. 29 In ADJUNCT ONE, 309 out of 1398 participants (22.2%) discontinued liraglutide prematurely, the majority within the first 8 weeks from randomization and in a dose-dependent manner.28,29 In ADJUNCT TWO, 140 out of 835 participants (16.8%) discontinued liraglutide prematurely and most frequently in the first 12 weeks of the trial, particularly for liraglutide 1.8 mg and 1.2 mg due to adverse events (AEs).28,29
To our knowledge, no studies have examined the factors affecting intolerability or increased risk for side effects leading to drug discontinuation of GLP-1 RA in individuals with T1D. Hence, we aimed to investigate baseline characteristics and the AE profiles for completers and non-completers from the ADJUNCT ONE and TWO studies.
Methods
Study Design
The ADJUNCT studies were randomized, placebo-controlled, double-blind, parallel-group, phase 3 studies performed in adults with T1D. In the 52-week ADJUNCT ONE trial (177 centers in 17 countries; 1398 participants; NCT01836523), once-daily subcutaneous injections of liraglutide were added to insulin, which was continued and titrated on a treat-to-target basis. In the 26-week ADJUNCT TWO trial (59 centers in 12 countries; 835 participants; NCT02098395), subcutaneous liraglutide was used as an adjunct to insulin, which was capped for each participant as the pre-randomization 7-day-average total daily dose.
In both studies, liraglutide was introduced at 0.6 mg and then randomized to either 0.6, 1.2, or 1.8 mg once daily; thereafter, the dose could not be reduced. Volume-matched placebo injections followed the same regimen. At randomization (week 0), the pre-trial total daily insulin dose was decreased by 25%, and further 10% reductions were implemented for a minimum of 24 hours on each dose-escalation day. Detailed descriptions of study design, sample size determination, randomization, blinding, participant flow, recruitment, baseline demographics, and results for all endpoints including interpretations, study limitations and generalizability, ethical approval, and comprehensive overview of unintended effects are all available in the primary publications.28,29
Participants
The key inclusion criteria were: T1D diagnosed ≥12 months prior to enrollment; body mass index (BMI) ≥20 kg/m2; HbA1C range of 7.0–10% (53-86 mmol/mol). Furthermore, pre-trial treatment for ≥six months (stable for ≥three months) with continuous subcutaneous insulin infusion (CSII) or basal-bolus insulin was required in ADJUNCT ONE and ADJUNCT TWO. Individuals with a history of severe hypoglycemia or hypoglycemia unawareness were not excluded, nor were those with a history of ketoacidosis. Key exclusion criteria were: prior use of GLP-1 RAs or dipeptidyl peptidase-4 inhibitors; use of medication that interfered with glycemic control (except insulins), including all anti-hyperglycemic agents or steroids; history of acute or chronic pancreatitis; severely decreased renal function (estimated glomerular filtration rate <30 mL/min/1.73 m2); calcitonin level >50 ng/L at screening; personal/family history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2; severe neuropathy. The full sets of eligibility criteria are described in the primary publications.28,29 All participants gave their informed consent before participating in either of the studies.
Stratification of Participants Into Completers and Non-completers
All randomized participants (including a minor subset not exposed) in both ADJUNCT studies were grouped post hoc based on study completion status. “Completers” finalized the studies without discontinuing treatment at any time point. The “non-completers” were here defined as the participants withdrawn prematurely from the studies due to an AE or lack of tolerance to treatment. We included participants who withdrew due to AEs and those who withdrew due to lack of tolerance as non-completers, as the studies did not formally differentiate between these two types of withdrawal. The number of “non-completers” was calculated by subtracting the number of non-completers withdrawn from the studies due to other reasons than AE/lack of tolerance to treatment from the total number of discontinued participants from the studies. A minor subset (ADJUNCT ONE, 8 participants and ADJUNCT TWO, 13 participants) discontinued treatment without being withdrawn from the studies, for example, due to logistic reasons, and these were excluded from the above analyses. Reasons for discontinuation in the studies were only well described by the AE/lack of tolerance data, which is the focus of this paper. Details of participant disposition and the basis of the utilized post hoc stratification in the present manuscript are available in the primary publications.28,29
Baseline Demographic Analysis—Completers vs Non-completers
The completers and non-completers were then stratified by baseline age, HbA1C and C-peptide levels (relative to the lower detection limit of 0.03 nmol/L), body weight, BMI, duration of T1D and choice of insulin treatment regimen using either CSII or multiple daily injections (MDI).
Adverse Events—Completers vs Non-completers
Tabulation of all AEs observed in both ADJUNCT studies was performed for all four dose groups (liraglutide 1.8 mg, 1.2 mg, or 0.6 mg, and placebo) stratified by completers and non-completers. Event rates were calculated for each dose group for all events and for the two system organ classes (SOC) showing the highest overall rates for the non-completers, that is, “Gastrointestinal disorders” and “Metabolism and Nutrition disorders.” Event rates were calculated as events per dose group/100 years of exposure for the entire dose group.
Statistical Analysis
All statistical analyses were conducted using Statistical Analysis System (SAS) version 9.4. One-way ANOVA was performed to compare completers vs non-completers for the baseline data sets for the continuous parameters: age, body weight, BMI, duration of T1D, HbA1C, and total daily insulin dose by CSII and MDI, respectively, for both ADJUNCT studies. No statistical analysis was possible to perform for the C-peptide proportion as this was a single digit. For ADJUNCT ONE, a two-way ANOVA was performed to analyze if treatment regimen (liraglutide vs placebo) showed statistically significant interaction with non-completion. This was not performed for ADJUNCT TWO, as there were only two participants in the placebo non-completer group.
