Abstract
Background
Pneumococcal disease, caused by Streptococcus pneumoniae, imposes a significant global health burden, particularly affecting vulnerable groups such as the elderly and immunocompromised. The 23-valent pneumococcal polysaccharide vaccine (PPV23) is designed to protect against 23 serotypes of Streptococcus pneumoniae. However, there is ongoing debate about its effectiveness in reducing all-cause mortality. This systematic review and meta-analysis aimed to evaluate the efficacy of PPV23 in reducing all-cause and pneumonia-related mortality among adults.
Methods
A systematic search was conducted across PubMed, Embase, and Web of Science, focusing on studies that evaluated the mortality outcomes of adults vaccinated with PPV23 compared to non-vaccinated adults. Both randomized controlled trials (RCTs) and observational studies were included, while case reports, case series, and non-human studies were excluded. Data extraction and quality assessment were facilitated by Nested Knowledge software, using the Newcastle-Ottawa Scale for observational studies and the Cochrane Risk of Bias tool for RCTs.
Results
The search yielded 826 records, with 19 studies meeting the inclusion criteria. The pooled analysis of four RCTs showed no significant reduction in all-cause mortality (RR = 1.030; 95% CI: 0.945, 1.122). However, analysis of pneumonia-related mortality across various studies indicated a significant reduction (HR = 0.504; 95% CI: 0.316, 0.693). Moderate to high heterogeneity was noted in mortality studies, and a potential publication bias was identified.
Conclusion
The findings suggest that while PPV23 may not significantly reduce all-cause mortality, it is effective in reducing pneumonia-related mortality among adults, particularly in those at higher risk. These results support the continued use of PPV23 in targeted adult populations, emphasizing the need for more primary studies to explore its effectiveness across diverse groups.
Supplementary Information
The online version contains supplementary material available at 10.1186/s41479-024-00149-5.
Keywords: Pneumonia, PPV23, Vaccination, Meta-analysis, Mortality
Introduction
Pneumococcal disease, caused by Streptococcus pneumoniae, presents a significant global health burden. It manifests in forms ranging from mild respiratory infections to severe diseases such as pneumonia, meningitis, and sepsis [1, 2]. Particularly vulnerable populations include young children, the elderly, and individuals with compromised immune systems [3]. The 23-valent pneumococcal polysaccharide vaccine (PPV23), also known as Pneumovax 23, targets 23 serotypes of the bacterium [4]. It has been a cornerstone in the prevention strategy against pneumococcal diseases for adults over 50 years of age and younger adults at increased risk of pneumococcal disease [5].
Despite the widespread use of PPV23, debates persist regarding its effectiveness, especially concerning mortality reduction in adult populations. While numerous studies have explored the vaccine’s efficacy in preventing invasive pneumococcal disease (IPD), fewer have specifically addressed its role in reducing mortality [6–12]. This research gap is significant given the critical role of mortality in evaluating the impact of vaccination programs on public health. Historically, the introduction of PPV23 was based on its potential to elicit a serotype-specific immune response capable of reducing the incidence of IPD, a major contributor to pneumococcal mortality [13, 14]. Initial studies demonstrated promising results, suggesting a significant reduction in IPD incidence among vaccinated individuals [15–17]. However, the translation of these findings into a demonstrable mortality benefit has been inconsistently reported, with studies yielding varying results, from substantial benefits to negligible effects [6, 7, 18–20]. This inconsistency may stem from several factors, including study design, population heterogeneity, differing definitions of outcomes, and the evolution of pneumococcal serotypes. Moreover, introducing pneumococcal conjugate vaccines (PCVs) for children has altered the epidemiological landscape of pneumococcal disease through herd immunity, indirectly affecting the serotype distribution and the disease burden in adults [21, 22]. These changes necessitate a reassessment of PPV23’s impact on mortality among different adult populations. Given the substantial investment in vaccination programs and the critical implications for public health policy, a systematic review and meta-analysis are warranted to consolidate the existing data on the effectiveness of PPV23 in reducing mortality due to pneumococcal disease. Such a synthesis can provide a clearer picture of the vaccine’s impact, inform stakeholders, and guide future vaccination strategies.
This systematic review and meta-analysis aimed to comprehensively evaluate the available evidence on the mortality risk reduction associated with PPV23. This investigation focused on adult populations globally, considering variations in vaccine performance across different demographic and clinical backgrounds. By integrating data from multiple studies, we aimed to address the following question: Did PPV23 reduce mortality in adults? This research systematically gathered and analyzed randomized controlled trials (RCTs) and observational studies that reported on mortality outcomes following vaccination with PPV23.
Method
This study was conducted by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guidelines (Table S1) [23]. The protocol was registered in PROSPERO under the registration number: CRD42024560236. We used a semi-automated software named Nested Knowledge for conducting this systematic review.
Eligibility criteria
For our systematic review and meta-analysis, we included studies that met specific eligibility criteria to capture a comprehensive and relevant dataset. The population of interest consisted of adults aged 18 years and older, reflecting the primary demographic targeted by the PPV23 immunization programs. Studies focused on the intervention of PPV23 administration, allowing us to assess the direct impact of this vaccine on adult populations. We considered studies that used either no vaccination as comparators to isolate the effects of PPV23 and differentiate its effectiveness from other interventions. The primary outcome of interest for this review was all-cause mortality, which provided a broad measure of the vaccine’s impact on survival. Secondary outcomes included pneumonia-related mortality. Eligible study designs encompassed randomized controlled trials (RCTs) and observational studies, including cohort and case-control studies, to ensure a robust analysis of available evidence across different research settings. Case reports, case series, and non-human studies were excluded. Articles not available in the English language were excluded.
