Abstract
v-Ha-ras encoded p21 protein (p21V), the cellular c-Ha-ras encoded protein (p21C) and its T24 mutant form p21T were produced in Escherichia coli under the control of the tac promoter. Large amounts of the authentic proteins in a soluble form can be extracted and purified without the use of denaturants or detergents. All three proteins are highly active in GDP binding, GTPase and, for p21V, autokinase activity. Inhibition of [3H]GDP binding to p21C by regio- and stereospecific phosphorothioate analogs of GDP and GTP was investigated to obtain a measure of the relative affinities of the three diphosphate and five triphosphate analogs of guanosine. p21 has a preference for the Sp isomers of GDP alpha S and GTP alpha S. It has low specificity for the Sp isomer of GTP beta S. Together with the data for GDP beta S and GTP gamma S these results are compared with those obtained for elongation factor (EF)Tu and transducin. This has enabled us to probe the structural relatedness of these proteins. We conclude that p21 seems to be more closely related to EF-Tu than to transducin.
Full text
PDF
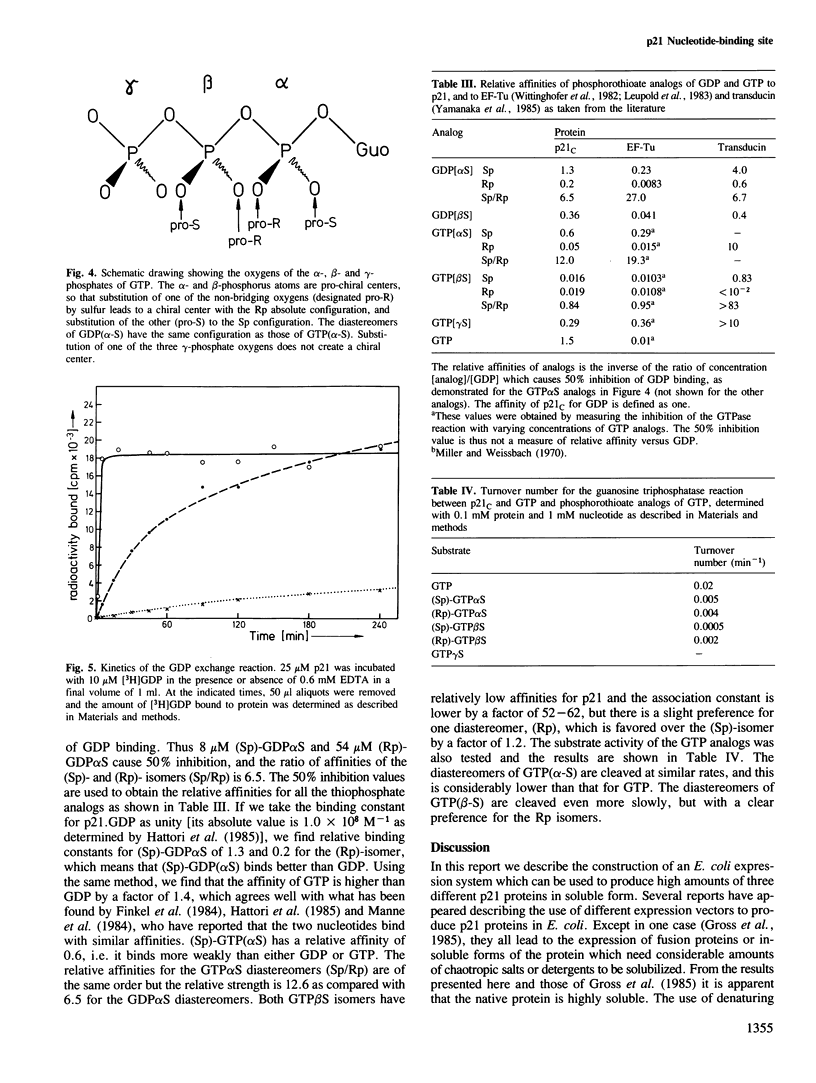
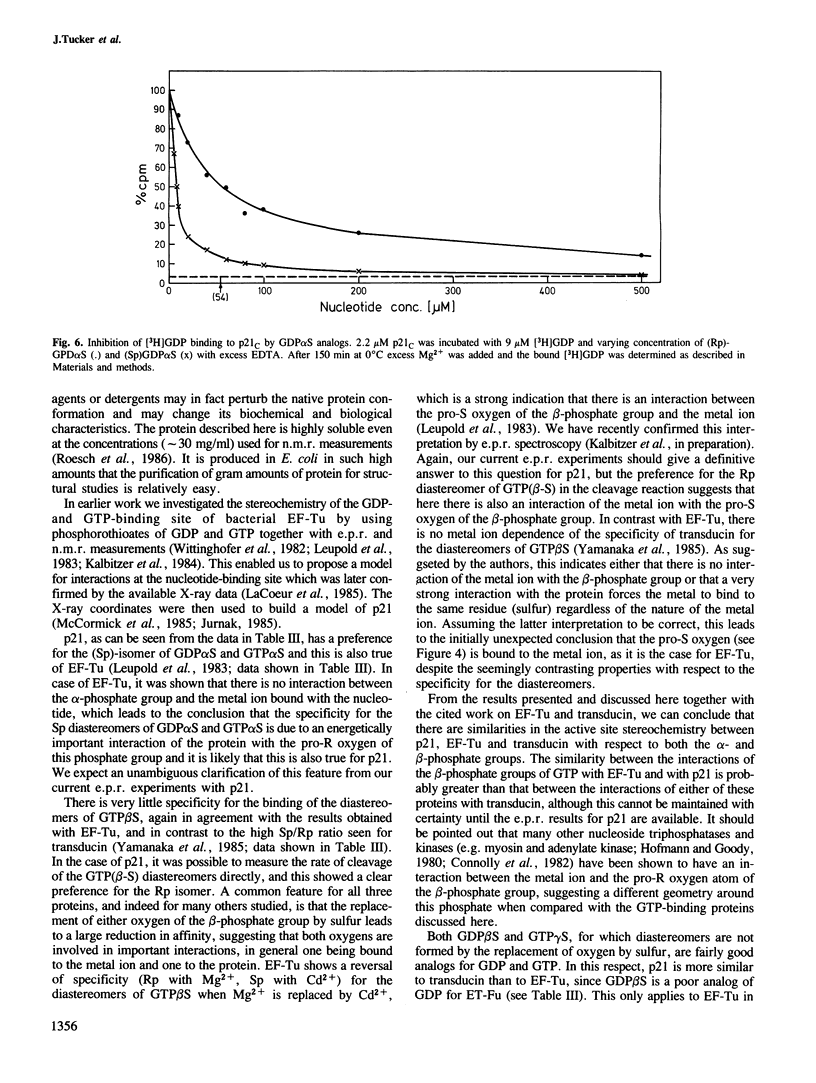


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckner S. K., Hattori S., Shih T. Y. The ras oncogene product p21 is not a regulatory component of adenylate cyclase. Nature. 1985 Sep 5;317(6032):71–72. doi: 10.1038/317071a0. [DOI] [PubMed] [Google Scholar]
- Birchmeier C., Broek D., Wigler M. ras proteins can induce meiosis in Xenopus oocytes. Cell. 1985 Dec;43(3 Pt 2):615–621. doi: 10.1016/0092-8674(85)90233-8. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Viral oncogenes. Cell. 1985 Aug;42(1):23–38. doi: 10.1016/s0092-8674(85)80098-2. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Connolly B. A., Romaniuk P. J., Eckstein F. Synthesis and characterization of diastereomers of guanosine 5'-O-(1-thiotriphosphate) and guanosine 5'-O-(2-thiotriphosphate). Biochemistry. 1982 Apr 27;21(9):1983–1989. doi: 10.1021/bi00538a002. [DOI] [PubMed] [Google Scholar]
- DeFeo-Jones D., Tatchell K., Robinson L. C., Sigal I. S., Vass W. C., Lowy D. R., Scolnick E. M. Mammalian and yeast ras gene products: biological function in their heterologous systems. Science. 1985 Apr 12;228(4696):179–184. doi: 10.1126/science.3883495. [DOI] [PubMed] [Google Scholar]
- Dhar R., Nieto A., Koller R., DeFeo-Jones D., Scolnick E. M. Nucleotide sequence of two rasH related-genes isolated from the yeast Saccharomyces cerevisiae. Nucleic Acids Res. 1984 Apr 25;12(8):3611–3618. doi: 10.1093/nar/12.8.3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccleston J. F. Spectroscopic studies of the nucleotide binding site of elongation factor Tu from Escherichia coli. An approach to characterizing the elementary steps of the elongation cycle of protein biosynthesis. Biochemistry. 1981 Oct 13;20(21):6265–6272. doi: 10.1021/bi00524a055. [DOI] [PubMed] [Google Scholar]
- Eckstein F. Nucleoside phosphorothioates. Annu Rev Biochem. 1985;54:367–402. doi: 10.1146/annurev.bi.54.070185.002055. [DOI] [PubMed] [Google Scholar]