Results
ADJUNCT Completers vs Non-completers
An overview of the analyzed baseline characteristics across the three liraglutide groups and placebo stratified by completers and non-completers is presented in Table 1.
Table 1.
Baseline Data for Completers and Non-completers inADJUNCT ONE and ADJUNCT TWO for the Continuous Parameters BMI, Body Weight, Age, Duration of T1D, MDI—Total Daily Insulin Dose, CSII—Total Daily Insulin Dose, and HbA1C Are Presented as Observed Means (SD)/Number of Participants Out of Randomized. For C-peptide, Instead of a Mean Value, the Data Show the Proportion of Participants out of 100% Below the LLOQ, while the data in Parenthesis Shows (the Number of Participants/Total Number of Participants Per Goup).
| Subgroup | ADJUNCT ONE |
ADJUNCT TWO |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Liraglutide | Placebo | Liraglutide | Placebo | ||||||
| 1.8 mg | 1.2 mg | 0.6 mg | 1.8 mg | 1.2 mg | 0.6 mg | ||||
| BMI (kg/m2) | Completers | 29.9 (5.2)/258 | 29.7 (5.1)/265 | 29.5 (5.3)/ 300 | 30.0 (5.6)/274 | 29.5 (5.6)/165 | 29.3 (5.1)/177 | 29.1 (5.0)/186 | 28.8 (4.8)/180 |
| Non-completers | 27.5 (4.7)/64 | 28.1 (4.7)/48 | 28.9 (5.9)/16 | 27.3 (4.6)/15 | 26.1 (4.6)/34 | 25.3 (2.9)/20 | 27.8 (3.6)/13 | 30.1 (0.0)/2 | |
| Body weight (kg) | Completers | 87.2 (17.7)/258 | 86.6 (16.8)/265 | 86.7 (17.5)/300 | 87.3 (17.8)/274 | 85.4 (17.8)/165 | 86.3 (18.4)/177 | 83.5 (16.2)/186 | 83.8(16.4)/180 |
| Non-completers | 81.5 (14.1)/64 | 79.2 (18.0)/48 | 83.9 (18.3)/16 | 80.9 (17.7)/15 | 73.6 (12.1)/34 | 74.2 (14.0)/20 | 76.5 (12.2)/13 | 85.7 (12.2)/2 | |
| Age (years) | Completers | 43.5 (12.9)/258 | 43.3 (12.5)/265 | 43.4 (12.4)/300 | 44.2 (12.5)/274 | 42.3 (12.2)/165 | 42.9 (13.0)/177 | 43.9 (13.1)/186 | 42.6 (12.8)/180 |
| Non-completers | 43.4 (13.9)/64 | 49.6 (13.4)/48 | 52.8 (14.4)/16 | 40.6 (14.5)/15 | 46.0 (14.7)/34 | 40.8 (15.3)/20 | 45.4 (12.8)/13 | 58.5 (13.4)/2 | |
| Duration of T1D (years) | Completers | 20.2 (12.1)/258 | 20.7 (12.2)/265 | 20.8 (11.9)/300 | 22.3 (12.2) /274 | 21.0 (11.6)/165 | 21.1 (11.3)/177 | 21.2 (12.5)/186 | 20.7 (11.5)/180 |
| Non-completers | 25.2 (13.4)/64 | 28.1 (13.2)/48 | 28.1 (9.9)/16 | 18.6 (8.1)/15 | 23.6 (11.8)/34 | 24.0 (13.0)/20 | 23.8 (11.7)/13 | 35.0 (2.8)/2 | |
| MDI—total daily insulin dose (units) | Completers | 72.1 (38.7)/173 | 68.9 (34.9)/186 | 65.5 (29.5)/240 | 68.6 (33.8)/198 | 66.3 (26.4)/125 | 65.3 (31.7)/129 | 64.3 (27.7)/139 | 67.2 (33.2)/135 |
| Non-completers | 60.9 (22.9)/39 | 54.5 (39.7)/33 | 59.2 (26.8)/13 | 60.1 (32.0)/11 | 50.4 (22.0)/23 | 61.4 (28.5)/12 | 53.4 (19.4)/11 | 103.2 (43.5)/2 | |
| CSII—total daily insulin dose (units) | Completers | 56.4 (24.7)/80 | 56.8 (24.6)/76 | 58.5 (25.1)/58 | 57.8 (29.0)/75 | 66.6 (86.8)/40 | 56.0 (25.1)/47 | 53.2 (22.0)/47 | 54.9 (25.5)/45 |
| Non-completers | 49.7 (23.2)/24 | 42.0 (16.8)/14 | 37.8 (10.8)/3 | 49.0 (17.0)/4 | 45.2 (15.5)/10 | 33.4 (13.9)/8 | 50.0 (4.0)/2 | No data | |
| HbA1C (%) | Completers | 8.2 (0.7)/258 | 8.2 (0.8)/265 | 8.2 (0.7)/300 | 8.2 (0.7)/274 | 8.1 (0.7)/165 | 8.1 (0.7)/177 | 8.1 (0.7)/186 | 8.1 (0.7)/180 |
| Non-completers | 8.1 (0.8)/64 | 8.1 (0.6)/47 | 8.1 (0.8)/16 | 8.0 (0.6)/15 | 8.0 (0.7)/34 | 7.9 (0.7)/20 | 8.5 (0.9)/13 | 8.3 (0.4)/2 | |
| C-peptide < LLOQ (%) | Completers | 80.1 (205/256) | 83.3 (220/264) | 77.5 (227/293) | 84.8 (229/270) | 82.9 (136/164) | 83.0 (146/176) | 83.2 (153/184) | 90.5 (162/179) |
| Non-completers | 92.1 (58/63) | 89.6 (43/48) | 100.0 (16/16) | 85.7 (12/14) | 88.2 (30/34) | 90.0 (18/20) | 84.6 (11/13) | 50 (1/2) | |
Abbreviations: BMI, body mass index; CSII, continuous subcutaneous insulin infusion; LLOQ, lower limit of quantification; MDI, multiple daily (insulin) injections; SD, standard deviation; T1D, type 1 diabetes.
