Abstract
OBJECTIVES
The efficacy of neoadjuvant therapy (NT) prior to angioplastic lobectomy (AL) in non-small cell lung cancer is unclear. This study assessed its impact on morbidity, mortality and 5-year survival.
METHODS
We retrospectively analysed 114 patients who underwent AL at 2 tertiary centres from January 2000 to December 2020. Comparisons were made between patients who received NT and those who did not.
RESULTS
Among the patients, 78 (68.4%) underwent upfront surgery, and 36 (31.6%) received NT. There were no significant differences in postoperative complications (46.2% vs 31.6%, P = 0.42) or mortality rates (0% vs 3.8%, P = 0.55). Pathological upstaging differed significantly (37.2% vs 5.6%, P = 0.0008). Five-year survival rates were comparable (54% vs 38%, P = 0.3).
CONCLUSIONS
Neoadjuvant therapy does not adversely affect morbidity, arterial repair complications or mortality in AL. There are no survival differences at 5 years. AL remains a safe option following NT.
Keywords: Lung cancer: combined treatment modalities, Lung cancer: surgical therapy
Surgery is the gold standard for locally advanced non-small cell lung cancer (NSCLC).
Graphical Abstract
INTRODUCTION
Surgery is the gold standard for locally advanced non-small cell lung cancer (NSCLC). For centrally located NSCLC, many expert centres prefer a sleeve lobectomy (SL) over a pneumonectomy (PN) to conserve lung tissue. An SL requires resecting and reconstructing the main bronchus and/or pulmonary artery, which has improved postoperative morbidity, respiratory function and quality of life [1–3]. Complete resections yield oncological outcomes similar to those obtained with PNs [1, 3, 4]. For arterial invasion, procedures like tangential resection, end-to-end anastomosis, arterial patching or conduit replacement are favoured [3, 5, 6]. These arterial reconstructions are performed less frequently than a bronchial SL. The decision to use neoadjuvant therapy (NT) before angioplastic lobectomies (AL) varies among teams and cases. No studies have thoroughly assessed how NT affects survival in patients with AL. Existing research on various SL types shows mixed results on postoperative morbidity and survival [7–9]. It is suggested that NT may impair healing and create inflammation, affecting oncological margins [7, 10]. Therefore, this bicentric study examines postoperative outcomes and survival for patients undergoing AL for centrally located NSCLC, comparing those with and without preoperative NT.
MATERIALS AND METHODS
This study was carried out at 2 university hospitals specializing in NSCLC. We utilized combined prospective databases focused on the surgical management of centrally located NSCLC. Uniform patient management protocols were followed, with operations conducted by a surgeon active at both centres. We performed a retrospective review of all patients who underwent an AL from January 2000 to December 2020. Each case was reviewed by a multidisciplinary team per lung cancer guidelines. Ethics approval was granted (ethics committee reference number: 2023 BS 547), with waivers for individual consent.
Eligibility criteria
Patients with an Eastern Cooperative Oncology Group performance status of 0–1 aiming for curative resection were included. Comprehensive staging involved computed tomography, positron emission tomography, brain imaging and bronchoscopy, adhering to the 8th edition of the TNM system [11]. Invasive nodal staging was undertaken for suspected N2 involvement, with restaging post-neoadjuvant treatment. Preoperative cardiorespiratory evaluations followed European Society of Thoracic Surgeons guidelines [12]. Decisions on induction treatment or surgery were made during weekly oncological multidisciplinary meetings. Induction therapy, typically a platinum-based doublet chemotherapy, was recommended based on lymph node invasion status, tumour stage or uncertain resectability. Only patients responding to neoadjuvant treatment without progression were surgical candidates. Surgery generally followed 3–6 weeks of post-induction therapy. Bronchial reconstruction was not an exclusion criterion.
Surgical protocol
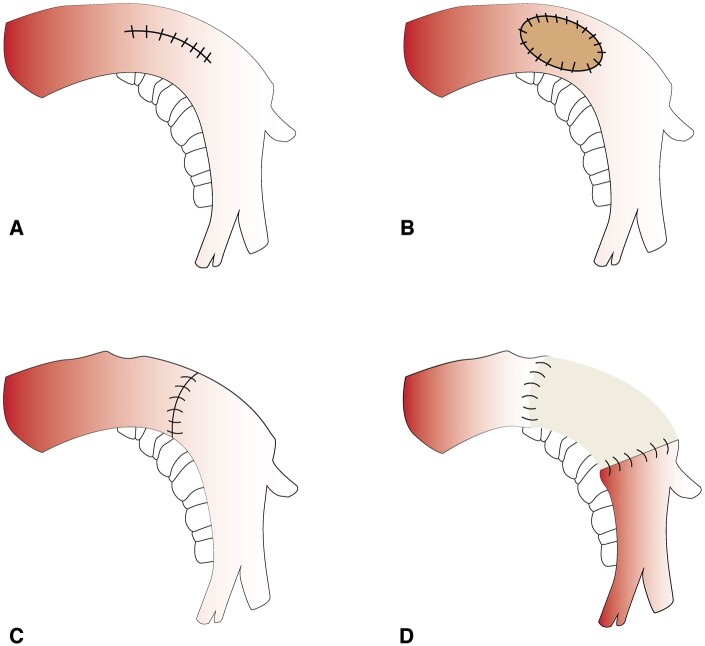
All operations were performed via a thoracotomy with the patient under general anaesthesia, with epidural analgesia offered. Thoracotomy included thorough exploration to confirm resectability. ALs involved pulmonary artery reconstruction tailored to the arterial involvement of the tumour—ranging from simple angioplasty to complex grafting using prosthetic, autologous pericardial or cryopreserved arterial grafts (Fig. 1) [5]. In the case of associated bronchial invasion, a bronchial end-to-end anastomosis was performed. Patients received intensive care postoperatively for at least 24 h and began curative anticoagulation, transitioning to antiplatelets and prophylactics.