Literature search
We conducted comprehensive searches across multiple databases including PubMed, Embase, and Web of Science through June 15, 2024. Our strategy utilized a combination of keywords: “23-valent pneumococcal polysaccharide vaccine,” “PPV23,” “Pneumovax 23” for vaccine-related terms and “mortality,” “death,” “deaths,” “fatal,” “fatality,” “survive*,” “survival*,” and “died” for mortality-related outcomes. These terms were linked using Boolean operators to ensure a comprehensive retrieval of relevant literature. Although we imposed no initial restrictions on publication date or language to broaden the search scope, only English-language articles were reviewed in detail due to the language capabilities of our team. The complete search strategy, including the exact Boolean combinations used, is detailed in Table S2.
Screening
Initially, all records identified from the database searches were compiled into a centralized database and screened based on their titles and abstracts to eliminate clearly irrelevant studies, such as those not involving adults, not using PPV23, or not focusing on mortality outcomes. Subsequently, studies that passed this preliminary screening underwent a detailed full-text review by two independent reviewers to assess eligibility based on predefined criteria regarding the study population, intervention, comparators, outcomes, and design. Discrepancies between reviewers were resolved through discussion or consultation with a third reviewer if necessary. Studies that met all inclusion criteria after this thorough review were selected for final analysis.
Data extraction and quality assessment
Data from the included studies were systematically extracted and managed using the semi-automated software tool, Nested Knowledge, to ensure accuracy and consistency [24]. We extracted a range of critical data from each study, including study identification details such as authors, publication year, and country. Study design elements, including whether the study was an RCT, cohort study, or case-control study, were noted. Detailed demographic information on the study populations, such as age, sex, and comorbidities, was gathered to evaluate the generalizability and relevance of the findings. Outcomes of interest, particularly all-cause mortality, and pneumonia-related mortality, were recorded, including the number of events, participant totals in each group, and statistical measures like hazard ratios (HR), odds ratios (OR), Relative risk (RR), and their confidence intervals (CI). The extraction process involved two independent reviewers to minimize errors, with any discrepancies resolved through discussion or by consulting a third reviewer, ensuring a thorough and precise data collection phase. The quality assessment of observational studies was carried out using the Newcastle-Ottawa Scale (NOS) [25], and the Cochrane Risk of Bias 2 (RoB 2) tool was used for RCTs.
Statistical analysis
In this meta-analysis, we aggregated HR and 95% CI for mortality data from the studies. A random-effects model was selected to synthesize these data due to the anticipated differences across the included studies. Heterogeneity was quantitatively assessed using I² and Tau² statistics, with Tau² computed using the maximum likelihood method [26, 27]. A threshold of p < 0.05 was set for statistical significance. Doi plots with the LFK index were used to detect any publication bias [28, 29]. 95% prediction interval is also calculated [30, 31]. The number of deaths in the vaccinated and unvaccinated groups were pooled along with the sample sizes from both groups from RCTs to calculate the RR. The statistical analyses, including meta-analysis and publication bias assessments, were conducted using R software, version 4.3 [32].
Results
Literature search
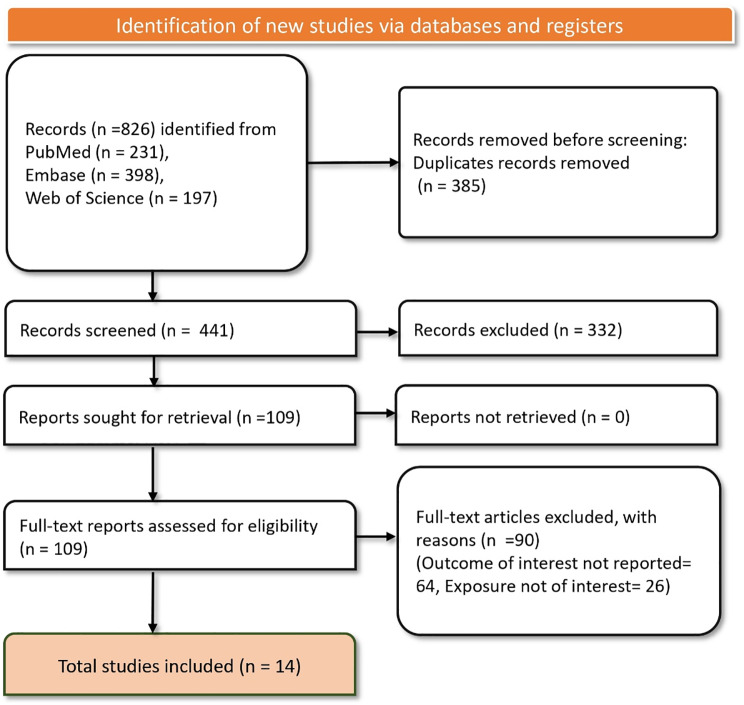
In the literature search across PubMed, Embase, and Web of Science databases yielded a total of 826 records. After the removal of 385 duplicate records, 441 studies remained for screening. Upon initial screening of titles and abstracts, 332 records were excluded, leaving 109 studies for full-text retrieval and detailed assessment. All 109 reports were successfully retrieved and assessed for eligibility based on our predefined criteria. During this phase, 90 studies were excluded for reasons such as not reporting the outcome of interest (64 studies) and not involving exposures of interest (26 studies). Ultimately, 19 studies [6–12, 18–20, 33–41] met al.l the inclusion criteria and were included in the final analysis (Fig. 1).
Fig. 1.