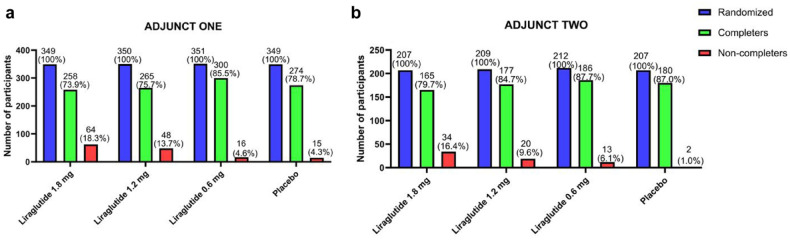
The rates of non-completion related to AEs/lack of tolerance of both ADJUNCT studies showed a dose-dependent increase for liraglutide, as seen from the red bars in Figure 1.
Figure 1.
Participant disposition of ADJUNCT ONE (A) and ADJUNCT TWO (B) across the three liraglutide dose groups (1.8 mg, 1.2 mg, 0.6 mg) and placebo. Bars show number N of all randomized participants (blue), completers defined as participants completing the last scheduled visit (week 52 in ADJUNCT ONE and week 26 in ADJUNCT TWO) (green), and non-completers defined as participants discontinuing treatment prematurely due to AEs/lack of tolerance before the last visit (red). The numbers (N) and percentages out of all randomized participants (%) were added above each graph. Note that non-completers in this study were defined as participants withdrawn from the study prematurely due to AEs/lack of tolerance only. Participants withdrawn from the study for other reasons and participants discontinued without being withdrawn were not included in the non-completer definition used here. The details of participant disposition are presented in section “Methods.”
Baseline Comparison of Completers vs Non-Completers
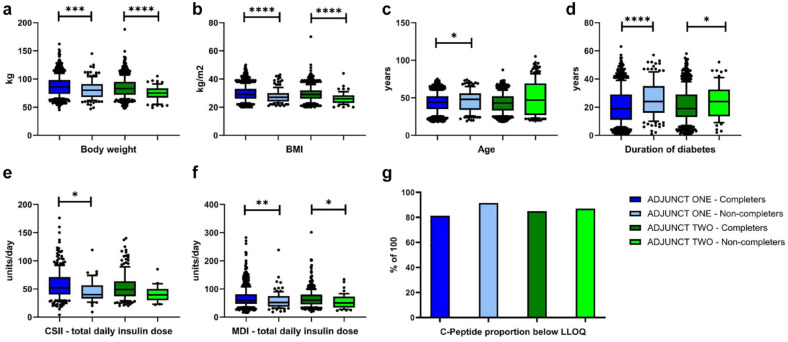
Comparison of baseline data with statistically significant differences between participants completing and those not completing ADJUNCT ONE and ADJUNCT TWO, respectively, is presented using box and whisker plots (10th/90th percentiles) in Figure 2. The figure includes total values for all four treatment groups (liraglutide: 1.8 mg, 1.2 mg, 0.6 mg and placebo).
Figure 2.
Box plots of ADJUNCT ONE completers (dark blue) vs ADJUNCT ONE non-completers (light blue) and for ADJUNCT TWO completers (dark green) and ADJUNCT TWO non-completers (light green) for body weight (A), BMI (B), age (C), duration of T1D (D), CSII—total daily insulin dose (E), MDI—total daily insulin dose, (F). Data were combined across all three liraglutide dose groups (1.8 mg, 1.2 mg, and 0.6 mg) and placebo. Lower and upper bounds of the boxes denote 25th and 75th percentiles of the data sets and the line inside the box denotes the median value (50th percentile). The whiskers mark the 10th and 90th percentiles, respectively. Values beyond these lower and upper bounds are denoted by individual dots. Proportion of C-peptide below the LLOQ is presented as a bar plot (G).
Statistically significant differences for non-completers vs completers within each of the ADJUNCT studies added according to: *P < .05, **P < .01, ***P < .001, ****P < .0001. No statistical analysis was possible to perform for the C-peptide proportion as this was a single digit.
Abbreviations: BMI, body mass index; CSII, continuous subcutaneous insulin infusions; LLOQ, lower level of quantification; MDI, multiple daily insulin injections; SD, standard deviation; T1D, type 1 diabetes.