- Ellis R. W., DeFeo D., Maryak J. M., Young H. A., Shih T. Y., Chang E. H., Lowy D. R., Scolnick E. M. Dual evolutionary origin for the rat genetic sequences of Harvey murine sarcoma virus. J Virol. 1980 Nov;36(2):408–420. doi: 10.1128/jvi.36.2.408-420.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano O., Aldrich T., Tamanoi F., Taparowsky E., Furth M., Wigler M. Analysis of the transforming potential of the human H-ras gene by random mutagenesis. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4008–4012. doi: 10.1073/pnas.81.13.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T., Der C. J., Cooper G. M. Activation of ras genes in human tumors does not affect localization, modification, or nucleotide binding properties of p21. Cell. 1984 May;37(1):151–158. doi: 10.1016/0092-8674(84)90310-6. [DOI] [PubMed] [Google Scholar]
- Furth M. E., Davis L. J., Fleurdelys B., Scolnick E. M. Monoclonal antibodies to the p21 products of the transforming gene of Harvey murine sarcoma virus and of the cellular ras gene family. J Virol. 1982 Jul;43(1):294–304. doi: 10.1128/jvi.43.1.294-304.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay N. J., Walker J. E. Homology between human bladder carcinoma oncogene product and mitochondrial ATP-synthase. Nature. 1983 Jan 20;301(5897):262–264. doi: 10.1038/301262a0. [DOI] [PubMed] [Google Scholar]
- Gibbs J. B., Sigal I. S., Poe M., Scolnick E. M. Intrinsic GTPase activity distinguishes normal and oncogenic ras p21 molecules. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5704–5708. doi: 10.1073/pnas.81.18.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goody R. S., Hofmann W. Stereochemical aspects of the interaction of myosin and actomyosin with nucleotides. J Muscle Res Cell Motil. 1980 Mar;1(1):101–115. doi: 10.1007/BF00711928. [DOI] [PubMed] [Google Scholar]
- Goody R. S., Leberman R. The preparation of thiophosphate analogs of GDP and their interaction with EF-Tu. FEBS Lett. 1979 Jun 15;102(2):269–272. doi: 10.1016/0014-5793(79)80016-2. [DOI] [PubMed] [Google Scholar]
- Gross M., Sweet R. W., Sathe G., Yokoyama S., Fasano O., Goldfarb M., Wigler M., Rosenberg M. Purification and characterization of human H-ras proteins expressed in Escherichia coli. Mol Cell Biol. 1985 May;5(5):1015–1024. doi: 10.1128/mcb.5.5.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday K. R. Regional homology in GTP-binding proto-oncogene products and elongation factors. J Cyclic Nucleotide Protein Phosphor Res. 1983;9(6):435–448. [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hattori S., Ulsh L. S., Halliday K., Shih T. Y. Biochemical properties of a highly purified v-rasH p21 protein overproduced in Escherichia coli and inhibition of its activities by a monoclonal antibody. Mol Cell Biol. 1985 Jun;5(6):1449–1455. doi: 10.1128/mcb.5.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurnak F. Structure of the GDP domain of EF-Tu and location of the amino acids homologous to ras oncogene proteins. Science. 1985 Oct 4;230(4721):32–36. doi: 10.1126/science.3898365. [DOI] [PubMed] [Google Scholar]