PRISMA flow diagram depicting the article selection and screening process
Characteristics of included studies
The important characteristics of the included studies are summarized in Table 1. The studies encompassed RCTs, prospective and retrospective cohort studies, and case-control studies, offering a comprehensive view of data derived from both experimental and observational research designs. Geographically, the research was conducted across multiple continents in countries such as Spain, China, Uganda, Taiwan, South Korea, Japan, Canada, Germany, Sweden, and France. Participant demographics were varied, targeting adults with specific health conditions such as chronic obstructive pulmonary disease (COPD), HIV-1 infection, chronic renal failure, and generally older adults in nursing homes and community settings. Sample sizes ranged dramatically, from small-scale studies with just over 500 participants to large-scale research involving over one million individuals, enhancing the robustness and applicability of the findings. The age groups in these studies mostly focused on older adults, with many specifically including participants aged 60 years and older, aligning with the primary demographic for whom PPV23 is recommended. The gender distribution varied, with some studies, like Alfageme 2006, predominantly involving male participants (96.6%), while others featured a more balanced gender mix. Effectiveness was quantified using HR and OR across various studies. The quality assessment of the studies is provided in Table S3.
Table 1.
Basic characteristics of the included studies
| Study | Study Design | Country | Study population | Sample size | Age | Male (%) | All-cause mortality with vaccination | Pneumonia related mortality |
|---|---|---|---|---|---|---|---|---|
| Alfageme 2006 [18] | RCT | Spain | Patients with COPD | 596 | 65.8 | 96.6 | NA | NA |
| Chan 2012 [6] | Prospective cohort study | China | Nursing home older adults | 532 | 85.7 | 39.8 | HR = 0.54 (95% CI: 0.35 to 0.84) | HR = 0.60 (95% CI: 0.35 to 0.99) |
| Córcoles AV 2006 [40] | Prospective cohort study | Spain | Community-dwelling individuals aged ≥ 65 years | 11,241 | ≥ 65 | 43 | HR = 0.97 (95% CI: 0.86–1.09) | HR = 0.41 (95%: 0.23 to 0.72) |
| Corcoles AV 2014 [41] | Population-based cohort study | Spain | Individuals aged ≥ 65 years | 27,204 | 71.7 | 44.6 | HR = 0.97 (95% CI: 0.89 to 1.05) | NA |
| French 2000 [33] | RCT | Uganda | HIV-1-infected adults | 1392 | 31 | 29 | HR = 1.08 (95% CI: 0.87 to 1.33) | NA |
| Gondar 2014 [20] | Population-based cohort study | Spain | Individuals aged ≥ 65 years | 27,204 | 71.7 | 44.6 | HR = 0.97 (95% CI: 0.89 to 1.05) | NA |
| Hsieh 2013 [7] | Case control study | Taiwan | Elderly aged 75 years and older | 1,063,116 | ≥ 75 | NA | NA | OR = 0.06 |
| Hsieh 2016 [19] | Case-control study | Taiwan | Patients with chronic renal failure | 545 | > 50 | NA | OR = 0.691 (95% CI: 0.460 to 1.039) | NA |
| Hung CC 2004 [8] | Prospective cohort study | Taiwan | HIV-1-infected patients | 508 | 37 | 8.8 | HR = 0.733 (95% CI: 0.236 to 2.274) | NA |
| Hyun 2023 [34] | Prospective cohort study | South Korea | Patients aged ≥ 19 years with CAP | 5009 | 70.3 | 63.1 | OR = 0.507 (95% CI: 0.267 to 0.961) | NA |
| Ihara 2019 [35] | Case-control study | Japan | Dialysis patients | 510 | 64 | 59.2 | HR = 0.62 (95% CI: 0.46 to 0.83) | HR = 0.91 (95% CI: 0.29 to 2.83) |
| Inoue S 2011 [9] | Prospective cohort study | Japan | Patients of chronic pulmonary diseases ≥ 60 years of age | 1378 | 73 | 58.4 | HR = 0.795 (95% CI: 0.499 to 1.264) | NA |
| Johnstone J 2010 [36] | Case-control study | Canada | Adults with CAP | 2950 | 68 | 52 | HR = 0.92 (95% CI: 0.79 to 1.06) | NA |
| Kolditz 2018 [10] | Retrospective cohort study | Germany | Individuals aged ≥ 65 years | 738,927 | 76 | 38 | Age 60 to 79 years: RR = 0.82 (95% CI: 0.73 to 0.91), Age ≥ 80 years: RR = 1.00 (95% CI: 0.94 to 1.07) | NA |
| Maruyama T 2010 [11] | RCT | Japan | Nursing home residents | 1006 | 84.7 | 22.1 | NA | NA |
| Ochoa-Gondar 2008 [12] | Prospective cohort study | Spain | Older adults with chronic respiratory diseases | 1298 | ≥ 75 | 73 | HR = 1.20 (95% CI: 0.91 to 1.59) | HR = 0.87 (95% CI: 0.33 to 2.28) |
| Ortqvist 1998 [37] | RCT | Sweden | Non-immunocompromised middle-aged and elderly people | 691 | 69·4 | 50 | RR = 0.95 (95% CI: 0.53 to 1.59) | NA |
| Pierre E 2023 [38] | Case-control study | France | Dialysis patients | 1849 | 68.5 | 64.7 | HR = 0.96 (95% CI: 0.78 to 1.19) | NA |
| Tsai YH 2015 [39] | Case-control study | Taiwan | Individuals aged ≥ 75 years | 1,078,955 | 81.67 | 52.16 | OR = 0.07 (95% CI: 0.069 to 0.072) | OR = 0.07 (95% CI: 0.059 to 0.082) |
Abbreviations CAP - Community-Acquired Pneumonia, CI - Confidence Interval, COPD - Chronic Obstructive Pulmonary Disease, HR - Hazard Ratio, NA - Not Available, NIDDM - Non-Insulin-Dependent Diabetes Mellitus, OR - Odds Ratio, RCT - Randomized Controlled Trial, RR - Relative Risk
All-cause mortality
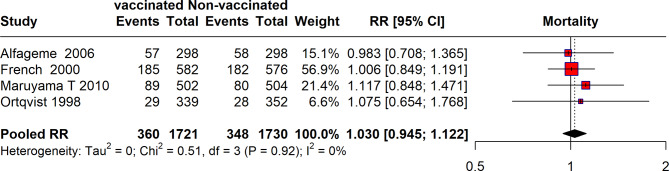
The meta-analysis of four RCTs reported the impact of vaccination on all-cause mortality. The analysis included data from studies conducted by Alfageme et al. [18], French et al. [33], Maruyama et al. [11], and Ortqvist et al. [37]. The pooled results from these four studies included a total of 360 events from 1,721 vaccinated participants and 348 events from 1,730 non-vaccinated participants, resulting in a pooled RR of 1.030 (95% CI: 0.945, 1.122). The heterogeneity among these studies was low, with an I² of 0%. Overall, the meta-analysis shows a pooled risk ratio slightly above 1, indicating no significant reduction in all-cause mortality among the vaccinated compared to the non-vaccinated groups across the studies analyzed (Fig. 2).
