Abstract
Purpose
Multiple sclerosis (MS) is a neurological disorder affecting almost 2.8 million people globally, approximately 80–85% of whom have the relapsing-remitting form of the disease (RRMS). There are several autoinjectors available for the administration of injectable disease-modifying therapies for the treatment of MS. The objective of the current study was to gain an understanding of factors related to patients’ and nurses’ autoinjector preferences, and to evaluate two autoinjectors for glatiramer acetate (MyJECT™ and CSYNC™) against those preferences.
Patients and Methods
Patients with RRMS and nurses experienced in training patients with an autoinjector were recruited from 12 health centers in Germany. Surveys were administered to patients and nurses and their answers to 13 questions over five categories (participants’ characteristics, important autoinjector attributes, autoinjector performance, satisfaction with the autoinjector devices and demographics) were scored, where appropriate, using a 5-point Likert scale.
Results
A total of 15 patients and 15 nurses were included in the study. Overall, the top four most important attributes, for both nurses and patients, were ease of handling, ability to use independently, ease of gripping the autoinjector and ease of self-injection. MyJECT™ received a mean score of at least 4.5 (out of 5) on more attributes than CSYNC™ and satisfaction with both autoinjectors was high.
Conclusion
Nurses and patients with RRMS were highly satisfied with both the MyJECT™ and CSYNC™ autoinjectors, with scores suggesting that MyJECT™ performs better on the attributes they identified as most important. All patients currently using the MyJECT™ were likely or highly likely to recommend it to another patient with RRMS.
Keywords: relapsing-remitting multiple sclerosis, disease-modifying therapies, glatiramer acetate, autoinjector, CSYNC™, MyJECT™
Plain Language Summary
Multiple sclerosis is a disorder of the brain and spinal cord that affects almost 3 million people worldwide. Some of the multiple sclerosis treatments may need patients to inject the medicine at regular intervals. There are several different injection devices to administer the drugs for the management of multiple sclerosis, these are called autoinjectors. MyJECT™ and CSYNC™ are two different autoinjectors that patients can use with a drug called glatiramer acetate.
The current study was done to look at what features of autoinjectors do patients and nurses think are most important. To do this, a survey was completed among “multiple sclerosis nurses” and “patients with a type of multiple sclerosis called relapsing-remitting multiple sclerosis” who visited one of 12 health centers in Germany. The survey had 13 questions covering patient background, important features of autoinjectors, autoinjector performance, satisfaction with the autoinjector, and patient demographics (age, gender and education).
Overall, 15 nurses and 15 patients took part. Both nurses and patients answered that the top four features of autoinjectors were ease of handling, ability to use independently, ease of gripping the autoinjector and ease of self-injection. The MyJECT™ autoinjector was given an average score of at least 4.5 out of 5 more often than the CSYNC™ autoinjector, although patients and nurses had high levels of satisfaction with both types of autoinjector.
The overall conclusion was that multiple sclerosis nurses and patients were highly satisfied with both the MyJECT™ and CSYNC™ autoinjectors, with higher scores for MyJECT™ for features considered to be most important. All patients currently using MyJECT™ were likely to recommend it to another patient with relapsing-remitting multiple sclerosis.
Introduction
Multiple sclerosis (MS) is one of the most common neurological disorders and a leading cause of nontraumatic neurologic disability in young adults.1 The disease is characterized by patchy loss of the myelin sheath that surrounds nerve fibers, that can be visualized as plaques or lesions in the brain and spinal cord on imaging scans.2 In 2020, MS was estimated to affect almost 2.8 million people worldwide (35.9 per 100,000 people) and to affect at least twice as many females as males.3 MS has a substantial influence on physical, financial, and psychological well-being, as well as the quality of life of both patients and caregivers.4,5 The most prevalent MS subtype (80%–85%) is relapsing–remitting multiple sclerosis (RRMS), which is characterised by flare-ups and remissions.6 Around 10–20% of MS patients can be classified as having a primary progressive or progressive relapsing course, and most untreated patients with RRMS eventually progress to secondary progressive MS.6 While some research points to viral infections, genetic predisposition, reduced sunlight exposure/reduced blood levels of vitamin D, smoking, and obesity as possible underlying contributing factors, the etiology and risk factors of RRMS have not been completely elucidated.7,8
Therapy for MS can be divided into treatments for management of acute relapses, disease-modifying treatments (DMTs) and symptomatic management.9 DMTs in MS are treatments that help to slow or prevent disease progression.10 Glatiramer acetate, administered by subcutaneous (SC) injection, is one of the longest established DMTs for MS, and has been shown to significantly reduce the annual rate of relapse and to decrease MRI-assessed disease activity and burden, compared with placebo.11 SC administration of biotherapeutics can be a valuable option, as it is generally preferred over intravenous administration by both patients and healthcare providers, and is associated with reduced drug-delivery-related healthcare costs and resource use.12
Glatiramer acetate is a first-line DMT for the treatment of RRMS and has been approved for self-administration by SC injection in both the US and Europe for over 20 years.13–15 Since these initial approvals, more recent data for glatiramer acetate, an immunomodulating agent, has shown that glatiramer acetate (40 mg) given three times per week is effective, safe, and well-tolerated for the treatment of RRMS,16 and this low-frequency regime has been approved in the US since 201417 and in Europe since 2015.13