Figure 1:
Different types of arterial reconstructions. (A) Tangential angioplasty; (B) patch angioplasty; (C) direct end-to-end anastomosis; and (D) conduit replacement.
Data collection
Data gathered included demographic and clinical details, type of arterial reconstruction and perioperative outcomes such as complications, reoperations and hospital length of stay. Perioperative mortality was defined as death within 30 days post-surgery. Follow-up data were collected from clinical notes and through direct contact with patients and physicians. Overall survival was defined as the time between the surgical procedure and the patient's last follow-up or death. Event-free survival was defined as the time between the surgical procedure and recurrence of the tumour or death.
Statistical analysis
Patient characteristics were described using appropriate descriptive statistics. Outcomes were analysed using the χ2 or the Fisher exact test, and survival outcomes were assessed with Kaplan–Meier analysis, considering death and recurrence as events. Survival and recurrence rates were compared across arterial reconstruction types using R software (R Foundation for Statistical Computing, Institute for Statistics and Mathematics, Vienna, Austria). A significance level of P < 0.05 was maintained.
RESULTS
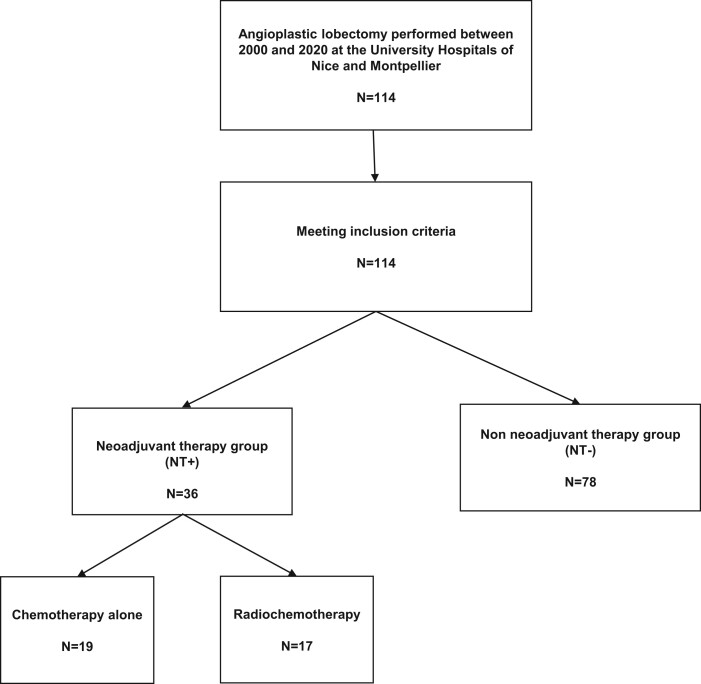
Between January 2000 and December 2020, a total of 114 patients underwent pulmonary artery reconstruction for centrally located NSCLC with pulmonary artery involvement at the University Hospitals of Nice and Montpellier, France. Patients were categorized into 2 groups: the non-NT group (NT−) and the NT group (NT+). Seventy-eight (68.4%) patients underwent surgery directly, whereas 36 (31.6%) received NT. Among the NT+ group, 17 (47.2%) patients received chemotherapy and radiotherapy at a dose of 45 Gray, and 19 (52.8%) received chemotherapy alone (Fig. 2). No patient received radiotherapy alone. Clinical characteristics are detailed in Table 1. The NT+ group had statistically higher proportions of advanced clinical stages and adenocarcinomas.
Figure 2:
Flow chart.