Fig. 2.
Forest plot depicting risk of all-cause mortality between vaccinated and unvaccinated Individuals from RCTs
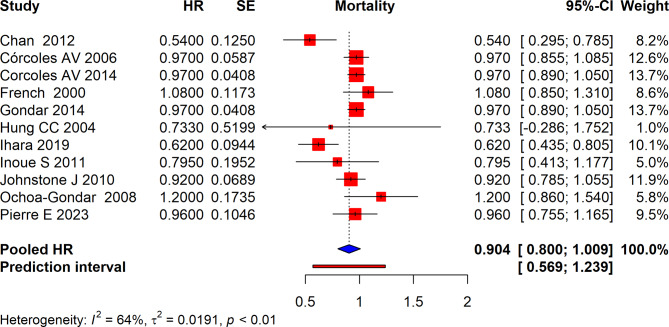
The meta-analysis of several studies assessed the effect of interventions on mortality which reported HR based on Cox regression. The pooled HR across all included studies was 0.904 (95% CI: 0.800 to 1.009) suggesting a non-significant reduction in mortality risk associated with the intervention. The prediction interval was [0.569, 1.239], indicating variability in the effect size across different settings and populations. The analysis showed high heterogeneity with an I² of 64% which suggests that the observed variability in HRs is not solely due to chance but may be influenced by differences in study design, populations, or interventions across the studies (Fig. 3).
Fig. 3.
Forest plot depicting pooled Hazard ratios for all-cause mortality with vaccination
Pneumonia related mortality
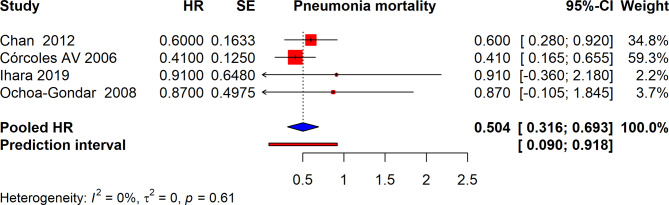
The meta-analysis summarized the impact of interventions on pneumonia-related mortality, evaluating hazard ratios (HR) from several studies. The pooled HR for these studies was 0.504 (95% CI: 0.316 to 0.693), indicating a significant reduction in pneumonia-related mortality across the included studies. The prediction interval ranged from 0.090 to 0.918, suggesting that in a new study, the actual HR might fall within this range. The heterogeneity among these studies was reported as I² = 0%, indicating negligible variability among the studies, and suggesting that the observed effects are consistent across different research settings and populations (Fig. 4).
Fig. 4.
Forest plot depicting pooled Hazard ratios for pneumonia-related mortality with vaccination
Publication bias
The assessment of publication bias in our meta-analysis was conducted using the Doi Plot and the LFK index. This approach is critical for identifying systematic discrepancies in the published literature concerning all-cause mortality outcomes associated with the interventions studied. The Doi Plot visualizes the relationship between the effect sizes of the included studies and their corresponding standard errors, providing a graphical representation of potential asymmetry in study reporting. Ideally, if no publication bias exists, the plot should display symmetry around the pooled effect estimate. However, our analysis revealed a significant deviation from this pattern. This asymmetry was quantitatively measured using the LFK index, which was calculated to be -2.25 for our dataset. An LFK index value outside the range of -1 to + 1, such as our finding of -2.25, indicates substantial asymmetry. This result suggests a significant presence of publication bias, potentially due to the underrepresentation of smaller studies or studies with non-significant, neutral, or negative results (See Fig. 5).
Fig. 5.