Injectable DMTs can be administered either by manual injection using a needle and syringe or by using an injection device (autoinjector).18 Many patients with MS have concerns associated with self-injection, such as needle phobia, with such patients less likely to be receiving treatment for their MS than those patients without injection concerns.19 Newer technologies that help these patients to overcome these concerns include the autoinjectors, which have been designed to improve the convenience and safety of self-injection and to reduce pain.20 In addition to their utility in overcoming injection concerns, autoinjector devices can be used by those who have not been medically trained and can help to simplify RRMS therapy, often providing additional features to improve patient comfort and adherence, such as dose reminder functions.21,22 These autoinjectors vary, with some employing a simple push-on-the-skin mechanism and others that are completely automated with button-activated technology.21,22
There are a variety of autoinjectors available for the administration of injectable MS medications, including the glatiramer acetate autoinjectors CSYNC™ by TEVA Pharmaceuticals23 and MyJECT™ by Mylan (now Viatris).24 Although there are several publications comparing various autoinjectors,25–28 largely confirming that the autoinjectors provide multiple benefits to patients in terms of their functionalities, little is known about the factors that patients and nurses consider most important for autoinjectors. Therefore, to address this knowledge gap, we conducted a survey of nurses and patients in Germany to determine the most important attributes of autoinjectors in RRMS, both from a patient perspective and from a nurse perspective, and to compare these characteristics and preferences between MyJECT™ and CSYNC™ autoinjectors.
Materials and Methods
Participants
Patients were recruited for this cross-sectional qualitative study from 12 MS centers in Germany (Bavaria, Baden-Württemberg, Hessen, North Rhine-Westphalia, Thuringia, Berlin, Brandenburg, Niedersachsen, Bayern, Hamburg, Schleswig-Holstein, Sachsen) between August and October 2022. Eligible patients were those who had visited the MS centres in the preceding month who had a diagnosis of RRMS and had been using an autoinjector for at least 2 months and who consented to participate in the survey; recruitment was stopped once a sample of 15 patients was obtained. Nurses included were those who volunteered to take part in the study and who had experience with the handling of the CSYNC™ or MyJECT™ autoinjectors and had at least three years’ experience working in MS centers, who had spent 80% of their time in clinical practice, and had experience in training patients on between two and six different autoinjectors.
Study Design
Standardized interviews and discussion guides were created separately for patients and for nurses and utilized to collect information for this study; these were first pilot tested with different healthcare providers. The discussion guide included 13 questions, split into five categories: (i) participants’ characteristics; (ii) important attributes of an autoinjector device; (iii) performance of the autoinjector devices on prompted attributes; (iv) satisfaction with the autoinjector devices; and (v) demographic information (Tables 1 and 2). Apart from questions on demographic information, all items were scored on a 5-point Likert scale, with 1 being the lowest score and 5 the highest (scores were categorized as 1: very unsatisfied and 5: very satisfied or 1: very poor and 5: very good or 1: very unimportant and 5: very important).
Table 1.
Discussion Guide for Nurses
| Sections | Questions | Scale of (1–5) |
|---|---|---|
| Background | Tell us about yourself. | Open-ended question |
| How long have you been managing patients with RRMS? | Open-ended question | |
| Between MyJECTTM and CSYNCTM, which autoinjector have you used for RRMS? How long have you been using autoinjectors on patients with RRMS? |
Open-ended question | |
| How long you have been delivering training on autoinjectors to patients with RRMS? | Open-ended question | |
| Important attributes of an autoinjector | What attributes are important to you for evaluating a new autoinjector for RRMS? E.g.- Ease of handling, ability to use independently, ease of preparing the injection, ease of self-injection, ease of gripping the autoinjector, fit of autoinjector, is intuitive to use, injection needle is concealed, locking mechanism, audible feedback after successful injection, thickness of needle, adjustable needle depth, length of needle, position of injection button, time taken for the entire injection process, visual feedback, size of autoinjector, shape of autoinjector, weight of autoinjector, attractive design. |
1 is “very unimportant” and 5 is “very important” |
| Performance of the autoinjector | What are the key strengths and weaknesses of both autoinjectors? E.g.- Ease of handling, ability to use independently, ease of preparing the injection, ease of self-injection, ease of gripping the autoinjector, fit of autoinjector, is intuitive to use, injection needle is concealed, locking mechanism, audible feedback after successful injection, thickness of needle, adjustable needle depth, length of needle, position of injection button, time taken for the entire injection process, visual feedback, size of autoinjector, shape of autoinjector, weight of autoinjector, attractive design. |
1 is “very poor” and 5 is “very good” |
| Satisfaction with the autoinjector | Which characteristics of the autoinjector do you find satisfying? E.g.- Time taken for the entire injection process, ease of use of autoinjector, number of steps it takes to complete injection, convenience of storing the autoinjector and overall experience of holding the autoinjector. |
1 is “very unsatisfied” and 5 is “very satisfied” |
| Likelihood to recommend autoinjector | How likely are you to recommend your current autoinjector to other nurses? | 1 is “very unlikely” and 5 is “very likely” |
| Demographic | How old are you? | Open ended question |
| What is your gender? | Male, female, prefer not to say | |
| What is your highest education level? | Secondary school, Undergraduate degree, Postgraduate degree, other |
Abbreviation: RRMS, relapsing-remitting multiple sclerosis.