Table 1:
Patient characteristics
| Variable | NT+ (n = 36) | NT− (n = 78) | P-value |
|---|---|---|---|
| Age, mean (year) | 58.7 | 65.2 | 0.001 |
| Gender, n | 23 M/13 F | 63 M/15 F | 0.087 |
| Active smoking, n (%) | 9 (26.5) | 22 (28.6) | 0.498 |
| Comorbidities, n (%) | |||
| Cardiovascular | 5 (13.9) | 27 (34.6) | 0.269 |
| Diabetes | 2 (5.6) | 9 (11.5) | 0.498 |
| COPD | 7 (19.4) | 31 (39.7) | 0.054 |
| FEV1 (%) | 85 | 81.2 | 0.256 |
| Tumour location | |||
| LUL | 22 (61.1%) | 53 (68%) | |
| LLL | 5 (13.9%) | 4 (18%) | |
| RUL | 9 (25%) | 15(19.2%) | |
| ML | 4 (11.1%) | 7 (9%) | |
| RLL | 0 | 0 | |
| Type of arterial reconstruction, n (%) | 0.285 | ||
| Tangential resection | 17 (47.2) | 23 (29.5) | |
| Patch | 4 (11.1) | 16 (20.5) | |
| End-to-end anastomosis | 6 (16.7) | 18 (23.1) | |
| Arterial graft placement | 9 (25) | 21 (26.9) | |
| Bronchial reconstruction | 9 (25) | 30 (38.5) | 0.232 |
| Clinical stage, n (%) | 0.001 | ||
| I–II | 3 (8.6) | 41 (54) | |
| III–IV | 32 (91.4) | 35 (46) | |
| Tumour histology, n (%) | 0.009 | ||
| Squamous cell carcinoma | 12 (33.3) | 48 (61.5) | |
| Adenocarcinoma | 23 (63.9) | 23 (29.5) | |
| Others | 1 (2.8) | 7 (9) |
COPD: chronic obstructive pulmonary disease; F: female; FEV1: forced expiratory volume in the first s; LLL: left lower lobe; LUL: left upper lobe; M: male; ML: middle lobe; NT−: non-neoadjuvant therapy; NT+: neoadjuvant therapy; RLL: right lower lobe; RUL: right upper lobe.Results in bold have a P-value under 0.05.
Postoperative outcomes
Postoperative morbidity rates were similar between the 2 groups, with 46.2% in the NT− group and 36.1% in the NT+ group (P = 0.42) (Table 2). Pneumonia was the most common complication in both groups, occurring in 20.8% and 13.9%, respectively (P = 0.45). Arterial thrombosis occurred in 1 (1.5%) patient in the NT− group and in 2 (6.5%) patients in the NT+ group (P = 0.18). One patient (2.8%) in the NT+ group required a second operation for a bronchial fistula on day 15. In the NT− group, 4 patients (5.1%) had a second operation, including 2 for a bronchopleural fistula, 1 for massive haemoptysis and 1 for decortication. The difference was not statistically significant between the 2 groups. Thirty-day mortality was reported in 3 cases in the NT− group and none in the NT+ group (not statistically different). The causes of death were bronchial fistula, massive haemoptysis and pneumonia.
Table 2:
Postoperative outcomes
| Variable | NT+ group (n = 36) | NT− group (n = 78) | P-value |
|---|---|---|---|
| Complications, n (%) | 13 (36.1) | 36 (46.2) | 0.421 |
| Supraventricular arrhythmias, n (%) | 2 (5.7) | 10 (12.8) | 0.336 |
| Pneumonia, n (%) | 5 (13.9) | 16 (20.8) | 0.446 |
| Recurrent nerve palsy, n (%) | 5 (13.9) | 4 (5.1) | 0.138 |
| Air leakage, n (%) | 1 (2.9) | 5 (6.8) | 0.661 |
| Arterial thrombosis, n (%) | 2 (6.5) | 1 (1.5) | 0.180 |
| Other, n (%) | 2 (5.6) | 7 (9.1) | 0.716 |
| Surgical revision, n (%) | 1 (2.8) | 4 (5.1) | 1 |
| 30-day mortality, n (%) | 0 | 3 (3.8) | 0.550 |
NT−: non-neoadjuvant therapy; NT+: neoadjuvant therapy;
Oncological outcomes
Nodal staging was compared between both groups. Twenty-nine (37.2%) patients in the NT− group and 2 (5.6%) in the NT+ group were upstaged, respectively (P = 0.0008). In the NT− group, 19 (24.4%) patients had lymph node upstaging from N0 to N1 and 10 (12.8%) patients had lymph node progression from N0 and/or N1 to N2 (Table 3). Microscopic margin invasion rates were similar between the 2 groups (11.4% vs 9.2%, P = 0.74). However, incomplete resection of the bronchus was higher in the NT+ group (8.3% vs 0%, P = 0.003).