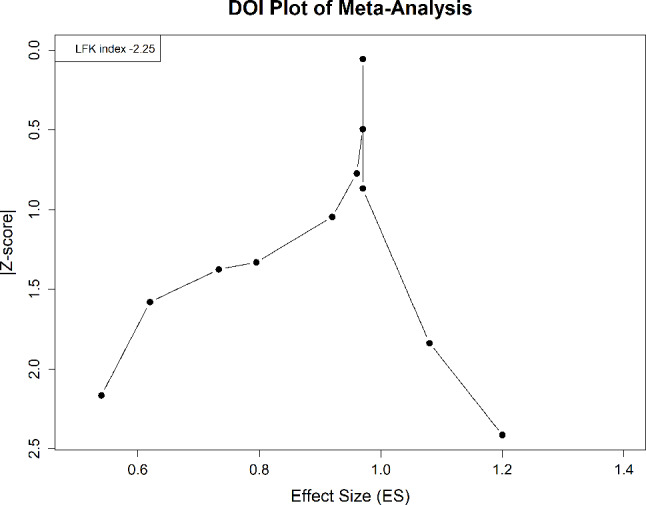
Forest plot depicting pooled Hazard ratios for pneumonia-related mortality with vaccination
Discussion
This systematic review and meta-analysis have evaluated the effectiveness of PPV23 in reducing all-cause and pneumonia-related mortality among adult populations globally. Our analysis included a diverse set of studies encompassing RCTs and observational studies from various geographical locations. The pooled results for all-cause mortality did not demonstrate a significant reduction among those vaccinated with PPV23. This finding is crucial as it suggests that while PPV23 may be effective in preventing invasive pneumococcal disease, its impact on reducing all-cause mortality is not pronounced as previously perceived. In contrast, the pooled analysis for pneumonia-related mortality showed a significant reduction in death rates among those receiving PPV23. This result indicates a more direct benefit of the vaccine in preventing deaths from pneumonia, particularly in older adults and those with pre-existing health conditions such as COPD or chronic renal failure. The substantial reduction in pneumonia-related mortality shows the importance of targeted vaccination programs within these high-risk groups.
Our analysis revealed moderate to high heterogeneity in the pooled estimates for all-cause mortality but found minimal heterogeneity in pneumonia-related mortality outcomes. This discrepancy may stem from differences in study populations, variations in vaccine administration protocols, or differences in healthcare settings across the included studies. For instance, higher heterogeneity in all-cause mortality could reflect the broader effects of various co-morbid conditions and lifestyle factors that are not directly influenced by pneumococcal vaccination. A prior systematic review indicated that both PCV13 and PPSV23 are effective in preventing vaccine-type invasive pneumococcal disease (VT-IPD) and vaccine-type pneumococcal pneumonia in adults [42]. Another meta-analysis demonstrated that the PPSV23 vaccination provides a significant protective benefit against invasive pneumococcal diseases (IPDs). However, this analysis did not show a significant impact of PPV23 on all-cause pneumonia or pneumococcal pneumonia [43].
Recent evidence shows the significant herd protection effects conferred by the widespread implementation of pneumococcal conjugate vaccines (PCVs) in national immunization programs (NIPs) for children since the 2000s [44]. Studies indicate that these effects extend beyond the pediatric population, impacting adult and elderly groups by reducing the circulation of vaccine-related serotypes (VRTs) and associated pneumococcal diseases [45, 46]. The reduction in pneumonia-related mortality among adults, as highlighted in our findings, may not solely be attributable to the direct effects of the PPV23. It is plausible that this reduction is also indirectly influenced by long-term PCV usage in children, which contributes to decreased transmission of virulent pneumococcal strains capable of causing severe disease in adults, particularly the elderly and immunocompromised. This phenomenon illustrates the broader, community-wide benefits of pediatric vaccination programs and supports the integration of both PPV23 and PCVs in comprehensive pneumococcal disease prevention strategies. By incorporating PCVs into childhood vaccination schedules, countries have effectively reduced the reservoir of pathogenic pneumococci, which might otherwise contribute to higher rates of morbidity and mortality in non-vaccinated adult populations. This discussion warrants further investigation into how pediatric vaccination impacts adult health outcomes and emphasizes the need for ongoing surveillance and research to optimize pneumococcal vaccination strategies across all age groups.
The findings from this review have significant implications for public health, especially concerning vaccination strategies in adults. While PPV23 shows limited efficacy in reducing all-cause mortality, its role in preventing pneumonia-related deaths is evident and supports continued use, particularly in populations at increased risk of severe pneumococcal infections. Health policymakers should consider these findings in the broader context of community health and resource allocation, emphasizing the need for targeted vaccination programs alongside other preventive health measures. Moreover, the introduction of PCVs and their effect on herd immunity might also influence the future role of PPV23, particularly in how both vaccines are integrated within national immunization schedules to maximize public health outcomes. Further research is needed to explore the long-term effectiveness of PPV23 in diverse populations, particularly in low and middle-income countries where the burden of pneumococcal disease is highest. Studies should also focus on evaluating the combined impact of PPV23 and PCVs in reducing mortality across different age groups and clinical conditions. Studies needed to assess how PPV23 can be delivered alongside other vaccines [47, 48]. Additionally, exploring patient-centered outcomes and quality of life post-vaccination could provide deeper insights into the benefits of pneumococcal vaccination beyond mortality reduction.
One of the key strengths of this study is its comprehensive and systematic approach to synthesizing existing data from a diverse range of RCTs and observational studies. Furthermore, the inclusion of a wide geographic and demographic variety of studies enhances the generalizability of the findings, making the conclusions relevant for public health strategies worldwide. Additionally, the critical assessment of publication bias and heterogeneity among the studies adds to the credibility and depth of the analysis, ensuring that the results reflect a balanced view of the available evidence.