The following data present mean values for completers vs non-completers and P-value of the one-way ANOVA. At baseline, non-completers had lower body weight (ADJUNCT ONE: 81.0 kg vs 87.0 kg, P = .001; ADJUNCT TWO: 74.7 kg vs 84.7 kg, P < .0001) and BMI (ADJUNCT ONE: 27.8 kg/m2 vs 29.8 kg/m2, P < .0001; ADJUNCT TWO: 26.3 kg/m2 vs 29.2 kg/m2, P < .0001) than completers. Total daily insulin doses at baseline were generally lower in the non-completers for both studies: CSII (ADJUNCT ONE: 46.4 U vs 57.3 U, P = .01; ADJUNCT TWO: 40.9 U vs 57.4 U, P = .12) and MDI (ADJUNCT ONE: 58.4 U vs 68.5 U, P = .006; ADJUNCT TWO: 56.0 U vs 65.8 U, P = .03). In contrast, duration of T1D at baseline (ADJUNCT ONE: 25.8 years vs 21.0 years, P < .0001; ADJUNCT TWO: 24.1 years vs 21.0 years, P = .04) was higher for the non-completers. A higher proportion of non-completers had baseline C-peptide levels below the detection limit of 0.03 nmol/L (ADJUNCT ONE: 91.5% vs 81.3% and ADJUNCT TWO: 87.0% vs 84.9%). Baseline age showed the same trend as duration of T1D, that is, higher values in the non-completers (ADJUNCT ONE: 46.2 years vs 43.6 years, P = .02; ADJUNCT TWO: 44.7 years vs 43.0 years, P = .28). No differences were observed between the two groups of participants for HbA1C baseline levels (ADJUNCT ONE: 8.1% vs 8.2%, P = .29; ADJUNCT TWO: 8.1% vs 8.1% P = .95).
Baseline Characteristics of Completers vs Non-completers in Liraglutide and Placebo Groups
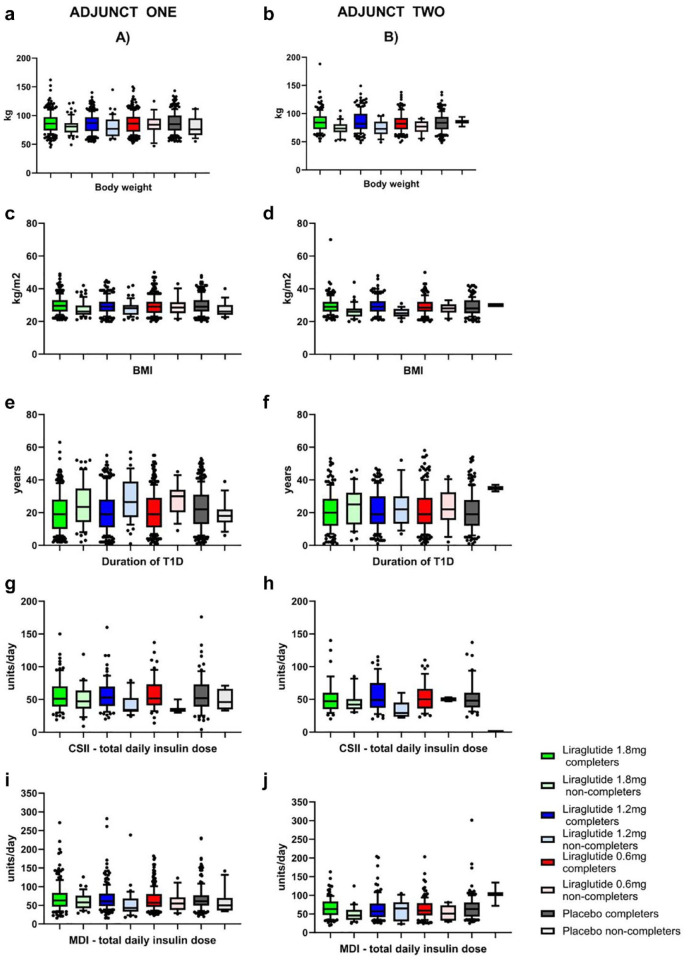
Figure 3 shows comparison using box and whisker plots (10th/90th percentiles) for all four treatment groups in ADJUNCT ONE and TWO for the continuous variables BMI, body weight, duration of T1D, and total daily insulin dosing by CSII and MDI. Details and results for the remaining parameters are presented in Table 1.
Figure 3.
Boxplots of completers vs non-completers for ADJUNCT ONE (left column of figures) and ADJUNCT TWO (right column of figures) across all three liraglutide dose groups (1.8 mg, 1.2 mg, and 0.6 mg) and placebo for BMI (A, B), body weight (C, D), duration of T1D (E, F), CSII—total daily insulin dose (G, H), and MDI—total daily insulin dose (I, J). Lower and upper bounds of the boxes denote 25th and 75th percentiles of the data sets, and the line inside the box denotes the median value (50th percentile). The whiskers mark the 10th and 90th percentiles, respectively. Values beyond these lower and upper bounds are denoted by individual dots.
Abbreviations: BMI, body mass index; CSII, continuous subcutaneous insulin infusions; MDI, multiple daily insulin injections; SD, standard deviation; T1D, type 1 diabetes.
The per dose group stratified data sets for ADJUNCT ONE demonstrated the trends described above for BMI, body weight, and both insulin regimens, that is, lower levels in the non-completing participants for all three liraglutide groups as well as for the placebo group. This was demonstrated by statistically non-significant interaction terms (P ≥ .05) of the two-way ANOVA for body weight, BMI, CSII, and MDI total daily insulin doses, age, and HbA1C (data not shown). In contrast, the duration of T1D was longer across all three doses of liraglutide in non-completing vs completing participants. This was not observed for the placebo group, which was also confirmed statistically by a significant interaction term (Pinteraction = .005) between the two factors completer/non-completer vs liraglutide/placebo. Similar comparisons were not possible for ADJUNCT TWO as there were only two participants in the non-completer placebo group.