- Kalbitzer H. R., Goody R. S., Wittinghofer A. Electron-paramagnetic-resonance studies of manganese(II) complexes with elongation factor Tu from Bacillus stearothermophilus. Observation of a GTP hydrolysis intermediate state complex. Eur J Biochem. 1984 Jun 15;141(3):591–597. doi: 10.1111/j.1432-1033.1984.tb08234.x. [DOI] [PubMed] [Google Scholar]
- Kaziro Y. The role of guanosine 5'-triphosphate in polypeptide chain elongation. Biochim Biophys Acta. 1978 Sep 21;505(1):95–127. doi: 10.1016/0304-4173(78)90009-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leberman R., Antonsson B., Giovanelli R., Guariguata R., Schumann R., Wittinghofer A. A simplified procedure for the isolation of bacterial polypeptide elongation factor EF-Tu. Anal Biochem. 1980 May 1;104(1):29–36. doi: 10.1016/0003-2697(80)90272-9. [DOI] [PubMed] [Google Scholar]
- Leberman R., Egner U. Homologies in the primary structure of GTP-binding proteins: the nucleotide-binding site of EF-Tu and p21. EMBO J. 1984 Feb;3(2):339–341. doi: 10.1002/j.1460-2075.1984.tb01808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leupold C. M., Goody R. S., Wittinghofer A. Stereochemistry of the elongation factor Tu X GTP complex. Eur J Biochem. 1983 Sep 15;135(2):237–241. doi: 10.1111/j.1432-1033.1983.tb07643.x. [DOI] [PubMed] [Google Scholar]
- Manne V., Bekesi E., Kung H. F. Ha-ras proteins exhibit GTPase activity: point mutations that activate Ha-ras gene products result in decreased GTPase activity. Proc Natl Acad Sci U S A. 1985 Jan;82(2):376–380. doi: 10.1073/pnas.82.2.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manne V., Yamazaki S., Kung H. F. Guanosine nucleotide binding by highly purified Ha-ras-encoded p21 protein produced in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6953–6957. doi: 10.1073/pnas.81.22.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick F., Clark B. F., la Cour T. F., Kjeldgaard M., Norskov-Lauritsen L., Nyborg J. A model for the tertiary structure of p21, the product of the ras oncogene. Science. 1985 Oct 4;230(4721):78–82. doi: 10.1126/science.3898366. [DOI] [PubMed] [Google Scholar]
- McGrath J. P., Capon D. J., Goeddel D. V., Levinson A. D. Comparative biochemical properties of normal and activated human ras p21 protein. Nature. 1984 Aug 23;310(5979):644–649. doi: 10.1038/310644a0. [DOI] [PubMed] [Google Scholar]
- Miller D. L., Weissbach H. Studies on the purification and properties of factor Tu from E. coli. Arch Biochem Biophys. 1970 Nov;141(1):26–37. doi: 10.1016/0003-9861(70)90102-5. [DOI] [PubMed] [Google Scholar]
- Murakami M. Mutations affecting the 12th and 61st amino acids of p21 protein result in decreased probability of beta-turn occurrence around the mutation positions: a prediction. J Theor Biol. 1985 May 21;114(2):193–198. doi: 10.1016/s0022-5193(85)80103-x. [DOI] [PubMed] [Google Scholar]