This review has some limitations that may impact the interpretation and generalizability of its findings. The variability in study designs and participant demographics across the included studies introduces potential inconsistencies. These variations can affect the applicability of the results to broader populations, as different study designs and population characteristics may influence the outcomes of interest. Observational studies, which form a significant portion of the data included in this analysis, are inherently susceptible to biases. These biases can arise from several sources, including selection bias, recall bias, and confounding factors, which may compromise the validity of the findings. While our methodological approach was rigorous entailing a comprehensive literature search and a critical appraisal of studies to minimize errors the analysis is potentially limited by publication bias. This bias was suggested by the Doi Plot and LFK index, which indicated an underreporting of non-significant or negative outcomes. Such a bias could lead to an overestimation of the benefits of the interventions being assessed. The scope of this review was restricted to articles published in English, which may have excluded relevant studies published in other languages. This language limitation could potentially introduce bias, limiting the comprehensiveness of the analyzed data and affecting the overall conclusions drawn from the review. These limitations necessitate a cautious interpretation of the results, suggesting that further studies, particularly those addressing the identified biases and including a broader range of languages and study designs, are needed to provide a more definitive conclusion on the impact of the PPV23 vaccine.
Conclusion
While the PPV23 may not significantly impact all-cause mortality, our findings confirm its efficacy in reducing pneumonia-related mortality, particularly in high-risk adult populations. This indicates the importance of targeted vaccination programs, which should be strategically tailored to maximize health outcomes for vulnerable groups. Policy frameworks should be revised to reflect these findings, prioritizing vaccine distribution to populations that stand to benefit most, such as the elderly and immunocompromised, and incorporating these insights to optimize resource allocation. Additionally, future research should focus on conducting large-scale studies across varied demographic settings to refine vaccination strategies further, investigate the long-term effects of PPV23, and provide valuable information to guide ongoing public health decisions and vaccination strategies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors acknowledge the Nested-Knowledge, MN, USA for providing the access to the software.
Author contributions
Muhammed Shabil and Shilpa Gaidhane: Conceptualization, review and editing of drafts, Methodology. Suhas Ballal, Sanjay Kumar, and Mahakshit Bhat: Data Curation, Formal Analysis. Shilpa Sharma and M Ravi Kumar: Investigation, Supervision. Sarvesh Rustagi and Mahalaqua Nazli Khatib: Project Administration, Resources. Nishant Rai, Kiran Bhopte, Rachna Kathuria, Sanjit Sah, Ambanna Yapparpalvi, and Edward Mawejje: Software, Validation. Ganesh Bushi: Writing - Original Draft, Writing - Review & Editing.
Funding
This study received no funding.
Data availability
The data is with the authors and available on request.
Declarations
Ethical approval
Not required.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Muhammed Shabil and Shilpa Gaidhane contributed equally to this work.
Contributor Information
Mahalaqua Nazli Khatib, Email: nazlikhatib@dmiher.edu.in.
Edward Mawejje, Email: emawejje62@gmail.com.
References
- 1.Wahl B, O’Brien KL, Greenbaum A, Majumder A, Liu L, Chu Y, et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000–15. Lancet Global Health. 2018;6(7):e744–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dhoubhadel BG, Morimoto K. Prevention of pneumococcal diseases: the challenge remains. Lancet Global Health. 2022;10(10):e1375–6. [DOI] [PubMed] [Google Scholar]
- 3.Kumar P, Neyazi A, Rillera Marzo R, Augusto Guimaraes C, Barboza J, Abu Serhan J. H, Mycoplasma pneumoniae returns: understanding its spread and growing impact. Evid. 2024;2(1).
- 4.Savoy M, Pneumococcal Vaccine MSD, Manual Professional, Edition. MSD Manual; 2024 [updated 2024/04. https://www.msdmanuals.com/en-in/professional/infectious-diseases/immunization/pneumococcal-vaccine
- 5.Lawrence H, Pick H, Baskaran V, Daniel P, Rodrigo C, Ashton D, et al. Effectiveness of the 23-valent pneumococcal polysaccharide vaccine against vaccine serotype pneumococcal pneumonia in adults: a case-control test-negative design study. PLoS Med. 2020;17(10):e1003326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan T-C, Hung IF-N, Luk JK-H, Shea Y-F, Chan FH-W, Woo PC-Y, Chu L-W. Prevention of mortality and pneumonia among nursing home older adults by dual pneumococcal and seasonal influenza vaccination during a pandemic caused by novel pandemic influenza A (H1N1). J Am Med Dir Assoc. 2012;13(8):698–703. [DOI] [PubMed] [Google Scholar]
- 7.Hsieh M-J, Tsai Y-H, Chang C-J, Wen Y-W, Hu H-C, Chao Y-N, et al. The efficacy of 23-valent pneumococcal polysaccharide vaccine in preventing pneumonia and invasive pneumococcal disease in the elderly aged 75 years and older in Taiwan. Chest. 2013;144(4):254A. [Google Scholar]