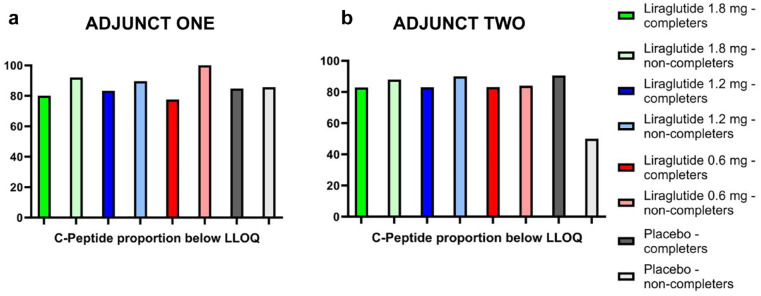
Figure 4 shows the proportion (out of 100%) of participants with C-peptide levels below the lower limit of quantification (LLOQ) for both studies. A higher proportion of non-completing participants showed C-peptide levels below LLOQ across all three liraglutide groups compared to both placebo and completing participants in ADJUNCT ONE. The same pattern was seen for liraglutide in ADJUNCT TWO, but with only N = 2 participants in placebo, comparisons are difficult.
Figure 4.
Percentage of participants in ADJUNCT ONE (a) and ADJUNCT TWO (b) with C-peptide below LLOQ of 0.03 nmol/L at baseline for completers vs non-completers.
Abbreviations: LLOQ, lower level of quantification.
Adverse Events
The AE rates comparing completers with non-completers for the two SOCs with the highest rates among the non-completers (Gastrointestinal and Metabolism and Nutrition SOCs) are presented in Table 2. For the completers, the two SOCs showing the highest AE rates were not identical, as these were “Gastrointestinal disorders” and “Infections and Infestations” (data not shown). In both ADJUNCT studies, for the completers, there were no differences in the proportion of people experiencing AEs, nor were there any dose-dependent differences in the observed AE rates within the SOC “Infections and Infestations.”
Table 2.
AE Rates for Completers and Non-Completers. The Presented Data Show Rates for All Events Cumulatively and the Two Highlighted System Organ Classes: Gastrointestinal Disorders and Metabolism and Nutrition Disorders. Rates are Presented as AE Rates Multiplied by 100/Number of Participants Experiencing AEs. AE Rates were Calculated as Events/Exposure Years for the Entire Dose Group.
| SOC | Subgroup | ADJUNCT ONE |
ADJUNCT TWO |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Liraglutide | Placebo | Liraglutide | Placebo | ||||||
| 1.8 mg | 1.2 mg | 0.6 mg | 1.8 mg | 1.2 mg | 0.6 mg | ||||
| All SOCs | Completers | 674/232 | 541/234 | 515/260 | 455/224 | 998/143 | 867/154 | 656/153 | 612/143 |
| Non-completers | 2384/63 | 2466/47 | 1631/16 | 1256/15 | 4831/33 | 6352/20 | 4260/13 | 2124/2 | |
| Gastrointestinal disorders | Completers | 205/168 | 154/155 | 116/150 | 67/95 | 388/102 | 304/112 | 163/81 | 112/60 |
| Non-completers | 1299/55 | 1366/42 | 893/12 | 341/7 | 3019/ 32 | 3042/17 | 2418/12 | 1352/2 | |
| Metabolism and Nutrition disorders | Completers | 54/81 | 35/64 | 34/65 | 31/49 | 75/47 | 60/46 | 53/37 | 34/25 |
| Non-completers | 236/25 | 159/11 | 185/6 | 106/4 | 637/15 | 1163/11 | 691/5 | 0/0 | |
Abbreviation: AE, adverse event.
The overall event rates as well as rates for both the Gastrointestinal and Metabolism and Nutrition disorders SOCs were higher in the non-completers across all four treatment groups (liraglutide 1.8, 1.2, and 0.6 mg, and placebo) for both ADJUNCT studies. Moreover, the rates for the Gastrointestinal, Metabolism, and Nutrition SOCs showed dose dependency for liraglutide in the completers but not for the non-completers.
Discussion
In the present post hoc study, we observed that participants who did not complete treatment in the ADJUNCT studies due to AEs/lack of tolerance were characterized at baseline by lower body weight/BMI, lower total daily insulin doses, longer duration of T1D, and a higher likelihood of having an undetectable C-peptide level (i.e., C-peptide below the detection limit of 0.03 nmol/L) compared to participants completing the studies.
As there were only two participants categorized as placebo non-completers in ADJUNCT TWO, meaningful comparisons between liraglutide and placebo could only be reasonably conducted for ADJUNCT ONE. In ADJUNCT ONE for daily insulin dose, body weight, and BMI, the differences were seen for both liraglutide and placebo, suggesting non-completion to be unrelated to liraglutide treatment for these participant characteristics. It is possible that individuals with lower BMI may exhibit reduced motivation to engage in a clinical study that involves attending visits with assessments and taking a second drug that induces the well-characterized gastrointestinal side effects of GLP-1 RA treatment.
There were no differences between non-completers and completers for HbA1C, suggesting glucose control to be of little relevance to premature GLP-1 discontinuation.
For duration of T1D and proportion of participants with fasting C-peptide levels below the LLOQ used at the time of study conduct, the observed baseline differences were exclusively seen for the liraglutide dose groups and not for placebo. This may indicate that individuals with longer standing and progressed undetectable insulin secretion could be more likely to discontinue adjunctive GLP-1 RA treatment, as opposed to individuals with residual β-cell function. Individuals with diabetes and suboptimally controlled blood glucose levels, as seen with inherently lower C-peptide levels and longer duration of T1D carry an increased risk for developing diabetic neuropathies. 30 Diabetic gastroparesis is a potential neuropathic complication manifested with gastrointestinal symptoms, for example, nausea, vomiting, and stomach pains. 30 It is possible that this population would report more gastrointestinal AEs and therefore carry a higher likelihood of premature treatment discontinuation.