Table 2.
Discussion Guide for Patients
| Sections | Questions | Scale of (1–5) |
|---|---|---|
| Background | Tell us about yourself. What was your age when you were diagnosed with RRMS? | Open-ended question |
| Which prescription medication are you currently using for RRMS? | Open-ended question | |
| Which of the two autoinjectors between MyJECTTM and CSYNCTM do you now use to manage your RRMS? How long have you been using an autoinjector on average? | Open-ended question | |
| Have you used any other autoinjector for managing your RRMS in the past? How long did you use it for approximately? | Open-ended question | |
| Important attributes of an autoinjector | What attributes are important to you for evaluating a new autoinjector for RRMS? E.g.- Ease of handling, ability to use independently, ease of preparing the injection, ease of self-injection, ease of gripping the autoinjector, fit of autoinjector, is intuitive to use, injection needle is concealed, locking mechanism, audible feedback after successful injection, thickness of needle, adjustable needle depth, length of needle, position of injection button, time taken for the entire injection process, visual feedback, size of autoinjector, shape of autoinjector, weight of autoinjector, attractive design. |
1 is “very unimportant” and 5 is “very important” |
| Performance of the autoinjector | What are the key strengths and weaknesses of both the autoinjectors? E.g.- Ease of handling, ability to use independently, ease of preparing the injection, ease of self-injection, ease of gripping the autoinjector, fit of autoinjector, is intuitive to use, injection needle is concealed, locking mechanism, audible feedback after successful injection, thickness of needle adjustable needle depth, length of needle, position of injection button, time taken for the entire injection process, visual feedback, size of autoinjector, shape of autoinjector, weight of autoinjector, attractive design. |
1 is “very poor” and 5 is “very good” |
| Satisfaction with the autoinjector | Which attributes of the autoinjector do you find satisfying? E.g.- Time taken for the entire injection process, ease of use of the autoinjector, convenience of storing the autoinjector, number of steps it takes to complete injection, overall experience of holding of autoinjector. |
1 is “very unsatisfied” and 5 is “very satisfied” |
| Likelihood to recommend the autoinjector | How likely are you to suggest your current autoinjector to another patient? | 1 is “very unlikely” and 5 is “very likely” |
| Demographic | How old are you? | Open-ended question |
| What is your gender? | Male, female, prefer not to say | |
| What is your highest education level? | Secondary school, Undergraduate degree, Postgraduate degree, other |
Abbreviation: RRMS, relapsing-remitting multiple sclerosis.
The aim of the study was clearly explained to each participant and 45-minute in-depth semi-structured virtual interviews were held with each participant; all explanations and interviews were conducted by the same researcher. The interview guide was sufficiently flexible to allow participants to introduce issues that they deemed important or relevant.
Statistical Analysis
Interviews were audio-recorded, transcribed and quality-checked by one researcher. The interview transcripts were then analyzed to find themes and a descriptive analysis was conducted. Following the scoring, a quantitative analysis was performed by calculating the mean value for each question/item. Attributes that scored a mean rating of at least 4.5 were considered the most important attributes.
Results
Baseline Demographics/Clinical Characteristics
The study included 15 female patients with a mean ± standard deviation (SD) age of 42.93 ± 8.3 years, and 15 nurses (13 females) with a mean ± SD age of 47.13 ± 10.58 years (Table 3). Of these 15 patients, eight were using the MyJECT™ autoinjector and seven were using the CSYNC™ device; three of the patients currently using the MyJECT™ autoinjector had previously used a CSYNC™ autoinjector. All 15 included nurses had experience with the CSYNC™ autoinjector, 11 of whom also had experience using the MyJECT™ autoinjector.
Table 3.