- 8.Hung C-C, Chen M-Y, Hsieh S-M, Hsiao C-F, Sheng W-H, Chang S-C. Clinical experience of the 23-valent capsular polysaccharide pneumococcal vaccination in HIV-1-infected patients receiving highly active antiretroviral therapy: a prospective observational study. Vaccine. 2004;22(15–16):2006–12. [DOI] [PubMed] [Google Scholar]
- 9.Inoue S, Watanuki Y, Kaneko T, Sato T, Miyazawa N, Kaneko T, et al. Heterogeneity of the efficacy of the 23-valent pneumococcal polysaccharide vaccine caused by various underlying conditions of chronic pulmonary disease in older patients: prospective cohort study. BMJ open. 2011;1(1):e000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kolditz M, Schmitt J, Pletz MW, Tesch F. Impact of pneumococcal polysaccharide vaccine on incidence and mortality after pneumonia in adults aged ≥ 60 years—a population-based retrospective cohort study. Clin Microbiol Infect. 2018;24(5):500–4. [DOI] [PubMed] [Google Scholar]
- 11.Maruyama T, Taguchi O, Niederman MS, Morser J, Kobayashi H, Kobayashi T et al. Efficacy of 23-valent pneumococcal vaccine in preventing pneumonia and improving survival in nursing home residents: double blind, randomised and placebo controlled trial. BMJ. 2010;340. [DOI] [PMC free article] [PubMed]
- 12.Ochoa-Gondar O, Vila-Corcoles A, Ansa X, Rodriguez-Blanco T, Salsench E, de Diego C, et al. Effectiveness of pneumococcal vaccination in older adults with chronic respiratory diseases: results of the EVAN-65 study. Vaccine. 2008;26(16):1955–62. [DOI] [PubMed] [Google Scholar]
- 13.Belmonti S, Rossetti B, Modica S, Paglicci L, Borghetti A, Ciccullo A, et al. Long-term serological response to 13-valent pneumococcal conjugate vaccine versus 23-valent polysaccharide vaccine in HIV-infected adults. Infect Dis Therapy. 2019;8:453–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ravichandran S, Erra-Diaz F, Karakaslar OE, Marches R, Kenyon-Pesce L, Rossi R, et al. Distinct baseline immune characteristics associated with responses to conjugated and unconjugated pneumococcal polysaccharide vaccines in older adults. Nat Immunol. 2024;25(2):316–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bukhanova D, Sergeeva M, Belov B, Tarasova G, Cherkasova M, Muraviev YA, et al. Immunogenicity and efficiency of a 23-valent pneumococcal vaccine in patients with rheumatoid arthritis: results of a 5-year follow up study. Mod Rheumatol J. 2018;12(4):85–8. [Google Scholar]
- 16.Shimbashi R, Suzuki M, Chang B, Watanabe H, Tanabe Y, Kuronuma K, et al. Effectiveness of 23-valent pneumococcal polysaccharide vaccine against invasive pneumococcal disease in adults, Japan, 2013–2017. Emerg Infect Dis. 2020;26(10):2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Azuma M, Oishi K, Akeda Y, Morino S, Motoki Y, Hanibuchi M, Nishioka Y. Safety and immunogenicity of sequential administration of PCV13 followed by PPSV23 in pneumococcal vaccine-naïve adults aged ≥ 65 years: comparison of booster effects based on intervals of 0.5 and 1.0 year. Vaccine. 2023;41(5):1042–9. [DOI] [PubMed] [Google Scholar]
- 18.Alfageme I, Vazquez R, Reyes N, Muñoz J, Fernández A, Hernández M, et al. Clinical efficacy of anti-pneumococcal vaccination in patients with COPD. Thorax. 2006;61(3):189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsieh M-J, Yu C-C, Hu H-C, Thai Y-H. No preventive effects of 23-valent pneumococcal polysaccharide vaccine in patients with chronic renal failure. Eur Respiratory Soc; 2016.
- 20.Ochoa-Gondar O, Vila-Corcoles A, Rodriguez-Blanco T, de Diego-Cabanes C, Hospital-Guardiola I, Jariod-Pamies M, Group ER. Evaluating the clinical effectiveness of pneumococcal vaccination in preventing myocardial infarction: the CAPAMIS study, three-year follow-up. Vaccine. 2014;32(2):252–7. [DOI] [PubMed] [Google Scholar]
- 21.Verma R, Khanna P. Pneumococcal conjugate vaccine: a newer vaccine available in India. Hum Vaccines Immunotherapeutics. 2012;8(9):1317–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wasserman M, Chapman R, Lapidot R, Sutton K, Dillon-Murphy D, Patel S, et al. Twenty-year public health impact of 7-and 13-valent pneumococcal conjugate vaccines in US children. Emerg Infect Dis. 2021;27(6):1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372. [DOI] [PMC free article] [PubMed]
- 24.Shabil M, Khatib MN, Banda GT, Zahiruddin QS, Ballal S, Bansal P, et al. Effectiveness of early Anakinra on cardiac function in children with multisystem inflammatory syndrome of COVID-19: a systematic review. BMC Infect Dis. 2024;24(1):847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shabil M, Khatib MN, Zahiruddin QS, Rekha M, Kaur M, Rani B, et al. Dengue infection during pregnancy and adverse birth outcomes: a systematic review and Meta-analysis. Rev Med Virol. 2024;34(5):e2582. [DOI] [PubMed] [Google Scholar]
- 26.Langan D, Higgins JP, Jackson D, Bowden J, Veroniki AA, Kontopantelis E, et al. A comparison of heterogeneity variance estimators in simulated random-effects meta‐analyses. Res Synthesis Methods. 2019;10(1):83–98. [DOI] [PubMed] [Google Scholar]
- 27.Gandhi AP, Shamim MA, Padhi BK. Steps in undertaking meta-analysis and addressing heterogeneity in meta-analysis. Evid. 2023;1(1):44–59. [Google Scholar]