Table 3:
Pre- and postoperative lymph node staging
| Variable | NT+ group | NT− group | P-value |
|---|---|---|---|
| Preoperative lymph node staging | 0.0005 | ||
| N0 | 8 (22.2) | 47 (61) | |
| N1 | 9 (25) | 25 (32.5) | |
| N2 | 18 (50) | 5 (6.5) | |
| Postoperative lymph node staging | 0.086 | ||
| N0 | 15 (41.7) | 23 (30.3) | |
| N1 | 11 (30.6) | 40 (52.6) | |
| N2 | 10 (27.8) | 13 (17.1) | |
| Upstaging | 2 (5.6) | 29 (37.2) | 0.0008 |
| N0 → N1 | 1 (2.8) | 19 (24.4) | |
| N0 → N2 | 1 (2.8) | 6 (7.7) | |
| N1 → N2 | 0 | 4 (5.1) |
N: node; NT−: non-neoadjuvant therapy; NT+: neoadjuvant therapy.Results in bold have a P-value under 0.05.
Follow-up and survival outcomes
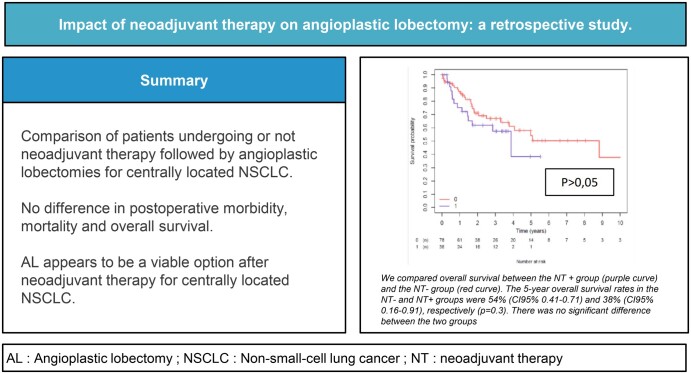
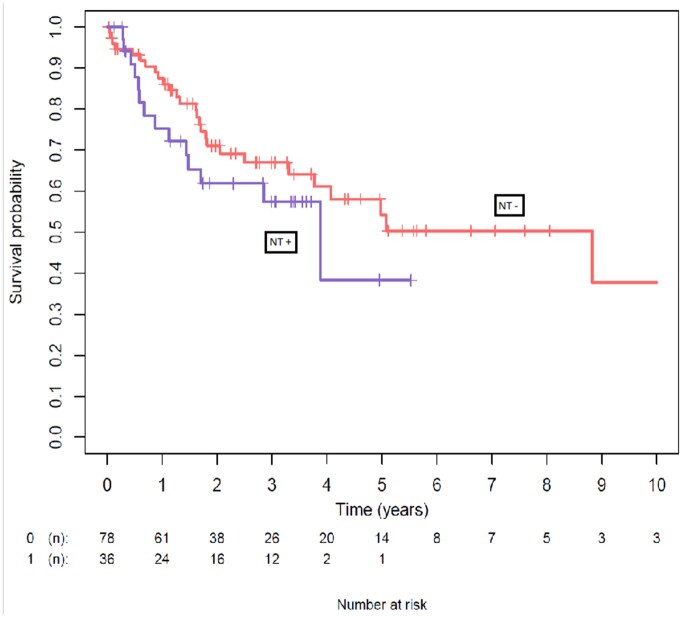
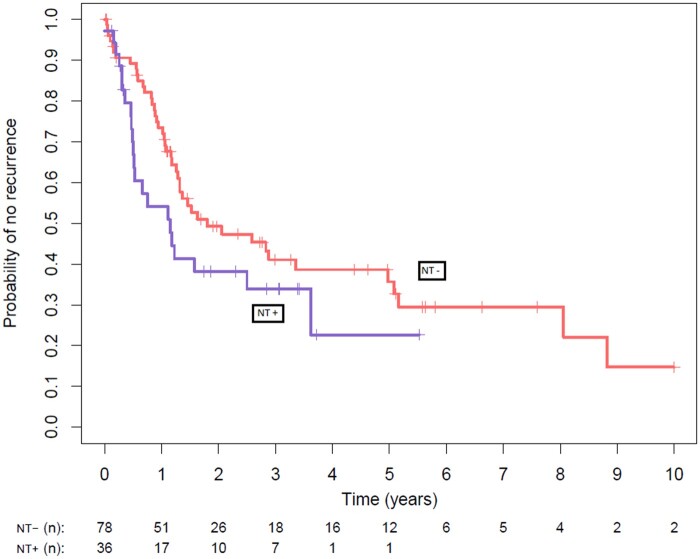
The median follow-up periods for recurrence and death were 14 months and 21 months, respectively. Fifty-five (62.7%) patients received adjuvant therapy. More patients received adjuvant chemotherapy in the NT− group (25.6% vs 25.7%, P = 0.023). Local recurrence rates were not statistically different between the 2 groups (25% vs 20.5%, P = 0.77). Distant metastases occurred in 26 (33.3%) patients in the NT− group and 18 (50%) occurred in the NT+ group (P = 0.13). The 5-year overall survival rates in the NT− and NT+ groups were 54% (CI 95% 0.41–0.71) and 38% (CI 95% 0.16–0.91), respectively (P = 0.3) (Fig. 3). The 5-year event-free survival rates in the NT− and NT+ groups were not statistically different, with 36% (CI 95% 0.25–0.51) and 23% (CI 95% 0.08–0.58), respectively (P = 0.1) (Fig. 4).