Low C-peptide levels and longer duration of T1D are both associated with an increased risk of diabetic ketoacidosis (DKA) 31 and more severe hypoglycemic episodes. 32 There were eight liraglutide-treated participants experiencing DKA in ADJUNCT ONE. All eight events were assessed as unrelated to liraglutide by the investigators and only one of the events was observed in a participant who discontinued the study prematurely. 28 In ADJUNCT TWO, there was one case of DKA observed with liraglutide. This participant did not discontinue the study prematurely. In ADJUNCT ONE, the rate of symptomatic hypoglycemic episodes was higher for liraglutide 1.8 mg and 1.2 mg compared to placebo; however, lower rates of severe hypoglycemic episodes were observed for all liraglutide groups compared to placebo. The rates of hypoglycemic episodes did not differ among the participants completing and those not completing the study. 28 Only one participant in the placebo group in ADJUNCT ONE discontinued potentially due to hypoglycemia (withdrawal criteria: hypo-/hyperglycemia posing safety concerns). 28 No participants discontinued ADJUNCT TWO due to hypoglycemic events. Based on the above, the presence of hypoglycemic events or DKA did not influence, whether a participant completed or discontinued any of the two studies.
It should be noted that dose de-escalation was not allowed in the ADJUNCT studies; hence, real-world discontinuation rates may be lower than observed here.
It was previously shown that general study discontinuation for both ADJUNCT studies was dose-dependent for liraglutide during the first months of the studies. 33 Comparison in the present post hoc study confirmed that this GLP-1 RA dose-dependent increase in study discontinuation was correlated with non-completion due to AEs/not tolerating treatment compared to the completing participants in both ADJUNCT ONE and ADJUNCT TWO. The analysis presented here excluded a minor subset of participants who discontinued but did not withdraw from the studies, thus focusing solely on discontinuation due to side effects and intolerance. This may be of clinical relevance, when deciding whether and how to initiate adjunct treatment in individuals with T1D.
Unsurprisingly, AE rates were higher for the non-completers, a trend observed across all four dose groups, including placebo. Dose dependency of the AE rates across liraglutide doses was observed for the completers only and not for the non-completers. The lack of dose dependency should be considered in relation to the bias that the non-completers were already preselected as discontinuing treatment due to AEs. Moreover, most discontinuations were due to the AEs within the SOC for gastrointestinal AEs, which was typically observed during the drug escalation phases in the first two to three months of the studies.
It is also noteworthy that the two SOCs having the highest rates across both studies were different between completers (Gastrointestinal and Infections and Infestations) and non-completers (Gastrointestinal and Metabolism and Nutrition disorders). The difference in the AE profiles may suggest physiological distinctions between completers and non-completers.
All in all, this potentially suggests an inherently lower threshold for liraglutide-induced AEs in the participants who discontinued treatment prematurely, regardless of dose. Whether this is potentially correlated with a more extensive disease progression at study entry, as indicated by the lower C-peptide and longer duration of T1D outlined above, is beyond the scope of this paper.
The use of GLP-1 RAs in T1D has been shown to have beneficial effects on both weight loss and insulin dose requirement, especially in individuals with residual β-cell function (detectable C-peptide) 33 and/or obesity. 34 Our data suggest that individuals with T1D who have residual β-cell function (albeit still low) and a shorter history of T1D are more likely to continue GLP-1 RA adjunct treatment than those with longer-standing disease and/or undetectable insulin production.
Substantial evidence supports the benefits of GLP-1 RA adjunctive therapy in T1D, not only for improving glycemic control but also for lowering body weight and blood pressure and therefore potentially reducing mortality associated with cardiovascular and renal comorbidities.35,36
Further studies are needed to investigate the potential impact of GLP-1 RAs on other clinical outcomes in connection with T1D, such as microvascular and macrovascular complications. Recent real-world evidence data confirms the observed efficacy and safety findings of both ADJUNCT studies, thus reinforcing the representativeness of the present study. 37
It is important to note that this post hoc study is purely exploratory and based on data not originally intended for this purpose. It is possible that unaccounted covariates in the selected analyses may significantly influence the decision to discontinue GLP-1 RA adjunct therapy prematurely. Therefore, while the presented findings are potentially interesting, they must be interpreted with caution.
Conclusions
Our data suggest that individuals with longer-standing T1D and impaired insulin production are more likely to discontinue adjunctive GLP-1 RA treatment due to AEs or lack of tolerance than individuals with shorter diabetes duration and residual insulin production from the β-cells of the pancreas. These new findings may be relevant for clinical practice.
Acknowledgments
The authors thank all participants, investigators and site staff who were involved in the conduct of the ADJUNCT ONE and ADJUNCT TWO studies. Medical writing and editorial support were provided by Anders Bergström, PhD, and Riia Karoliina Sustarsic, PhD, both employees of Novo Nordisk A/S.