Baseline Demographics and Clinical Characteristics
| Characteristic | Nurses (N=15) | Patients (N=15) |
|---|---|---|
| Gender | ||
| Female | 13 | 15 |
| Male | 2 | |
| Age (years) | ||
| 30–40 | 4 | 7 |
| 41–50 | 5 | 6 |
| 51–60 | 4 | 1 |
| 61–70 | 2 | 1 |
| Autoinjector: experience (nurses), current (patients) | ||
| MyJECTTM | 11 | 8 |
| CSYNCTM | 15 | 7 |
| Location of center | ||
| Bavaria | – | 3 |
| Baden-Württemberg | 3 | 4 |
| Hessen | 3 | 1 |
| North Rhine-Westphalia | 1 | 2 |
| Thuringia | - | 1 |
| Berlin | 2 | 2 |
| Brandenburg | - | 1 |
| Niedersachsen | - | 1 |
| Bayern | 2 | - |
| Hamburg | 1 | - |
| Schleswig-Holstein | 1 | - |
| Sachsen | 2 | - |
Most Important Attributes of Autoinjectors
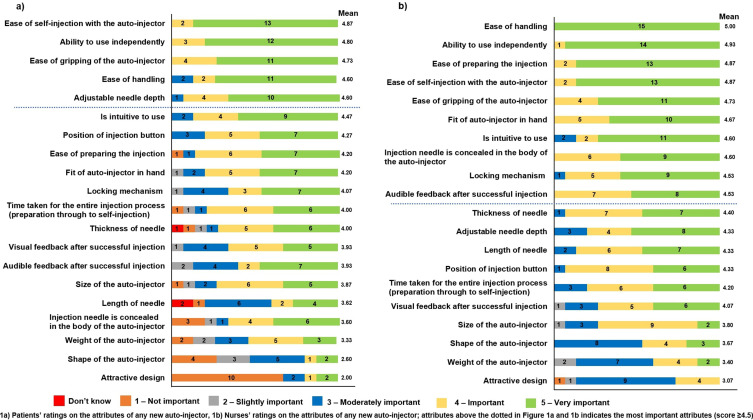
Patient scores for all 20 attributes of autoinjectors are shown in Figure 1a, with the top five most important attributes for patients found to be ease of self-injection (mean score on the 5-point Likert scale of 4.87), ability to use independently (4.80), ease of gripping of the autoinjector (4.73), ease of handling (4.60), and adjustable needle depth (4.60). Patients commented that these usability attributes were important for them, especially given the likelihood of progressive disease.
Figure 1.
Patients’ & Nurses’ ratings on the attributes of any new autoinjector. (1a), by patients; (1b), by nurses.
Four of these top five attributes were also in the top five for nurses (Figure 1b): ease of handling (5.00), ability to use independently (4.93), ease of gripping the autoinjector (4.73), and ease of self-injection with the autoinjector (4.87), with the other attribute in the top five for nurses being ease of preparing the injection (4.87), with adjustable needle depth further down the list (4.33).
The lowest scoring autoinjector attribute for both patients and nurses was for an attractive design, rated 3.07 by nurses and 2.00 by patients. One patient (using the MyJECT™ autoinjector) said “for me, the administration is more important and that it fits well in the hand but what it looks like is unimportant for me. It’s only me who sees it. It is more important that the administration is premium or highly developed”. One nurse noted that an attractive design may be more important for younger patients.
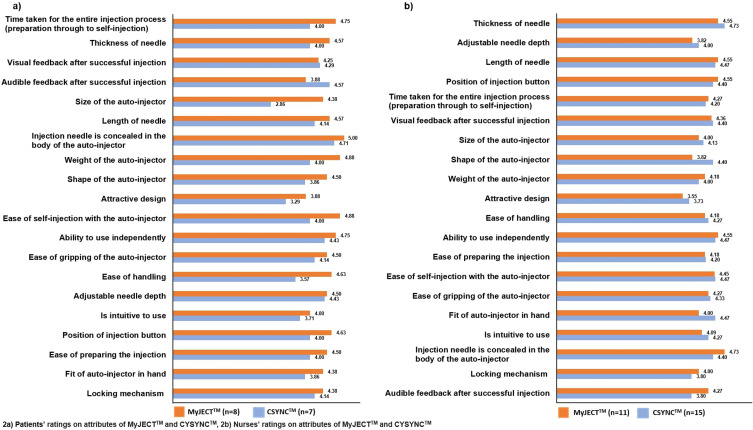
Autoinjector Performance
Figure 2 shows how patients (Figure 2a) and nurses (Figure 2b) rate each autoinjector on each of the 20 attributes. For patients, of the 20 attributes, the highest rated attributes of the MyJECT™ were injection needle is concealed in the body of the autoinjector (5.00), ease of self-injection with the autoinjector (4.88), weight of the autoinjector (4.88), time taken for the entire injection process (preparation through to self-injection; 4.75), ability to use independently (4.75), position of injection button (4.63), thickness of needle (4.57), length of needle (4.57), ease of gripping of the autoinjector (4.50), adjustable needle depth (4.50) and shape of the autoinjector (4.50); all other attributes received a mean score of less than 4.5. In contrast, for CYNC™ only injection needle is concealed in the body of the autoinjector (4.71) and audible feedback after successful injection (4.57) received a rating above 4.5. For the attributes of autoinjectors that patients rated as most important, as described above and shown in Figure 1a, patient ratings were consistently higher for MyJECT™ than CSYNC™ (Figure 2a) with scores for ease of self-injection of 4.88 and 4, respectively, for ability to use independently of 4.75 and 4.43, respectively, for ease of gripping of the autoinjector of 4.5 and 4.14, respectively, for ease of handling of 4.63 and 3.57, respectively, and for adjustable needle depth of 4.5 and 4.43, respectively.