Figure 3:
Comparison of overall survival between the neoadjuvant (NT + curve) and the non-neoadjuvant therapy (Nt - curve) groups. The 5-year overall survival rates in the non-neoadjuvant therapy and neoadjuvant therapy groups were 54% (95% confidence interval 0.41–0.71) and 38% (95% confidence interval 0.16–0.91), respectively (P = 0.3). There was no significant difference between the 2 groups.
Figure 4:
Comparison of overall event-free survival between neoadjuvant therapy (NT + curve) and non-neoadjuvant therapy (NT - curve) groups. The 5-year event-free survival rates in the non-neoadjuvant therapy and the neoadjuvant therapy groups were not statistically different, with 36% (95% confidence interval 0.25–0.51) and 23% (95% confidence interval 0.08–0.58), respectively (P = 0.1).
Subgroup analysis based on the type of neoadjuvant therapy
We conducted a subgroup analysis comparing patients treated with neoadjuvant chemotherapy alone (n = 19) and patients treated with neoadjuvant radiochemotherapy (n = 17). There was no difference in postoperative complications, with 6 (31.6%) patients in the first group and 7 (41.2%) in the second group. Five-year overall survival and 5-year event-free survival were both similar between the 2 groups (P = 0.3).
DISCUSSION
The impact of NT on AL remains uncertain. The goal of this study was to compare the postoperative courses and 5-year survival outcomes in patients with central NSCLC who underwent AL with or without NT. Our findings indicate no significant differences in terms of postoperative morbidity, mortality and 5-year overall survival. However, there was a difference in favour of the NT− group in terms of 5-year recurrence-free survival.
AL is a technically demanding procedure that requires a high level of oncological expertise, surgical skill and access to suitable technical resources. Its functional and oncological outcomes appear to be superior to those of PN. Indeed, multiple studies have reported promising results for AL, particularly in terms of long-term survival [3]. However, it is important to note that AL is associated with a relatively high rate of postoperative morbidity, which can range from 13% to 39% [3, 13–16].
The most frequently encountered complications include pneumonia and supraventricular arrhythmias [16]. Postoperative mortality ranged from 1% to 17% in various studies [3, 13–15]. In our cohort, the rates of postoperative morbidity and mortality were 43% and 2.6%, respectively. It is worth noting that the higher morbidity rate in our study may be attributed to the inclusion of mild complications and to the fact that only 6.4% required a second operation. In recent years, data on NT before bronchial sleeve lobectomy (SL) or bronchial and arterial (double) SL have confirmed its feasibility and safety [7, 8, 17–20]. Induction therapy is often recommended for such patients, who are frequently classified as being stages III and IV, partly to enhance resectability. In our study, 51 (45.9%) and 16 (14.4%) patients were staged as cIII and cIV oligometastatic, respectively. In cases of PN, induction therapy is known to significantly increase postoperative morbidity and mortality [21–24]. In the most recent series, the mortality rate following PN after induction therapy has been reported to be as high as 43% [21–24]. For this reason, and when oncological safety is attainable, it is imperative to consider parenchymal-sparing strategies.
The role of induction therapy in SL remains controversial. NT can cause tissue fibrosis, which increases vascular fragility and complicates surgical dissection. In bronchial SL, Rodriguez et al. [9] found that radiochemotherapy adversely affected bronchial anastomosis. In contrast, Gomez-Caro et al. [8] reported that neoadjuvant chemoradiotherapy did not exacerbate surgical morbidity, complications at anastomotic sites or mortality rates of patients who had SL. Similarly, Bao et al. [7] observed no significant differences in perioperative outcomes between patients who received neoadjuvant chemotherapy and those who did not, prior to a double SL.
To date, no studies have assessed the local complications of vascular reconstruction following AL. Although our findings were focused exclusively on AL, we did not identify any differences in terms of morbidity and mortality. This difference might be explained by the small number of patients who underwent associated bronchial SL in our cohort and the fact that neoadjuvant radiotherapy was not consistently administered. In contrast to bronchial reconstruction alone, the correlation between NT and an increased risk of postoperative complications in arterial reconstruction is less clear [17]. The most concerning local complications after arterial reconstruction are arterial thrombosis and bleeding [16, 25]. Such complications should be treated with utmost caution, because they occurred in 4 of our cases, resulting in 1 fatality. Although standardized surgical techniques for AL are currently lacking, some recommendations may help reduce the incidence of arterial complications: systemic and local heparinization during arterial clamping, confirmation of arterial patency after reperfusion of the remaining parenchyma and the appropriate interposition of a flap [16, 17]. It is widely accepted that downstaging and achieving a complete lymph node response (ypN0) are strong predictors of survival. Gomez-Caro et al. [8] reported a 5-year survival rate ranging from 35% to 46% when a complete postoperative lymph node response is achieved. From another perspective, NT may help prevent postoperative upstaging. Interestingly, we observed that postoperative upstaging occurred in as many as 37.2% of cases in the NT− group, whereas it occurred in only 5.6% in the NT+ group. These results can be attributed to the fact that only patients who responded to neoadjuvant treatment and had negative restaging were included in the NT+ group. It is important to note that of the 29 patients upstaged in the NT− group, 19 patients had lymph node upstaging from N0 to N1. The rate of patients with upstaging in the NT− group may be explained by the preoperative difficulty in differentiating hilar lymph node invasion from hilar tumour extension, which may bias preoperative lymph node staging.