Footnotes
Abbreviations: AE, adverse event; ANOVA, analysis of variance; BMI, body mass index; CSII, continuous subcutaneous insulin infusion; CVD, cardiovascular disease; DKA, diabetic ketoacidosis; GI, gastrointestinal; GLP-1 RA, GLP-1 receptor agonist; LLOQ, lower limit of quantification; MDI, multiple daily injections; SOC, system organ class; T1D, type 1 diabetes.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The authors disclose the following competing interests: Rikke M. Agesen, Lars Bardtrum, and Erik Christiansen are all employees and shareholders of Novo Nordisk A/S. Jennifer Snaith and Jerry R. Greenfield have received semaglutide and matched placebo from Novo Nordisk A/S for an investigator-initiated study in T1D. Jennifer Snaith is supported by JDRF Australia (Grant# 3-SRA-2023-1296-M-N), the recipient of the Commonwealth of Australia grant for Accelerated Research under the Medical Research Future Fund. Jerry R. Greenfield has received honorarium from the following companies for delivering talks to health professionals over the last 10 years: Novartis, Novo Nordisk A/S, Amgen, Allergan, Boehringer Inglheim, Lilly and Abbott. Viral N. Shah’s institution has received research funding from Alexion, Enable Bioscience, JDRF (Breakthrough T1D), and NIH (NIDDK). Viral N. Shah’s institution has received drug support for an investigator-initiated trial of semaglutide in adults with T1D from Novo Nordisk A/S. Viral N. Shah has received honoraria for consulting, advising, or speaking from Dexcom, Insulet, Tandem Diabetes Care, Sanofi, Eli Lilly, Novo Nordisk A/S, Embecta, and Ascensia Diabetes Care, unrelated to this submitted work.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was sponsored by Novo Nordisk A/S.
ORCID iD: Viral N. Shah  https://orcid.org/0000-0002-3827-7107
https://orcid.org/0000-0002-3827-7107
Rikke Agesen  https://orcid.org/0000-0002-7649-7928
https://orcid.org/0000-0002-7649-7928
Jennifer Snaith  https://orcid.org/0000-0001-8559-8387
https://orcid.org/0000-0001-8559-8387
References
- 1. Ogle G, Wang F, Gregory G, Maniam J. IDF Diabetes Atlas. Published 2022. Accessed December 3, 2024. https://diabetesatlas.org/
- 2. Ebekozien O, Mungmode A, Sanchez J, et al. Longitudinal trends in glycemic outcomes and technology use for over 48,000 people with type 1 diabetes (2016-2022) from the T1D exchange quality improvement collaborative. Diabetes Technol Ther. 2023;25(11):765-773. [DOI] [PubMed] [Google Scholar]
- 3. Karakus KE, Akturk HK, Alonso GT, Snell-Bergeon JK, Shah VN. Association between diabetes technology use and glycemic outcomes in adults with type 1 diabetes over a decade. Diabetes Care. 2023;46(9):1646-1651. [DOI] [PubMed] [Google Scholar]
- 4. Kueh MTW, Chew NWS, Al-Ozairi E, le Roux CW. The emergence of obesity in type 1 diabetes. Int J Obes (Lond). 2024;48(3):289-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fang M, Echouffo-Tcheugui JB, Selvin E. Prevalence and management of obesity in U.S. adults with type 1 diabetes. Ann Intern Med. 2023;176(9):eL230228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chillarón JJ, Flores Le-Roux JA, Benaiges D, Pedro-Botet J. Type 1 diabetes, metabolic syndrome and cardiovascular risk. Metabolism. 2014;63(2):181-187. [DOI] [PubMed] [Google Scholar]
- 7. Chiesa ST, Marcovecchio ML. Preventing cardiovascular complications in type 1 diabetes: the need for a lifetime approach. Front Pediatr. 2021;9:696499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shah VN, Bailey R, Wu M, et al. Risk factors for cardiovascular disease (CVD) in adults with type 1 diabetes: findings from prospective real-life T1D exchange registry. J Clin Endocrinol Metab. 2020;105(5):e2032-e2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shah VN, Grimsmann JM, Foster NC, et al. Undertreatment of cardiovascular risk factors in the type 1 diabetes exchange clinic network (United States) and the prospective diabetes follow-up (Germany/Austria) registries. Diabetes Obes Metab. 2020;22(9):1577-1585. [DOI] [PubMed] [Google Scholar]
- 10. Harding JL, Shaw JE, Peeters A, Guiver T, Davidson S, Magliano DJ. Mortality trends among people with type 1 and type 2 diabetes in Australia: 1997-2010. Diabetes Care. 2014;37(9):2579-2586. [DOI] [PubMed] [Google Scholar]
- 11. Hill CJ, Cardwell CR, Maxwell AP, et al. Obesity and kidney disease in type 1 and 2 diabetes: an analysis of the National Diabetes Audit. QJM. 2013;106(10):933-942. [DOI] [PubMed] [Google Scholar]
- 12. Wallace AS, Chang AR, Shin JI, et al. Obesity and chronic kidney disease in US adults with type 1 and type 2 diabetes mellitus. J Clin Endocrinol Metab. 2022;107(5):1247-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Akturk HK, Dong F, Snell-Bergeon JK, Karakus KE, Shah VN. Efficacy and safety of tirzepatide in adults with type 1 diabetes: a proof of concept observational study. J Diabetes Sci Technol. 2024 February 5. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vergès B. Cardiovascular disease in type 1 diabetes, an underestimated danger: epidemiological and pathophysiological data. Atherosclerosis. 2024;394:117158. [DOI] [PubMed] [Google Scholar]