Figure 2.
Patients’ and Nurses’ ratings on attributes of MyJECTTM and CSYNCTM,(2a), by patients; (2b), by nurses.
For nurses, the highest rated attributes of MyJECT™ (those that had a mean score of at least 4.5 on the 5-point Likert scale) were injection needle is concealed in the body of the autoinjector (mean score 4.73), ability to use independently (4.55), position of injection button (4.55), thickness of needle (4.55) and length of needle (4.55). The only attribute of the CSYNC™ autoinjector that had a mean score of at least 4.5 was thickness of needle (4.73). Scores for the attributes of autoinjectors considered by nurses to be the most important, as described above and shown in Figure 1b, were comparable between MyJECT™ and CSYNC™ (Figure 2b), with scores for ease of handling of 4.18 and 4.27, respectively, for ability to use independently of 4.55 and 4.47, respectively, for ease of preparing the injection of 4.18 and 4.20, respectively, for ease of self-injection with the autoinjector of 4.45 and 4.47, respectively and for ease of gripping the autoinjector of 4.27 and 4.33, respectively.
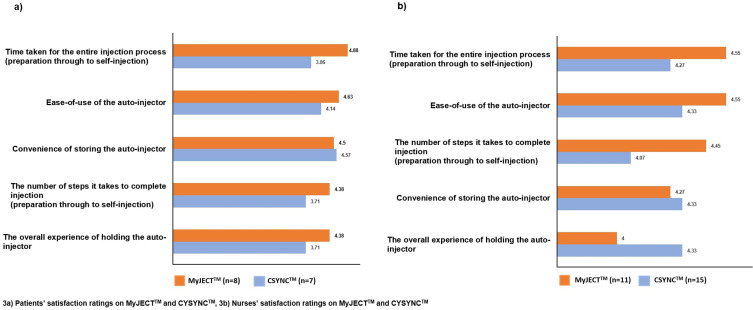
Satisfaction with Each Autoinjector
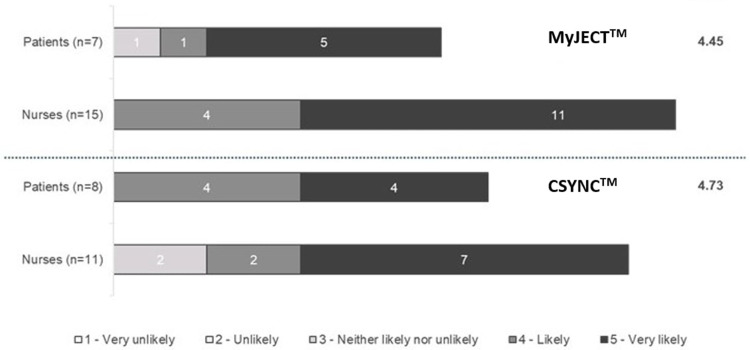
For the five questions used to determine satisfaction with each of the autoinjectors, mean patient scores were higher for MyJECT™ than CSYNC™ for all measures (Figure 3a). Results were similar for nurses (Figure 3b), although the differences in ratings between the two autoinjectors were smaller than for patients, and nurses rated both the convenience of storing the autoinjector and the overall experience of holding the autoinjector higher for CSYNC™ than for MyJECT™ (4.33 vs 4.27 and 4.33 vs 4.00, respectively); all other ratings were higher for MyJECT™. The likelihood of patients and nurses recommending each of the autoinjectors to another patient with RRMS or nurse had mean scores of 4.45 and 4.50, respectively for MyJECT™, compared with 4.73 and 4.57, respectively for CSYNC™. However, when looking at the individual scores, five of seven patients and 11 of 15 nurses reported that they were very likely to recommend MyJECT™ compared with four of eight patients and seven of 11 nurses stating that they were very likely to recommend CSYNC™ (Figure 4).
Figure 3.
Patients’ satisfaction ratings on MyJECTTM and CSYNCTM. (3a), by patients; (3b), by nurses.
Figure 4.