There was no difference in overall survival between the 2 groups. Induction therapy should be considered for patients with preoperative lymph node invasion or doubt of complete resectability. Additionally, it is worth noting that, for patients with preoperative negative lymph nodes (cN0), induction therapy may also play a role in the treatment of initially unresectable patients [19].
Furthermore, the addition of radiotherapy is not necessarily justified for resectable central NSCLC. Jaradeh et al. [26] reported equivalent survival outcomes between neoadjuvant chemotherapy and neoadjuvant radiochemotherapy. Our results support the same conclusion because we found no difference in survival outcomes in the subgroup analysis, despite the small number of patients. In our current clinical practice, there is no longer a role for radiation therapy in the neoadjuvant treatment of locally advanced NSCLC. However, it remains relevant for patients receiving systemic treatment with radiochemotherapy. Currently, and since the positive outcomes observed in the CheckMate816 trial regarding complete pathological response and relapse-free survival, immunotherapy holds great promise in thoracic oncology surgery, especially for stage III patients [27]. Chen et al. [18] have demonstrated the feasibility of SL following chemo-immunotherapy. Recent studies have even reported a superior pathological response following chemo-immunotherapy in comparison to chemotherapy alone [28, 29]. These encouraging results should motivate us in the coming years to expand the surgical boundaries for these central tumours as part of a more effective multimodal treatment.
Our study has several limitations. Firstly, it is a retrospective study, but conducting a prospective study appears impractical due to the varying clinical stages of the patients and the treatments offered. Indeed, it is difficult to be certain of tumour staging and resectability preoperatively. Secondly, our results should be confirmed by a larger data set through a national database analysis or by employing propensity score matching. We acknowledge that our study may have limitations in its ability to detect small differences or differences in less-studied variables, such as postoperative mortality. It is possible that our sample size may not be large enough to identify all possible variations. Lastly, due to the variability in neoadjuvant treatments over time and across different centres, we were unable to perform further stratified analyses that would consider different neoadjuvant regimens.
CONCLUSION
The scarcity of data on AL in the literature has not led to the development of guidelines on induction therapy. Despite the rarity of this indication, this study brings together a considerable number of patients with central lung cancer, from 2 centres with a high level of expertise. When we compared whether induction therapy was performed or not, we found no additional morbidity and mortality during the period following the operation. In our opinion, the use of induction therapy should not be a limitation or a cause for concern in the performance of AL.
Glossary
ABBREVIATIONS
- AL
Angioplastic lobectomy
- NSCLC
Non-small cell lung cancer
- NT
Neoadjuvant therapy
- PN
Pneumonectomy
- SL
Sleeve lobectomy
Contributor Information
Tayeb Benkiran, Department of Thoracic Surgery, Pasteur 1 Hospital, University Hospital of Nice, Nice, France; University of Cote d’Azur, Nice, France.
Kheira Hireche, Department of Thoracic and Vascular Surgery, Arnaud de Villeneuve Hospital, University Hospital of Montpellier, Montpellier, France.
Sebastien Frey, University of Cote d’Azur, Nice, France; Department of General Surgical Emergency, Pasteur 2 Hospital, University Hospital of Nice, Nice, France.
Adeline Morisot, Department of Public Health, L’Archet Hospital, University Hospital of Nice, Nice, France.
Aude Nguyen, Department of Thoracic and Vascular Surgery, Arnaud de Villeneuve Hospital, University Hospital of Montpellier, Montpellier, France.
Quentin Rudondy, Department of Thoracic Surgery, Pasteur 1 Hospital, University Hospital of Nice, Nice, France; University of Cote d’Azur, Nice, France.
Florent Alcaraz, Department of Thoracic Surgery, Pasteur 1 Hospital, University Hospital of Nice, Nice, France; University of Cote d’Azur, Nice, France.
Mauro Guarino, Department of Thoracic Surgery, Pasteur 1 Hospital, University Hospital of Nice, Nice, France; University of Cote d’Azur, Nice, France.
Charlotte Cohen, Department of Thoracic Surgery, Pasteur 1 Hospital, University Hospital of Nice, Nice, France.
Abel Gomez-Caro, Department of Thoracic Surgery, Pasteur 1 Hospital, University Hospital of Nice, Nice, France.