- 15. Rawshani A, Franzén S, Sattar N, et al. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2018;379(7):633-644. [DOI] [PubMed] [Google Scholar]
- 16. Frampton R, Snaith JR, Hocking S, Holmes-Walker J, Olsen N, Greenfield JR. Reducing cardiometabolic risk with semaglutide in type 1 diabetes (RESET1): study protocol of a phase 2 double-blinded randomised placebo-controlled trial. Diabet Med. 2024;41(10):e15377. [DOI] [PubMed] [Google Scholar]
- 17. Singh S, Garg A, Tantry US, Bliden K, Gurbel PA, Gulati M. Safety and efficacy of glucagon-like peptide-1 receptor agonists on cardiovascular events in overweight or obese non-diabetic patients. Curr Probl Cardiol. 2024;49(3):102403. [DOI] [PubMed] [Google Scholar]
- 18. Davies MJ, Drexel H, Jornayvaz FR, Pataky Z, Seferović PM, Wanner C. Cardiovascular outcomes trials: a paradigm shift in the current management of type 2 diabetes. Cardiovasc Diabetol. 2022;21(1):144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang W, Liu H, Xiao S, Liu S, Li X, Yu P. Effects of insulin plus glucagon-like peptide-1 receptor agonists (GLP-1RAs) in treating type 1 diabetes mellitus: a systematic review and meta-analysis. Diabetes Ther. 2017;8(4):727-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dimitrios P, Michael D, Vasilios K, et al. Liraglutide as adjunct to insulin treatment in patients with type 1 diabetes: a systematic review and meta-analysis. Curr Diabetes Rev. 2020;16(4):313-326. [DOI] [PubMed] [Google Scholar]
- 22. Goyal I, Sattar A, Johnson M, Dandona P. Adjunct therapies in treatment of type 1 diabetes. J Diabetes. 2020;12(10):742-753. [DOI] [PubMed] [Google Scholar]
- 23. Karakus KE, Klein MP, Akturk HK, Shah VN. Changes in basal and bolus insulin requirements with tirzepatide as an adjunctive therapy in adults with type 1 diabetes using tandem control-IQ. Diabetes Ther. 2024;15(7):1647-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Garg SK, Kaur G, Haider Z, Rodriquez E, Beatson C, Snell-Bergeon J. Efficacy of semaglutide in overweight and obese patients with type 1 diabetes. Diabetes Technol Ther. 2024;26(3):184-189. [DOI] [PubMed] [Google Scholar]
- 25. Garg SK, Akturk HK, Kaur G, Beatson C, Snell-Bergeon J. Efficacy and safety of tirzepatide in overweight and obese adult patients with type 1 diabetes. Diabetes Technol Ther. 2024;26(6):367-374. [DOI] [PubMed] [Google Scholar]
- 26. Corbin KD, Driscoll KA, Pratley RE, et al. Obesity in type 1 diabetes: pathophysiology, clinical impact, and mechanisms. Endocr Rev. 2018;39(5):629-663. [DOI] [PubMed] [Google Scholar]
- 27. Mottalib A, Kasetty M, Mar JY, Elseaidy T, Ashrafzadeh S, Hamdy O. Weight management in patients with type 1 diabetes and obesity. Curr Diab Rep. 2017;17(10):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mathieu C, Zinman B, Hemmingsson JU, et al. Efficacy and safety of liraglutide added to insulin treatment in type 1 diabetes: the ADJUNCT ONE treat-to-target randomized trial. Diabetes Care. 2016;39(10):1702-1710. [DOI] [PubMed] [Google Scholar]
- 29. Ahrén B, Hirsch IB, Pieber TR, et al. Efficacy and safety of liraglutide added to capped insulin treatment in subjects with type 1 diabetes: the ADJUNCT TWO randomized trial. Diabetes Care. 2016;39(10):1693-1701. [DOI] [PubMed] [Google Scholar]
- 30. Feldman EL, Callaghan BC, Pop-Busui R, et al. Diabetic neuropathy. Nat Rev Dis Primers. 2019;5(1):41. [DOI] [PubMed] [Google Scholar]
- 31. Umpierrez GE, Kitabchi AE. Diabetic ketoacidosis: risk factors and management strategies. Treat Endocrinol. 2003;2(2):95-108. [DOI] [PubMed] [Google Scholar]
- 32. McCrimmon RJ, Sherwin RS. Hypoglycemia in type 1 diabetes. Diabetes. 2010;59(10):2333-2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dejgaard TF, von Scholten BJ, Christiansen E, et al. Efficacy and safety of liraglutide in type 1 diabetes by baseline characteristics in the ADJUNCT ONE and ADJUNCT TWO randomized controlled trials. Diabetes Obes Metab. 2021;23(12):2752-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kuhadiya ND, Malik R, Bellini NJ, et al. Liraglutide as additional treatment to insulin in obese patients with type 1 diabetes mellitus. Endocr Pract. 2013;19(6):963-967. [DOI] [PubMed] [Google Scholar]
- 35. Avgerinos I, Manolopoulos A, Michailidis T, et al. Comparative efficacy and safety of glucose-lowering drugs as adjunctive therapy for adults with type 1 diabetes: a systematic review and network meta-analysis. Diabetes Obes Metab. 2021;23(3):822-831. [DOI] [PubMed] [Google Scholar]
- 36. Freeby M, Lane K. Treating obesity in type 1 diabetes mellitus—review of efficacy and safety. Curr Opin Endocrinol Diabetes Obes. 2024;31(1):1-7. [DOI] [PubMed] [Google Scholar]
- 37. Edwards K, Li X, Lingvay I. Clinical and safety outcomes with GLP-1 receptor agonists and SGLT2 inhibitors in type 1 diabetes: a real-world study. J Clin Endocrinol Metab. 2023;108(4):920-930. [DOI] [PubMed] [Google Scholar]