Likelihood of recommending each autoinjector to another nurse or another patient with relapsing remitting multiple sclerosis.
Discussion
Concerns associated with injections, as well as physical limitations make treatment adherence to parenterally administered DMTs challenging for many patients. The use of autoinjectors provides functional and emotional benefits for these patients and hence may improve patient adherence to MS treatment. In our study, nurses and patients were aligned on the four most important attributes of autoinjectors for the management of RRMS, namely, ease of handling, ability to use independently, ease of self-injection with the autoinjector and ease of gripping the autoinjector – all attributes associated with usability. This focus on usability is similar to the findings of a study in patients with rheumatoid arthritis and associated healthcare providers, in which patients reported that the most important attributes of an autoinjector were use without assistance, ease of administration, ease of operation and ease of grip, with similar responses for nurses and physicians.29 A survey of US patients with MS also showed similar results, with ease of the overall injection process, comfortable to hold, and easy to push the start button being the most important attributes for patients using an autoinjector.26 This focus on usability is considered to be reflective of the patients’ knowledge of the likelihood of progressive disease leading to declining fine motor skills in their hands and an associated diminishing ability to self-administer injections.30 We found that patients also considered it important for autoinjectors to have adjustable needle depth, a locking mechanism, and be intuitive to use and to fit in the hand, again, similar to previous findings.26,27,29 For both patients and nurses, an attractive design was of least importance, a finding that has been reported previously,26,27,29 though one nurse mentioned that this may be important to younger patients.
While most of the strengths and weaknesses of MyJECTTM and CSYNCTM autoinjectors were similar, mean ratings by nurses were higher for MyJECTTM performance than CSYNCTM for ability to use independently, concealed injection needle, locking mechanism, audible feedback after successful injection, length of the needle, the position of injection button, time taken for the entire injection process, and weight of the autoinjector. Similarly, patients using MyJECTTM were more satisfied with the performance of their autoinjector on 10 important attributes of an autoinjector for the management of RRMS.
Previous studies have reported that audible feedback, such as an audible click, is important, as it reassures patients that the injection has been correctly administered.20,26,27,29,31 Many of these previous studies have also reported that patients find visual feedback with a viewing window to be important.26,27,29,31 However, compared to audible feedback, visual feedback was rated low in our study.
Several previous surveys have evaluated patient satisfaction with various autoinjectors.26–28,32,33 In our study, nurses satisfaction ratings were higher for MyJECTTM than for CSYNCTM on the following attributes: time taken for the entire process, ease of use, and the number to steps required to complete the injection. However, these differences were small, and nurses rated CSYNCTM over MyJECTTM for two attributes: the overall experience of holding the autoinjector, and the convenience of storing the autoinjector. In contrast, patients rated their satisfaction on all five of these attributes higher for MyJECTTM than CSYNCTM. The difference between patients’ and nurses’ preferences may stem from the narrower design of MyJECT™ as nurses may consider a smaller autoinjector to be less suitable for holding in the hand, whereas patients may prefer a smaller autoinjector as it is easy to handle and carry. All eight patients currently using MyJECTTM in this study reported they were likely or very likely to recommend it to another patient with RRMS, compared with six of seven patients who were likely or highly likely to recommend CSYNCTM.
Strengths of this study include the length of each interview, lasting about 45 minutes for each patient and nurse, as well as the prior validation of the questions through pilot testing, to ensure the relevance of the attributes to the practical use of autoinjectors in MS. Additional strengths include the consistency of approach achieved by having only one researcher conduct all patient and nurse interviews and discussions, as well as the requirement for all included nurses to have extensive experience with autoinjectors in MS patients. We consider the results of our study may be broadly generalizable to the German population, as the study participants were recruited from across various cities in Germany. However, there are some important limitations of our study, in particular the small sample size, and that all patients included were female. Additionally, only 11 of the 15 included nurses and three patients had experience with both autoinjectors. These findings need to be confirmed in larger cohorts of both nurses and patients before they can be considered generalizable to a broad population of MS patients.
Conclusion
The results of this survey conducted in Germany suggest that nurses and patients with RRMS were highly satisfied with both the MyJECT™ and CSYNC™ autoinjectors, with some evidence, limited by the small sample size, to suggest that both performance and satisfaction is greater with MyJECT™ than CSYNCTM. Most patients and nurses would recommend MyJECT™ to other patients and nurses, and our findings suggest that the use of the MyJECT™ autoinjector may increase patient satisfaction, with the potential to increase overall medication adherence. Overall, we recommend that patients with MS are engaged in the decision-making process when selecting an autoinjector.
Acknowledgments
We thank Marie Cheeseman, on behalf of Research Review, who provided medical writing support funded by Viatris. We would also like to thank Phamax for support in the development of the questionnaire and for input into the study design. We thank Pramod Mallikarjuna and Shantha Kumar V from Viatris for providing editorial support.