Jean-Phillippe Berthet, Department of Thoracic Surgery, Pasteur 1 Hospital, University Hospital of Nice, Nice, France; University of Cote d’Azur, Nice, France.
FUNDING
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Conflict of interest: All the authors declare that they have no conflicts of interest.
DATA AVAILABILITY
The data underlying this article will be shared on reasonable request to the corresponding author.
Author contributions
Tayeb Benkiran: Conceptualization; Data curation; Formal analysis; Methodology; Writing—original draft; Writing—review & editing. Kheira Hireche: Conceptualization; Data curation; Formal analysis; Methodology; Validation; Visualization; Writing—original draft; Writing—review & editing. Sebastien Frey: Conceptualization; Supervision; Writing—original draft; Writing—review & editing. Adeline Morisot: Formal analysis; Methodology. Aude Nguyen: Conceptualization; Data curation. Quentin Rudondy: Conceptualization; Data curation. Florent Alcaraz: Data curation; Methodology. Mauro Guarino: Visualization. Charlotte Cohen: Visualization. Abel Gomez-Caro: Visualization; Writing—review & editing. Jean-Phillippe Berthet: Conceptualization; Supervision; Writing—original draft; Writing—review & editing
Reviewer information
Interdisciplinary CardioVascular and Thoracic Surgery thanks Noriyoshi Sawabata, Ahmed Boseila, Joao Santos Silva and the other anonymous reviewers for their contributions to the peer review process of this article.
REFERENCES
- 1. Deslauriers J, Grégoire J, Jacques LF, Piraux M, Guojin L, Lacasse Y.. Sleeve lobectomy versus pneumonectomy for lung cancer: a comparative analysis of survival and sites or recurrences. Ann Thorac Surg 2004;77:1152–6; discussion 1156. [DOI] [PubMed] [Google Scholar]
- 2. Gómez-Caro A, Garcia S, Reguart N, Cladellas E, Arguis P, Sanchez M et al Determining the appropriate sleeve lobectomy versus pneumonectomy ratio in central non-small cell lung cancer patients: an audit of an aggressive policy of pneumonectomy avoidance. Eur J Cardiothorac Surg 2011;39:352–9. [DOI] [PubMed] [Google Scholar]
- 3. Ma Z, Dong A, Fan J, Cheng H.. Does sleeve lobectomy concomitant with or without pulmonary artery reconstruction (double sleeve) have favorable results for non-small cell lung cancer compared with pneumonectomy? A meta-analysis. Eur J Cardiothorac Surg 2007;32:20–8. [DOI] [PubMed] [Google Scholar]
- 4. Pagès PB, Mordant P, Renaud S, Brouchet L, Thomas PA, Dahan M et al; Epithor Project (French Society of Thoracic and Cardiovascular Surgery). Sleeve lobectomy may provide better outcomes than pneumonectomy for non-small cell lung cancer. A decade in a nationwide study. J Thorac Cardiovasc Surg 2017;153:184–95.e3. [DOI] [PubMed] [Google Scholar]
- 5. Berthet JP, Boada M, Paradela M, Molins L, Matecki S, Marty-Ané CH et al Pulmonary sleeve resection in locally advanced lung cancer using cryopreserved allograft for pulmonary artery replacement. J Thorac Cardiovasc Surg 2013;146:1191–7. [DOI] [PubMed] [Google Scholar]
- 6. Yang M, Zhong Y, Deng J, She Y, Zhang L, Wang Y et al Comparison of bronchial sleeve lobectomy with pulmonary arterioplasty versus pneumonectomy. Ann Thorac Surg 2022;113:934–41. [DOI] [PubMed] [Google Scholar]
- 7. Bao Y, Jiang C, Wan Z, Wang Y, Zhong Y, Deng J et al Feasibility of double sleeve lobectomy after neoadjuvant chemotherapy in patients with non-small-cell lung cancer. Interact CardioVasc Thorac Surg 2022;35:ivac103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gómez-Caro A, Boada M, Reguart N, Viñolas N, Casas F, Molins L.. Sleeve lobectomy after induction chemoradiotherapy. Eur J Cardiothorac Surg 2012;41:1052–8. [DOI] [PubMed] [Google Scholar]
- 9. Rodriguez M, Dezube AR, Bravo-Iniguez CE, Fox S, De León LE, Tarascio J et al Impact of neoadjuvant chemoradiation on adverse events after bronchial sleeve resection. Ann Thorac Surg 2021;112:890–6. [DOI] [PubMed] [Google Scholar]
- 10. Venuta F, Ciccone AM, Anile M, Ibrahim M, De Giacomo T, Coloni GF et al Reconstruction of the pulmonary artery for lung cancer: long-term results. J Thorac Cardiovasc Surg 2009;138:1185–91. [DOI] [PubMed] [Google Scholar]
- 11. Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WEE et al; International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee Advisory Boards and Participating Institutions. The IASLC Lung Cancer Staging Project: proposals for revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39–51. [DOI] [PubMed] [Google Scholar]