- Pincus M. R., Brandt-Rauf P. W. Structural effects of substitutions on the p21 proteins. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3596–3600. doi: 10.1073/pnas.82.11.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poe M., Scolnick E. M., Stein R. B. Viral Harvey ras p21 expressed in Escherichia coli purifies as a binary one-to-one complex with GDP. J Biol Chem. 1985 Apr 10;260(7):3906–3909. [PubMed] [Google Scholar]
- Powers S., Kataoka T., Fasano O., Goldfarb M., Strathern J., Broach J., Wigler M. Genes in S. cerevisiae encoding proteins with domains homologous to the mammalian ras proteins. Cell. 1984 Mar;36(3):607–612. doi: 10.1016/0092-8674(84)90340-4. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryer L. Transducin and the cyclic GMP phosphodiesterase: amplifier proteins in vision. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 2):841–852. doi: 10.1101/sqb.1983.048.01.087. [DOI] [PubMed] [Google Scholar]
- Sweet R. W., Yokoyama S., Kamata T., Feramisco J. R., Rosenberg M., Gross M. The product of ras is a GTPase and the T24 oncogenic mutant is deficient in this activity. Nature. 1984 Sep 20;311(5983):273–275. doi: 10.1038/311273a0. [DOI] [PubMed] [Google Scholar]
- Toda T., Uno I., Ishikawa T., Powers S., Kataoka T., Broek D., Cameron S., Broach J., Matsumoto K., Wigler M. In yeast, RAS proteins are controlling elements of adenylate cyclase. Cell. 1985 Jan;40(1):27–36. doi: 10.1016/0092-8674(85)90305-8. [DOI] [PubMed] [Google Scholar]
- Wierenga R. K., Hol W. G. Predicted nucleotide-binding properties of p21 protein and its cancer-associated variant. Nature. 1983 Apr 28;302(5911):842–844. doi: 10.1038/302842a0. [DOI] [PubMed] [Google Scholar]
- Wittinghofer A., Goody R. S., Roesch P., Kalbitzer H. R. The structure of the EF-Tu . GDP . Me2+ complex. Eur J Biochem. 1982 May;124(1):109–115. doi: 10.1111/j.1432-1033.1982.tb05912.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Koch N., Steinmetz M., Hämmerling G. J. One gene encodes two distinct Ia-associated invariant chains. J Immunol. 1985 May;134(5):3461–3467. [PubMed] [Google Scholar]
- Yamanaka G., Eckstein F., Stryer L. Stereochemistry of the guanyl nucleotide binding site of transducin probed by phosphorothioate analogues of GTP and GDP. Biochemistry. 1985 Dec 31;24(27):8094–8101. doi: 10.1021/bi00348a039. [DOI] [PubMed] [Google Scholar]
- Yasuda S., Furuichi M., Soeda E. An altered DNA sequence encompassing the ras gene of Harvey murine sarcoma virus. Nucleic Acids Res. 1984 Jul 25;12(14):5583–5588. doi: 10.1093/nar/12.14.5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer H. A., Comstock L. J., Vasser M. The tac promoter: a functional hybrid derived from the trp and lac promoters. Proc Natl Acad Sci U S A. 1983 Jan;80(1):21–25. doi: 10.1073/pnas.80.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- la Cour T. F., Nyborg J., Thirup S., Clark B. F. Structural details of the binding of guanosine diphosphate to elongation factor Tu from E. coli as studied by X-ray crystallography. EMBO J. 1985 Sep;4(9):2385–2388. doi: 10.1002/j.1460-2075.1985.tb03943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]