Funding Statement
This manuscript has been supported by Viatris.
Data Sharing Statement
The participants of this study did not give written consent for their data to be shared publicly. Therefore, any supplementary and person-level data that were generated by the study and used in this analysis are not publicly available.
Ethical Approval and Consent
This study was conducted in accordance with the principles of the Declaration of Helsinki. The study was conducted as market research, in full compliance with the European Pharmaceutical Market Research Association (EPHMRA) Code of Conduct (September 2022). The code specifies in Section 1.3 that Market Research as defined by the Code does not require Clinical Research Ethics Committee or Independent Review Board approval. Accordingly, this research was exempt from ethical review.
Zoom interviews were employed for the remote, online research. We obtained telephonic informed consent observing principles similar to the guidance issued to researchers by the University of Oxford on governance and informed consent, which states where an oral consent process could be practical (for research conducted via remote video conferencing software). Each record of the oral telephonic informed consent process is held by our recruitment partner, and it documents the introduction, purpose, process, respondents’ rights including their ability to withdraw consent; their acknowledgement and understanding of the survey; and their consent to the research.
Disclosure
Dr Santosh Shirol is an employee of Viatris. The author(s) report no conflicts of interest in this work.
References
- 1.Browne P, Chandraratna D, Angood C, et al. Atlas of multiple sclerosis 2013: a growing global problem with widespread inequity. Neurology. 2014;83(11):1022–1024. doi: 10.1212/WNL.0000000000000768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Filippi M, Bar-Or A, Piehl F, et al. Multiple sclerosis. Nature Reviews Disease Primers. 2018;4(1):43. doi: 10.1038/s41572-018-0041-4 [DOI] [PubMed] [Google Scholar]
- 3.Walton C, King R, Rechtman L, et al. Rising prevalence of multiple sclerosis worldwide: insights from the Atlas of MS, third edition. Mult Scler. 2020;26(14):1816–1821. doi: 10.1177/1352458520970841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amtmann D, Bamer AM, Kim J, Chung H, Salem R. People with multiple sclerosis report significantly worse symptoms and health related quality of life than the US general population as measured by PROMIS and NeuroQoL outcome measures. Disabil Health J. 2018;11(1):99–107. doi: 10.1016/j.dhjo.2017.04.008 [DOI] [PubMed] [Google Scholar]
- 5.Sadigh G, Lava N, Switchenko J, et al. Patient-reported financial toxicity in multiple sclerosis: predictors and association with care non-adherence. Mult Scler J. 2020;27(3):453–464. doi: 10.1177/1352458520913977 [DOI] [PubMed] [Google Scholar]
- 6.Klineova S, Lublin FD. Clinical course of multiple sclerosis. Cold Spring Harb Perspect Med. 2018;8(9):a028928. doi: 10.1101/cshperspect.a028928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodin DS. The epidemiology of multiple sclerosis. Handbook Clin Neurol. 2014;122:231–266. [DOI] [PubMed] [Google Scholar]
- 8.Xie Y, Tian Z, Han F, Liang S, Gao Y, Wu D. Factors associated with relapses in relapsing-remitting multiple sclerosis: a systematic review and meta-analysis. Medicine. 2020;99(27):e20885–e20885. doi: 10.1097/MD.0000000000020885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doshi A, Chataway J. Multiple sclerosis, a treatable disease. Clin Med Lond. 2016;16(Suppl 6):s53–s59. doi: 10.7861/clinmedicine.16-6-s53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Travers BS, Tsang BK, Barton JL. Multiple sclerosis: diagnosis, disease-modifying therapy and prognosis. Aust J Gen Pract. 2022;51(4):199–206. doi: 10.31128/ajgp-07-21-6103 [DOI] [PubMed] [Google Scholar]
- 11.Simpson D, Noble S, Perry C. Glatiramer acetate: a review of its use in relapsing-remitting multiple sclerosis. CNS Drugs. 2002;16(12):825–850. doi: 10.2165/00023210-200216120-00004 [DOI] [PubMed] [Google Scholar]
- 12.Bittner B, Richter W, Schmidt J. Subcutaneous administration of biotherapeutics: an overview of current challenges and opportunities. BioDrugs. 2018;32(5):425–440. doi: 10.1007/s40259-018-0295-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.European Medicines Agency. List of nationally authorised medicinal products: active substance Glatiramer (Procedure no.: PSUSA/00001529/202011). 2021.
- 14.Medicines Evaluation Board (MEB). Glatiramer acetate: public assessment report, scientific discussion. 2016.
- 15.US Food and Drug Administration. Glatiramer Acetate Injection, 40 mg/mL - ANDA Approval. 2017.