- 12. Brunelli A, Charloux A, Bolliger CT, Rocco G, Sculier JP, Varela G et al; European Respiratory Society and European Society of Thoracic Surgeons Joint Task Force on Fitness for Radical Therapy. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy). Eur Respir J 2009;34:17–41. [DOI] [PubMed] [Google Scholar]
- 13. Alifano M, Cusumano G, Strano S, Magdeleinat P, Bobbio A, Giraud F et al Lobectomy with pulmonary artery resection: morbidity, mortality, and long-term survival. J Thorac Cardiovasc Surg 2009;137:1400–5. [DOI] [PubMed] [Google Scholar]
- 14. Cerfolio RJ, Bryant AS.. Surgical techniques and results for partial or circumferential sleeve resection of the pulmonary artery for patients with non-small cell lung cancer. Ann Thorac Surg 2007;83:1971–6; discussion 1976–7. [DOI] [PubMed] [Google Scholar]
- 15. Shrager JB, Lambright ES, McGrath CM, Wahl PM, Deeb ME, Friedberg JS et al Lobectomy with tangential pulmonary artery resection without regard to pulmonary function. Ann Thorac Surg 2000;70:234–9. [DOI] [PubMed] [Google Scholar]
- 16. Menna C, Rendina EA, D'Andrilli A.. Parenchymal sparing surgery for lung cancer: focus on pulmonary artery reconstruction. Cancers (Basel) 2022;14:4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. D'Andrilli A, Venuta F, Maurizi G, Rendina EA.. Bronchial and arterial sleeve resection after induction therapy for lung cancer. Thorac Surg Clin 2014;24:411–21. [DOI] [PubMed] [Google Scholar]
- 18. Chen Y, Zhang L, Yan B, Zeng Z, Hui Z, Zhang R et al Feasibility of sleeve lobectomy after neo-adjuvant chemo-immunotherapy in non-small cell lung cancer. Transl Lung Cancer Res 2020;9:761–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bagan P, Berna P, Brian E, Crockett F, Le Pimpec-Barthes F, Dujon A et al Induction chemotherapy before sleeve lobectomy for lung cancer: immediate and long-term results. Ann Thorac Surg 2009;88:1732–5. [DOI] [PubMed] [Google Scholar]
- 20. Koryllos A, Lopez-Pastorini A, Zalepugas D, Galetin T, Ludwig C, Hammer-Hellmig M et al Optimal timing of surgery for bronchial sleeve resection after neoadjuvant chemoradiotherapy. J Surg Oncol 2020;122:328–35. [DOI] [PubMed] [Google Scholar]
- 21. Maurizi G, D'Andrilli A, Anile M, Ciccone AM, Ibrahim M, Venuta F et al Sleeve lobectomy compared with pneumonectomy after induction therapy for non-small-cell lung cancer. J Thorac Oncol 2013;8:637–43. [DOI] [PubMed] [Google Scholar]
- 22. Albain KS, Swann RS, Rusch VW, Turrisi AT, Shepherd FA, Smith C et al Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet 2009;374:379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Venuta F, Anile M, Diso D, Ibrahim M, De Giacomo T, Rolla M et al Operative complications and early mortality after induction therapy for lung cancer. Eur J Cardiothorac Surg 2007;31:714–7. [DOI] [PubMed] [Google Scholar]
- 24. Pisters KMW, Vallières E, Crowley JJ, Franklin WA, Bunn PA, Ginsberg RJ et al Surgery with or without preoperative paclitaxel and carboplatin in early-stage non-small-cell lung cancer: Southwest Oncology Group Trial S9900, an intergroup, randomized, phase III trial. J Clin Oncol 2010;28:1843–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vannucci J, Matricardi A, Potenza R, Ragusa M, Puma F, Cagini L.. Lobectomy with angioplasty: which is the best technique for pulmonary artery reconstruction? J Thorac Dis 2018;10:S1892–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jaradeh M, Vigneswaran WT, Raad W, Lubawski J, Freeman R, Abdelsattar ZM.. Neoadjuvant chemotherapy vs chemoradiation therapy followed by sleeve resection for resectable lung cancer. Ann Thorac Surg 2022;114:2041–7. [DOI] [PubMed] [Google Scholar]
- 27. Forde PM, Spicer J, Lu S, Provencio M, Mitsudomi T, Awad MM et al; CheckMate 816 Investigators. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med 2022;386:1973–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen T, Ning J, Shen J, Pan H, Fu L, Xu E et al Sleeve lobectomy after neoadjuvant chemoimmunotherapy versus chemotherapy for squamous cell lung cancer: a multicenter, retrospective study. JTO Clin Res Rep 2023;4:100472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li X, Li Q, Yang F, Gao E, Lin L, Li Y et al Neoadjuvant therapy does not increase postoperative morbidity of sleeve lobectomy in locally advanced non–small cell lung cancer. J Thorac Cardiovasc Surg 2023;166:1234–44.e13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.