- 28.Shabil M, Yadav A, Shamim MA, Ahmed M, Satapathy P, Zaidan AA, et al. Prevalence of hepatitis B and C infections among HIV-positive men who have sex with men: a systematic review and meta‐analysis. Health Sci Rep. 2024;7(6):e2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shabil M, Bushi G, Khatib MN. A commentary on psychological health among healthcare professionals during COVID-19 pandemic: an updated meta-analysis. Indian J Psychiatry. 2024;66(8):763–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.IntHout J, Ioannidis JP, Rovers MM, Goeman JJ. Plea for routinely presenting prediction intervals in meta-analysis. BMJ open. 2016;6(7):e010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pandey P, Shabil M, Bushi G. Comment on Sodium fluorescein and 5-aminolevulinic acid fluorescence-guided biopsy in brain lesions: a systematic review and meta-analysis. J Neurooncol. 2024:1–2. [DOI] [PubMed]
- 32.Wen X, Thomas MA, Liu L, Moe AA, Duong PH, Griffiths ME, Munlyn AL. Association between maternal e-cigarette use during pregnancy and low gestational weight gain. Int J Gynaecol Obstet. 2023;162(1):300–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.French N, Nakiyingi J, Carpenter L, Lugada E, Watera C, Moi K, et al. 23–valent pneumococcal polysaccharide vaccine in HIV-1-infected Ugandan adults: double-blind, randomised and placebo controlled trial. Lancet. 2000;355(9221):2106–11. [DOI] [PubMed] [Google Scholar]
- 34.Hyun H, Jang A-Y, Suh JW, Bae I-G, Choi WS, Seo YB et al. Community-acquired pneumococcal pneumonia in highly vaccinated Population: analysis by Serotypes, Vaccination Status, and Underlying Medical conditions. J Korean Med Sci. 2023;38(42). [DOI] [PMC free article] [PubMed]
- 35.Ihara H, Kikuchi K, Taniguchi H, Fujita S, Tsuruta Y, Kato M, et al. 23-valent pneumococcal polysaccharide vaccine improves survival in dialysis patients by preventing cardiac events. Vaccine. 2019;37(43):6447–53. [DOI] [PubMed] [Google Scholar]
- 36.Johnstone J, Eurich DT, Minhas JK, Marrie TJ, Majumdar SR. Impact of the pneumococcal vaccine on long-term morbidity and mortality of adults at high risk for pneumonia. Clin Infect Dis. 2010;51(1):15–22. [DOI] [PubMed] [Google Scholar]
- 37.Örtqvist Å, Hedlund J, Burman L-Å, Elbel E, Höfer M, Leinonen M, et al. Randomised trial of 23-valent pneumococcal capsular polysaccharide vaccine in prevention of pneumonia in middle-aged and elderly people. Lancet. 1998;351(9100):399–403. [DOI] [PubMed] [Google Scholar]
- 38.Pierre É, Pladys A, Bayat-Makoei S, Tattevin P, Vigneau C. Pneumococcal vaccination coverage at the initiation of chronic dialysis, and its association with mortality during the first year. Vaccine. 2023;41(24):3655–62. [DOI] [PubMed] [Google Scholar]
- 39.Tsai Y-H, Hsieh M-J, Chang C-J, Wen Y-W, Hu H-C, Chao Y-N, et al. The 23-valent pneumococcal polysaccharide vaccine is effective in elderly adults over 75 years old—Taiwan’s PPV vaccination program. Vaccine. 2015;33(25):2897–902. [DOI] [PubMed] [Google Scholar]
- 40.Vila-Córcoles A, Ochoa-Gondar O, Hospital I, Ansa X, Vilanova A, Rodríguez T, et al. Protective effects of the 23-valent pneumococcal polysaccharide vaccine in the elderly population: the EVAN-65 study. Clin Infect Dis. 2006;43(7):860–8. [DOI] [PubMed] [Google Scholar]
- 41.Vila-Corcoles A, Ochoa-Gondar O, Rodriguez-Blanco T, de Diego-Cabanes C, Satue-Gracia E, Vila-Rovira A, et al. Evaluating clinical effectiveness of pneumococcal vaccination in preventing stroke: the CAPAMIS Study, 3-year follow-up. J Stroke Cerebrovasc Dis. 2014;23(6):1577–84. [DOI] [PubMed] [Google Scholar]
- 42.Farrar JL, Childs L, Ouattara M, Akhter F, Britton A, Pilishvili T, Kobayashi M. Systematic review and Meta-analysis of the efficacy and effectiveness of Pneumococcal vaccines in adults. Pathogens. 2023;12(5):732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Latifi-Navid H, Latifi-Navid S, Mostafaiy B, Jamalkandi SA, Ahmadi A. Pneumococcal disease and the effectiveness of the PPV23 vaccine in adults: a two-stage bayesian meta-analysis of observational and RCT reports. Sci Rep. 2018;8(1):11051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Werkhoven CH. Herd effects of child vaccination with pneumococcal conjugate vaccine against pneumococcal non-invasive community-acquired pneumonia: what is the evidence? Hum Vaccin Immunother. 2017;13(5):1177–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weinberger B. Vaccination of older adults: Influenza, pneumococcal disease, herpes zoster, COVID-19 and beyond. Immun Ageing. 2021;18(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palmborg A, Skovdal M, Molden T, Åhman H, Chen L, Banefelt J. Invasive pneumococcal disease among the elderly in the later era of paediatric pneumococcal conjugate vaccination-A longitudinal study over 10 years based on public surveillance data in the nordics. PLoS ONE. 2023;18(6):e0287378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gupta S, Basu S, Banerjee R. Efficacy of single-dose HPV vaccine over no vaccination and standard multiple dose regimen: a systematic review and meta-analysis of available evidence. Evid. 2024;2(2).
- 48.Bonanni P, Steffen R, Schelling J, Balaisyte-Jazone L, Posiuniene I, Zatoński M, Van Damme P. Vaccine co-administration in adults: an effective way to improve vaccination coverage. Hum Vaccines Immunotherapeutics. 2023;19(1):2195786. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data is with the authors and available on request.