- 16.Khan O, Rieckmann P, Boyko A, Selmaj K, Zivadinov R. Three times weekly glatiramer acetate in relapsing-remitting multiple sclerosis. Ann. Neurol. 2013;73(6):705–713. doi: 10.1002/ana.23938 [DOI] [PubMed] [Google Scholar]
- 17.US Food and Drug Administration. Copaxone (glatiramer acetate): approval package. 2014.
- 18.Kozubski W. Autoinjector improves injection-related tolerability issues in patients with multiple sclerosis – exploring the new extaviJect™ 30G system for the injection of interferon Beta-1b. European Neurol Rev. 2010;5(2):77–81. doi: 10.17925/ENR.2010.05.02.77 [DOI] [Google Scholar]
- 19.Narayanan S, Hautamaki E, Khan H, Gabriele S, White J. Needle phobia and associated clinical practice patterns among patients with multiple sclerosis (ms) in Europe and the United States. Value Health. 2014;17(3):A63. doi: 10.1016/j.jval.2014.03.371 [DOI] [Google Scholar]
- 20.Verdun Di Cantogno E, Russell S, Snow T. Understanding and meeting injection device needs in multiple sclerosis: a survey of patient attitudes and practices. Patient Prefer Adherence. 2011;5:173–180. doi: 10.2147/PPA.S14903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roy A, Geetha RV, Magesh A, Vijayaraghavan R, Ravichandran V. Autoinjector - A smart device for emergency cum personal therapy. Saudi Pharm J. 2021;29(10):1205–1215. doi: 10.1016/j.jsps.2021.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bayas A. Improving adherence to injectable disease-modifying drugs in multiple sclerosis. Expert Opin Drug Delivery. 2013;10(3):285–287. doi: 10.1517/17425247.2013.763793 [DOI] [PubMed] [Google Scholar]
- 23.Glatiramer acetate - copaxone - CSYNC autoinjector user guide. 2014. Available from: https://products.tevauk.com/media/download/45818. Accessed December 04, 2024.
- 24.Health products regulatory authority - Ireland. brabio 20 mg/mL solution for injection, pre-filled syringe. Available from: https://www.hpra.ie/img/uploaded/swedocuments/Licence_PA23266-010-001_17112023150542.pdf. Accessed December 04, 2024.
- 25.Wray S, Hayward B, Dangond F, Singer B. Ease of use of two autoinjectors in patients with multiple sclerosis treated with interferon beta-1a subcutaneously three times weekly: results of the randomized, crossover REDEFINE study. Expert Opin Drug Delivery. 2018;15(2):127–135. doi: 10.1080/17425247.2018.1407755 [DOI] [PubMed] [Google Scholar]
- 26.Barone DA, Singer BA, Merkov L, Rametta M, Suarez G. Survey of US patients with multiple sclerosis: comparison of the new electronic interferon beta-1b autoinjector (BETACONNECT™) with mechanical autoinjectors. Neurol Ther. 2016;5(2):155–167. doi: 10.1007/s40120-016-0047-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Limmroth V, Reischl J, Mann B, et al. Autoinjector preference among patients with multiple sclerosis: results from a national survey. Patient Prefer Adherence. 2017;11:1325–1334. doi: 10.2147/PPA.S137741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tischer B, Mehl A. Patients’ and nurses’ preferences for autoinjectors for rheumatoid arthritis: results of a European survey. Patient Prefer Adherence. 2018;12:1413–1424. doi: 10.2147/PPA.S169339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rekaya N, Vicik SM, Hulesch BT, McDonald LL. Enhancement of an auto-injector device for self-administration of etanercept in patients with rheumatoid arthritis confers emotional and functional benefits. Rheumatol Ther. 2020;7(3):537–552. doi: 10.1007/s40744-020-00216-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Costello K, Kennedy P, Scanzillo J. Recognizing nonadherence in patients with multiple sclerosis and maintaining treatment adherence in the long term. Medscape J Med. 2008;10(9):225. [PMC free article] [PubMed] [Google Scholar]
- 31.Lange J, Richard P, Bradley N. Usability of a new disposable autoinjector platform device: results of a formative study conducted with a broad user population. Med Devices. 2015;8:255–264. doi: 10.2147/MDER.S85938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phillips JT, Fox E, Grainger W, Tuccillo D, Liu S, Deykin A. An open-label, multicenter study to evaluate the safe and effective use of the single-use autoinjector with an Avonex® prefilled syringe in multiple sclerosis subjects. BMC Neurol. 2011;11(1):126. doi: 10.1186/1471-2377-11-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weller I, Saake A, Schreiner T, Vogelreuter J, Petroff N. Patient satisfaction with the BETACONNECT™ autoinjector for interferon beta-1b. Patient Prefer Adherence. 2015;9:951–959. doi: 10.2147/PPA.S85917 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The participants of this study did not give written consent for their data to be shared publicly. Therefore, any supplementary and person-level data that were generated by the study and used in this analysis are not publicly available.